Abstract
Purpose
Thoracic radiotherapy is a major treatment modality of stage III non-small cell lung cancer. The normal lung tissue is sensitive to radiation and radiation pneumonitis is the most important dose-limiting complication of thoracic radiation therapy. This study was performed to identify the clinical and dosimetric parameters related to the risk of radiation pneumonitis after definitive radiotherapy in stage III non-small cell cancer patients.
Materials and Methods
The medical records were reviewed for 49 patients who completed definitive radiation therapy for locally advanced non-small cell lung cancer from August 2000 to February 2010. Radiation therapy was delivered with the daily dose of 1.8 Gy to 2.0 Gy and the total radiation dose ranged from 50.0 Gy to 70.2 Gy (median, 61.2 Gy). Elective nodal irradiation was delivered at a dose of 45.0 Gy to 50.0 Gy. Seven patients (14.3%) were treated with radiation therapy alone and forty two patients (85.7%) were treated with chemotherapy either sequentially or concurrently.
Results
Twenty-five cases (51.0%) out of 49 cases experienced radiation pneumonitis. According to the radiation pneumonitis grade, 10 (20.4%) were grade 1, 9 (18.4%) were grade 2, 4 (8.2%) were grade 3, and 2 (4.1%) were grade 4. In the univariate analyses, no clinical factors including age, sex, performance status, smoking history, underlying lung disease, tumor location, total radiation dose and chemotherapy were associated with grade ≥2 radiation pneumonitis. In the subgroup analysis of the chemotherapy group, concurrent rather than sequential chemotherapy was significantly related to grade ≥2 radiation pneumonitis comparing sequential chemotherapy. In the univariate analysis with dosimetric factors, mean lung dose (MLD), V20, V30, V40, MLDipsi, V20ipsi, V30ipsi, and V40ipsi were associated with grade ≥2 radiation pneumonitis. In addition, multivariate analysis showed that MLD and V30 were independent predicting factors for grade ≥2 radiation pneumonitis.
Conclusion
Concurrent chemotherapy, MLD and V30 were statistically significant predictors of grade ≥2 radiation pneumonitis in patients with stage III non-small cell lung cancer undergoing definitive radiotherapy. The cutoff values for MLD and V30 were 16 Gy and 18%, respectively.
Keywords: Non-small cell lung cancer, Radiation therapy, Radiation pneumonitis, Dosimetric factor
Introduction
The incidence of lung cancer in Korea is 11%, ranking fourth, and mortality due to lung cancer continued to increase to rank first position in cancer mortality in 2008 [1]. Non-small cell lung cancer (NSCLC) accounts for about 75% of lung cancer and 25-40% of cases are locally advanced disease, not amenable for curative resection at the time of diagnosis [2]. Thoracic radiotherapy was considered the standard treatment for patients with unresectable and locally advanced NSCLC. However, due to poor 5-year survival with standard radiotherapy [3], altered fraction and dose escalated radiotherapy or addition of chemotherapy to radiotherapy were attempted in order to improve the survival rate and local control rate. Recently, concurrent chemoradiotherapy has been demonstrated to increase survival to a greater degree than induction chemotherapy followed by radiotherapy. Therefore, concurrent chemoradiotherapy is currently considered as the standard of care for locally advanced stage III NSCLC [4,5].
When radiotherapy is used to treat tumors within or adjacent to the thorax, the dose-limiting organs of primary concern are the lungs and the spinal cord. The lungs are sensitive to the effects of both short term and long term radiation at a lower dose than other structures in the chest, such as the esophagus, heart and spinal cord [6,7]. Therefore, radiation pneumonitis is the major side effect of thoracic radiation therapy that can impact the clinical course of the patients. Discontinuation of treatment or limiting the amount of radiation dose due to radiation pneumonitis leads to reduction in the therapeutic effect and decreases both the local control rate and survival rate [8].
Radiation pneumonitis usually occurs within 1-8 weeks after irradiation and its incidence ranges widely from 0% to 58%. Clinical manifestation is usually mild dry cough, fever or mild dyspnea. However, severe respiratory failure can leading to death, and pulmonary fibrosis may also lead to chronic dyspnea. Radiation pneumonitis is classified in three forms; acute radiation pneumonitis within 2 months, subacute radiation pneumonitis within 2-6 months and chronic radiation pneumonitis more than 6 months after completion of radiation therapy. Mostly it appears within 2-3 months but is unlikely to occur in less than a month or after six months [9].
The factors related to the occurrence of radiation pneumonitis can be divided into clinical factors and dosimetric factors. Clinical factors associated with radiation pneumonitis are performance status [10], tumor location [11], smoking history [12] and concurrent chemotherapy [11,13]. Among dosimetric factors, V20 (volume of total lung receiving 20 Gy or more), V30 (volume of total lung receiving 30 Gy or more) and mean lung dose (MLD) have been used to predict the occurrence of radiation pneumonitis [14-20].
Materials and Methods
1. Patients
We retrospectively reviewed the records of patients who completed at our institution definitive radiotherapy for locally advanced NSCLC from August 2000 to February 2010. Because most of radiation pneumonitis cases occur within 6 months of radiotherapy, a minimum of 6 months follow-up was required for inclusion in this study. In all patients, history was obtained and physical examination and staging exams were performed, including chest, upper abdomen and brain computed tomography (CT). Bronchoscopy and biopsy were performed for pathological diagnosis. Clinical evaluation of staging was based on the 2002 American Joint Committee on Cancer (AJCC) staging classification and the performance status was assessed according to Eastern Cooperative Oncology Group (ECOG) performance scale.
2. Radiotherapy
All patients underwent three-dimensional treatment planning and radiotherapy was delivered using a linear accelerator (Primus, Siemens, German) with 6 MV or 10 MV photon beam, with a daily dose 1.8 to 2.0 Gy. The total radiation dose ranged from 50.0 Gy to 70.2 Gy (median, 61.2 Gy). Elective nodal irradiation was delivered at a total dose of 45.0 Gy to 50.0 Gy.
Patients were placed in the treatment position (generally supine, with arms above the head) and immobilized in a custom-made device to improve the setup reproducibility during planning and treatment. Planning CT scan was performed in the immobilization device, with the patients breathing freely, and included the entire lung volume. A scan thickness of 3 mm was used. Gross tumor volume (GTV) was defined as all gross disease defined by the planning CT and clinical information (GTV primary), as well as the enlarged regional lymph nodes over 1.0 cm in the short-axis (GTV node). The clinical target volume (CTV) included the GTV with 2.0-2.5 cm margin in all directions, plus the ipsilateral hilum and the entire mediastinum and supraclavicular area, for patients with apical tumors and with enlargement of the supraclavicular lymph nodes. The planning target volume (PTV) was defined as CTV plus 0.5-1.0 cm margin in all directions. The prescribed dose was mostly 61.2 Gy in 34 fractions. The heterogeneity correction was used and the dose was prescribed at the isocenter. PTV was encouraged to cover 90 % of isodose surface. Normal tissue constraints were prioritized for treatment planning, in the following order: the maximum spinal cord dose did not exceed 40 Gy and the volume of total lung receiving 20 Gy or more (V20) was below 35%.
3. Dosimetric factors
Radiation doses were extracted only from the cumulative dose-volume histograms. Vn was defined as the volume of total lung receiving at least n Gy of radiation. Data points extracted from the dose-volume histograms were obtained, including V10, V20, V30, V40, and MLD. The same parameters were evaluated ipsilaterally and were recorded as V20ipsi, V30ipsi, V40ipsi, and MLDipsi, respectively. Total lung was defined as (right lung + left lung) - GTV. Accordingly, the ipsilateral lung was defined as right lung/left lung - PTV.
4. Chemotherapy
Forty-two patients received chemotherapy, i.e., 14 patients concurrently, and 28 sequentially. Chemotherapy regimens were paclitaxel + carboplatin in 20 patients, gemcitabine + cisplatin in 9 patients, gemcitabine + carboplatin in 5 patients, paclitaxel + cispltin in four patients, docetaxel + cisplatin in 2 patients, docetaxel + carboplatin in one patient and vinorebine + cisplatin in one patient.
5. Follow-up and evaluation of radiation pneumonitis
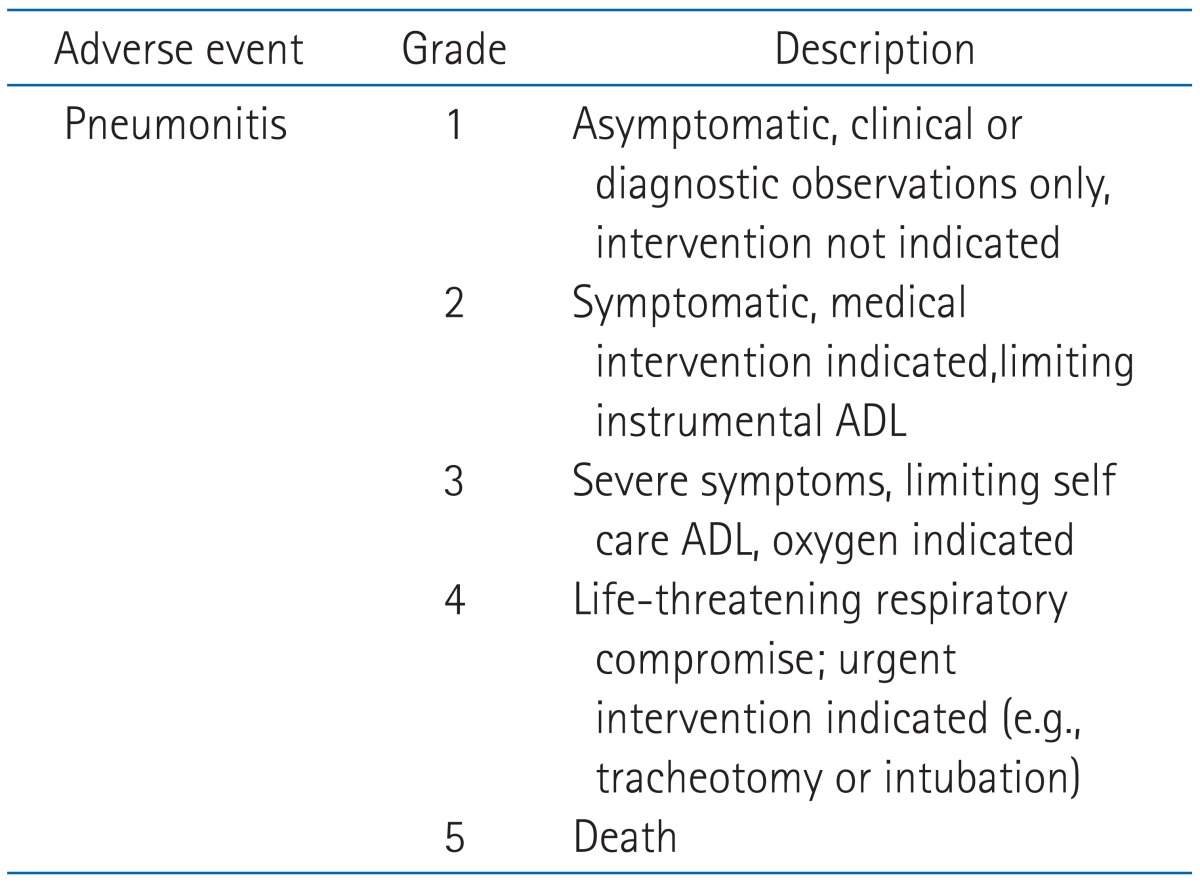
Patients were evaluated for follow-up with weekly chest X-rays and when clinically indicated during radiation treatment. The first follow-up visit was one month after completion of treatment, every 3 months for the first 2 years and every 6 months thereafter. At the time of the regular follow-ups, routine laboratory studies,chest X-ray and chest CT scans were taken. Radiation pneumonitis was graded according to the National Cancer Institute of the Common Toxicity Criteria for Adverse Events version 4.0 (CTCAE) (Table 1).
Table 1.
Common Toxicity Criteria for Adverse Events v4.0
ADL, activities of daily life.
Available from: http://ctep.cancer.gov/reporting/ctc.html.
6. Statistical analysis
Univariate logistic regression analysis was used to test the association between Vn/MLD and radiation pneumonitis. For the multivariate analysis, forward stepwise logistic regression analysis was adopted. Patients were grouped based on the development of grade 0 to 1 vs. grade 2 to 5 pneumonitis. Receiver-operating characteristic (ROC) curves were generated to define the cutoff value. Statistical tests were performed using SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA) and statistical significance was defined as a p-value ≤ 0.05.
Results
1. Patients' characteristics
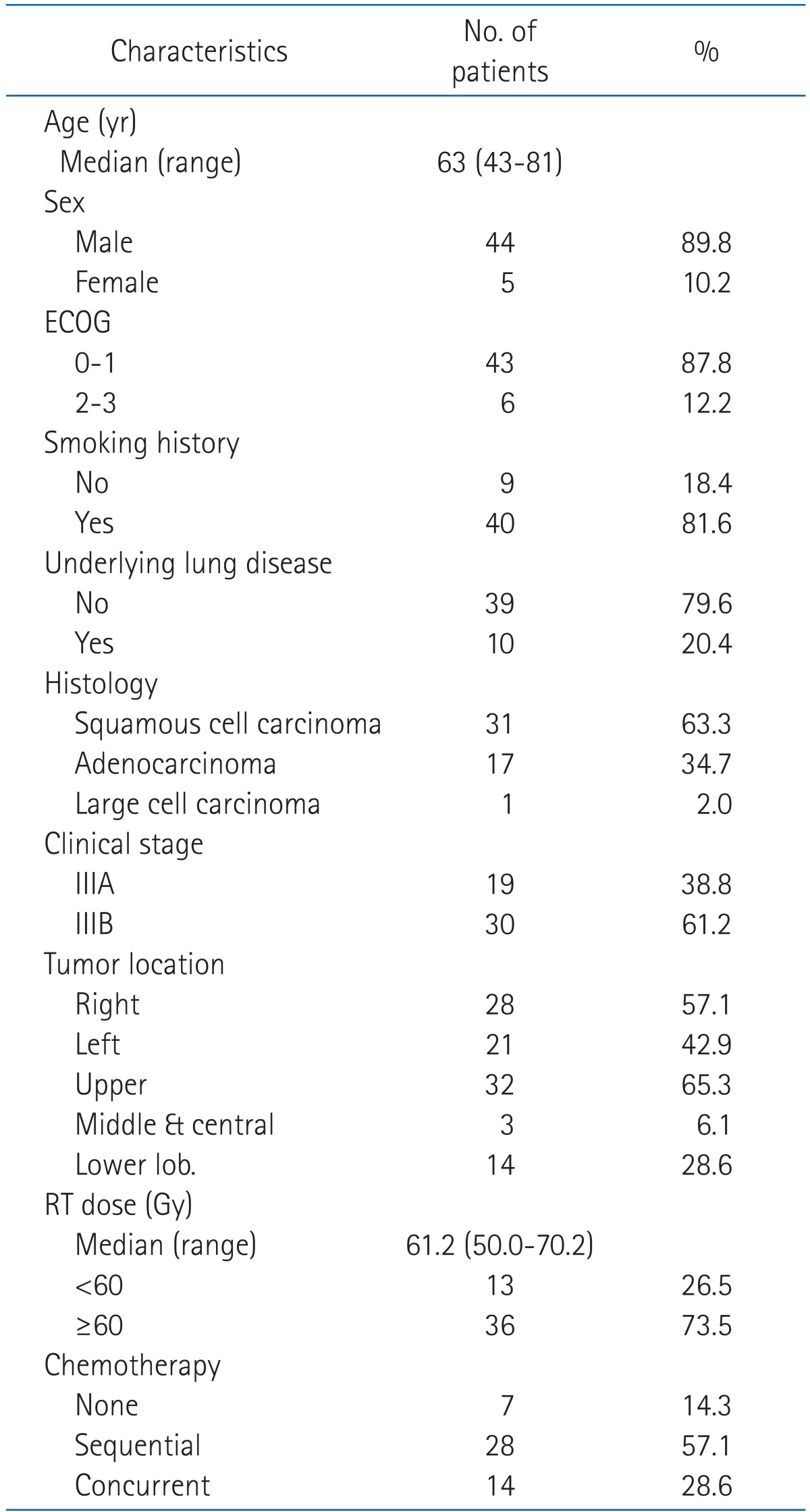
From August 2000 to February 2010, 79 patients with biopsy proven NSCLC stage III underwent definitive radiotherapy at our institution. Of these, we retrospectively analysed data for 49 patients who completed radiotherapy and had a minimum of 6 months follow-up duration. The characteristics of patients are summarized in Table 2. There was a male predominance (44 male [89.8%] vs. 5 female [10.2%]). The patients' age ranged from 43 to 81 years (median, 63 years) and the majority of patients were in their 50s or 60s. Pathologically, 31 patients (63.3%) had squamous cell carcinoma, 17 patients (34.7%) had adenocarcinoma and 1 patient (2.0%) had large cell carcinoma. 40 patients (81.6%) had past smoking history. 10 patients (20.4%) had underlying lung disease, 5 (10.2%) were pulmonary tuberculosis, 3 (6.1%) were chronic obstructive pulmonary disease (COPD) and 2 patients (4.1%) had both diseases. ECOG performance status was 1 in 42 patients (85.7%), 2 in 6 patietns (12.2%) and 0 in 1 patient (2.1%). Seven patients (14.3%) were treated with radiation therapy alone and forty two patients (85.7%) were treated with chemotherapy either sequentially or concurrently. Of forty two patients who received chemotherapy, 28 patients (57.1%) received sequential chemoradiotherapy and 14 (28.6%) received concurrent chemoradiotherapy; 11 of this 14 patients also received induction chemotherapy. The prescribed dose for primary lesions ranged from 50 Gy to 70.2 Gy (median, 61.2 Gy). 36 patients (73.5%) received 60.0 Gy or more, whereas 13 patients (26.5%) received less than 60 Gy. The mean duration of radiotherapy was 50.9 days (range, 37 to 73 days).
Table 2.
Patient characteristics (n = 49)
ECOG, Eastern Cooperative Oncology Group; RT, radiation therapy.
2. Clinical manifestations of radiation pneumonitis
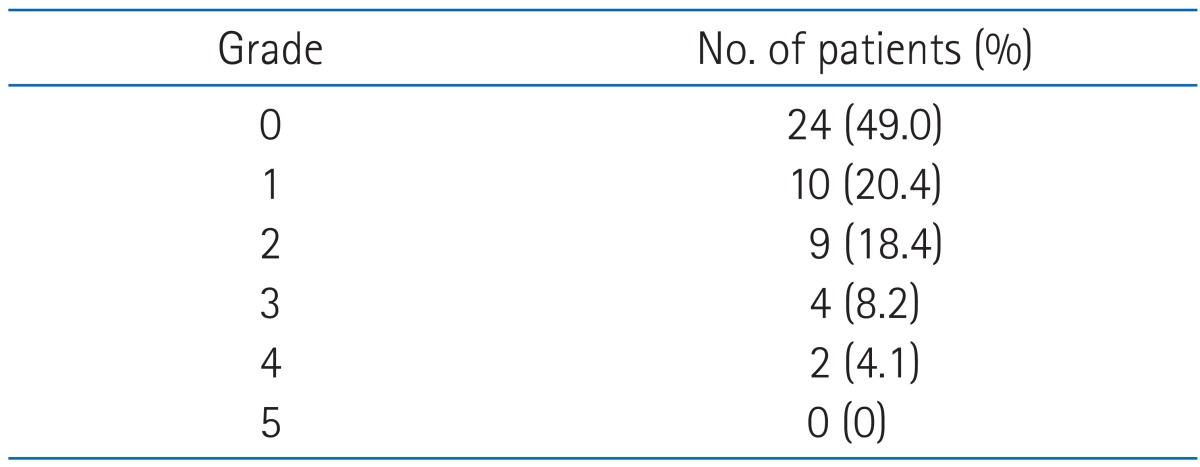
Of 49 investigated patients, 25 (51.0%) developed radiation pneumonitis: 10 (20.4%) grade 1, 9 (18.4%) grade 2, 4 (8.2%) grade 3, and 2 (4.1%) grade 4, according to the CTCAE ver. 4.0 classification. There was no death related to radiation pneumonitis (Table 3). The median time to diagnosis of pneumonitis was 3.0 months from initiation of radiotherapy and 1.3 months from completion of radiotherapy. Two patients developed radiation peumonitis during radiotherapy. According to the onset of radiation pneumonitis, acute radiation pneumonitis (occuring within 2 months after radiotherapy), subacute radiation pneumonitis (occuring 2 to 6 months after radiotherapy), and chronic radiation pneumonitis cases (occuring 6 months after radiotherapy) were 20 (80.0 %), 5 (20.0%), and 0 (0%), respectively.
Table 3.
Pulmonary toxicity according to Common Toxicity Criteria for Adverse Events v4.0 grading scale
Treatment using corticosteroid was performed in 14 of 15 patients (93.3%) with grade ≥2 radiation peumonitis.
3. Analysis of factors predicting radiation pneumonitis
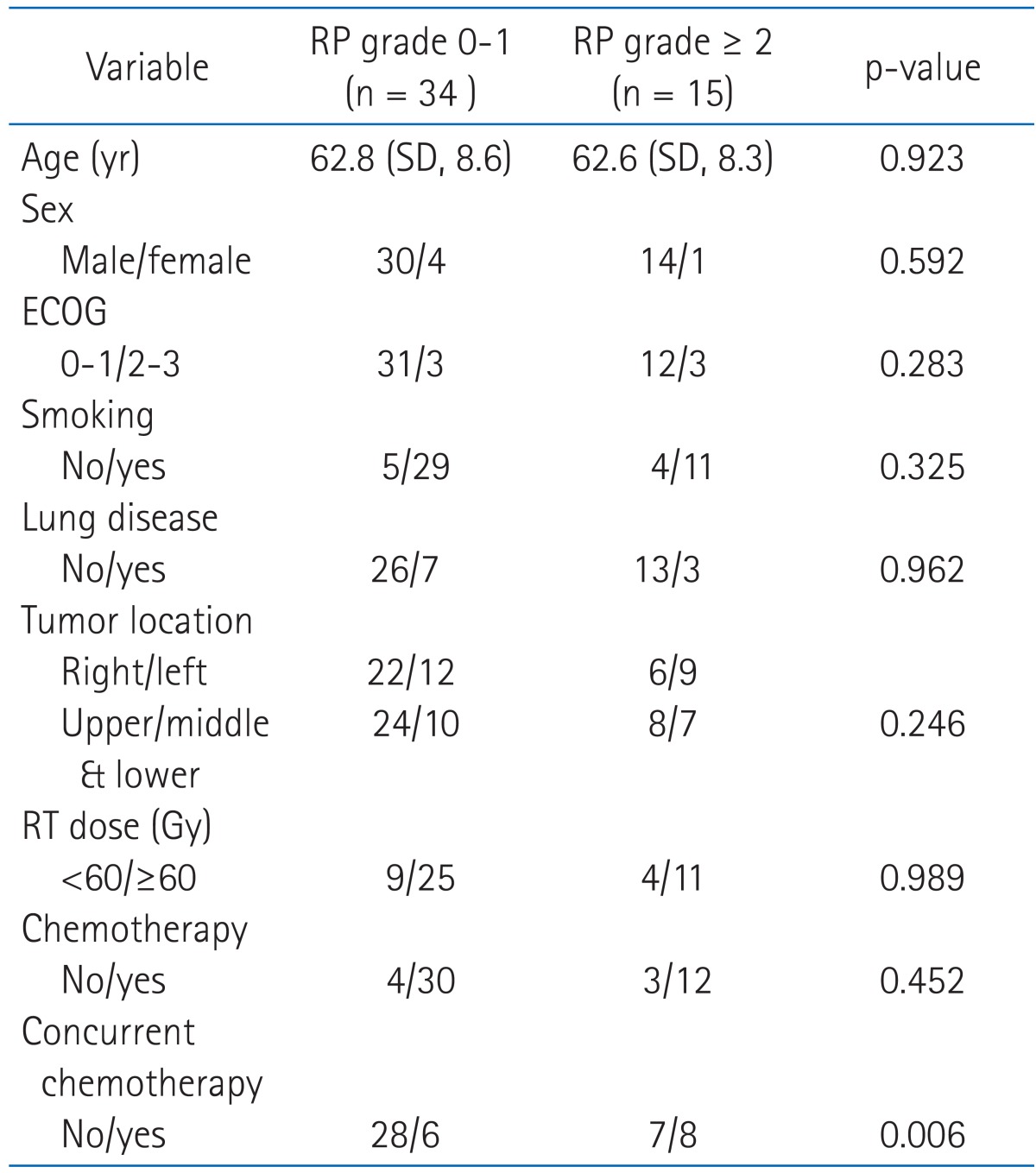
In the univariate analysis, no clinical factors (including age, sex, performance status, smoking history, underlying lung disease, tumor location, total radiation dose and chemotherapy) were associated with grade ≥2 radiation pneumonitis. In the subgroup analysis of chemotherapy group, concurrent rather than sequential chemotherapy was significantly related with grade ≥2 radiation pneumonitis (p = 0.006) (Table 4).
Table 4.
Patient and tumor characteristics according to radiation pneumonitis grade
RP, radiation pneumontis; SD, standard deviation; ECOG, Eastern Cooperative Oncology Group.
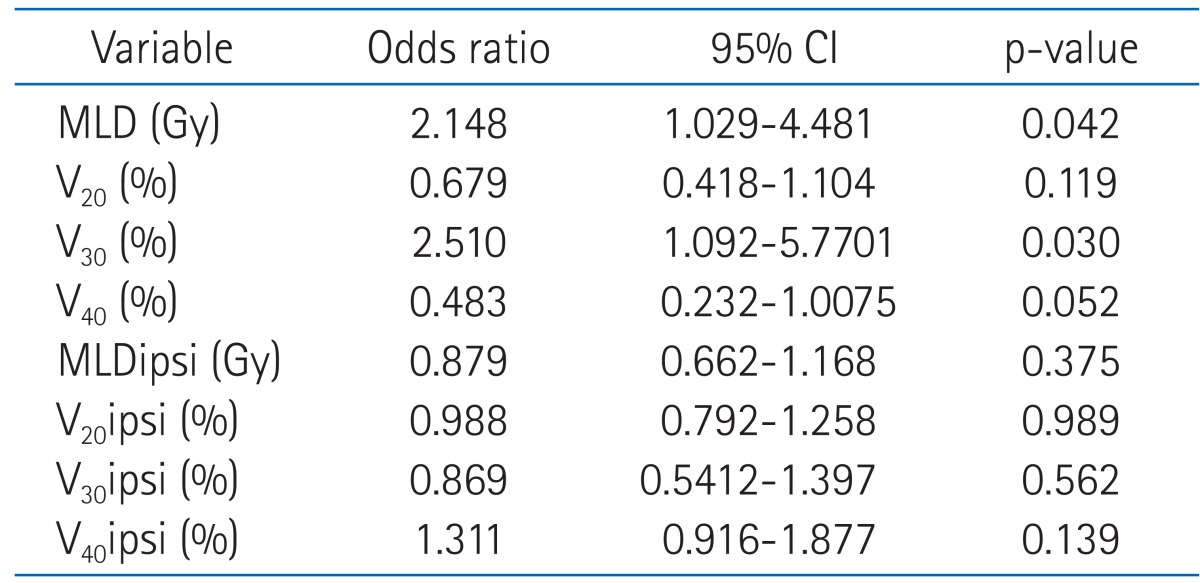
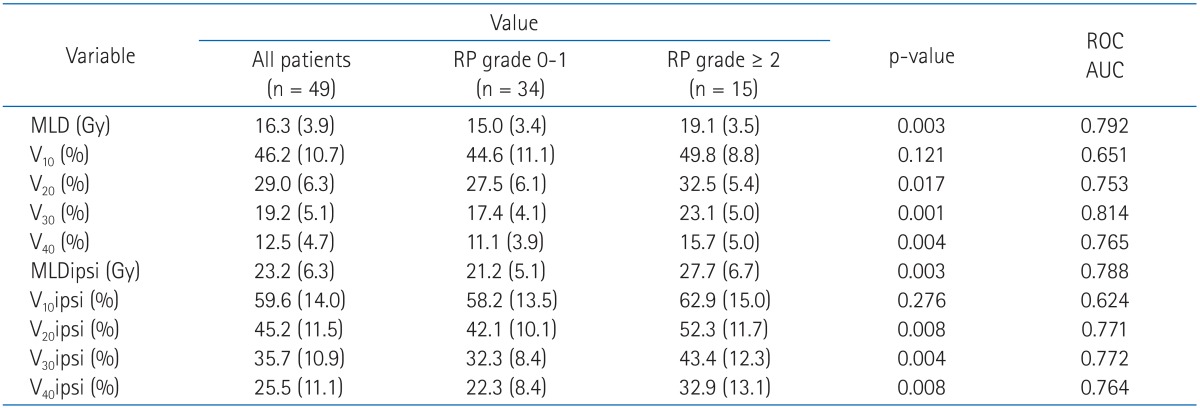
Table 5 shows the mean MLD, V10, V20, V30, V40, MLDipsi (ipsilateral MLD), V10ipsi, V20ipsi, V30ipsi and V40ipsi for all patients and in patients with grade 0-1 and grade ≥2 radiation pneumonitis. In the univariate analysis with dosimetric factors, MLD (p = 0.003), V20 (p = 0.017), V30 (p = 0.001), V40 (p = 0.004), MLDipsi (p = 0.003), V20ipsi (p = 0.008), V30ipsi (p = 0.004), and V40ipsi (p = 0.008) were all associated with highergrade radiation pneumonitis. In contrast, multivariate analysis showed that only MLD (odds ratio [OR], 2.148; 95% confidence interval [CI], 1.029 to 4.481; p = 0.042) and V30 (OR, 2.510; 95% CI, 1.092 to 5.771; p = 0.030) were independent predicting factors for grade ≥2 radiation pneumonitis (Table 6). The mean value of MLD was 16.3 Gy for all patients, i.e., 15.0 Gy for patients who developed grade 0 to 1 radiation pneumonitis and 19.1 Gy for patients who developed grade ≥2 radiation pneumonitis. The mean value of V30 was 19.2% for all patients, i.e., 17.4% for patients who developed grade 0 to 1 radiation pneumonitis and 23.1% for patientswho developed grade ≥2 radiation pneumonitis.
Table 6.
Multivariate analysis of dosimetric factors for predicting development of grade ≥2 radiation pneumonits
CI, confidence interval; MLD, mean lung dose; V20, volume of total lung receiving 20 Gy or more; MLDipsi, ipsilateral mean lung dose; V20ipsi, volume of ipsilateral lung receiving 20 Gy or more.
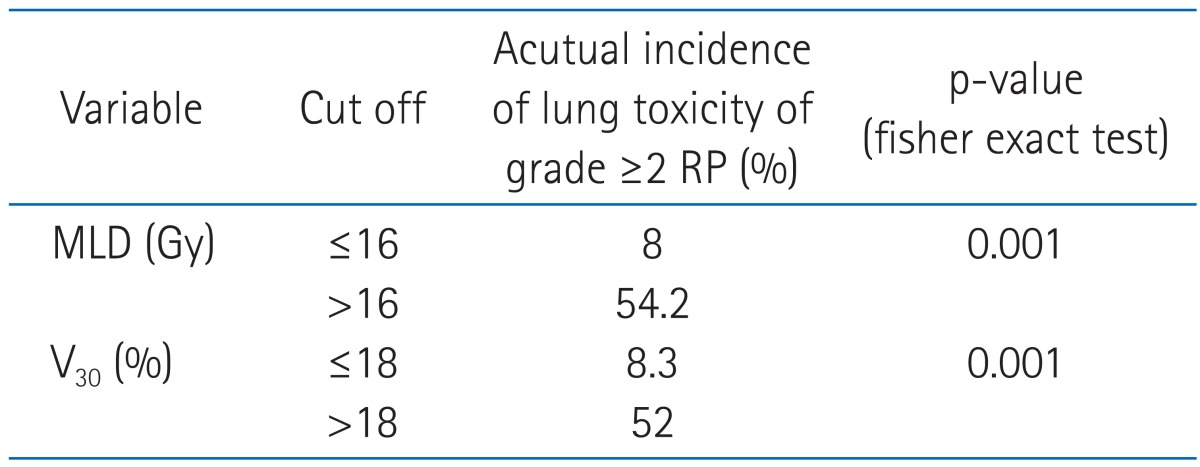
The cutoff values of MLD and V30, as calculated by the ROC curves, were 16 Gy and 18%, respectively. The incidence of grade ≥2 radiation pneumonitis was 8% if MLD was ≤16 Gy and 54.2% if MLD was >16 Gy (p = 0.001). Similarly, the incidence of grade ≥2 radiation pneumonitis was 8.3 % if V30 was ≤18% and 52% if V30 was >18% (p = 0.001) (Table 7).
Table 7.
Risk groups of patients according to dosimetric constraints
RP, radiation pneumonitis; MLD, mean lung dose; V30, volume of total lung receiving 30 Gy or more.
Discussion and Conclusion
Radiation pneumonitis is one of the most significant complications associated with thoracic radiotherapy. It affects the respiratory function and quality of life and may also lead to death in severe cases. The incidence of radiation pneumonits varies widely between reports, ranging from 0 to 58%. Differences in radiation technique, awareness, method of reporting and evaluation of the symptoms themselves may account for this variability [9,21-23]. In this study, in 49 patients receiving thoracic radiation therapy and chemotherapy, the incidence of radiation pneumonitis was 30.6%, which was higher than 10-20% reported in the study of Roach et al. [24]. Tsoutsou and Koukourakis [21] reported that both the rate and severity of symptoms increased when large lung volumes were included or high doses (>50 Gy) were applied, especially when combined with chemotherapy. They showed that 50-90% of patients undergoing irradiation to the lung developed radiographic and pulmonary function test abnormalities within a time frame of 12 weeks after radiation therapy (RT).
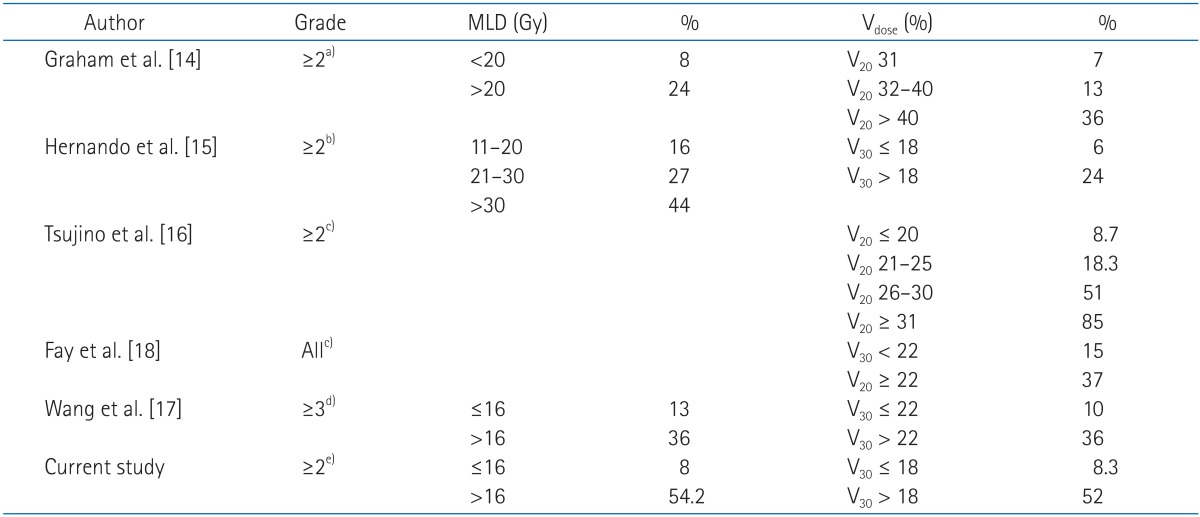
A review of published studies showed that the ideal dose-volume histogram metric for predicting the risk of radiation pneumonitis has not yet been identified. In the study of Graham et al. [14], half of the patients were treated with radiotherapy and half with radiochemotherapy. The incidence of pneumonitis was up to 20%, but only 7% if the V20 value was 31% or lower. Similarly, in the trial of Tsujino et al. [16], all patients were treated by adding chemotherapy to radiotherapy in a concomitant set, and the incidence of pneumonitis increased up to 51% with the standard dosimetric constraints. Only with very low values of V20 did the pneumonitis risk become acceptable (8.7% for V20 ≤ 20%). This means that the use of constraints lower than V20 is paramount to avoid pulmonary toxicity. In our study, the mean value of V20 was 29.0% for all patients, i.e., 27.5% for patients who developed grade 0 to 1 radiation pneumonitis, and 32.5% for patients who developed grade ≥2 radiation pneumonitis. The difference was statistically significant in univariate, but not multivariate analysis.
Wang et al. [17] reported that the incidence of radiation pneumonitis was 10% if V30 < 22% and increased to 36% if V30 ≥22%. In the experience of Hernando et al. [15], the global rate of pulmonary toxicity was 19%, but it was only 6% when V30 was 18% or lower; thus, the authors suggested the cutoff value of V30 as 18%. Fay et al. [18] also reported that the incidence of radiation pneumonitis was 15 % if V30 <22% and 37% if V30 ≥22%. In our study, V30 was a statistically significant predictor of grade ≥2 radiation pneumonitis and the mean value in all patients was 19.2%, i.e., 17.4% for grade 0-1 pneumonitis, and 23.1% for grade ≥2 radiation pneumonitis. In our study, the cutoff value of V30 was 18%, similar to the results of Hernando et al. [15].
Graham et al. [14] reported that MLD was a statistically significant predictor of radiation pneumonitis. The incidence of grade ≥2 radiation pneumonitis was 8% if MLD < 20 Gy and 24% if MLD ≥20 Gy. Wang et al. [17] also reported that the incidence of radiation pneumonitis was 13% if MLD ≤16 Gy and 36% if MLD > 16 Gy. In our study MLD was also a statistically significant predictor of grade ≥2 radiation pneumonitis and its mean value in all patients was 16.3 Gy, i.e., 15.0 Gy for grade 0-1 and 19.2 Gy for grade ≥2 radiation pneumonitis. The cutoff value of MLD was 18% in our study, similar to the results of Wang et al. [17] (Table 8).
Table 8.
Predictive dosimetric factors of radiation pneumonitis
MLD, mean lung dose; Vdose, volume of total lung receiving (dose) Gy or more.
a)RTOG score for acute toxicity. b)Institutional criteria (similar to Common Toxicity Criteria [CTC]). c)CTC, ver. 2.0. d)CTC, ver. 3.0. e)CTC, ver. 4.0.
Pulmonary function results from the function of the right and left lung; therefore, in investigations related to toxicity, the lungs were usually considered as a unique organ unit. To date and to the best of our knowledge, only few authors have examined the effect of the radiation dose applied to the ipsilateral and contralateral lungs separately, with respect to tumor location. In the study of Oetzel et al. [25] the lungs were analyzed as separate organs. The toxicity rate was 0% if MLDipsi was 15 Gy or lower, 13% if MLDipsi was 17.5 to 20 Gy, 21% if MLDipsi was 22.5 to 25 Gy and 43% if MLDipsi was 27.5 Gy or higher. Correlation between the irradiation dose to the ipsilateral lung and pneumonitis has been reported in two studies, i.e., Seppenwoolde et al. [26] and Yorke et al. [27]. In the latter study, the strongest correlation with pneumonitis was found for V5 through V30 in the ipsilateral lung, but the authors did not suggest a constraint. In the study of Ramella et al. [28], V20ipsi was proven as an effective classification criterion, substantially dividing the patients into two groups. The V20ipsi threshold value was 52%, with toxicity rate ranging from 9% (if V20ipsi was ≤52%) to 46% (if V20ipsi was >52%) (p < 0.05). V20ipsi and V30ipsi were important if the V20 to the total lung, V30 to the total lung and MLD have not exceeded the constraints of 31%, 18%, and 20 Gy, respectively. In this study, MLDipsi, V10ipsi, V20ipsi, V30ipsi and V40ipsi were calculated and all of them, except V10ipsi, were significantly associated with radiation pneumonitis in the univariate, but not multivariate analysis.
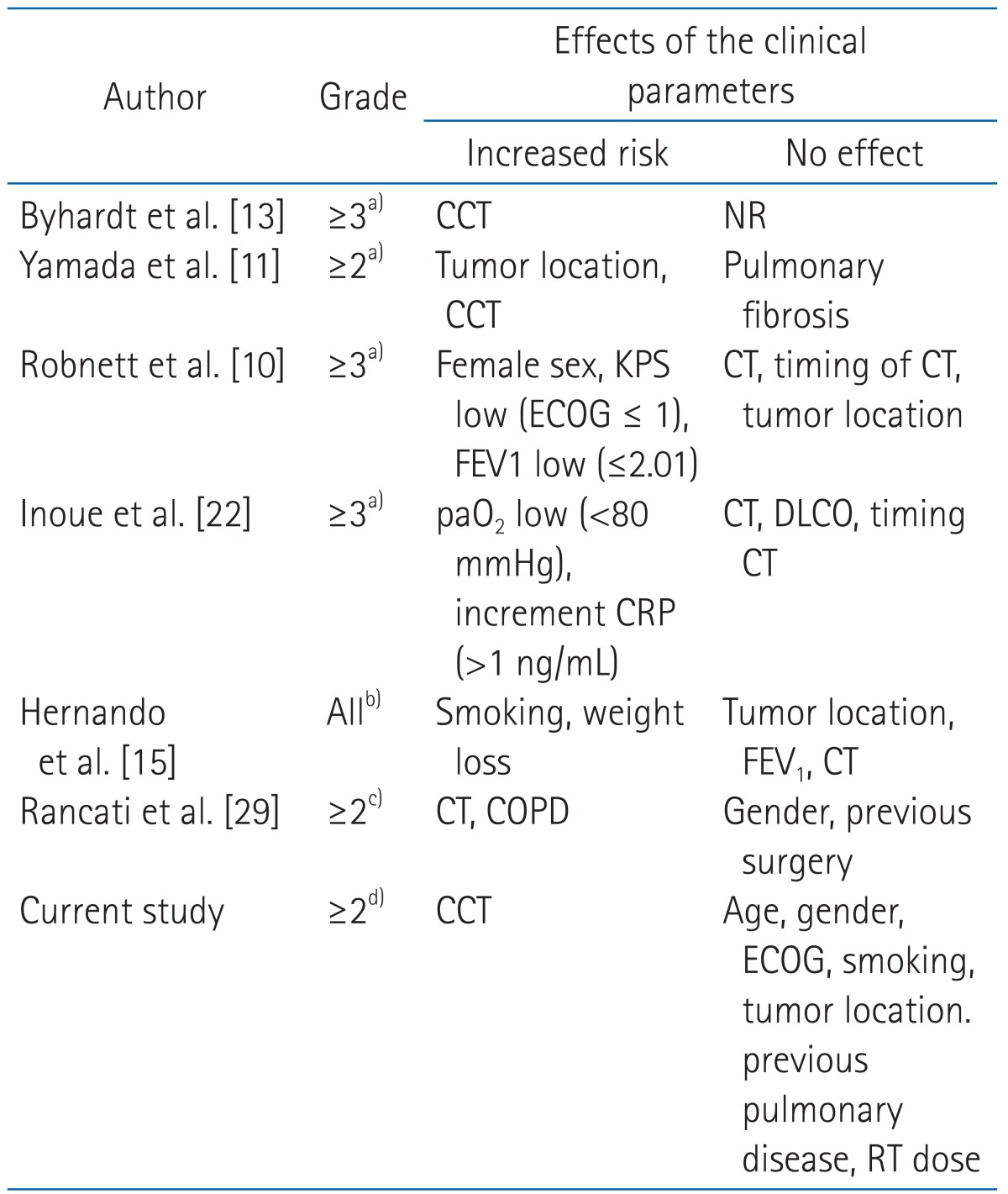
Several clinical factors, such as old age (≥60 years), female, underlying lung disease and pulmonary function, have been previously reported as independent predictors for radiation pneumonitis. Treatment related factors included the total radiation dose, treatment field, chemotherapy regimen and concurrent chemoradiotherapy [10,11,13,15,22,29] (Table 9). In our univariate analysis, no clinical factors (including age, sex, performance status, smoking history, underlying lung disease, tumor location, total radiation dose and chemotherapy) were associated with grade ≥2 radiation pneumonitis. However, in the subgroup analysis of chemotherapy group, concurrent rather than sequential chemotherapy was significantly related to grade ≥2 radiation pneumonitis (p = 0.006) (Table 4).
Table 9.
Clinical prognostic factors related to radiation pneumonitis
CCT, concurrent chemotherapy; NR, not reported; KPS, Karnofsky performance status; ECOG, Eastern Cooperative Oncology Group; CT, chemotherapy; FEV1, forced expiratory volume in 1 sec; paO2, arterial oxygen pressure; DLCO, diffusion capacity for carbon monoxide; CRP, C-reactive protein; COPD, chronic obstructive pulmonary disease; RT, radiation therapy.
a)Radiation Therapy Oncology Group score for acute toxicity. b)Institutional criteria (similar to Common Toxicity Criteria [CTC]), c)Southwest Oncology Group score. d)CTC, ver. 4.0.
Long-standing efforts have been made to develop pharmacologic agents that protect normal tissues from the effects of radiation. One very promising radioprotector emerging from these efforts was amifostine, an organic thiophosphate. The active metabolite is a free thiol thought to provide an alternative target for reactive species from alkylating agents that would otherwise target DNA. The free thiol is also believed to scavenge the free radicals released during the interaction between ionizing radiation and water [30,31]. In the study of Komaki et al. [31], 62 patients received hyperfractionated thoracic radiotherapy with chemotherapy, and there was a statistically significant reduction in the incidence of severe (grade 3) pneumonitis in the patients who received amifostine, compared to those who did not. (16% vs. 0%, p = 0.020).
According to Mehta's hypothesis [32], the safest approach is likely to be limiting both the amount of radiation and the volume of normal lung that is irradiated. Improvement of local tumor control and consecutively overall survival by dose escalation has become an important issue. Inverse treatment planning and intensity-modulated radiation therapy (IMRT) enables dose escalation at specific tumor sites and provides a potential for sparing organs in the vicinity, especially the concave targets, to an extent that was not possible before. Volume restriction seems to be the most important issue in dose escalation. Irradiation of normal tissue can be reduced by omission of clinically uninvolved lymph-node areas, maximal set-up accuracy and decreased tumor mobility through respiratory gating and image guidance. In patients with inoperable stage III NSCLC treated with concurrent chemoradiotherapy, Yuan et al. [33] reported that the rate of radiation pneumonitis was lower in patients with involved-field irradiation (IFI) than in patients with elective nodal irradiation (ENI) (17% vs. 29%, p = 0.044). In addition, IMRT could deliver 25-30% higher dose within the target and achieve improved critical structure sparing, compared to 3D radiotherapy [34]. Murshed et al. [35] compared IMRT and 3D-conformal radiation therapy (CRT) plans in 41 NSCLC patients and reported that IMRT planning improved target conformity, without significantly sacrificing the homogeneity of the tumor dose. The median absolute reduction in the percentage of lung volume irradiated to > 10 and > 20 Gy were 7% and 10%, respectively. This corresponded to a decrease of > 2 Gy in the total lung mean dose and of 10% in the risk of radiation pneumonitis. Kim [36] reported that of 105 NSCLC patients treated with IMRT, 21 patients (20%) had abnormal radiological findings, but only seven patients (6.7%) required treatment for radiation pneumonitis and suggested that IMRT could be a beneficial treatment modality for the reduction of radiation pneumonitis in NSCLC patients.
In conclusion, twenty-five cases (51.0%) out of 49 cases experienced radiation pneumonitis and 15 cases were grade ≥2 radiation pneumonitis. Among clinical factors, only concurrent rather than sequential chemotherapy was significantly related to grade ≥2 radiation pneumonitis. The dosimetric factors MLD and V30 were statistically significant predictors of grade ≥2 radiation pneumonitis. The cutoff values for MLD and V30 were 16 Gy and 18%, respectively. Toxicities related to radiotherapy depend on total radiation dose and treatment field; therefore, V20, V30, and MLD should be below 25%, 18%, and 16 Gy, respectively. For reducing irradiation of normal tissue, omission of clinically uninvolved lymph-node areas, and more advanced treatment technique, such as IMRT, respiratory gating and image guidance, could be recommended. In addition, radiation protector to prevent radiation pneumonitis should be investigated.
Table 5.
Patient characteristics
Values are presented as mean (standard deviation).
RP, radiation pneumonitis; ROC, receiver operating characteristic; AUC, area under ROC curve; MLD, mean lung dose; V10, volume of total lung receiving 10 Gy or more; MLDipsi, ipsilateral mean lung dose; V10ipsi, volume of ipsilateral lung receiving 10 Gy or more.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Ministry for Health, Welfare and Family Affairs. Annual report of cancer incidence (2007), cancer prevalence (2007) and survival (1993-2007) in Korea. Seoul: Ministry for Health, Welfare and Family Affairs; 2009. [Google Scholar]
- 2.Morton RF, Jett JR, McGinnis WL, et al. Thoracic radiation therapy alone compared with combined chemoradiotherapy for locally unresectable non-small cell lung cancer: a randomized, phase III trial. Ann Intern Med. 1991;115:681–686. doi: 10.7326/0003-4819-115-9-681. [DOI] [PubMed] [Google Scholar]
- 3.Onn A, Vaporciyan AA, Chang JY, Komaki R, Roth JA, Herbst RS. Cancer of the lung. In: Kufe DW, Bast RC, Hait WN, editors. Cancer medicine. 7th ed. London: BC Decker Inc; 2006. pp. 1179–1224. [Google Scholar]
- 4.Furuse K, Fukuoka M, Kawahara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17:2692–2699. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 5.Curran WJ, Scott CB, Langer CJ, et al. Long-term benefit is observed in a phase III comparison of sequential vs concurrent chemo-radiation for patients with unresected stage III nsclc: RTOG 9410. Proc Am Soc Clin Oncol. 2003;22:2499. [Google Scholar]
- 6.Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21:109–122. doi: 10.1016/0360-3016(91)90171-y. [DOI] [PubMed] [Google Scholar]
- 7.Salinas FV, Winterbauer RH. Radiation pneumonitis: a mimic of infectious pneumonitis. Semin Respir Infect. 1995;10:143–153. [PubMed] [Google Scholar]
- 8.Gross NJ. Pulmonary effects of radiation therapy. Ann Intern Med. 1977;86:81–92. doi: 10.7326/0003-4819-86-1-81. [DOI] [PubMed] [Google Scholar]
- 9.Stover DE, Devita VT, Hellman S, Rosenberg SA, editors. Cancer: principles and practice of oncology. 7th ed. Philadelphia: J. B. Lippincott; 2005. [Google Scholar]
- 10.Robnett TJ, Machtay M, Vines EF, McKenna MG, Algazy KM, McKenna WG. Factors predicting severe radiation pneumonitis in patients receiving definitive chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2000;48:89–94. doi: 10.1016/s0360-3016(00)00648-9. [DOI] [PubMed] [Google Scholar]
- 11.Yamada M, Kudoh S, Hirata K, Nakajima T, Yoshikawa J. Risk factors of pneumonitis following chemoradiotherapy for lung cancer. Eur J Cancer. 1998;34:71–75. doi: 10.1016/s0959-8049(97)00377-8. [DOI] [PubMed] [Google Scholar]
- 12.Segawa Y, Takigawa N, Kataoka M, Takata I, Fujimoto N, Ueoka H. Risk factors for development of radiation pneumonitis following radiation therapy with or without chemotherapy for lung cancer. Int J Radiat Oncol Biol Phys. 1997;39:91–98. doi: 10.1016/s0360-3016(97)00297-6. [DOI] [PubMed] [Google Scholar]
- 13.Byhardt RW, Scott C, Sause WT, et al. Response, toxicity, failure patterns, and survival in five Radiation Therapy Oncology Group (RTOG) trials of sequential and/or concurrent chemotherapy and radiotherapy for locally advanced non-small-cell carcinoma of the lung. Int J Radiat Oncol Biol Phys. 1998;42:469–478. doi: 10.1016/s0360-3016(98)00251-x. [DOI] [PubMed] [Google Scholar]
- 14.Graham MV, Purdy JA, Emami B, et al. Clinical dose-volume histogram analysis for pneumonitis after 3D treatment for non-small cell lung cancer (NSCLC) Int J Radiat Oncol Biol Phys. 1999;45:323–329. doi: 10.1016/s0360-3016(99)00183-2. [DOI] [PubMed] [Google Scholar]
- 15.Hernando ML, Marks LB, Bentel GC, et al. Radiation-induced pulmonary toxicity: a dose-volume histogram analysis in 201 patients with lung cancer. Int J Radiat Oncol Biol Phys. 2001;51:650–659. doi: 10.1016/s0360-3016(01)01685-6. [DOI] [PubMed] [Google Scholar]
- 16.Tsujino K, Hirota S, Endo M, et al. Predictive value of dosevolume histogram parameters for predicting radiation pneumonitis after concurrent chemoradiation for lung cancer. Int J Radiat Oncol Biol Phys. 2003;55:110–115. doi: 10.1016/s0360-3016(02)03807-5. [DOI] [PubMed] [Google Scholar]
- 17.Wang S, Liao Z, Wei X, et al. Analysis of clinical and dosimetric factors associated with treatment-related pneumonitis (TRP) in patients with non-small-cell lung cancer (NSCLC) treated with concurrent chemotherapy and three-dimensional conformal radiotherapy (3D-CRT) Int J Radiat Oncol Biol Phys. 2006;66:1399–1407. doi: 10.1016/j.ijrobp.2006.07.1337. [DOI] [PubMed] [Google Scholar]
- 18.Fay M, Tan A, Fisher R, Mac Manus M, Wirth A, Ball D. Dose-volume histogram analysis as predictor of radiation pneumonitis in primary lung cancer patients treated with radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:1355–1363. doi: 10.1016/j.ijrobp.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 19.Schallenkamp JM, Miller RC, Brinkmann DH, Foote T, Garces YI. Incidence of radiation pneumonitis after thoracic irradiation: Dose-volume correlates. Int J Radiat Oncol Biol Phys. 2007;67:410–416. doi: 10.1016/j.ijrobp.2006.09.030. [DOI] [PubMed] [Google Scholar]
- 20.Bradley JD, Hope A, El Naqa I, et al. A nomogram to predict radiation pneumonitis, derived from a combined analysis of RTOG 9311 and institutional data. Int J Radiat Oncol Biol Phys. 2007;69:985–992. doi: 10.1016/j.ijrobp.2007.04.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsoutsou PG, Koukourakis MI. Radiation pneumonitis and fibrosis: mechanisms underlying its pathogenesis and implications for future research. Int J Radiat Oncol Biol Phys. 2006;66:1281–1293. doi: 10.1016/j.ijrobp.2006.08.058. [DOI] [PubMed] [Google Scholar]
- 22.Inoue A, Kunitoh H, Sekine I, Sumi M, Tokuuye K, Saijo N. Radiation pneumonitis in lung cancer patients: a retrospective study of risk factors and the long-term prognosis. Int J Radiat Oncol Biol Phys. 2001;49:649–655. doi: 10.1016/s0360-3016(00)00783-5. [DOI] [PubMed] [Google Scholar]
- 23.Rodrigues G, Lock M, D'Souza D, Yu E, Van Dyk J. Prediction of radiation pneumonitis by dose - volume histogram parameters in lung cancer: a systematic review. Radiother Oncol. 2004;71:127–138. doi: 10.1016/j.radonc.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 24.Roach M, 3rd, Gandara DR, Yuo HS, et al. Radiation pneumonitis following combined modality therapy for lung cancer: analysis of prognostic factors. J Clin Oncol. 1995;13:2606–2612. doi: 10.1200/JCO.1995.13.10.2606. [DOI] [PubMed] [Google Scholar]
- 25.Oetzel D, Schraube P, Hensley F, Sroka-Perez G, Menke M, Flentje M. Estimation of pneumonitis risk in three-dimensional treatment planning using dose-volume histogram analysis. Int J Radiat Oncol Biol Phys. 1995;33:455–460. doi: 10.1016/0360-3016(95)00009-N. [DOI] [PubMed] [Google Scholar]
- 26.Seppenwoolde Y, De Jaeger K, Boersma LJ, Belderbos JS, Lebesque JV. Regional differences in lung radiosensitivity after radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;60:748–758. doi: 10.1016/j.ijrobp.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 27.Yorke ED, Jackson A, Rosenzweig KE, Braban L, Leibel SA, Ling CC. Correlation of dosimetric factors and radiation pneumonitis for non-small-cell lung cancer patients in a recently completed dose escalation study. Int J Radiat Oncol Biol Phys. 2005;63:672–682. doi: 10.1016/j.ijrobp.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 28.Ramella S, Trodella L, Mineo TC, et al. Adding ipsilateral V20 and V30 to conventional dosimetric constraints predicts radiation pneumonitis in stage IIIA-B NSCLC treated with combined-modality therapy. Int J Radiat Oncol Biol Phys. 2010;76:110–115. doi: 10.1016/j.ijrobp.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 29.Rancati T, Ceresoli GL, Gagliardi G, Schipani S, Cattaneo GM. Factors predicting radiation pneumonitis in lung cancer patients: a retrospective study. Radiother Oncol. 2003;67:275–283. doi: 10.1016/s0167-8140(03)00119-1. [DOI] [PubMed] [Google Scholar]
- 30.Antonadou D, Coliarakis N, Synodinou M, et al. Randomized phase III trial of radiation treatment +/- amifostine in patients with advanced-stage lung cancer. Int J Radiat Oncol Biol Phys. 2001;51:915–922. doi: 10.1016/s0360-3016(01)01713-8. [DOI] [PubMed] [Google Scholar]
- 31.Komaki R, Lee JS, Milas L, et al. Effects of amifostine on acute toxicity from concurrent chemotherapy and radiotherapy for inoperable non-small-cell lung cancer: report of a randomized comparative trial. Int J Radiat Oncol Biol Phys. 2004;58:1369–1377. doi: 10.1016/j.ijrobp.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Mehta V. Radiation pneumonitis and pulmonary fibrosis in non-small-cell lung cancer: pulmonary function, prediction, and prevention. Int J Radiat Oncol Biol Phys. 2005;63:5–24. doi: 10.1016/j.ijrobp.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 33.Yuan S, Sun X, Li M, et al. A randomized study of involved-field irradiation versus elective nodal irradiation in combination with concurrent chemotherapy for inoperable stage III nonsmall cell lung cancer. Am J Clin Oncol. 2007;30:239–244. doi: 10.1097/01.coc.0000256691.27796.24. [DOI] [PubMed] [Google Scholar]
- 34.Beckmann GK, Kölbl O, Krieger T, Wulf J, Flentje MP. How can we further improve radiotherapy for stage-III non-small-cell lung cancer? Lung Cancer. 2004;45(Suppl 2):S125–S132. doi: 10.1016/j.lungcan.2004.07.982. [DOI] [PubMed] [Google Scholar]
- 35.Murshed H, Liu HH, Liao Z, et al. Dose and volume reduction for normal lung using intensity-modulated radiotherapy for advanced-stage non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2004;58:1258–1267. doi: 10.1016/j.ijrobp.2003.09.086. [DOI] [PubMed] [Google Scholar]
- 36.Kim MS. Incidence and prognostic factors of radiation pneumonitisin NSCLC treated with intensity modulated radiation therapy (IMRT) J Korean Soc Ther Radiol Oncol. 2008;26:35–44. [Google Scholar]