Abstract
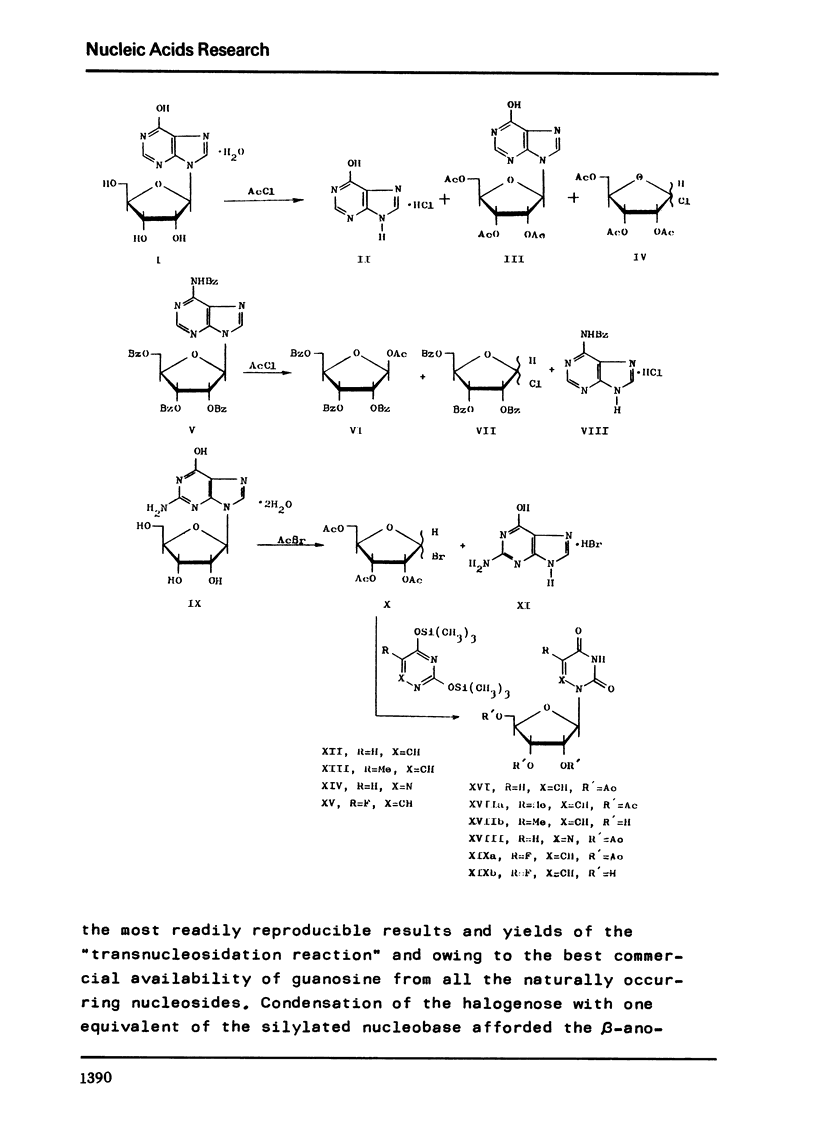
Inosine (I) when acetylated with acetic anhydride in the presence of acetyl chloride in acetic acid solution (the so called "acid acetylation"), affords an acetylated nucleoside III (75%) along with cleavage products of the nucleoside (hypoxanthine, 19%). The reaction of I with acetyl chloride (7 days) results in the formation of hypoxanthine (95%) and triacetylribofuranosyl chloride (IV) isolated in the form of tetraacetylribofuranose (47%). The acetylated purine nucleoside affords a similar result by reaction with acetyl chloride or acetyl bromide. 2'-Deoxyuridine gives a diacetyl derivative (80%) by reaction with acetyl bromide. On treatment with acetyl bromide, the nucleoside bond of purine nucleosides is quantitatively cleavaged (4 h, 20 degrees C) with the formation of tri-O-acetyl-D-ribofuranosyl bromide (X). The halogenose X affords pure beta-anomers, namely, 1,2,3,5-tetra-O-acetyl-beta-D-ribofuranose (75%), the triacetyl derivatives of 5-methyluridine (XVIIa; 75%, referred to guanosine), 6-azauridine (XVIII; 71%), and 5-fluorouridine (XIXa; 75%).
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Calabresi P., Turner R. W. Beneficial effects of triacetyl azauridine in psoriasis and mycosis fungoides. Ann Intern Med. 1966 Feb;64(2):352–371. doi: 10.7326/0003-4819-64-2-352. [DOI] [PubMed] [Google Scholar]
- Robins M. J., Robins R. K. Purine nucleosides. XI. The synthesis of 2'-deoxy-9-alpha- and-beta-D-ribofuranosylpurines and the correlation of their anomeric structure with proton magnetic resonance spectra. J Am Chem Soc. 1965 Nov 5;87(21):4934–4940. doi: 10.1021/ja00949a042. [DOI] [PubMed] [Google Scholar]
- Winkley M. W., Robins R. K. Pyrimidine nucleosides. I. The synthesis of 6-methylcytidine, 6-methyluridine, and related 6-methylpyrimidine nucleosides. J Org Chem. 1968 Jul;33(7):2822–2827. doi: 10.1021/jo01271a046. [DOI] [PubMed] [Google Scholar]



