Abstract
Galectins constitute an evolutionary conserved family of β-galactoside-binding proteins, ubiquitous in mammals and other vertebrate taxa, invertebrates, and fungi. Since their discovery in the 1970s, their biological roles, initially understood as limited to recognition of carbohydrate ligands in embryogenesis and development, have expanded in recent years by the discovery of their immunoregulatory activities. A gradual paradigm shift has taken place in the past few years through the recognition that galectins also bind glycans on the surface of potentially pathogenic microbes, and function as recognition and effector factors in innate immunity. Further, an additional level of functional complexity has emerged with the most recent findings that some parasites “subvert” the recognition roles of the vector/host galectins for successful attachment or invasion.
Keywords: Pattern recognition receptors, Galectins, β-Galactoside, Carbohydrate recognition domain, Glycans, Structure, Function, Proto-type, Chimera, Tandem-repeat
1 Introduction
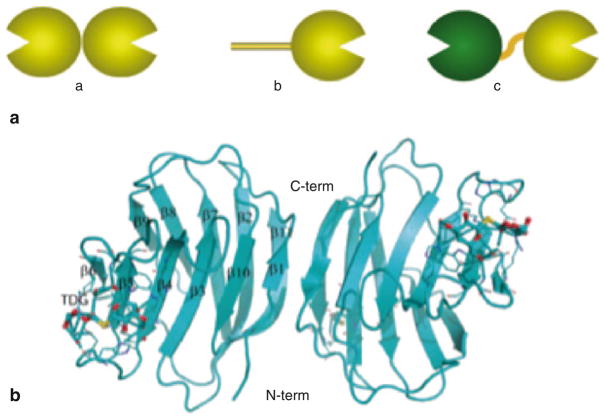
Complex carbohydrate structures encode information that modulates interactions between cells, or cells and the ECM, by specifically binding to carbohydrate-binding proteins such as galectins, formerly known as S-type lectins [Gabius 1997, and references therein]. Galectins constitute an evolutionary conserved family of β-galactoside-binding proteins, ubiquitous in eukaryotic taxa, including the parazoa (sponges) and both protostome and deuterostome lineages of metazoans, and fungi (Cooper 2002; Vasta et al. 1999). Two properties are required in a protein for its inclusion in the galectin family: (a) a characteristic affinity for β-galactosides, and (b) a conserved carbohydrate recognition domain (CRD) sequence motif. Based on structural features, galectins have been classified in three types: “proto”, “chimera”, and “tandem-repeat” (TR) (Fig. 1) (Hirabayashi and Kasai 1993). Proto-type galectins (Fig. 1a(a); Fig. 1b) contain one CRD per subunit and are non-covalently linked homodimers. The chimera galectins (Fig 1a(b)) have a C-terminal CRD and an N-terminal domain rich in proline and glycine. In TR galectins (Fig. 1a(c)) two CRDs are joined by a functional linker peptide. Recently, a novel TR-type galectin with four CRDs has been described (Tasumi and Vasta 2007). The dimerization of proto-type galectins is critical for their function in mediating cell–cell or cell–ECM interactions (Gabius 1997), and similar interactions via the N-terminus domain have been proposed for the chimera galectins (Colnot et al. 1997; Rabinovich et al. 2002). Proto- and TR-types comprise several distinct galectin subtypes. Galectin subtypes have been numbered following the order of their discovery, and so far, 15 have been described in mammals. Galectins-1, -2, -5, -7, -10, -11, -13, -14, and -15 are proto-type. Galectin-3 is the only chimera-type. Galectins-4, -6, -8, -9, and -12 are TR-type. Lower vertebrates and invertebrates appear to have a smaller galectin repertoire. Although galectins lack a typical secretion signal peptide, they are present not only in the cytosol and the nucleus, but also in the extracellular space (Cooper 2002) (Fig. 2). From the cytosol, galectins may be targeted for secretion by non-classical mechanisms, possibly by direct translocation across the plasma membrane (Cho and Cummings 1995; Patterson et al. 1997; Sato and Hughes 1994; Cleves et al. 1996).
Fig. 1. Galectin types and structure of the galectin-1/LacNAc complex.
a Galectins are classified in three types: “proto” (a), “chimera” (b), and “tandem-repeat” (c); b The structure of the galectin-1/thiodigalactoside (TDG) complex reveals a dimer, which each globular subunit binding a single oligosaccharide
Fig. 2. Subcellular and extracellular localization of galectins.
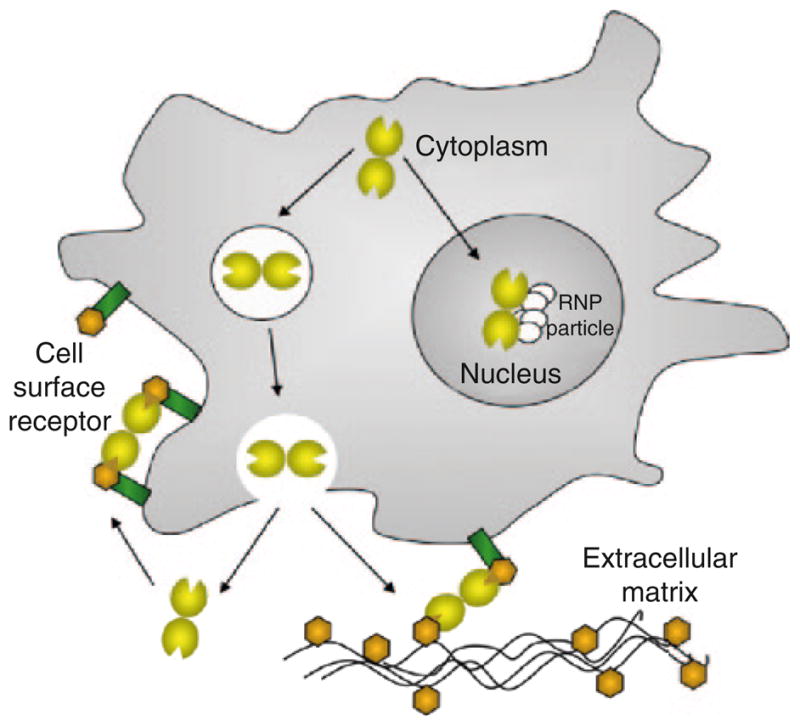
Galectins are synthesized in the cytosol, and can be translocated into the nucleus or secreted to the extracellular space, where they bind to glycans of the extracellular matrix or the cell surface
2 Structure and Biochemical Properties of Galectins
The structure of galectin-1 (Liao et al. 1994; Bianchet et al. 2000) complexed with a di-galactoside (Fig. 1b) shows a jellyroll topology typical of legume lectins. The subunit of galectin is composed of an 11-strand antiparallel β-sandwich and contains one CRD. The 3-D structure of the galectin–ligand complex allowed us to identify amino acids that participate in interactions with ligands, as well as the position and orientation of the sugar hydroxyls that interact with the amino acids (Liao et al. 1994; Bianchet et al. 2000).
Most galectins are non-glycosylated soluble proteins, although a few recently discovered exceptions have transmembrane domains (Lipkowitz et al. 2004; Gorski et al. 2002). The presence of a galectin fold in the protistan parasite Toxoplasma gondii, and galectin-like proteins in the fungus Coprinopsis cinerea and in the sponge Geodia cydonium reveals the early emergence and structural conservation of galectins in eukaryotic evolution (Saouros et al. 2005; Walser et al. 2005; Stalz et al. 2006). In contrast, galectin-like proteins such as the lens crystallin protein galectin-related inter-fiber protein (GRIFIN) and the galectin-related protein (GRP) (previously HSPC159; hematopoietic stem cell precursor) lack carbohydrate-binding activity, and are considered products of evolutionary co-option (Ogden et al. 1998; Ahmed and Vasta 2008). The primary structures and gene organization of mammalian galectins are substantially conserved. Prior to or during early in chordate evolution, duplication of a mono-CRD galectin gene would have led to a bi-CRD galectin gene, in which the N- and C-terminal CRDs subsequently diverged into two different subtypes, defined by exon–intron structure (F4-CRD and F3-CRD). All vertebrate single-CRD galectins belong to either the F3- (e.g., gal-1, -2, -3, -5) or F4- (e.g., gal-7, -10, -13, -14) subtype, whereas TR galectins such as gal-4, -6, -8, -9, and -12 contain both F4 and F3 subtypes (Houzelstein et al. 2004).
Galectins are β-galactoside-binding lectins, and their preferred ligands are N-acetyllactosamine (LacNAc; Galβ1,4GlcNAc) and related disaccharides, with dissociation constants in the order of 10−5 M (Schwarz et al. 1998; Dam and Brewer 2008). Binding specificities of galectins for lactose (Lac), LacNAc, T-disaccharide (Galβ1,3GalNAc) and the human blood group A-tetrasaccharide, together with the presence of amino acid residues that interact with the carbohydrate ligands, have enabled classification of their CRDs into “conserved” or “variable” types (Ahmed and Vasta 1994). The crystal structure of the galectin-1 (conserved type) complexed with a di-galactoside determined at 1.9 Å resolution (Fig. 1b) revealed the galectin structural fold, and allowed the identification of the amino acids involved and the hydroxyl groups of the ligands that participate in protein–carbohydrate interactions (Liao et al. 1994; Bianchet et al. 2000; Lobsanov et al. 1993). The carbohydrate-binding site is formed by three continuous concave strands (β4–β6) containing all residues involved in direct interactions with LacNac. Additional interactions involving a water molecule that bridges the nitrogen of the NAc group with His52, Asp54, and Arg73 explains the higher affinity of LacNAc over Lac. Unlike galectin-1, galectin-3 has an extended carbohydrate-binding site formed by a cleft open at both ends, in which the LacNAc is positioned in such a way that the reducing end of the LacNAc (GlcNAc) is open to solvent, but the non-reducing moiety (Gal) is in close proximity to residues in the β3 strand (Seetharaman et al. 1998). The extended binding site leads to increased affinity for glycans with multiple lactosamine units, and with their substitution of the non-reducing terminal galactose moiety with ABH blood group oligosaccharides [Fucα1, 2; GalNAcα1,3(Fucα1,2); and Galα1,3(Fucα1,2)]. For the nematode Caenorhabditis elegans 16-kDa galectin (variable type), the shorter length of the loops connecting the three β4–β6 strands determines its broader binding specificity for blood group precursor oligosaccharides. Therefore, although galectins are considered a conserved lectin family, most metazoans are endowed of a complex galectin repertoire, with members exhibiting multiple isoforms and more or less subtle variations in carbohydrate specificity, which together with a certain degree of plasticity in sugar binding of each CRD, suggests a substantial diversity in recognition properties (Sparrow et al. 1987; Sato and Hughes 1992; Ahmed et al. 2002; Shoji et al. 2003; Zhou and Cummings 1990; Fang et al. 1993).
Thermodynamic approaches have been used not only to assess the galectins’ carbohydrate-binding properties, but also the oligomeric organization of the protein. On microcalorimetric studies, the dissociation constants for the interactions of bovine galectin-1 with the preferred ligands (Lac, N-acetyllactosamine, thiodigalactoside) were in the range of 10−5 M, with two binding sites per molecule (Schwarz et al. 1998). Although galectin and legume lectins display a striking similarity in their 3-D structures, the thermal stability of the galectin is different from that of concanavalin A (Con A). Like Con A, the bovine galectin exists as a tetramer at the denaturation temperature, but, unlike Con A, it does not dissociate upon unfolding (Schwarz et al. 1998).
3 Biological Roles of Galectins in Development and Regulation of Immune Homeostasis
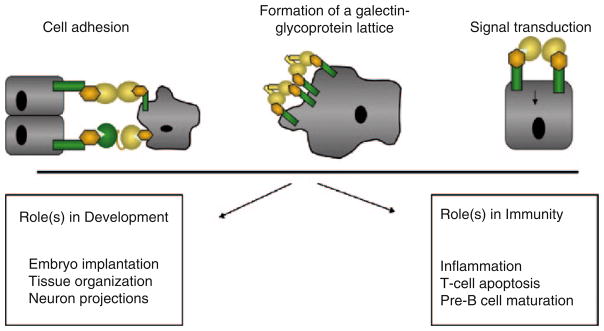
Galectins were initially thought to only bind endogenous (“self”) glycans and mediate developmental processes, including cell differentiation and tissue organization, and more recently, regulation of immune homeostasis (Leffler et al. 2004, Yang et al. 2008) (Fig. 3). In the past few years, however, it has become clear that galectins also bind glycans on the surface of potentially pathogenic microbes and parasitic worms, and mediate recognition and effector functions in innate immunity (Sato and Nieminen 2004). Glycans that contain N-acetyllactosamine and polylactosamine chains [(Galβ1,4GlcNAc)n], such as laminin, fibronectin, lysosome-associated membrane proteins, and mucins, are the preferred endogenous ligands for mammalian, bird, and amphibian galectins (Seetharaman et al. 1998; Sparrow et al. 1987; Sato and Hughes 1992; Ahmed et al. 2002; Shoji et al. 2003; Zhou and Cummings 1990; Fang et al. 1993). The biological function of a particular galectin, however, may vary from site to site, depending on the availability of suitable ligands. The binding properties and biological functions of galectins in the oxidative extracellular environment, however, may depend on their immediate binding to ligand, which prevents the oxidation of free cysteine residues, as well as galectin susceptibility to proteolysis (Liao et al. 1994; Lobsanov et al. 1993). The binding of galectins to cell surface β-galactoside-containing glycolipids and glycoproteins can lead to the formation of lattices that cluster these ligands into lipid raft micro-domains required for optimal transmission of signals relevant to cell function (Rabinovich et al. 2007b; Brewer et al. 2002; Partridge et al. 2004) (Fig. 4). In solution galectins can form multivalent species in a concentration-dependent equilibrium (Morris et al. 2004). Proto-type galectins associate as non-covalently bound dimers via a hydrophobic interphase, whereas galectin-3 associates via its N-terminal domain to form oligomers that in the presence of multivalent oligosaccharides in solution or at the cell surface display binding cooperativity (Dam and Brewer 2008; Brewer et al. 2002). The bivalent TR-type galectins can recognize different saccharide ligands with a single polypeptide, although they can also form higher order aggregates that enhances their avidity. Galectin-mediated lipid raft assembly may modulate turnover of endocytic receptors, signal transduction pathways leading to T-cell activation and cytokine secretion, or apoptosis, B-cell maturation, activation and tolerance, and neutrophil activation leading to phagocytosis, oxidative burst, and protease and cytokine release. Thus, galectin-glycoprotein lattices at the cell surface have been proposed to function as an “on-an-off switch” that regulates cell proliferation, differentiation and survival, including immune cell responsiveness and tolerance (Dam and Brewer 2008; Brewer et al. 2002).
Fig. 3. Biological roles of galectins upon binding to the cell surface.
Galectins can bind to glycans on neighboring cells or to cells and the extracellular matrix, leading to cell adhesion, or to the surface of a single cell resulting in the formation of lattices, and the activation of signaling pathways
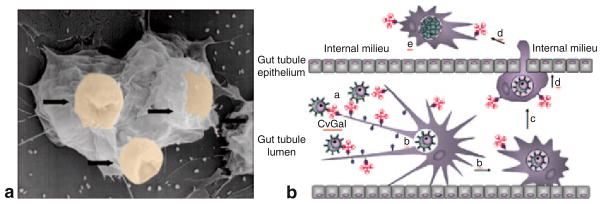
Fig. 4. The role of hemocyte galectins on parasite host invasion.
a: SEM of oyster hemocytes in the process of engulfing Perkinsus marinus trophozoites (indicated by arrows) in vitro (Gauthier and Vasta; 71). b: Cartoon of the hypothetical process that takes place in the gut and other epithelial surfaces: The oyster galectin CvGal mediates recognition and phagocytosis of P. marinus trophozoites by hemocytes; the trophozoites survive inside the oyster hemocytes, which migrate from the gut via a trans-epithelia route and are transported to other tissues where the parasite proliferates (Tasumi and Vasta 2007)
Since their discovery, galectins have been proposed to participate in embryogenesis, development, and neoplasia. This has been based on their binding to “self” carbohydrate moieties, such as polylactosamine-containing glycans, abundant at the cell surface and the ECM (Fig 3). Chicken galectins have been proposed to participate in myoblast fusion, whereas murine galectin-1 and galectin-3 would have roles in notochord development, somitogenesis, and development of muscle tissue and central nervous system (Cooper et al. 1991; Watt et al. 2004; Georgiadis et al. 2007; Fowlis et al. 1995). Despite the increasing availability of genetically modified mice, however, strains carrying null mutations for some galectins have failed to display overt developmental phenotypes (Colnot et al. 1998; Puche et al. 1996; Colnot et al. 2001). Thus, other genetically tractable model organisms endowed with a less diversified galectin repertoire such as Drosophila and zebrafish have become attractive alternatives for these selected galectins, with promising results (Pace et al. 2002; Ahmed et al. 2004).
In the past few years it has been shown that galectins participate in regulation of both innate and adaptive immunity (Vasta 2009; Rabinovich et al. 2002; van Die and Cummings 2010). The recently proposed roles of galectins in immune functions have been further supported by their ability to directly recognize microbial pathogens (Vasta 2009), a property well characterized for other lectin types, such as C- and F-lectins, ficolins, and pentraxins. Although the roles of lectins in non-self recognition are particularly critical in invertebrates, since these organisms lack immunoglobulins and rely solely in innate immune mechanisms for recognition of potential microbial pathogens (Vasta et al. 1999), susceptibility/resistance to several infectious diseases in humans are determined by the presence of certain lectin alleles (Dias-Baruffi et al. 2010). Galectins from both invertebrates and vertebrates recognize a variety of viral and bacterial pathogens and protozoan parasites (Reviewed in Vasta 2009).
Galectins are ubiquitously expressed and distributed in mammalian tissues, including most cells of the innate (dendritic cells, macrophages, mast cells, natural killer cells, gamma/delta T cells, and B-1 cells) and adaptive (activated B and T cells) immune system, and as in other cell types (Stowell et al. 2008; Rabinovich et al. 2007a). Since the early 1990s a growing body of experimental (in vivo and in vitro) evidence has accumulated to support the roles of galectins expressed by these cells and neighboring stromal cells in the development and regulation of innate and adaptive immunity homeostasis as well as responses to infectious and allergic challenge, and cancer. Galectins released by stromal cells in central compartments contribute to the differentiation of immune cell precursors. Immune challenge and several pathological conditions, may lead to further activation and differentiation of immune cells, and modulate the expression and release of galectins to the extracellular space where they may have autocrine or paracrine effects on immune regulation. Galectins released by immune cells can oligomerize and form lattices at the cell surface leading to activation of transmembrane signaling pathways that modulate immune cell functions, including for example, cell adhesion and migration, T-cell apoptosis, and the Th1/Th2 cytokine balance (Rabinovich et al. 2002, 2007a, 2007b). Further, galectins released into the extracellular environment under abnormal situations may constitute “danger signals”, or by exerting their activities on other cells, such as mast cells, induce degranulation and release of factors (e.g., histamine) that represent the “danger signals” leading to activation of immune mechanisms in the absence of antigenic challenge (Sato and Nieminen 2004).
Galectins have diverse effects on cells involved in innate immune responses, including macrophages and dendritic cells, neutrophils, eosinophils, and mast cells. Galectin-1 participates in acute and allergic inflammation and displays anti-inflammatory activities by blocking or attenuating signaling events that lead to leukocyte infiltration, migration, and recruitment (Stowell et al. 2008). It also displays various other effects on innate immunity, including cell surface exposure of phosphatidyl-serine in activated neutrophils, a process that leads to neutrophil removal by phagocytic cells without causing apoptosis, and activation/deactivation of macrophages on a concentration-dependent manner. In contrast to the anti-inflammatory effects of galectin-1, galectin-3 shows pro-inflammatory activity. Galectin-3 is normally expressed in various epithelia and inflammatory cells, such as activated macrophages, dendritic cells, and Kupffer cells, and is upregulated during inflammation, cell proliferation, and cell differentiation. Galectin-3 also exhibits anti-apoptotic activity for macrophages and enhances their interactions with basal lamina glycans, such as laminin and fibronectin. Taken together, these observations strongly suggest that galectin-3 enhances macrophage survival, and positively modulates their recruitment and anti-microbial activity. Galectin-9 is a selective chemoattractant for eosinophils, highly expressed in various tissues of the immune system, such as bone marrow, spleen, thymus, and lymph nodes. Gal-9 released from activated T cells induces chemotaxis, activation, oxidative activity, and degranulation of eosinophils, and monocyte-derived DC maturation (Stowell et al. 2007; Zuñiga et al. 2001; Liu and Hsu 2007; Hirashima et al. 2004).
Concerning adaptive immune responses, galectins have been proposed as regulators of immune cell homeostasis (Rabinovich et al. 2002). Interactions between stromal cells from the bone marrow and thymic compartments and lymphocyte precursors are critical to their development, selection, and further progression to the periphery. In this regard, interactions mediated by galectins can modulate B-cell maturation and differentiation both at the central and peripheral immune compartments (Rossi et al. 2006) Similarly, from their early developmental stages in the thymic compartment to the removal of the mature activated T cells in the periphery, the regulation of T-cell survival is critical to a controlled immune response. Galectin-1 can regulate T-cell proliferation and apoptosis through binding and clustering of lactosamine-rich cell surface glycoconjugates into segregated membrane micro-domains (Rabinovich et al. 2007b). Galectin-1 may have pro- or anti-apoptotic effects on T cells depending on the developmental stage and activation status of the cell, and the microenvironment in which the exposure takes place. The effects of galectin-3 in T-cell survival, however, are dependent on whether protein is produced endogenously (anti-apoptotic) or by exogenous exposure (pro-apoptotic) (Liu and Hsu 2007). Galectins also exert regulatory functions in T-cell homeostasis, and signaling cascades triggered by their binding and lattice formation at the T-cell surface has implications in a variety of downstream events that modulate their differentiation, functional activation, and production of pro- and anti-inflammatory cytokines. The effects of galectins on T-cell cytokine synthesis and secretion ultimately determines the Th1/Th2 polarization of the immune response. By reducing IFN-γ and IL-2 and enhancing IL-5, IL-10, and TGF-β production, galectin-1 skews the balance from a Th1- toward a Th2- polarized response, whereas by reducing IL-5 levels, galectin-3 has the opposite effect (Yang et al. 2008). Finally, given the regulatory roles of galectins on cells that mediate both innate and adaptive immune responses, their effects can be beneficial or detrimental to pathological conditions that have a basis on exacerbated or depressed immune function, such as inflammatory, allergic and autoimmune disorders, and cancer (Yang et al. 2008).
4 Galectins as Pattern Recognition Receptors
Recently, galectins have been discovered to bind glycans on the surface of viruses, bacteria, protista, and fungi (reviewed in Vasta 2009). Thus, the potential role of galectins as pattern recognition receptors (PRRs) has become an area of increased attention. Furthermore, the considerable diversity of the galectin repertoire in each organism and the substantial or subtle variations in the specificity of each galectin towards the target glycans, which are determined by oligosaccharide repeats, branchings or substitutions, suggest that there is extensive diversity and plasticity in the capacity of galectins for non-self recognition. The presence of canonical and extended CRDs, and the carbohydrate-independent binding properties of the N-terminus region of galectin-3, further suggests that galectins have a substantially diversified recognition capacity. Moreover, because galectins from all three types (proto, chimera, or TR) can form oligomers, their multivalent binding properties, including increased avidity, clearly enable galectins to participate effectively both in direct recognition of pathogens and parasites, and downstream processes that lead to modulation of innate and adaptive immune responses. Whether galectin-mediated recognition is an effective defence mechanism with a clear benefit for the host is not entirely clear, except for a few examples. It is noteworthy that a particular glycan on the surface of a microorganism or parasite can be recognized by multiple galectins, and that the outcome of the interaction differs considerably depending on the galectin type involved and the concentration of the galectin in a particular cell surface or extracellular microenvironment. This, in turn, determines the level of oligomerization and cooperative binding to ligand, and the potentially antagonistic or synergistic activation of pathogen signaling pathways (e.g., modulation of immune activation, or cytokine production and secretion) (Rabinovich et al. 2007b).
5 Some Microbial Pathogens and Parasites Subvert the Role of Galectins as PRRs
In some cases, the microbe’s recognition by the vector or host galectins promote its adhesion, host cell entry, or infection persistence, in addition to modulating the host’s immune responses. Thus, these pathogens and parasites would “subvert” the roles of host or vector galectins as PRRs, to attach to or gain entry into their cells. This is clearly illustrated by the participation of galectin interactions in the infection mechanisms of HIV. In contrast to the inhibitory role of galectin-1 in paramixovirus-mediated cell fusion, galectin-1, which is abundant in organs that represent major reservoirs for HIV-1, such as the thymus and lymph nodes, promotes infection by HIV-1 by facilitating viral attachment to CD4 receptor, and increasing infection efficiency (Ouellet et al. 2005; Mercier et al. 2008). Recent studies showed that galectin-1 enhances HIV adsorption kinetics on monocyte-derived host macrophages, which facilitates HIV-1 infectivity by shortening the time required to establish an infection. Further, galectin-1 would also function as a soluble scavenger receptor and enhance the uptake of the virus by macrophages, which together with evidence that galectin-1 is present in the ejaculate and the heads and tails of late spermatids, led to extend the proposal that galectin-1 may also facilitate sexual transmission of HIV-1 (Mercier et al. 2008). This would take place through enhancement of viral adsorption kinetics on the target cells’ surface by the galectin-1 released by sheared fibroblasts and epithelial cells following sex-related micro-abrasions. Gal-3 has no effect on HIV-1 adsorption, entry, or infection, although its expression is upregulated by the HIV Tat protein in several human cell lines, and in cells infected with other retroviruses, suggesting that it may participate in regulation of antiviral immunity (Fogel et al. 1999; Schroder et al. 1995; Hsu et al. 1996). This underscores the relevance of the subtle differences in galectin specificity and affinity that may determine very different recognition and effector outcomes. It is noteworthy that HIV also uses recognition by DC-SIGN, a C-type lectin, to enter dendritic cells, thereby underscoring the multiple adaptations of the viral glycome for host infection (Ouellet et al. 2005; Mercier et al. 2008).
Leishmania species, which spend part of their life cycle in phlebotomine sandflies that constitute vectors for transmission to the vertebrate hosts, are also illustrative examples. Upon the sandfly feeding on blood from an infected host, the ingested amastigotes mature into promastigotes, which attach to the insect midgut epithelium to prevent their excretion along with the digested bloodmeal, and undergo numerous divisions before differentiating into free-swimming infective metacyclics (Kamhawi 2006). Although the involvement of the parasite LPG in this interaction had been suspected from prior studies, the specific Phlebotomus papatasi sandfly midgut receptor for the procyclic L. major LPG was identified as a 35.4-kDa TR galectin (PpGalec) only expressed by epithelial midgut cells, and upregulated in the blood-feeding females (Kamhawi et al. 2004). Because the binding specificity of PpGalec is restricted to Leishmania promastigotes bearing poly-Gal(β1–3) side chains on their LPG, it was proposed that it is the carbohydrate moiety responsible for specific binding of L. major to P. papatasi midgut linings. The assembly of poly-galactose epitopes is downregulated during L. major metacyclogenesis, and thus, unable to bind to rPpGalec the free-swimming infective metacyclic promastigotes are released from the midgut for transmission from the sandfly to the mammalian host (Kamhawi et al. 2004).
The protozoan parasite Perkinsus marinus is a facultative intracellular parasite that causes “Dermo” disease in the eastern oyster Crassostrea virginica, and is responsible for catastrophic damage to shellfisheries and the estuarine environment in North America (Harvell et al. 1999). The infection mechanism remains unclear, but it is likely that while filter feeding, the healthy oysters ingest P. marinus trophozoites released to the water column by the infected neighboring individuals. Inside oyster phagocytic cells (hemocytes), trophozoites resist oxidative killing, proliferate, and spread throughout the host. It was recently discovered that oyster hemocytes recognize P. marinus via a novel galectin (CvGal) that displays four canonical galectin CRDs, a domain organization unlike any of the known galectin types (Tasumi and Vasta 2007). Two amino acid residues (His53 and Asp55) that interact with the NAc group via a water molecule are missing in all four CvGal CRDs resulting in broader carbohydrate specificity. CvGal is present in the cytoplasm of circulating granulocytes, and upon their attachment and spreading it is translocated to the periphery, secreted, and binds to the cell surface. The remaining galectin is released to the extracellular environment, where it may bind to all other circulating (non-activated) granulocytes and hyalinocytes. The most surprising observation, however, was that the soluble CvGal also binds in a carbohydrate-specific manner to a wide variety of microorganisms, phytoplankton components, and preferentially, to Perkinsus spp trophozoites, suggesting a direct role in recognition and opsonization of potential microbial pathogens, as well as algal food. The partial inhibition of phagocytosis of P. marinus trophozoites by pre-treatment of hemocytes with anti-CvGal revealed that the hemocyte surface-associated CvGal is a phagocytosis receptor for P. marinus. Thus, P. marinus may have evolved to adapt the trophozoite’s glycocalyx to be selectively recognized by the oyster hemocyte CvGal, thereby subverting the oyster’s innate immune/feeding recognition mechanism to gain entry into the host cells (Tasumi and Vasta 2007) (Fig. 4).
A recent study identified galectin-1 as the receptor for the protozoan parasite T. vaginalis (Okumura et al. 2008) the causative agent of the most prevalent non-viral sexually transmitted human infection in both women and men. As an obligate extracellular parasite, establishment and persistence of T. vaginalis infection requires adherence to the host epithelial cell surface. Like Leishmania spp, T. vaginalis displays a surface LPG rich in galactose and N-acetyl glucosamine, which is recognized in a carbohydrate-dependent manner by galectin-1 expressed by the epithelial cells in the cervical linings, as well as placenta, prostate, endometrial, and decidual tissue, also colonized by the parasite (Okumura et al. 2008).
6 Conclusions
Recent studies clearly indicate that galectins can function as PRRs that target lactosamine-containing oligosaccharides on the surface of virus, bacteria, protista, and helminth pathogens and parasites. A perplexing paradox arises, however, by the fact that galectins also recognize lactosamine-containing glycans on the cell surface of the host for development and regulation of immune homeostasis. According to the Medzhitov and Janeway model (2002) for non-self recognition, PRRs recognize pathogens via highly conserved microbial surface molecules of wide distribution such as lipopolysaccharide or peptidoglycan (pathogen-associated molecular patterns [PAMPs]), which are absent in the host. Hence, this would not rigorously apply to galectins, which apparently bind the same self/non-self molecular pattern. This paradox underscores first, an oversimplification in the use of the PRR/PAMP terminology, which although it has been useful and is currently widespread, it should be use with great caution. Second, and most importantly, it reveals the significant gaps in our knowledge about the actual diversity in recognition of the host galectin repertoire, and the dynamic and mechanistic aspects of the subcellular compartamentalization and secretion of its components, as well as the detailed structural and biophysical aspects of their interactions with the microbial carbohydrate moieties. The microbial and host glycomes and their receptors continuously evolve to escape mutual recognition, a process known as the “Red Queen effect” (Varki 2006), by which the microbe avoids recognition by the host innate immune receptors (PRRs) and, the host by the microbial colonization factors (agglutinins, adhesins, and lectins). Given the key roles played by galectins in host development and immunoregulation by the recognition of “self” lactosamine moieties, strong functional constraints would prevent galectins from dramatic evolutionary changes in carbohydrate specificity, which is to some extent supported by the apparent structural conservation within this lectin family. Further, with the current evidence about how pathogens and parasites, which display a remarkable evolutionary plasticity, efficiently subvert the roles of galectins to attach or gain entrance into the host cells, it seems more plausible that instead of avoiding recognition by the host, they would have evolved their glycocomes to mimic their hosts’ in a “Trojan horse” model (Tasumi and Vasta 2007), and rely on the host’s self-recognition molecules such as galectins for attachment to the vector or host invasion. It is noteworthy that most of (if not all) these pathogens and parasites are endowed with diverse and powerful mechanisms to evade intra-cellular killing by the host, and/or down-regulate downstream immune responses. The complex strategies developed by microbial pathogens to successfully colonize, enter, proliferate, and disseminate within and among their vectors or hosts, are the products of strong selective pressures that have led to adaptations that ensure their survival in the most hostile environment of all, and thus represent a significant challenge for the development of novel strategies for intervention in human disease.
Acknowledgments
Research in the author’s lab reviewed here was supported by grant 5R01GM070589-06 from the National Institutes of Health, and grants IOB-0618409 and IOS0822257 from the National Science Foundation.
References
- Ahmed H, Vasta GR. Galectins: conservation of functionally and structurally relevant amino acid residues defines two types of carbohydrate recognition domains. Glycobiology. 1994;4:548. doi: 10.1093/glycob/4.5.545. [DOI] [PubMed] [Google Scholar]
- Ahmed H, Vasta GR. Unlike mammalian GRIFIN, the zebrafish homologue (DrG-RIFIN) represents a functional carbohydrate-binding galectin. Biochem Biophys Res Commun. 2008;371:355. doi: 10.1016/j.bbrc.2008.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed H, Bianchet MA, Amzel LM, Hirabayashi J, Kasai KI, Giga-Hama Y, Tohda H, Vasta GR. Novel carbohydrate specificity of the 16-kDa galectin from Caenorhabditis elegans: binding to blood group precursor oligosaccharides (type 1, type 2, Talpha, and Tbeta) and gangliosides. Glycobiology. 2002;12:461. doi: 10.1093/glycob/cwf052. [DOI] [PubMed] [Google Scholar]
- Ahmed H, Du SJ, O’Leary N, Vasta GR. Biochemical and molecular characterization of galectins from zebrafish (Danio rerio): notochord-specific expression of a prototype galectin during early embryogenesis. Glycobiology. 2004;14:232. doi: 10.1093/glycob/cwh032. [DOI] [PubMed] [Google Scholar]
- Bianchet MA, Ahmed H, Vasta GR, Amzel LM. Soluble beta-galactosyl-binding lectin (galectin) from toad ovary: crystallographic studies of two protein-sugar complexes. Proteins. 2000;40:388. doi: 10.1002/1097-0134(20000815)40:3<378::aid-prot40>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Brewer CF, Miceli MC, Baum LG. Clusters, bundles, arrays and lattices: novel mechanisms for lectin-saccharide-mediated cellular interactions. Curr Opin Struct Biol. 2002;12:623. doi: 10.1016/s0959-440x(02)00364-0. [DOI] [PubMed] [Google Scholar]
- Cho M, Cummings RD. Galectin-1, a beta-galactoside -binding lectin in Chinese hamster ovary cells. II. Localization and biosynthesis. J Biol Chem. 1995;270:5212. doi: 10.1074/jbc.270.10.5207. [DOI] [PubMed] [Google Scholar]
- Cleves AE, Cooper DN, Barondes SH, Kelly RB. A new pathway for protein export in Saccharomyces cerevisiae. J Cell Biol. 1996;133:1026. doi: 10.1083/jcb.133.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colnot C, Fowlis D, Ripoche MA, Bouchaert I, Poirier F. Embryonic implantation in galectin 1/galectin 3 double mutant mice. Dev Dyn. 1998;211:306–313. doi: 10.1002/(SICI)1097-0177(199804)211:4<306::AID-AJA2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Colnot C, Ripoche M, Fowlis D, Cannon V, Scaerou F, Cooper DNW, Poirier F. The role of galectins in mouse development. Trends Glycosci Glycotechnol. 1997;9:31–40. [Google Scholar]
- Colnot C, Sidhu SS, Balmain N, Poirier F. Uncoupling of chondrocyte death and vascular invasion in mouse galectin 3 null mutant bones. Dev Biol. 2001;229:214. doi: 10.1006/dbio.2000.9933. [DOI] [PubMed] [Google Scholar]
- Cooper DNW. Galectinomics: finding themes in complexity. Biochim Biophys Acta. 2002;1572:231. doi: 10.1016/s0304-4165(02)00310-0. [DOI] [PubMed] [Google Scholar]
- Cooper DN, Massa SM, Barondes SH. Endogenous muscle lectin inhibits myoblast adhesion to laminin. J Cell Biol. 1991;115:1448. doi: 10.1083/jcb.115.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam TK, Brewer CF. Effects of clustered epitopes in multivalent ligand-receptor interactions. Biochemistry. 2008;47:8476. doi: 10.1021/bi801208b. [DOI] [PubMed] [Google Scholar]
- Dias-Baruffi M, Stowell SR, Song SC, Arthur CM, Cho M, Rodrigues LC, Montes MAB, Rossi MA, James JA, McEver RP, Cummings RD. Differential expression of immunomodulatory galectin-1 in peripheral leukocytes and adult tissues and its cytosolic organization in striated muscle. Glycobiology. 2010;20:507. doi: 10.1093/glycob/cwp203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang R, Mantle M, Ceri H. Characterization of quail intestinal mucin as a ligand for endogenous quail lectin. Biochem J. 1993;293:867. doi: 10.1042/bj2930867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel S, Guittaut M, Legrand A, Monsigny M, Hebert E. The tat protein of HIV-1 induces galectin-3 expression. Glycobiology. 1999;9:383–387. doi: 10.1093/glycob/9.4.383. [DOI] [PubMed] [Google Scholar]
- Fowlis D, Colnot C, Ripoche MA, Poirier F. Galectin-3 is expressed in the notochord, developing bones, and skin of the postimplantation mouse embryo. Dev Dyn. 1995;203:251. doi: 10.1002/aja.1002030211. [DOI] [PubMed] [Google Scholar]
- Gabius HJ. Animal lectins. Eur J Biochem. 1997;243:543. doi: 10.1111/j.1432-1033.1997.t01-1-00543.x. [DOI] [PubMed] [Google Scholar]
- Georgiadis V, Stewart HJS, Pollard HJ, Tavsanoglu Y, Prasad R, Horwood J, Deltour L, Goldring K, Poirier F, Lawrence-Watt DJ. Lack of galectin-1 results in defects in myoblast fusion and muscle regeneration. Dev Dyn. 2007;236:1024. doi: 10.1002/dvdy.21123. [DOI] [PubMed] [Google Scholar]
- Gorski JP, Liu FT, Artigues A, Castagna LF, Osdoby P. New alternatively spliced form of galectin-3, a member of the beta-galactoside -binding animal lectin family, contains a predicted transmembrane-spanning domain and a leucine zipper motif. J Biol Chem. 2002;277:18848. doi: 10.1074/jbc.M109578200. [DOI] [PubMed] [Google Scholar]
- Harvell CD, Kim K, Burkholder JM, Colwell RR, Epstein PR, Grimes DJ, Hofmann EE, Lipp EK, Osterhaus AD, Overstreet RM, Porter JW, Smith GW, Vasta GR. Emerging marine diseases–climate links and anthropogenic factors. Science. 1999;285:1510. doi: 10.1126/science.285.5433.1505. [DOI] [PubMed] [Google Scholar]
- Hirabayashi J, Kasai K. The family of metazoan metal-independent beta-galactoside -binding lectins: structure, function and molecular evolution. Glycobiology. 1993;3:304. doi: 10.1093/glycob/3.4.297. [DOI] [PubMed] [Google Scholar]
- Hirashima M, Kashio Y, Nishi N, Yamauchi A, Imaizumi TA, Kageshita T, Saita N, Nakamura T. Galectin-9 in physiological and pathological conditions. Glycoconj J. 2004;19:593. doi: 10.1023/B:GLYC.0000014090.63206.2f. [DOI] [PubMed] [Google Scholar]
- Houzelstein D, Goncalves IR, Fadden AJ, Sidhu SS, Cooper DNW, Drickamer K, Leffler H, Poirier F. Phylogenetic analysis of the vertebrate galectin family. Mol Biol Evol. 2004;21:1187. doi: 10.1093/molbev/msh082. [DOI] [PubMed] [Google Scholar]
- Hsu DK, Hammes SR, Kuwabara I, Greene WC, Liu FT. Human T lymphotropic virus-I infection of human T lymphocytes induces expression of the beta-galactoside -binding lectin, galectin-3. Am J Pathol. 1996;148:1670. [PMC free article] [PubMed] [Google Scholar]
- Kamhawi S. Phlebotomine sand flies and Leishmania parasites: friends or foes? Trends Parasitol. 2006;22:439. doi: 10.1016/j.pt.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Kamhawi S, Ramalho-Ortigao M, Pham VM, Kumar S, Lawyer PG, Turco SJ, Barillas-Mury C, Sacks DL, Valenzuela JG. A role for insect galectins in parasite survival. Cell. 2004;119:329. doi: 10.1016/j.cell.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Leffler H, Carlsson S, Hedlund M, Qian Y, Poirier F. Introduction to galectins. Glycoconj J. 2004;19:440. doi: 10.1023/B:GLYC.0000014072.34840.04. [DOI] [PubMed] [Google Scholar]
- Liao DI, Kapadia G, Ahmed H, Vasta GR, Herzberg O. Structure of S-lectin, a developmentally regulated vertebrate beta-galactoside -binding protein. Proc Natl Acad Sci. 1994;91:1432. doi: 10.1073/pnas.91.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipkowitz MS, Leal-Pinto E, Cohen BE, Abramson RG. Galectin 9 is the sugarregulated urate transporter/channel UAT. Glycoconj J. 2004;19:491. doi: 10.1023/B:GLYC.0000014078.65610.2f. [DOI] [PubMed] [Google Scholar]
- Liu FT, Hsu DK. The role of galectin-3 in promotion of the inflammatory response. Drug News Perspect. 2007;20:460. doi: 10.1358/dnp.2007.20.7.1149628. [DOI] [PubMed] [Google Scholar]
- Lobsanov YD, Gitt MA, Leffler H, Barondes SH, Rini JM. X-ray crystal structure of the human dimeric S-Lac lectin, L-14-II, in complex with lactose at 2.9-A resolution. J Biol Chem. 1993;268:27038. doi: 10.2210/pdb1hlc/pdb. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA. Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- Mercier S, St-Pierre C, Pelletier I, Ouellet M, Tremblay MJ, Sato S. Galectin-1 promotes HIV-1 infectivity in macrophages through stabilization of viral adsorption. Virology. 2008;371:129. doi: 10.1016/j.virol.2007.09.034. [DOI] [PubMed] [Google Scholar]
- Morris S, Ahmad N, Andre S, Kaltner H, Gabius HJ, Brenowitz M, Brewer F. Quaternary solution structures of galectins -1, -3, and -7. Glycobiology. 2004;14:300. doi: 10.1093/glycob/cwh029. [DOI] [PubMed] [Google Scholar]
- Ogden AT, Nunes I, Ko K, Wu S, Hines CS, Wang AF, Hegde RS, Lang RA. GRIFIN, a novel lens-specific protein related to the galectin family. J Biol Chem. 1998;273:28896. doi: 10.1074/jbc.273.44.28889. [DOI] [PubMed] [Google Scholar]
- Okumura CY, Baum LG, Johnson PJ. Galectin-1 on cervical epithelial cells is a receptor for the sexually transmitted human parasite Trichomonas vaginalis. Cell Microbiol. 2008;10:2078. doi: 10.1111/j.1462-5822.2008.01190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouellet M, Mercier S, Pelletier I, Bounou S, Roy J, Hirabayashi J, Sato S, Tremblay MJ. Galectin-1 acts as a soluble host factor that promotes HIV-1 infectivity through stabilization of virus attachment to host cells. J Immunol. 2005;174:4126. doi: 10.4049/jimmunol.174.7.4120. [DOI] [PubMed] [Google Scholar]
- Pace KE, Lebestky T, Hummel T, Arnoux P, Kwan K, Baum LG. Characterization of a novel Drosophila melanogaster galectin. Expression in developing immune, neural, and muscle tissues. J Biol Chem. 2002;277:13091. doi: 10.1074/jbc.M112105200. [DOI] [PubMed] [Google Scholar]
- Partridge EA, Le Roy C, Di Guglielmo GM, Pawling J, Cheung P, Granovsky M, Nabi IR, Wrana JL, Dennis JW. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science. 2004;306:120. doi: 10.1126/science.1102109. [DOI] [PubMed] [Google Scholar]
- Patterson RJ, Dagher SF, Vyakarnam A, Wang JL. Nuclear galectins: functionally redundant components in processing of pre-mRNA. Trends Glycosci Glycotechnol. 1997;9:77. [Google Scholar]
- Puche AC, Poirier F, Hair M, Bartlett PF, Key B. Role of galectin-1 in the developing mouse olfactory system. Dev Biol. 1996;179:274. doi: 10.1006/dbio.1996.0257. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Liu FT, Hirashima M, Anderson A. An emerging role for ga-lectins in tuning the immune response: lessons from experimental models of inflammatory disease, autoimmunity and cancer. Scand J Immunol. 2007a;66:143. doi: 10.1111/j.1365-3083.2007.01986.x. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Rubinstein N, Toscano MA. Role of galectins in inflammatory and immunomodulatory processes. Biochim Biophys Acta. 2002;1572:274. doi: 10.1016/s0304-4165(02)00314-8. [DOI] [PubMed] [Google Scholar]
- Rabinovich GA, Toscano MA, Jackson SS, Vasta GR. Functions of cell surface galectin-glycoprotein lattices. Curr Opin Struct Biol. 2007b;17:520. doi: 10.1016/j.sbi.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi B, Espeli M, Schiff C, Gauthier L. Clustering of pre-B cell integrins induces galectin-1-dependent pre-B cell receptor relocalization and activation. J Immunol. 2006;177:796. doi: 10.4049/jimmunol.177.2.796. [DOI] [PubMed] [Google Scholar]
- Saouros S, Edwards-Jones B, Reiss M, Sawmynaden K, Cota E, Simpson P, Dowse TJ, Jakle U, Ramboarina S, Shivarattan T, Matthews S, Soldati-Favre D. A novel galectin-like domain from Toxoplasma gondii micronemal protein 1 assists the folding, assembly, and transport of a cell adhesion complex. J Biol Chem. 2005;280:38591. doi: 10.1074/jbc.C500365200. [DOI] [PubMed] [Google Scholar]
- Sato S, Hughes RC. Binding specificity of a baby hamster kidney lectin for H type I and II chains, polylactosamine glycans, and appropriately glycosylated forms of laminin and fibronectin. J Biol Chem. 1992;267:6990. [PubMed] [Google Scholar]
- Sato S, Hughes RC. Regulation of secretion and surface expression of Mac-2, a ga-lactoside -binding protein of macrophages. J Biol Chem. 1994;269:4424. [PubMed] [Google Scholar]
- Sato S, Nieminen J. Seeing strangers or announcing “danger”: Galectin-3 in two models of innate immunity. Glycoconj J. 2004;19:583. doi: 10.1023/B:GLYC.0000014089.17121.cc. [DOI] [PubMed] [Google Scholar]
- Schroder HC, Ushijima H, Theis C, Seve AP, Hubert J, Muller WE. Expression of nuclear lectin carbohydrate-binding protein 35 in human immunodeficiency virus type 1-infected Molt-3 cells. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:340. [PubMed] [Google Scholar]
- Schwarz FP, Ahmed H, Bianchet MA, Amzel LM, Vasta GR. Thermodynamics of bovine spleen galectin-1 binding to disaccharides: correlation with structure and its effect on oligomerization at the denaturation temperature. Biochemistry. 1998;37:5877. doi: 10.1021/bi9716478. [DOI] [PubMed] [Google Scholar]
- Seetharaman J, Kanigsberg A, Slaaby R, Leffler H, Barondes SH, Rini JM. X-ray crystal structure of the human galectin-3 carbohydrate recognition domain at 2.1-A resolution. J Biol Chem. 1998;273:13047. doi: 10.1074/jbc.273.21.13047. [DOI] [PubMed] [Google Scholar]
- Shoji H, Nishi N, Hirashima M, Nakamura T. Characterization of the Xenopus ga-lectin family. Three structurally different types as in mammals and regulated expression during embryogenesis. J Biol Chem. 2003;278:12293. doi: 10.1074/jbc.M209008200. [DOI] [PubMed] [Google Scholar]
- Sparrow CP, Leffler H, Barondes SH. Multiple soluble beta-galactoside -binding lectins from human lung. J Biol Chem. 1987;262:7390. [PubMed] [Google Scholar]
- Stalz H, Roth U, Schleuder D, Macht M, Haebel S, Strupat K, Peter-Katalinic J, Hanisch FG. The Geodia cydonium galectin exhibits prototype and chimera -type characteristics and a unique sequence polymorphism within its carbohydrate recognition domain. Glycobiology. 2006;16:414. doi: 10.1093/glycob/cwj086. [DOI] [PubMed] [Google Scholar]
- Stowell SR, Karmakar S, Stowell CJ, Dias-Baruffi M, McEver RP, Cummings RD. Human galectin-1, -2, and -4 induce surface exposure of phosphatidylserine in activated human neutrophils but not in activated T cells. Blood. 2007;109:227. doi: 10.1182/blood-2006-03-007153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowell SR, Qian Y, Karmakar S, Koyama NS, Dias-Baruffi M, Leffler H, McEver RP, Cummings RD. Differential roles of galectin-1 and galectin-3 in regulating leuko-cyte viability and cytokine secretion. J Immunol. 2008;180:3102. doi: 10.4049/jimmunol.180.5.3091. [DOI] [PubMed] [Google Scholar]
- Tasumi S, Vasta GR. A galectin of unique domain organization from hemocytes of the Eastern oyster (Crassostrea virginica) is a receptor for the protistan parasite Perkinsus marinus. J Immunol. 2007;179:3098. doi: 10.4049/jimmunol.179.5.3086. [DOI] [PubMed] [Google Scholar]
- van Die I, Cummings RD. Glycan gimmickry by parasitic helminths: a strategy for modulating the host immune response? Glycobiology. 2010;20:2. doi: 10.1093/glycob/cwp140. [DOI] [PubMed] [Google Scholar]
- Varki A. Nothing in glycobiology makes sense, except in the light of evolution. Cell. 2006;126:841. doi: 10.1016/j.cell.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Vasta GR. Roles of galectins in infection. Nat Rev Microbiol. 2009;7:424. doi: 10.1038/nrmicro2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasta GR, Quesenberry M, Ahmed H, O’Leary N. C-type lectins and galectins mediate innate and adaptive immune functions: their roles in the complement activation pathway. Dev Comp Immunol. 1999;23:401. doi: 10.1016/s0145-305x(99)00020-8. [DOI] [PubMed] [Google Scholar]
- Walser PJ, Kues U, Aebi M, Kunzler M. Ligand interactions of the Coprinopsis cinerea galectins. Fungal Genet Biol. 2005;42:305. doi: 10.1016/j.fgb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Watt DJ, Jones GE, Goldring K. The involvement of galectin-1 in skeletal muscle determination, differentiation and regeneration. Glycoconj J. 2004;19:615. doi: 10.1023/B:GLYC.0000014093.23509.92. [DOI] [PubMed] [Google Scholar]
- Yang RY, Rabinovich GA, Liu FT. Galectins: structure, function and therapeutic potential. Expert Rev Mol Med. 2008;10 doi: 10.1017/S1462399408000719. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Cummings RD. The S-type lectin from calf heart tissue binds selectively to the carbohydrate chains of laminin. Arch Biochem Biophys. 1990;281:35. doi: 10.1016/0003-9861(90)90408-q. [DOI] [PubMed] [Google Scholar]
- Zuñiga E, Gruppi A, Hirabayashi J, Kasai KI, Rabinovich GA. Regulated expression and effect of galectin-1 on Trypanosoma cruzi-infected macrophages: modulation of microbicidal activity and survival. Infect Immun. 2001;69:6804. doi: 10.1128/IAI.69.11.6804-6812.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]