Abstract
Objective
Evaluate the mechanism(s) for improved exercise capacity after endurance exercise training (ET) in elderly patients with heart failure and preserved ejection fraction (HFPEF). Background: Exercise intolerance, measured objectively by reduced peak oxygen consumption (VO2), is the primary chronic symptom in HFPEF and is improved by ET. However, the mechanism(s) are unknown.
Methods
Forty stable, compensated HFPEF outpatients (mean age 69 ± 6 yrs) were examined at baseline and after 4 months of ET (n=22) or attention control (n=18). VO2 and its determinants were assessed during rest and peak upright cycle exercise.
Results
Following ET, peak VO2 was higher than controls (16.3 ± 2.6 vs. 13.1 ± 3.4 ml/kg/min; p=0.002). This was associated with higher peak heart rate (139 ± 16 vs. 131 ± 20 beats/min; p=0.03), but no difference in peak end-diastolic volume (77 ± 18 vs. 77 ± 17 ml; p=0.51), stroke volume (48 ± 9 vs. 46 ± 9 ml; p=0.83), or cardiac output (6.6 ± 1.3 vs. 5.9 ± 1.5 L/min; p=0.32). However, estimated peak arterial-venous oxygen difference (A-VO2 Diff) was significantly higher in ET (19.8 ± 4.0 vs. 17.3 ± 3.7 ml/dl; p=0.03). The effect of ET on cardiac output was responsible for < 15% of the improvement in peak VO2.
Conclusions
In elderly stable compensated HFPEF patients, peak A-VO2 Diff was higher following ET and was the primary contributor to improved peak VO2. This suggests that peripheral mechanisms (improved microvascular and/or skeletal muscle function) contribute to the improved exercise capacity after ET in HFPEF.
Keywords: Heart failure, preserved ejection fraction, exercise, elderly, peripheral
Introduction
Approximately 50% or more of heart failure (HF) patients living in the community have preserved left ventricular ejection fraction (HFPEF), and the proportion is higher among women and the very elderly1, 2. As in patients with HF and reduced EF (HFREF), the primary chronic symptoms in HFPEF, even when well compensated, are those of severe exercise intolerance3-8 - dyspnea on exertion and exertional fatigue -and reduced quality of life4. However, the pathophysiology of exercise intolerance in this large and growing group of patients is not well understood and there are relatively few data regarding its treatment9.
Exercise intolerance can be objectively measured as reduced peak exercise oxygen consumption (peak VO2) by expired gas analysis, a technique that is valid and reproducible, including in elderly patients with HFPEF10, 11. In accordance with the Fick equation, reduced peak exercise VO2 results from either reduced cardiac output (CO), peripheral arterial-venous oxygen difference (A-VO2 Diff), or both. Results from several cross-sectional studies have suggested that decreased peak VO2 in elderly HFPEF patients is due to reduced peak CO secondary to blunted chronotropic, inotropic and vasodilator reserve6, 7, while others have suggested that it is due to reductions in both peak CO and A-VO2 Diff 3, 12, 13 or primarily due to reduced peak A-VO Diff secondary to impaired skeletal muscle oxidative metabolism.14
Many studies have reported that endurance exercise training improves peak VO2 in patients with HFREF,15-18 and that this improvement results from favorable changes in cardiac15,19-21, peripheral vascular19, and skeletal muscle function20, 22-24, that increase oxygen delivery to and utilization by the active muscles (i.e. increased A-VO2 Diff). In contrast, there have been only 4 studies of exercise training in patients with HFPEF25-28. In our recent report of the first randomized controlled, single-blind trial of exercise training in elderly patients with HFPEF26, we found that 4-months of endurance exercise training significantly increased peak VO2, and this was recently confirmed in a multicenter trial28. However, the mechanism(s) responsible for the improvement in peak VO2 with exercise training in HFPEF have not been examined. This can be challenging since it requires simultaneous measurement of VO2 and its determinants during peak exercise29. Thus, the aim of this study was to evaluate the acute cardiovascular and metabolic responses during upright cycle exercise before and after 4-months of endurance exercise training in elderly patients with chronic HFPEF. Our goal was to determine the mechanism(s) of the improvement in peak VO2 after exercise training, and particularly the relative contributions of the components of the Fick equation, CO and A-VO2 Diff.
Methods
Study design and subjects
The study design, subjects, and inclusion criteria have previously been described4, 26. Briefly, HFPEF patients were recruited by review of clinic visits and hospital discharge records from Wake Forest Medical Center. The subjects in this report are a subset of those from our recently reported randomized, single-blind trial of exercise training26 who had adequate acoustic windows for the evaluation of left ventricular (LV) volumes during upright rest and cycle exercise by two-dimensional echocardiographic imaging. At baseline, subjects performed a cardiopulmonary exercise test with two-dimensional echocardiography and were then randomized to either 4-months of endurance exercise training or attention control.
Cardiopulmonary Exercise Testing Protocol
Exercise testing was performed as previously described in the upright position on an electrically braked cycle ergometer with expired gas analysis (CPX-2000, MedGraphics, Minneapolis, Mn)4, 26. The initial power output was set at 12.5 watts for 2 min, increased to 25 watts for 3 min, followed thereafter by 25 watt increments every 3 min until volitional exhaustion. Peak VO2 was calculated as the highest oxygen consumed over the last 30 seconds of peak exercise.4, 26
Rest and Exercise Echocardiography
Resting and exercise echocardiograms were performed as previously described4, 13, 26 by an experienced, registered echosonographer using a Philips Sonos 5500 (Andover, MA) ultrasound imaging system fitted with multi-frequency transducer. Standard two-dimensional images were obtained in the parasternal long and short axes, and apical four and two chamber views. During exercise, the sonographer focused on capture of optimal apical 4-chamber views for LV volume assessment13. Left ventricular end-diastolic and end-systolic volumes were calculated using the single plane ellipsoid apical four-chamber area-length method30, 31 by a second experienced echosonographer who did not participate in image acquisition and who was blinded to the subject’s randomization group allocation31. Adequate images for evaluation of LV volumes were defined as a well-aligned LV without apical foreshortening and with the ventricular endocardial contours well visualized for tracing30, 32. All results were averaged from 3 single-beat digital cineloops4, 13. We have previously validated 2-D echocardiographic volume measurements of end-diastolic volume against end-diastolic volume derived from radionuclide angiography (Fick stroke volume ÷ radionuclide angiography ejection fraction) in elderly persons and reported excellent day-to-day re producibility and intra-and inter-observer variability13.
Continuous heart rate and non-invasive blood pressure (cuff sphygmomanometer) were acquired at rest and exercise. Stroke volume, CO, and systemic vascular resistance were derived from standard equations13. The A-VO2 Diff was calculated by using the Fick equation (VO2 ÷ CO)13. Circulatory power was calculated as VO2 x systolic blood pressure33.
Endurance exercise training
As previously described, medically supervised endurance exercise training was performed 3 days/week for 4 months during which time the exercise intensity progressively increased from 40% to 70% heart rate reserve26. Exercise training sessions consisted of walking on a track and cycling on a Schwinn Airdyne (Louisville, Colo) for up to as much as 60 min/session including warm-up and cool-down. Any missed sessions were made up so all subjects completed a minimum of 40 out of 48 training sessions26.
Attention control group
The attention control subjects were contacted every two weeks during which time the conversations focused on study retention, reminders and encouragement regarding attendance of upcoming study visits, and collection information on any new medical events they experienced since prior contact26. The subjects were not provided with information regarding exercise.
Statistical Analysis
Comparison of the baseline characteristics of the attention control and endurance exercise trained randomized groups were made by showing the mean and standard deviation and tested using the two-sample t-test for continuous data and percentages and tested using the Fisher’s exact test for dichotomous data or chi-square test for categorical data. Testing for the statistical significance of the effect of the exercise training was performed by analysis of covariance (ANCOVA) using the 4 month follow-up value of each outcome with the baseline measure of that outcome as a covariate. The assumption of normality of the residuals and equal variances for the ANCOVA model were checked. Because peak VO2 was positively skewed, analyses of this variable were based on logarithmic transformation. A mediation analysis was performed to estimate the effect of exercise training on peak CO and its relative contribution to the change in peak VO2 following exercise training.36 The mediation analysis consisted of fitting three ANCOVA models: Model 1: VO2=a1+b1 BVO2+c1EX, where BVO2 is baseline peak VO2 and EX is an indicator indicating being assigned to the exercise training group. The coefficient c1 estimates the overall (or total) effect being randomized to the exercise group had on peak VO2. Model 2: Mediator=a2+b2 BVO2+c2 EX+ d2VO2. If EX does not have an independent effect on the Mediator, then the variable cannot be a significant mediator for VO2. Assuming it is then Model 3: VO2=a3+b3 BVO2 + c3EX+d4Mediator. The coefficient c3 is an estimate of the independent effect of EX on peak VO2 not accounted for by EX’s effect on the mediator and its association with peak VO2. The mediation effect is estimated by c1 – c3. The percent of the effect EX had on peak VO2 that was explained by the mediator is expressed as (1- c3/c1)x100%. Goodman’s unbiased estimated of the standard error was used34. All statistical tests were performed at the 5% two-sided level of significance.
Results
Baseline characteristics
As previously reported26, a total of 46 participants completed the 4 month study (24 randomized to endurance exercise training and 22 randomized to attention control). Among these, there were 40 subjects (22 endurance exercise trained and 18 attention control) who had adequate rest and exercise echocardiographic images for the determination of LV volumes during rest and exercise and these subjects are the focus of the present report. There were no significant baseline differences in key demographic or clinical characteristics between the 2 treatment groups (Table 1). Further, there were no significant differences in any key variables in the subset of subjects in this report compared to the larger trial including age (69 vs. 71 yrs, p= 0.24); BMI (30.4 vs. 30.4, p = 0.93); weight (79.4 kg. vs. 79.2 kg, p=0.97); and percent females (88% vs. 85%, p=1.0).
Table 1. Participant Characteristics.
| Characteristic | Exercise (N=22) | Control (N=18) | P-Value |
|---|---|---|---|
| Age (years) | 70 ± 6 | 68 ± 5 | 0.43 |
| Women | 18 (82) | 17 (94) | 0.36 |
| Caucasian | 20 (91) | 14 (78) | 0.38 |
| Body Weight (kg) | 79 ± 17 | 79 ± 17 | 0.94 |
| BSA (m2) | 1.85 ± 0.21 | 1.80 ± 0.18 | 0.44 |
| BMI (kg/m2) | 29.6 ± 5.7 | 31.3 ± 7.1 | 0.40 |
| NYHA class | |||
| II | 14 (64) | 12 (67) | 1.0 |
| III | 8 (36) | 6 (33) | 1.0 |
| Diastolic Function | |||
| Normal | 5 (25) | 3 (17) | 0.70 |
| Abnormal relaxation | 13 (65) | 11 (61) | 1.0 |
| Pseudonormal | 2 (10) | 4 (22) | 0.39 |
| Hx pulmonary edema | 2 (9) | 5 (28) | 0.21 |
| Diabetes mellitus | 2 (9) | 4 (22) | 0.38 |
| Hx hypertension | 18(82) | 13 (72) | 0.71 |
| Current medication | |||
| ACE-inhibitors | 7 (32) | 3 (17) | 0.46 |
| Digoxin | 4 (18) | 3 (17) | 1.0 |
| Diuretics | 12 (55) | 11 (61) | 1.0 |
| Beta-blockers | 7 (32) | 3 (17) | 0.46 |
| Calcium Antagonists | 10 (45) | 8 (44) | 1.0 |
| Nitrates | 2 (9) | 0 (0) | 0.49 |
Data are presented as mean (SD), or count (%); BSA- body surface area; BMI- body mass index; NYHA- New York Heart Association; ACE-angiotensin converting enzyme.
Effects of Endurance Training on Variables Obtained at Rest
There were no significant differences between groups after the 4-month intervention in resting heart rate, end-diastolic volume, end-systolic volume, stroke volume, CO, systolic, diastolic or mean arterial blood pressures, systemic vascular resistance, or estimated A-VO2 Diff (Tables 2 and 3).
Table 2. Rest and Peak Exercise Oxygen Consumption and Its Determinant at Baseline and Follow-up after Exercise Training.
| Rest | Peak Exercise | Reserve (Peak - Rest) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Grp | Baseline | Follow-up | P value |
Baseline | Follow-up | P value |
Baseline | Follow-up | P value |
| VO2, ml/min | CON | 261 (51) | 256(59) | 0.68 | 998 (211) | 997 (255) | 0.003 | 735 (207) | 734 (230) | 0.001 |
| EX | 289 (100) | 271(55) | 1095 (250) | 1285 (313) | 806 (226) | 1014 (286) | ||||
| VO2, ml/kg/min |
CON | 3.4 (0.7) | 3.4 (0.9) | 0.96 | 12.9 (2.7) | 13.1 (3.4) | 0.002 | 9.4 (2.6) | 9.5 (3.0) | <0.001 |
| EX | 3.8 (1.4) | 3.5 (0.8) | 14.0 (2.5) | 16.3 (2.6) | 10.3 (2.3) | 12.8 (2.4) | ||||
| Heart Rate, b/min | CON | 77 (13) | 74 (13) | 0.62 | 133 (22) | 131 (20) | 0.03 | 56 (17) | 57 (17) | 0.01 |
| EX | 75 (17) | 71 (16) | 133 (21) | 139 (16) | 59 (19) | 69 (17) | ||||
| End Diastolic Volume, ml |
CON | 70(22) | 70 (22) | 0.41 | 76 (22) | 77 (17) | 0.51 | 6 (7) | 8 (9) | 0.20 |
| EX | 71 (22) | 72 (20) | 79 (25) | 77 (18) | 9 (9) | 5 (9) | ||||
| End Systolic Volume, ml |
CON | 31 (14) | 31 (15) | 0.61 | 31 (16) | 31(13) | 0.58 | 0 (5) | 0 (4) | 0.98 |
| EX | 30 (12) | 30 (11) | 30 (13) | 30 (11) | 0 (5) | 0 (6) | ||||
| Stroke Volume, ml | CON | 39 (11) | 39 (10) | 0.059 | 45 (10) | 46 (9) | 0.83 | 6 (5) | 8 (7) | 0.09 |
| EX | 41 (12) | 43 (10) | 49 (13) | 48 (9) | 8 (8) | 5 (5) | ||||
| Cardiac Output, L/min |
CON | 3.1 (0.7) | 2.8 (0.7) | 0.24 | 5.9 (1.4) | 5.9 (1.5) | 0.32 | 3.1 (0.9) | 3.4 (1.1) | 0.56 |
| EX | 3.1 (1.0) | 3.0 (0.8) | 6.5 (2.0) | 6.6 (1.3) | 3.4 (1.6) | 3.8 (1.0) | ||||
| Circulatory Power, ml/kg/min*mmHg |
CON | 492 (133) | 478 (180) | 0.65 | 2337 (525) | 2295 (687) | 0.002 | 1846 (523) | 1817 (608) | <0.001 |
| EX | 546 (172) | 484(102) | 2609 (643) | 3080 (712) | 2084 (592) | 2596 (670) | ||||
| A-VO2 Difference, ml/dL |
CON | 8.6 (2.0) | 9.2 (2.3) | 0.99 | 17.4 (3.9) | 17.3 (3.7) | 0.03 | 8.2 (3.0) | 7.2 (3.3) | 0.01 |
| EX | 9.7 (2.7) | 9.8 (2.7) | 17.7 (4.4) | 19.8 (4.0) | 8.3 (4.2) | 10.5 (4.2) | ||||
Data in the 4 blocks to the left of each p-value are raw means ± SD for each group at baseline and follow-up. The p-value represents comparison between the groups of the follow-up least square means after adjustment for baseline. Grp = group assignment; CON = attention control; EX= endurance exercise training.
Table 3. Rest and Peak Exercise Hemodynamics at Baseline and Following Exercise Training.
| Rest | Peak Exercise | ||||||
|---|---|---|---|---|---|---|---|
| Variable | Grp | Baseline | Follow-up | P value |
Baseline | Follow-up | P value |
| Systolic blood pressure, mmHg |
CON | 146 (22) | 140 (21) | 0.49 | 184 (25) | 178 (28) | 0.19 |
| EX | 147 (20) | 138 (16) | 186 (29) | 187 (22) | |||
| Diastolic blood pressure, mmHg |
CON | 80 (9) | 78 (9) | 0.89 | 84 (9) | 84 (8) | 0.43 |
| EX | 82 (7) | 80 (10) | 88 (9) | 89 (10) | |||
| Mean arterial pressure, mmHg |
CON | 102 (12) | 99 (13) | 0.77 | 117 (14) | 116 (14) | 0.22 |
| EX | 104 (9) | 99 (10) | 121 (13) | 122 (12) | |||
| Systemic vascular resistance, dyn-sec/cm5 |
CON | 2824 (941) | 2929 (654) | 0.24 | 1632 (374) | 1631 (440) | 0.32 |
| EX | 2973 (1009) | 2784 (753) | 1569 (417) | 1499 (303) | |||
| Pulse pressure, mmHg | CON | 66 ± 17 | 62 ± 15 | 0.28 | 99 ± 19 | 94 ± 24 | 0.26 |
| EX | 65 ± 18 | 58 ± 12 | 97 ± 25 | 99 ± 19 | |||
| Pulse pressure/Stroke Volume, mmHg/ml |
CON | 1.83 ± 0.77 | 1.72 ± 0.56 | 0.053 | 2.32 ± 0.78 | 2.11 ± 0.56 | 0.41 |
| EX | 1.68 ± 0.57 | 1.42 ± 0.42 | 2.08 ± 0.68 | 2.07 ± 0.55 | |||
Data are presented as mean (SD). P-value represents comparison of least square means at final visit following adjustment for baseline values. Grp = group assignment; CON = attention control; EX= endurance exercise training.
Effects of Endurance Training on Peak Exercise Variables
Peak VO2 was significantly higher following 4 months of endurance exercise training compared to attention control (16.3 ± 2.6 vs. 13.1 ± 3.4 ml/kg/min; Table 2). The change in peak VO2 and heart rate for the subjects in this study (+2.3 ml/kg/min and +6 beats/min, respectively) was similar to that previously reported for the entire cohort of endurance exercise trained subjects.26 The respiratory exchange ratio was similar between groups (1.15 ± 0.09 vs. 1.13 ± 0.11; p =0.18) and in both was > 1.10 indicating an exhaustive level of effort. Peak and reserve (peak exercise minus rest) heart rate were significantly higher after 4 months of endurance training compared to attention controls (139 ± 16 vs. 131 ± 20 beats/min, p=0.03; and, 69 ± 17 vs. 57 ± 17 beats/min, p=0.01, respectively).
After 4-months of endurance training, there were no significant differences between groups for peak exercise LV end-diastolic volume (77 ± 18 vs. 77 ± 17 ml, p=0.51), end-systolic volume (30 ± 11 vs. 31 ± 13 ml, p=0.58), stroke volume (48 ± 9 vs. 46 ± 9 ml, p=0.83), CO (6.6 ± 1.3 vs. 5.9 ± 1.5 L/min, p=0.32), systolic (187 ± 22 vs. 178 ± 28 mmHg, p=0.19), diastolic (89 ± 10 vs. 84 ± 8 mmHg, p=0.43) or mean arterial pressures (122 ± 12 vs. 116 ± 14 mmHg, p=0.22), or systemic vascular resistance (1499 ± 303 vs. 1631 ± 440 dyns/sec/cm5, p=0.32 (Tables 2 and 3, Figure 1). The percent reduction in systemic vascular resistance from rest to peak exercise in exercise group compared to controls was also unchanged after training (44 + 13% vs 45 + 14%; p=0.46). However, estimated peak and reserve A-VO2 Diff were significantly greater after endurance exercise training compared to attention controls (19.8 ± 4.0 vs. 17.3 ± 3.7 ml/dl, p=0.03, and 10.5 ± 4.2 vs. 7.2 ± 3.3 ml/dl, p=0.01, Table 2 and Figure 1). Finally, peak and reserve circulatory power were significantly greater after endurance training (Table 2).
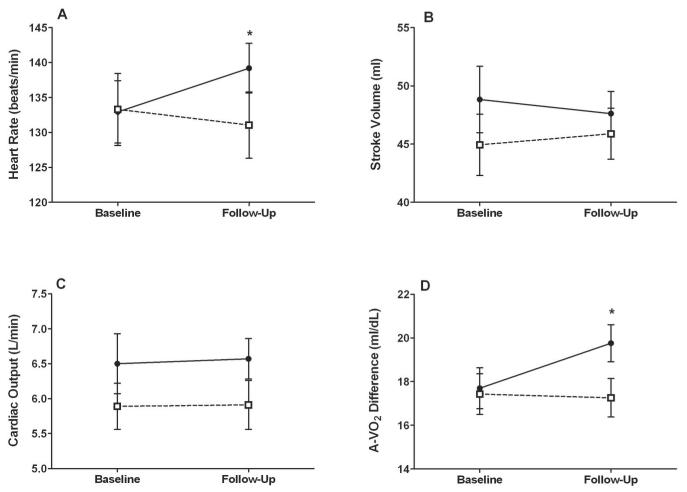
Figure 1.
Peak exercise data at baseline and 4 month follow-up. Solid circles with solid line represent the group randomized to exercise training; open squares with dashed line represent those randomized to the attention control group. Values displayed at each time point are raw mean +/− SE. *= p-value of 0.03. P-values are from the analysis of covariance model based on comparison of least square means at follow-up following adjustment for baseline values.
Relative Contributions of CO and A-VO2 Diff to the Improvement in Peak Exercise VO2 following Endurance Training
We performed a mediation analysis in order to 1) estimate the effect of endurance exercise training on peak exercise VO2; 2) estimate the contribution of CO to the increase in peak exercise VO2 resulting from endurance training; and 3) estimate the contribution of factors other than CO to the increase in peak VO2 from endurance training. Based on ANCOVA, endurance training increased peak VO2 by 19.8% compared to attention control. Considering CO as a mediator of the effect of training on peak VO2, a model estimating the effect on peak VO2 while controlling for CO indicated that the magnitude of the effect of training on peak VO2 that was explained by its effect on CO was 3.2% (or in relative terms 3.2/19.8 = 16% of the total improvement in peak VO2). Thus, 84% (16.6÷ 19.8 = 84%) of the training-related improvement in peak VO2) was due to factors other than cardiac output (i.e. improved A-V02 Diff).
Discussion
HFPEF is the most common form of HF in the growing population of elderly persons1, 2. Exercise intolerance is the primary manifestation in outpatients with chronic HFPEF even when patients are stable and compensated, however its pathophysiology and treatment are not well understood9. This study evaluated the effect of 4- months of endurance exercise training on cardiovascular and metabolic responses to acute cycle exercise in older HFPEF patients. The main new finding of this study was that increased peak exercise A-VO2 Diff estimated from the Fick equation was the primary contributor to the increase in peak VO2 that resulted from endurance exercise training, whereas peak exercise stroke volume and CO were not significantly changed (Table 2 and Figure 1). These findings provide potential insight into the pathophysiology of exercise intolerance in HFPEF and the design of future treatments specifically focused on addressing it.
Although there have been many reports of the effect of exercise training in patients with HFREF18, 35-38, nearly all of which have shown substantial improvements in exercise capacity, to date there have been only four reported studies of exercise training in patients with HFPEF25-28. A critical question in such studies is the mechanism(s) underlying the training-related improvement in exercise capacity. However, this requires measurement of VO2 and its determinants during peak exercise which can be challenging in humans9, 29. As a result, a relatively small number of the training studies in HFREF patients have been able to examine this question15-17, 19, 21, 22, 39, 40, and to our knowledge this is the first to do so in elderly patients with stable compensated HFPEF.
Given that there are no comparable data on the determinants of the training-related improvement in exercise capacity in HFPEF, a review of data in HFREF patients is instructive. In HFREF patients, Hambrecht et al.19, 22 reported that 6 months of endurance exercise training increased peak exercise heart rate and CO. In contrast, Sullivan et al.16 and others17, 39, 40 reported no significant change in peak stroke volume and CO after endurance exercise training in HFREF patients, and that increased peak A-VO2 Diff measured invasively by direct oximetry was the major contributor to improved exercise capacity. Dubach et al.21 found that 2 months of high-intensity endurance training improved both peak exercise CO and A-VO2 Diff in HFREF patients. Taken together, studies performed in HFREF patients suggest that the increased peak VO2 after 2 to 6 months of endurance exercise training is due to increased peak CO and/or A-VO2 Diff. When present, increased peak CO was due primarily to increased peak heart rate as peak stroke volume was not significantly increased after training in any of these studies in HFREF patients16, 17, 19-21, 40, 41.
These prior investigations in patients with HFREF discussed above16, 17, 39 and others in healthy subjects42-44, including several that measured peak A-VO2 by direct oximetry, support the plausibility of our results indicating that the improvement in peak exercise VO2 in elderly HFPEF patients following 4 months of endurance training was due primarily to an increased peak A-VO2 Diff. Of note this is contrary to our a priori hypothesis during the design of the trial. We had hypothesized, based upon our earlier report in a small number of patients3, that increased peak LV stroke volume and cardiac output would be the primary contributors to an endurance training related increase in peak VO2. However, our recently published cross-sectional analysis of the baseline exercise test results indicated that the strongest determinant of the severely reduced exercise capacity in HFPEF patients compared to age-matched, healthy control subjects was reduced peak A-VO2 Diff13. It is noteworthy that even in our early cross-sectional comparison study, an unheralded finding was that A-VO2 Diff, measured invasively by direct oximetry, was impaired during exercise in HFPEF patients.3 an observation recently confirmed by Bhella and associates14. Thus, three published studies from three separate laboratories, using three different techniques, have shown that at baseline before exercise training, HFPEF patients have reduced peak exercise A-VO2 difference and this contributes to their reduced peak VO2. To measure peak A-VO2 diff, one used direct oximetry, one used the Fick technique with acetylene rebreathing measurements of cardiac output, and one used the Fick technique with cardiac output measured by two-dimensional echo volumes.3, 13, 14
The finding of increased peak A-VO2 Diff indicates that after exercise training there was an improvement in either diffusive oxygen transport via improved peripheral vascular, microvascular function and/or increased oxygen utilization by the active muscles. The available data do not allow us to discern which of these was operational in the present study. In HFREF patients, peripheral arterial dilation is impaired and improves following exercise training45. However, we have previously reported that resting and flow-mediated increases in leg blood flow in elderly HFPEF patients are not significantly impaired and are similar to those of age-matched healthy subjects5. We and others have shown in cross-sectional analyses that arterial stiffness is markedly increased in HFPEF patients and is related to their reduced exercise capacity6,46, 47. In the present study, we did not perform formal measurements of arterial stiffness at rest or during exercise. However, after exercise training peak and the percent change in systemic vascular resistance were unchanged and the ratio of pulse pressure to stroke volume, a crude measure of arterial stiffness, although borderline reduced at rest (p = 0.053; Table 3), was not different at peak exercise. This suggests that the increase in peak A-VO2 Diff may be due to improved microvascular function and/or skeletal muscle adaptations that results in increased oxygen utilization by the active muscles.
The suggestion that improved skeletal muscle adaptations may have contributed to the training-related improvement in peak VO2 is supported by a variety of other data. Several cross-sectional studies have shown that HFREF patients and healthy elderly subjects have alterations in skeletal muscle bulk, composition, and function that correlate with their reduced exercise capacity48-51. Bhella et al. also recently reported that elderly HFPEF patients have impaired skeletal muscle oxidative metabolism14. Studies in a wide variety of animal models and human subjects indicate that skeletal muscle has a large capacity for favorable remodeling induced by exercise. Most relevant to elderly HFPEF patients are several studies in HFREF patients and in healthy elderly subjects which have demonstrated that exercise training produces a variety of favorable skeletal muscle adaptations, including increased percent oxidative fibers22, oxidative enzyme activity20, 22-24, 50 and capillary density23. Further studies will be required to determine the specific vascular and/or skeletal muscle mechanism(s) underlying the finding in the present study of a training-mediated increase in peak A-VO2 Diff in HFPEF patients.
The results of prior mechanistic studies of endurance training in patients with HFREF16, 17 and in healthy elderly subjects42, 44 also support the plausibility of our results indicating that endurance exercise training did not significantly enhance peak exercise left ventricular end-diastolic volume, end-systolic volume, or stroke volume in elderly HFPEF patients. As seen in patients with HFREF22, 41, we found that training did increase peak heart rate, representing a possible mitigation of the chronotropic incompetence that was described by our group3, 29, 52 and subsequently confirmed by others6, 7 in patients with HFPEF. However, the increased peak exercise heart rate did not translate into a significant training-related increase in peak exercise CO.
The lack of an increase in peak exercise stroke volume with endurance exercise training may be related to sex-related differences in the effect of endurance exercise training on cardiac function. Specifically, Spina et al. reported that the increase in peak VO2 after endurance training in healthy elderly men was primarily due to an augmented peak stroke volume and CO and to a lesser extent to increased A-VO2 Diff42, 43. In contrast, the increased peak VO2 in healthy elderly women was due entirely to an increase in peak A-VO2 Diff as peak exercise stroke volume, heart rate and CO were unchanged after training42, 43. Of note, nearly all of the HFREF patients in prior mechanistic exercise trials were men18 whereas the majority of patients in the present study of HFPEF were women, reflecting the sex-distribution of HFPEF in the general population2.
The intensity of the prescribed training program can also impact rest and exercise cardiovascular function. Wisloff et al. reported that high-intensity interval aerobic training was superior to continuous moderate intensity endurance exercise training for improving resting ejection fraction, peripheral vascular function and skeletal muscle mitochondrial biogenesis in elderly men with HRFEF53. Nechwatal et al. found that short-term high-intensity aerobic interval training significantly increased peak exercise stroke volume and CO and reduced systemic vascular resistance but there was no change in these outcomes after moderate intensity continuous endurance training in patients with HFREF40. Thus, we cannot exclude the possibility that high-intensity aerobic interval training could result in significant improvements in peak exercise cardiac function in HFPEF.
The type of exercise training can also impact results. Our study utilized endurance training only. However, resistance training has been shown to significantly increase skeletal muscle mass and strength as well as improve aerobic capacity specifically in elderly men and elderly women54, 55. Our observations regarding the contribution of peripheral mechanisms to the training-related improvements in exercise capacity suggest that this modality should be specifically examined in future training studies of elderly HFPEF patients.
Cohen-Solal et al.33 previously reported that peak exercise circulatory power was the single best predictor of survival in HFREF patients. Specifically, over a 25-month follow-up period, HFREF patients with a peak circulatory power >3,047 mmHg had significantly greater event-free survival compared to HFREF patients whose circulatory power below this value. Our finding that 16-weeks of endurance training increased peak circulatory power above this threshold value may have important prognostic implications for elderly HFPEF patients.
Study Limitations
Two-dimensional echocardiography can systematically underestimate LV volumes such that our rest and peak exercise CO values may have been underestimated30, 31, 56. However, our baseline peak CO values are similar to that reported by Ennezat et al. in older HFPEF patients during cycle exercise at a similar peak power output to our subjects8, although they are slightly lower than the peak exercise values reported by Borlaug et al. in HFPEF patients6.
A-VO2 Diff was not measured by direct oximetry. Although desirable, this would have involved invasive catheterization of pulmonary and systemic arteries during exhaustive upright exercise at baseline and again at follow-up in elderly outpatients which would have involved signficant participant burden and risk. Instead, A-VO2 Diff was estimated using the Fick equation where VO2 measured by expired gas analysis is divided by measured CO. The Fick technique has been used to calculate A-VO2Diff in number of recent physiologic studies investigating mechanisms of exercise intolerance including HFPEF patients13, 14, 56-58. Our peak A-VO2 Diff values were somewhat higher than reported by others14, 59, 60, possibly due to under-estimation of CO. However, the absolute change in peak A-VO2 Diff with exercise training in our HFPEF patients is similar to that found after exercise training in HFREF patients in studies in which it was measured by direct oximetry using invasively obtained systemic and pulmonary arterial blood samples16, 21. Most importantly, because key variables were measured at all testing times using identical methods in both groups, and because we assessed changes in reserve capacity (rest minus peak values) within individuals, comparisons of CO and estimated A-VO2 Diff between groups are valid.
Although the Doppler indexes at supine rest in the overall group indicated the presence of abnormal LV diastolic filling13, due to technical limitations including merging of the E and A wave, these were not measured during upright exercise. Tissue Doppler was not performed; therefore, changes in LV systolic and diastolic function may have occurred that we were unable to detect. However, any physiologically signficant changes in this regard may have been expected to impact rest and peak exercise stroke volume which was not changed after the 4 month training program. Although all patients had normal mitral valve morphology and function at rest, we cannot exclude the possibility that exercise-induced mitral regurgitation could have impacted peak stroke volume. Intracardiac hemodynamics were not measured, as these require invasive techniques which are challenging in studies such as ours that require serial assessments in elderly outpatients and involve signficant human research subject risk57.
Our patients met objective, standardized criteria for heart failure, and had features that closely match patients with HFPEF in other published studies, including in the characteristics of diastolic filling, BMI, and diuretic use. Because our study involved serial, exhaustive exercise tests and four months of exercise training, patients were required to be stable and compensated with no recent hospitalizations or medication changes. Thus, they would be expected to have less severe symptoms and biomarker abnormalities than patients with high rates of recent hospitalization and decompensation. Nevertheless, as recently reviewed by others the diagnosis of HFPEF can be difficult61, 62, and this remains a limitation to this and most HFPEF investigations.
In the mediation analysis, measurement error of cardiac output could potentially introduce positive bias. This would be inversely proportional to the coefficient of variation. The reliability of cardiac output measurement in our lab is > 90%. Thus, we believe that the potential for positive bias is relatively modest.
Conclusions
The improvement in peak exercise capacity after 4 months of endurance exercise training in elderly stable compensated HFPEF patents was due primarily to increased peak A-VO2 Diff. This suggests that peripheral vascular, micro-vascular, and/or skeletal muscle function were improved and resulted in increased diffusive oxygen transport or greater oxygen utilization by the active skeletal muscle.
Acknowledgments
This study was supported by the following research grants: N.I.H. Grants R37 AG18915 and RO1AG12257; The Claude D. Pepper Older Americans Independence Center of Wake Forest University N.I.H. Grant P30AG21332; and the General Clinical Research Center (grant # MO1RR07122) of the Wake Forest University School of Medicine
Abbreviations
- A-VO2 Diff
arterial-venous oxygen difference.
- CO
cardiac output.
- HF
Heart failure.
- HFPEF
Heart failure and preserved ejection fraction.
- Peak VO2
Peak exercise oxygen consumption.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Dr. Kitzman declares a minor (<$10,000) potential conflict of interest as a consultant for Boston Scientific Corporation; we have no other conflicts of interest to report.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Kitzman DW, Gardin JM, Gottdiener JS, Arnold A, Boineau R, Aurigemma G, Marino EK, Lyles M, Cushman M, Enright PL, CHS Research Group. Cardiovascular Health Study Importance of heart failure with preserved systolic function in patients > or = 65 years of age. Am J Cardiol. 2001;87(4):413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 3.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17(5):1065–1072. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- 4.Kitzman DW, Little WC, Brubaker PH, Anderson RT, Hundley WG, Marburger CT, Brosnihan B, Morgan TM, Stewart KP. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288(17):2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 5.Hundley WG, Bayram E, Hamilton CA, Hamilton EA, Morgan TM, Darty SN, Stewart KP, Link KM, Herrington DM, Kitzman DW. Leg flow-mediated arterial dilation in elderly patients with heart failure and normal left ventricular ejection fraction. Am J Physiol Heart Circ Physiol. 2007;292(3):H1427–1434. doi: 10.1152/ajpheart.00567.2006. [DOI] [PubMed] [Google Scholar]
- 6.Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56(11):845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, Kass DA. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114(20):2138–2147. doi: 10.1161/CIRCULATIONAHA.106.632745. [DOI] [PubMed] [Google Scholar]
- 8.Ennezat PV, Lefetz Y, Marechaux S, Six-Carpentier M, Deklunder G, Montaigne D, Bauchart JJ, Mounier-Vehier C, Jude B, Neviere R, Bauters C, Asseman P, de Groote P, Lejemtel TH. Left ventricular abnormal response during dynamic exercise in patients with heart failure and preserved left ventricular ejection fraction at rest. J Card Fail. 2008;14(6):475–480. doi: 10.1016/j.cardfail.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Kitzman DW. Understanding results of trials in heart failure with preserved ejection fraction: remembering forgotten lessons and enduring principles. J Am Coll Cardiol. 2011;57(16):1687–1689. doi: 10.1016/j.jacc.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bensimhon DR, Leifer ES, Ellis SJ, Fleg JL, Keteyian SJ, Pina IL, Kitzman DW, McKelvie RS, Kraus WE, Forman DE, Kao AJ, Whellan DJ, O’Connor CM, Russell SD. Reproducibility of peak oxygen uptake and other cardiopulmonary exercise testing parameters in patients with heart failure (from the Heart Failure and A Controlled Trial Investigating Outcomes of exercise traiNing) Am J Cardiol. 2008;102(6):712–717. doi: 10.1016/j.amjcard.2008.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marburger CT, Brubaker PH, Pollock WE, Morgan TM, Kitzman DW. Reproducibility of cardiopulmonary exercise testing in elderly patients with congestive heart failure. Am J Cardiol. 1998;82(7):905–909. doi: 10.1016/s0002-9149(98)00502-5. [DOI] [PubMed] [Google Scholar]
- 12.Maeder MT, Thompson BR, Brunner-La Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56(11):855–863. doi: 10.1016/j.jacc.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 13.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of Exercise Intolerance in Elderly Heart Failure Patients with Preserved Ejection Fraction. J Am Coll Cardiol. 2011;58(3):265–274. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhella PS, Prasad A, Heinicke K, Hastings JL, Arbab-Zadeh A, Adams-Huet B, Pacini EL, Shibata S, Palmer MD, Newcomer BR, Levine BD. Abnormal hemodynamic response to exercise in heart failure with preserved ejection fraction. European Journal of Heart Failure. 2011;13(12):1296–1304. doi: 10.1093/eurjhf/hfr133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coats AJ, Adamopoulos S, Radaelli A, McCance A, Meyer TE, Bernardi L, Solda PL, Davey P, Ormerod O, Forfar C, et al. Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation. 1992;85(6):2119–2131. doi: 10.1161/01.cir.85.6.2119. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan MJ, Higginbotham MB, Cobb FR. Exercise training in patients with severe left ventricular dysfunction. Hemodynamic and metabolic effects. Circulation. 1988;78(3):506–515. doi: 10.1161/01.cir.78.3.506. [DOI] [PubMed] [Google Scholar]
- 17.Belardinelli R, Georgiou D, Cianci G, Purcaro A. Effects of exercise training on left ventricular filling at rest and during exercise in patients with ischemic cardiomyopathy and severe left ventricular systolic dysfunction. Am Heart J. 1996;132(1):61–70. doi: 10.1016/s0002-8703(96)90391-9. [DOI] [PubMed] [Google Scholar]
- 18.Haykowsky MJ, Liang Y, Pechter D, Jones LW, McAlister FA, Clark AM. A meta-analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefit depends on the type of training performed. J Am Coll Cardiol. 2007;49(24):2329–2336. doi: 10.1016/j.jacc.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 19.Hambrecht R, Gielen S, Linke A, Fiehn E, Yu J, Walther C, Schoene N, Schuler G. Effects of exercise training on left ventricular function and peripheral resistance in patients with chronic heart failure: A randomized trial. JAMA. 2000;283(23):3095–3101. doi: 10.1001/jama.283.23.3095. [DOI] [PubMed] [Google Scholar]
- 20.Hambrecht R, Niebauer J, Fiehn E, Kalberer B, Offner B, Hauer K, Riede U, Schlierf G, Kubler W, Schuler G. Physical-Training in Patients with Stable Chronic Heart-Failure - Effects on Cardiorespiratory Fitness and Ultrastructural Abnormalities of Leg Muscles. Journal of the American College of Cardiology. 1995;25(6):1239–1249. doi: 10.1016/0735-1097(94)00568-B. [DOI] [PubMed] [Google Scholar]
- 21.Dubach P, Myers J, Dziekan G, Goebbels U, Reinhart W, Muller P, Buser P, Stulz P, Vogt P, Ratti R. Effect of high intensity exercise training on central hemodynamic responses to exercise in men with reduced left ventricular function. J Am Coll Cardiol. 1997;29(7):1591–1598. doi: 10.1016/s0735-1097(97)82540-5. [DOI] [PubMed] [Google Scholar]
- 22.Hambrecht R, Fiehn E, Yu J, Niebauer J, Weigl C, Hilbrich L, Adams V, Riede U, Schuler G. Effects of endurance training on mitochondrial ultrastructure and fiber type distribution in skeletal muscle of patients with stable chronic heart failure. J Am Coll Cardiol. 1997;29(5):1067–1073. doi: 10.1016/s0735-1097(97)00015-6. [DOI] [PubMed] [Google Scholar]
- 23.Magnusson G, Gordon A, Kaijser L, Sylven C, Isberg B, Karpakka J, Saltin B. High intensity knee extensor training, in patients with chronic heart failure. Major skeletal muscle improvement. Eur Heart J. 1996;17(7):1048–1055. doi: 10.1093/oxfordjournals.eurheartj.a015001. [DOI] [PubMed] [Google Scholar]
- 24.Tyni-Lenne R, Gordon A, Jansson E, Bermann G, Sylven C. Skeletal muscle endurance training improves peripheral oxidative capacity, exercise tolerance, and health-related quality of life in women with chronic congestive heart failure secondary to either ischemic cardiomyopathy or idiopathic dilated cardiomyopathy. Am J Cardiol. 1997;80(8):1025–1029. doi: 10.1016/s0002-9149(97)00597-3. [DOI] [PubMed] [Google Scholar]
- 25.Gary R. Exercise self-efficacy in older women with diastolic heart failure: results of a walking program and education intervention. J Gerontol Nurs. 2006;32(7):31–39. doi: 10.3928/00989134-20060701-05. quiz 40-31. [DOI] [PubMed] [Google Scholar]
- 26.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail. 2010;3(6):659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smart N, Haluska B, Jeffriess L, Marwick TH. Exercise training in systolic and diastolic dysfunction: effects on cardiac function, functional capacity, and quality of life. Am Heart J. 2007;153(4):530–536. doi: 10.1016/j.ahj.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Edelmann F, Gelbrich G, Dungen HD, Frohling S, Wachter R, Stahrenberg R, Binder L, Topper A, Lashki DJ, Schwarz S, Herrmann-Lingen C, Loffler M, Hasenfuss G, Halle M, Pieske B. Exercise Training Improves Exercise Capacity and Diastolic Function in Patients With Heart Failure With Preserved Ejection Fraction Results of the Ex-DHF (Exercise training in Diastolic Heart Failure) Pilot Study. J Am Coll Cardiol. 2011;58(17):1780–1791. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 29.Brubaker PH, Kitzman DW. Chronotropic incompetence: causes, consequences, and management. Circulation. 2011;123(9):1010–1020. doi: 10.1161/CIRCULATIONAHA.110.940577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2(5):358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 31.Gottdiener JS, Bednarz J, Devereux R, Gardin J, Klein A, Manning WJ, Morehead A, Kitzman D, Oh J, Quinones M, Schiller NB, Stein JH, Weissman NJ. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;17(10):1086–1119. doi: 10.1016/j.echo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Kuecherer HF, Kee LL, Modin G, Cheitlin MD, Schiller NB. Echocardiography in serial evaluation of left ventricular systolic and diastolic function: importance of image acquisition, quantitation, and physiologic variability in clinical and investigational applications. J Am Soc Echocardiogr. 1991;4(3):203–214. doi: 10.1016/s0894-7317(14)80020-5. [DOI] [PubMed] [Google Scholar]
- 33.Cohen-Solal A, Tabet JY, Logeart D, Bourgoin P, Tokmakova M, Dahan M. A non-invasively determined surrogate of cardiac power (‘circulatory power’) at peak exercise is a powerful prognostic factor in chronic heart failure. Eur Heart J. 2002;23(10):806–814. doi: 10.1053/euhj.2001.2966. [DOI] [PubMed] [Google Scholar]
- 34.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7(1):83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2009;301(14):1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Tol BA, Huijsmans RJ, Kroon DW, Schothorst M, Kwakkel G. Effects of exercise training on cardiac performance, exercise capacity and quality of life in patients with heart failure: a meta-analysis. Eur J Heart Fail. 2006;8(8):841–850. doi: 10.1016/j.ejheart.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 37.van der Meer S, Zwerink M, van Brussel M, van der Valk P, Wajon E, van der Palen J. Effect of outpatient exercise training programmes in patients with chronic heart failure: a systematic review. Eur J Cardiovasc Prev Rehabil. 2011 May 18; doi: 10.1177/1741826711410516. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 38.Davies EJ, Moxham T, Rees K, Singh S, Coats AJ, Ebrahim S, Lough F, Taylor RS. Exercise training for systolic heart failure: Cochrane systematic review and meta-analysis. Eur J Heart Fail. 2010;12(7):706–715. doi: 10.1093/eurjhf/hfq056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jette M, Heller R, Landry F, Blumchen G. Randomized 4-week exercise program in patients with impaired left ventricular function. Circulation. 1991;84(4):1561–1567. doi: 10.1161/01.cir.84.4.1561. [DOI] [PubMed] [Google Scholar]
- 40.Nechwatal RM, Duck C, Gruber G. Physical training as interval or continuous training in chronic heart failure for improving functional capacity, hemodynamics and quality of life--a controlled study. Z Kardiol. 2002;91(4):328–337. doi: 10.1007/s003920200034. [DOI] [PubMed] [Google Scholar]
- 41.Keteyian SJ, Levine AB, Brawner CA, Kataoka T, Rogers FJ, Schairer JR, Stein PD, Levine TB, Goldstein S. Exercise training in patients with heart failure. A randomized, controlled trial. Ann Intern Med. 1996;124(12):1051–1057. doi: 10.7326/0003-4819-124-12-199606150-00004. [DOI] [PubMed] [Google Scholar]
- 42.Spina RJ, Ogawa T, Miller TR, Kohrt WM, Ehsani AA. Effect of exercise training on left ventricular performance in older women free of cardiopulmonary disease. Am J Cardiol. 1993;71(1):99–104. doi: 10.1016/0002-9149(93)90718-r. [DOI] [PubMed] [Google Scholar]
- 43.Spina RJ, Ogawa T, Kohrt WM, Martin WH, 3r, Holloszy JO, Ehsani AA. Differences in cardiovascular adaptations to endurance exercise training between older men and women. J Appl Physiol. 1993;75(2):849–855. doi: 10.1152/jappl.1993.75.2.849. [DOI] [PubMed] [Google Scholar]
- 44.Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation. 1999;100(10):1085–1094. doi: 10.1161/01.cir.100.10.1085. [DOI] [PubMed] [Google Scholar]
- 45.Hornig B, Maier V, Drexler H. Physical training improves endothelial function in patients with chronic heart failure. Circulation. 1996;93(2):210–214. doi: 10.1161/01.cir.93.2.210. [DOI] [PubMed] [Google Scholar]
- 46.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107(5):714–720. doi: 10.1161/01.cir.0000048123.22359.a0. [DOI] [PubMed] [Google Scholar]
- 47.Hundley WG, Kitzman DW, Morgan TM, Hamilton CA, Darty SN, Stewart KP, Herrington DM, Link KM, Little WC. Cardiac cycle-dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38(3):796–802. doi: 10.1016/s0735-1097(01)01447-4. [DOI] [PubMed] [Google Scholar]
- 48.Wilson JR, Mancini DM, Dunkman WB. Exertional fatigue due to skeletal muscle dysfunction in patients with heart failure. Circulation. 1993;87(2):470–475. doi: 10.1161/01.cir.87.2.470. [DOI] [PubMed] [Google Scholar]
- 49.Duscha BD, Kraus WE, Keteyian SJ, Sullivan MJ, Green HJ, Schachat FH, Pippen AM, Brawner CA, Blank JM, Annex BH. Capillary density of skeletal muscle: a contributing mechanism for exercise intolerance in class II-III chronic heart failure independent of other peripheral alterations. J Am Coll Cardiol. 1999;33(7):1956–1963. doi: 10.1016/s0735-1097(99)00101-1. [DOI] [PubMed] [Google Scholar]
- 50.Stratton JR, Dunn JF, Adamopoulos S, Kemp GJ, Coats AJ, Rajagopalan B. Training partially reverses skeletal muscle metabolic abnormalities during exercise in heart failure. J Appl Physiol. 1994;76(4):1575–1582. doi: 10.1152/jappl.1994.76.4.1575. [DOI] [PubMed] [Google Scholar]
- 51.Sullivan MJ, Green HJ, Cobb FR. Skeletal muscle biochemistry and histology in ambulatory patients with long-term heart failure. Circulation. 1990;81(2):518–527. doi: 10.1161/01.cir.81.2.518. [DOI] [PubMed] [Google Scholar]
- 52.Brubaker PH, Joo KC, Stewart KP, Fray B, Moore B, Kitzman DW. Chronotropic incompetence and its contribution to exercise intolerance in older heart failure patients. J Cardiopulm Rehabil. 2006;26(2):86–89. doi: 10.1097/00008483-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 53.Wisloff U, Stoylen A, Loennechen JP, Bruvold M, Rognmo O, Haram PM, Tjonna AE, Helgerud J, Slordahl SA, Lee SJ, Videm V, Bye A, Smith GL, Najjar SM, Ellingsen O, Skjaerpe T. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115(24):3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 54.Frontera WR, Meredith CN, O’Reilly KP, Evans WJ. Strength training and determinants of VO2max in older men. J Appl Physiol. 1990;68(1):329–333. doi: 10.1152/jappl.1990.68.1.329. [DOI] [PubMed] [Google Scholar]
- 55.Haykowsky M, McGavock J, Vonder Muhll I, Koller M, Mandic S, Welsh R, Taylor D. Effect of exercise training on peak aerobic power, left ventricular morphology, and muscle strength in healthy older women. J Gerontol A Biol Sci Med Sci. 2005;60(3):307–311. doi: 10.1093/gerona/60.3.307. [DOI] [PubMed] [Google Scholar]
- 56.Tischler MD, Plehn JF. Applications of stress echocardiography: beyond coronary disease. J Am Soc Echocardiogr. 1995;8(2):185–197. doi: 10.1016/s0894-7317(05)80407-9. [DOI] [PubMed] [Google Scholar]
- 57.Stickland MK, Welsh RC, Petersen SR, Tyberg JV, Anderson WD, Jones RL, Taylor DA, Bouffard M, Haykowsky MJ. Does fitness level modulate the cardiovascular hemodynamic response to exercise? J Appl Physiol. 2006;100(6):1895–1901. doi: 10.1152/japplphysiol.01485.2005. [DOI] [PubMed] [Google Scholar]
- 58.Fujimoto N, Prasad A, Hastings JL, Arbab-Zadeh A, Bhella PS, Shibata S, Palmer D, Levine BD. Cardiovascular effects of 1 year of progressive and vigorous exercise training in previously sedentary individuals older than 65 years of age. Circulation. 2010;122(18):1797–1805. doi: 10.1161/CIRCULATIONAHA.110.973784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kugler J, Maskin C, Frishman WH, Sonnenblick EH, LeJemtel TH. Regional and systemic metabolic effects of angiotensin-converting enzyme inhibition during exercise in patients with severe heart failure. Circulation. 1982;66(6):1256–1261. doi: 10.1161/01.cir.66.6.1256. [DOI] [PubMed] [Google Scholar]
- 60.Maurer M, Katz SD, LaManca J, Manandhar M, Mancini D. Dissociation between exercise hemodynamics and exercise capacity in patients with chronic heart failure and marked increase in ejection fraction after treatment with beta-adrenergic receptor antagonists. Am J Cardiol. 2003;91(3):356–360. doi: 10.1016/s0002-9149(02)03171-5. [DOI] [PubMed] [Google Scholar]
- 61.From AM, Borlaug BA. Heart failure with preserved ejection fraction: pathophysiology and emerging therapies. Cardiovasc Ther. 2011;29(4):e6–21. doi: 10.1111/j.1755-5922.2010.00133.x. [DOI] [PubMed] [Google Scholar]
- 62.Bhuiyan T, Maurer MS. Heart Failure with Preserved Ejection Fraction: Persistent Diagnosis, Therapeutic Enigma. Curr Cardiovasc Risk Rep. 2011;5(5):440–449. doi: 10.1007/s12170-011-0184-2. [DOI] [PMC free article] [PubMed] [Google Scholar]