Abstract
Proteolytic cleavage of procollagen I to collagen I is essential for the formation of collagen fibrils in the extracellular matrix of vertebrate tissues. Procollagen is cleaved by the procollagen N- and C-proteinases, which remove the respective N- and C-propeptides from procollagen. Procollagen processing is initiated within the secretory pathway in tendon fibroblasts, which are adept in assembling an ordered extracellular matrix of collagen fibrils in vivo. It was thought that intracellular processing was restricted to the TGN (trans-Golgi network). In the present study, brefeldin A treatment of tendon explant cultures showed that N-proteinase activity is present in the resulting fused ER (endoplasmic reticulum)–Golgi compartment, but that C-proteinase activity is restricted to the TGN in embryonic chick tendon fibroblasts. In late embryonic and postnatal rat tail and postnatal mouse tail tendon, C-proteinase activity was detected in TGN and pre-TGN compartments. Preventing activation of the procollagen N- and C-proteinases with the furin inhibitor Dec-RVKR-CMK (decanoyl-Arg-Val-Lys-Arg-chloromethylketone) indicated that only a fraction of intracellular procollagen cleavage was mediated by newly activated proteinases. In conclusion, the N-propeptides are removed earlier in the secretory pathway than the C-propeptides. The removal of the C-propeptides in post-Golgi compartments most probably indicates preparation of collagen molecules for fibril formation at the cell–matrix interface.
Keywords: C-proteinase, electron microscopy, endoplasmic reticulum, furin, N-proteinase, trans-Golgi network (TGN)
Abbreviations: ADAMTS, a disintegrin and metalloprotease with thrombospondin motifs; BFA, brefeldin A; BMP1, bone morphogenetic protein 1; *collagen, collagen intermediate (a collective term for all procollagen, pCcollagen, pNcollagen and collagen molecules); COPI, coatomer protein 1; Dec-RVKR-CMK, decanoyl-Arg-Val-Lys-Arg-chloromethylketone; E, embryonic day; ER, endoplasmic reticulum; ERGIC, ER–Golgi intermediate compartment; mTLD, mammalian tolloid; MT1-MMP, membrane type 1 matrix metalloproteinase; NP40, Nonidet P40; PBS-T, PBS with 0.1% Tween; pCcollagen, procollagen lacking the N-propeptides but retaining the C-propeptides; pNcollagen, procollagen lacking the C-propeptides but retaining the N-propeptides; QL, quantum level; TEM, transmission electron microscopy; TGFβ, transforming growth factor β; TGN, trans-Golgi network; TLL, tolloid-like
INTRODUCTION
Collagen fibrils are the principal source of tensile strength of vertebrate connective tissues, where they are positioned in the extracellular matrix between cells. The fibrils comprise collagen molecules packed together in a quarter-staggered arrangement to produce a distinctive 67 nm (D) periodic banding pattern that is readily observed by electron microscopy [1]. Collagen molecules are synthesized inside the cell as procollagen that has N- and C-propeptides flanking the collagenous domain [2]. The propeptides, especially the C-propeptides, prohibit collagen fibril formation (see [3] and references therein). Procollagen is synthesized in the ER (endoplasmic reticulum), where it undergoes post-translational modification, and is then transported through the Golgi complex without leaving the lumen of the cisternae [4]. Transport of procollagen to the plasma membrane is facilitated by the detachment of large GPCs (Golgi-to-plasma membrane carriers) from the TGN (trans-Golgi network) [5]. We have reported that the N- and C- propeptides can be cleaved from procollagen within the confines of the cell membrane, in tendon fibroblasts in situ [6]. Knowledge of where procollagen is processed to collagen within the cell is needed for a more complete understanding of the mechanism of cell-mediated assembly of collagen fibrils.
Removal of the type I procollagen C-propeptides is mediated by members of the tolloid family of metalloproteinases: BMP1 (bone morphogenetic protein 1), mTLD (mammalian tolloid), TLL (tolloid-like) 1 and, in the presence of an enhancer protein, TLL2 [7,8]. The N-propeptides are removed by ADAMTS (a disintegrin and metalloprotease with thrombospondin motifs) 2, 3 and 14 [9,10]. The tolloid metalloproteinases and the ADAMTSs are themselves synthesized as pro-enzymes that are activated by removal of an inhibitory prodomain. In the case of BMP1 and ADAMTS2, prodomain removal has been found to be accomplished by furin-like convertases [11,12] and the RXXR furin-cleavage sites are conserved throughout mTLD (a splice variant of BMP1), TLL1, TLL2, ADAMTS3 and ADAMTS14. The furin-like convertases are themselves synthesized as inactive precursors and comprise a family of nine enzymes [13]. Furin undergoes its second and final autocatalytic activation step in the TGN and is able to cycle between the TGN and the cell surface via the endosomal system [14]. Furin-like convertases can be localized to the TGN, within granules of the regulated secretory pathway, bound to the cell surface or potentially in the extracellular matrix [15]. Therefore the formation of fully processed collagen molecules, and the concomitant ability of the cells to fabricate collagen fibrils, requires the controlled activation and localization of a cascade of upstream proteases.
It is conceivable that intracellular processing of procollagen could be mediated by newly synthesized procollagen-processing enzymes that are concomitantly synthesized and transported with procollagen through the secretory pathway and activated by furin-like convertases before secretion. In fact, BMP1 is activated in the TGN by furin-like proprotein convertases [12] and may therefore concurrently cleave the C-propeptide from type I procollagen during secretion. In an alternative scenario, intracellular processing could result from the action of pre-activated N- and C-proteinases, which might be targeted to specific locations via a retrograde transport step from the TGN or plasma membrane. The identification of intracellular collagen fibrils in post-Golgi compartments suggested that intracellular procollagen processing occurred in the TGN, at least in embryonic tendon [6]. However, the discovery of efficient intracellular procollagen processing in the absence of intracellular fibrils in postnatal tendon illustrates the ability of cells to prevent the premature assembly of processed collagen monomers into collagen fibrils [16].
In the present study, we tested the extent of intracellular procollagen processing in the presence of BFA (brefeldin A), which inhibits protein transport through the secretory pathway without disrupting procollagen trimerization and folding. BFA prevents the assembly of COPI (coatomer protein 1) protein coats by interfering with the activation of ARF1 (ADP-ribosylation factor 1) [17–19]. This prevents the formation of COPI-coated vesicles and rapidly results in Golgi complex disassembly. Golgi membrane tubulation is followed by the absorption of Golgi membranes and redistribution of Golgi enzymes into the ER [20,21]. Further protein export from the fused compartment does not occur. Components of the TGN do not fuse with the ER, but are thought to become connected to the recycling endosomal system in the presence of BFA [22,23]. We also used a synthetic peptide-based competitive furin inhibitor to determine the extent to which intracellular procollagen cleavage is mediated by newly activated procollagen N- and C-proteinases. It was not possible to localize the proteinases directly in tissue sections, either by light or electron microscopy (see the Results and Discussion section), therefore it was necessary to adopt an indirect approach measuring the conversion of procollagen into collagen via the intermediates pNcollagen, from which the C-propeptide has been removed, and pCcollagen, from which the N-propeptide has been removed (see Figure 3B). This approach allowed us to identify the sites at which the enzymes were active, rather than where the gene products accumulated. The results show that the procollagen propeptides are removed earlier in the secretory pathway than was suggested previously. The N-propeptides are removed first, most probably in the Golgi or in the ERGIC (ER–Golgi intermediate compartment). In contrast, the C-propeptides are cleaved in a post-Golgi compartment. We propose that the N-propeptides are dispensable for the secretion of procollagen, but that the C-propeptides are required for efficient transport from the ER to the Golgi. The removal of the C-propeptides in post-Golgi compartments probably indicates preparation of collagen molecules for fibril formation at the cell–matrix interface.
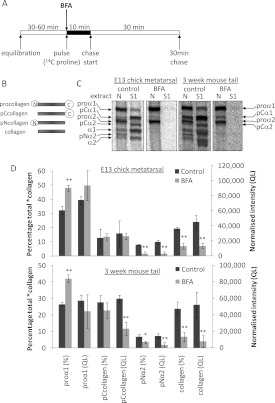
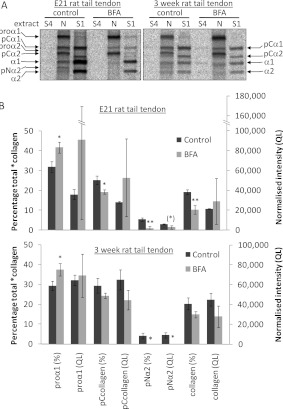
Figure 3. BFA treatment of embryonic and postnatal tendon explants results in the intracellular retention of newly synthesized procollagen and reduces the extent of intracellular processing at the procollagen C-propeptide.
(A) Schematic diagram of the pulse–chase protocol used to label, and then to follow the fate of, newly synthesized type I procollagen in tendon explants. (B) Schematic diagram of the intermediates obtained on removal of the N- and/or C-propeptides from type I procollagen. (C) Embryonic chick metatarsal tendons and postnatal CD1 mouse tail tendons, incubated according to the protocol shown in (A), were subjected to sequential salt and NP40 detergent extractions, and the extracts were analysed by electrophoresis and autoradiography as described in the Experimental section. Single representative images are shown. ‘S1’ denotes the first extracellular salt extract and ‘N’ is the intracellular NP40 detergent extract. Bands corresponding to each of the type I procollagen-processing intermediates are indicated. (D) The relative (percentage total) and absolute [normalized intensity (QL)] amounts of each *collagen were determined as described in the Experimental section (n=4). *P<0.05; **P<0.01 (Student's t test).
EXPERIMENTAL
Materials
Reagents were obtained from Invitrogen or Sigma unless stated otherwise.
In situ analysis of procollagen processing and secretion
Fertile hens' eggs were supplied by Joice and Hill Poultry and metatarsal tendons were obtained from E (embryonic day) 13 chick embryos. Tail tendons were obtained from E21 Sprague–Dawley rat embryos, 3-week-old CD1 mice and 3-week-old Sprague–Dawley rats. Pulse–chase and labelling were performed at 37°C in Dulbecco's minimal essential medium/nutrient mixture with Ham's F12 containing penicillin/streptomycin (1%, v/v), L-glutamine (2 mM), L-ascorbic acid 2-phosphate (200 μM), β-aminopropionitrile (400 μM) and 2.5 μCi/ml [14C]proline (GE Healthcare), and supplemented with furin inhibitor I [Dec-RVKR-CMK (decanoyl-Arg-Val-Lys-Arg-chloromethylketone), 40 μM] (Calbiochem), cycloheximide (175 μM) or BFA (3.5 μM), as required. Collagenase (type IV, Worthington, 200 units) was added 30 min before the end of labelling as required in postnatal murine tail tendons. Tissue extraction resulting in extracellular (S1–S4) and intracellular (N) fractions, electrophoresis and imaging were carried out as described in [24], except for 3-week-old rat tail tendon in which the second (S2) and third (S3) extraction volumes were increased from 100 μl to 200 μl each, to ensure complete extraction of extracellular material in the sequential salt extracts. For postnatal rat tail tendon extracts, the volume of the N and S4 extracts analysed by electrophoresis was halved and only one-tenth of the S1 extract (compared with the E21 rat, E13 chick and 3-week-old mouse tendon extracts) was loaded; these differences were taken into account during quantification.
TEM (transmission electron microscopy)
Metatarsal tendons obtained from E13 chick embryos or tail tendons obtained from 3-week-old CD1 mice were incubated in Dulbecco's minimal essential medium/nutrient mixture with Ham's F12 containing penicillin/streptomycin (1%, v/v), L-glutamine (2 mM), L-ascorbic acid 2-phosphate (200 μM) and β-aminopropionitrile (400 μM), and supplemented with BFA (3.5 μM) as required, for 30 min or 1 h before fixation. Whole tails from E21 rat embryos were transferred directly to fixative. Fixation, embedding and sectioning were performed as described previously [24]. Briefly, all tissues were fixed in glutaraldehyde (2%, v/v) in phosphate buffer (100 mM, pH 7.0) for 30 min at room temperature (21°C) and then for 2 h at 4°C in fresh fixative. After washing in phosphate buffer (200 mM), they were fixed in glutaraldehyde (2%, v/v) with osmium tetroxide (1%, w/v) in phosphate buffer (50 or 100 mM, pH 6.2) for 40–50 min at 4°C. After extensive washing in distilled water, they were stained en bloc with aqueous uranyl acetate (1%, w/v) at 4°C overnight. The samples were then dehydrated in a graded acetone series, treated with propylene oxide for 10 min at room temperature and embedded in either Spurr's or TAAB LV resin. Ultrathin (60–80 nm) sections were collected on 0.25% formvar coated copper 200 mesh grids and stained with lead citrate (0.3% in 0.1 M sodium hydroxide) for 1 min. Sections were examined on an FEI Tecnai 12 Biotwin transmission electron microscope, images recorded on 4498 film (Kodak) and digitized using an Imacon Flextight 848 scanner (Precision Camera and Video).
Western blotting
Samples were resolved by electrophoresis under reducing conditions on NuPAGE Novex 4–12% Bis-Tris gels using NuPAGE Mops running buffer and then subjected to Western immunoblotting. For detection of MT1-MMP (membrane type 1 matrix metalloproteinase), the primary antibody, mouse monoclonal LEM-2/15.8 (MAB3328, Millipore/Chemicon), directed against the catalytic domain of MT1-MMP was used, and for detection of β-actin, the primary antibody mouse monoclonal AC-15 (A5441, Sigma) was used. For film-based detection, the secondary antibody was anti-mouse HRP (horseradish peroxidase)-conjugated IgG (Thermo Scientific/Pierce) and the signal was detected by the enhanced chemiluminescence method (SuperSignal West Dura extended duration, Thermo Scientific/Pierce) using Fuji medical X-ray film (Fujifilm). For IR imaging, nitrocellulose membranes were blocked with Odyssey Blocking Buffer and incubated with the primary antibody mouse monoclonal antibody AC-15 (A5441, Sigma) in 5% BSA (fraction V) in PBS. The membranes were washed in PBS-T (PBS with 0.1% Tween) and incubated with Alexa Fluor® 680-conjugated anti-(mouse IgG) (A21058, Invitrogen) in 1:1 Odyssey blocking buffer/PBS-T. After washing with PBS, the signal was detected using the Odyssey Infrared Imaging System (LI-COR Biosciences).
Densitometry and statistical analysis
The relative amounts of unprocessed and cleaved procollagen intermediates (*collagens) in the detergent (N) and salt (S1) extracts were determined using AIDA 2.0 (Raytek) and PeakFit v4.12 (Systat) software. To reduce variability due to co-migration of the proα2(I) and pNα1(I) chains, the proα1(I) and pNα2(I) chains, rather than the total of the α1 and α2 chains, were used to represent of the amounts of procollagen and pNcollagen respectively. The relative amounts of intracellular or extracellular *collagens, as well as the relative proportions of each intracellular *collagen, were expressed as means±S.D. To obtain absolute amounts of each *collagen, the intensity signal for each band [in QL (quantum levels)] was normalized to the amount of β-actin in each extract, as determined by Western blotting using the Odyssey Infrared Imaging System and by calibration with serial dilutions of designated standards. Data were analysed using a Student's t test or by two-way ANOVA as appropriate.
RESULTS AND DISCUSSION
BFA treatment of tendon explant cultures: an explanation and validation of the experimental strategy
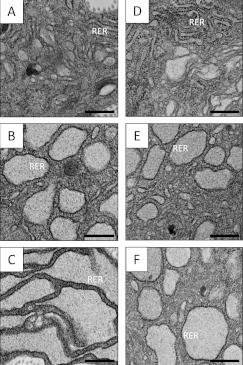
BFA splits the secretory pathway into a closed ERGIC and a functional TGN/endosomal compartment (Figure 1). To determine whether intracellular procollagen processing occurs solely within the TGN, BFA was used to inhibit protein transport through the secretory pathway in cells in tendon explants. We first confirmed the efficacy of BFA treatment in whole tendon explants, using TEM. BFA treatment resulted in the production of distended compartments studded with densely stained ribosomes, indicating that BFA effectively induces the accumulation of secretory proteins within the ER (Figure 2).
Figure 1. BFA treatment protocols.
(A) Schematic diagram of the secretory pathway components: RER (rough ER), ERGIC, cis-, medial- and trans-Golgi stacks, TGN, p.m. (plasma membrane) and ECM (extracellular matrix). (B) BFA treatment results in Golgi complex disassembly and formation of a fused ER/Golgi compartment. The TGN becomes connected to the recycling endosomal system. (C) A 10-min pulse results in labelled *collagens in the RER and early secretory pathway. After 30 min of chase, these *collagens would be approximately equally distributed between the secretory pathway and ECM (not shown). (D) A 10-min pulse in BFA-treated samples results in *collagens in the fused ER/Golgi compartment. Retained *collagens would not be secreted during the chase (not shown). (E) Continuous labelling floods the secretory pathway and ECM with labelled *collagens. (F) BFA treatment after continuous labelling results in retrieval of *collagens from the Golgi stacks to the intracellular fused ER/Golgi compartment. *collagens in the TGN at the time of BFA treatment are secreted.
Figure 2. BFA treatment of whole tendon explants results in the expansion and progressive in situ distension of rough ER.
Tendon explants were incubated in medium containing BFA for 30 min or 1 h. Samples were fixed, embedded and analysed by TEM as described in the Experimental section. RER denotes regions of the section showing ribosome-studded regions of rough ER. RER distension is most pronounced in samples treated with BFA for 1 h. (A–C) Chick metatarsal tendon (E13); (D–F) CD1 mouse tail tendon (3-week-old postnatal). (A and D) Control incubation; (B and E) BFA incubation (30 min); (C and F) BFA incubation (1 h). Scale bars, 500 nm.
We used a conventional pulse–chase experiment to label (with [14C]proline) newly synthesized procollagen (Figure 1C) and BFA to split the secretory pathway (Figure 1D). We could therefore determine whether procollagen cleavage occurred in pre- or post-Golgi compartments. We used three BFA protocols: we added BFA (i) before the start of [14C]proline labelling in a pulse–chase experiment, (ii) at the start of labelling, or (iii) during labelling. We observed, as reported previously [25], that pre-incubation with BFA reduced procollagen synthesis (results not shown). Nevertheless, sufficient label was incorporated when BFA was added at the start of labelling to detect the newly synthesized procollagen chains, as shown below. Our previous studies have shown that a 10-min pulse and 30-min chase is an effective protocol to obtain a representative snapshot of intracellular and extracellular procollagen intermediates in tendon [6,16,24]. In the third protocol, tendons were incubated with [14C]proline for 1 h to flood the secretory pathway and extracellular compartment with [14C]collagen before BFA treatment. This protocol allowed the retrieval of 14C-labelled procollagen-processing intermediates from all pre-TGN compartments (Figures 1E and 1F).
Pre-TGN type I procollagen N-propeptide processing
In an initial experiment to identify the sites of intracellular procollagen cleavage, we simply included BFA in the pulse buffer only. A schematic diagram of the experimental approach is shown in Figure 3(A). E13 chick tendons and 3-week-old postnatal mouse tail tendons were examined. We detected the 14C-labelled proteins in the intracellular and extracellular compartments by differential ‘salt’ and ‘detergent’ extraction followed by SDS/PAGE and radiography [6]. The extracellular proteins were contained with the ‘salt extracts’ (S1) and the intracellular proteins were contained with the ‘NP40 (Nonidet P40) extracts’ (N). With this approach, we obtained a first insight into the effects of BFA on procollagen secretion and intracellular processing. The results are summarized in Figure 3. All eight polypeptide chains of procollagen, pNcollagen, pCcollagen and collagen were represented between the intracellular (N) and extracellular (S1) extracts. Procollagen chains predominated in the intracellular extracts and collagen chains predominated in the extracellular extracts. However, BFA had a profound effect on the intracellular conversion of procollagen into collagen and the secretion of both procollagen and collagen (Table 1). In both chick and mouse samples, BFA stopped procollagen secretion. In E13 chick samples, BFA virtually abolished intracellular conversion of procollagen, with the exception of a small amount of N-propeptide cleavage resulting in a small amount of intracellular pCcollagen. In mouse samples, pCcollagen was clearly present in BFA-treated samples (Figure 3C). The results suggest that processing at the C-propeptide was inhibited and N-propeptide cleavage occurred at near normal levels in the presence of BFA. These experiments provide the first hints that cleavage of the N- and C-propeptides occurred at different points in the secretory pathway.
Table 1. Effect of BFA on the relative amounts of intracellular and extracellular *collagen.
The relative amounts of labelled procollagen, procollagen-processing intermediates and fully processed collagen (collectively denoted *collagen) in the first extracellular salt extract (S1) and in the corresponding intracellular detergent tendon extract (N) were quantified by densitometry and expressed as a percentage of 14C-labelled *collagen in the intracellular extract. BFA treatment significantly decreased the percentage of *collagens in the extracellular extract.
| Intracellular *collagen (%, mean±S.D.) | |||
|---|---|---|---|
| Sample | Control | BFA | P value (Student's t test) |
| Embryonic chick metatarsal tendon | 39.2±3.0 | 81.2±0.6 | P<0.01 (n=3) |
| Postnatal CD1 mouse tail tendon | 46.5±3.3 | 78.9±3.3 | P<0.01 (n=3) |
Quantification of procollagen cleavage
To quantify the cleavage of intracellular procollagen the amounts of labelled *collagens were normalized to β-actin by Western blotting. *collagen is a collective term for all procollagen, pCcollagen, pNcollagen and collagen molecules. Fluorophore-conjugated secondary antibodies were used and signals were detected using a LI-COR Odyssey Infrared Imaging System. In all samples, pNcollagen and collagen levels were decreased, consistent with a reduction in processing at the C-propeptide [normalized intensity (QL), Figure 3D]. In embryonic chick tendon, the total amount of intracellular *collagen was unchanged by BFA treatment and pCcollagen levels remained constant, consistent with proteolytic removal of the N-propeptide. In postnatal mouse tail tendon, pCcollagen levels were significantly decreased; however, this is likely to reflect the significant decrease in total *collagen levels following BFA treatment (QL/105: control, 2.17±0.28; BFA, 1.06±0.51; P<0.05, n=4), rather than a reduction in N-propeptide removal. It is possible that feedback inhibition prevents complete conversion of procollagen into pCcollagen, but the production of intracellular pCcollagen in the presence of BFA implies that the procollagen N-proteinases are active either within the ER or within the fused ERGIC in BFA-treated cells. Active N-proteinases are therefore able to cleave procollagen before the procollagen becomes accessible to BMP1/tolloid metalloproteinases.
Intracellular C-proteinase activity is localized to the TGN in embryonic chick metatarsal tendon
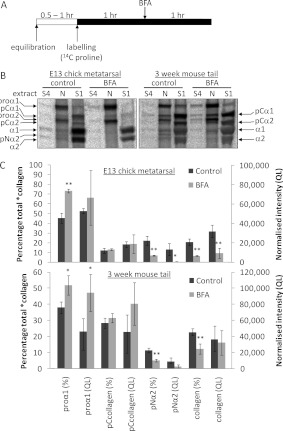
In further experiments, we saturated the pool of procollagen with [14C]proline and added BFA to split the secretory pathway (shown schematically in Figure 4A). The ‘metabolic labelling before BFA treatment’ protocol was used to identify procollagen-processing intermediates generated in the Golgi stacks (Figures 1E and 1F). The cleavage of procollagen to collagen was examined by SDS/PAGE (Figure 4B). The results show that BFA significantly reduced pNcollagen and fully processed collagen in the intracellular extracts of E13 chick metatarsal tendon, but pCcollagen was unchanged (Figures 4B and 4C), consistent with the results obtained using the pulse–chase protocol.
Figure 4. Intracellular retention of fully processed collagen is triggered by post-labelling BFA treatment in postnatal CD1 mouse tail tendon, but not in embryonic chick metatarsal tendon.
(A) Schematic diagram of the retrieval protocol used to label all *collagens within the secretory pathway of embryonic chick and postnatal mouse tail tendon fibroblasts in situ, before subsequent disruption of the secretory apparatus using BFA. (B) Embryonic chick metatarsal and postnatal mouse tail tendons incubated according to the protocol shown in (A) were subjected to sequential salt and NP40 detergent extractions, and the extracts were analysed by electrophoresis and autoradiography as described in the Experimental section. Single representative images are shown. ‘S1’ denotes the first extracellular salt extract, ‘S4’ represents the fourth and final salt extract, and ‘N’ is the intracellular NP40 detergent extract. Bands corresponding to each of the type I procollagen-processing intermediates are indicated. (C) The relative (% total) and absolute [normalized intensity (QL)] amounts of each *collagen were determined as described in the Experimental section (n=3). *P<0.05; **P<0.01 (Student's t test).
Identification of pre-TGN type I procollagen C-propeptide processing in murine tail tendon
Bacterial collagenase digestion after labelling and BFA treatment was used to confirm that the fully processed collagen in the N extract of postnatal mouse tail tendon was protected from proteolysis and therefore present in an intracellular membrane-enclosed compartment (Figure 5A). The efficacy of collagenase treatment is presented in Table 2. BFA treatment reduced the relative amount of the pNcollagen α2 chain, but the reduction in the absolute amount of pNα2 was not significant (Figures 4B and 4C).
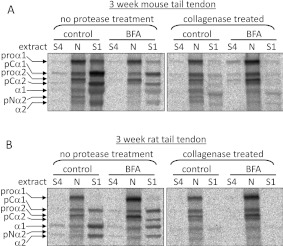
Figure 5. *collagens present in the intracellular ‘N’ extract are protected from collagenase digestion.
Postnatal mouse tail tendons (A) and postnatal rat tail tendons (B) were incubated according to the protocol shown in Figure 3(A) and treated with collagenase as described in the Experimental section. Tendons incubated according to the protocol shown in Figure 3(A) were subjected to sequential salt and NP40 detergent extractions, and the extracts were analysed by electrophoresis and autoradiography. Single representative images are shown. ‘S1’ denotes the first extracellular salt extract, ‘S4’ represents the fourth and final salt extract, and ‘N’ is the intracellular NP40 detergent extract. Bands corresponding to each of the type I procollagen-processing intermediates are indicated. Fully processed collagen is present in the intracellular (N) extract following BFA and/or collagenase treatment.
Table 2. Effect of collagenase on the relative amounts of intracellular and extracellular *collagens.
The relative amounts of labelled *collagens in the first extracellular salt extract (S1) and in the intracellular detergent extract (N) were quantified by densitometry and expressed as a percentage of 14C *collagen in the extracellular extract. Collagenase treatment significantly decreased the percentage of *collagens in the extracellular extract.
| Extracellular *collagen (%, mean±S.D.) | |||||
|---|---|---|---|---|---|
| Sample | Control | BFA | Control+collagenase | BFA+collagenase | P value (two-way ANOVA) |
| Postnatal CD1 mouse tail tendon | 66.8±5.3 | 61.6±3.0 | 34.2±1.0 | 35.4±7.9 | P<0.01 for collagenase factor (n=3) |
| Postnatal rat tail tendon | 85.6±1.6 | 83.9±4.5 | 64.2±17.7 | 42.4±4.0 | P<0.01 for collagenase factor (n=3) |
To compare similar embryonic and postnatal tendons from the same species, the metabolic labelling/BFA treatment protocol was applied to rat tail tendon explants (embryonic mouse tail tendons cannot be dissected rapidly enough or in sufficient quantities for analysis, and mineralization of postnatal chick tendons might be expected to complicate interpretation of the findings). It was possible to isolate small quantities of tendon from E21 rat tails such that pooled tendons from 12–17 embryos per pregnant rat were sufficient for one or two labelled samples. E21 rat tail tendons displayed an embryonic phenotype characterized by intercellular plasma membrane channels and cell-associated fibrils in fibricarriers and fibripositors [6,26–29] by TEM (Figure 6). In E21 rat tail tendon, some intracellular collagen persisted following BFA treatment (see lanes marked ‘BFA, N’ in Figure 7A), even though the proportion of fully processed collagen was significantly decreased (Figure 7B). In embryonic samples, absolute quantification was hindered by the small quantities of extractable protein, leading to larger error bars for normalized intensity (QL). In postnatal rat tail tendon, the metabolic labelling/BFA treatment protocol did not result in a loss of intracellular collagen (Figure 7). Collagenase digestion was used to confirm the intracellular location of fully processed collagen in the N extracts (Figure 5 and Table 2). There was no significant change in total intracellular *collagen levels (QL) between control and BFA-treated samples subjected to the metabolic labelling/BFA treatment protocol, indicating that C-proteinase activity is not restricted to the TGN.
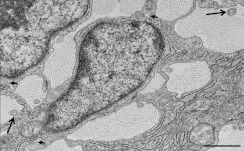
Figure 6. Transmission electron micrograph of embryonic E21 rat tail tendon.
The image shows fibricarriers (collagen fibrils within the main body of the cells) (short arrows) and fibripositors (collagen fibrils located within cellular projections) (long arrows), in transverse view. Fibripositors and fibricarriers are characteristic features of embryonic tendon. Scale bar, 1 μm.
Figure 7. Intracellular retention of fully processed collagen in rat tail tendon following post-labelling BFA treatment.
(A) Embryonic and postnatal rat tail tendons incubated according to the protocol shown in Figure 3(A) were subjected to sequential salt and NP40 detergent extractions, and the extracts were analysed by electrophoresis and autoradiography as described in the Experimental section. Single representative images are shown. ‘S1’ denotes the first extracellular salt extract, ‘S4’ represents the fourth and final salt extract, and ‘N’ is the intracellular NP40 detergent extract. Bands corresponding to each of the type I procollagen-processing intermediates are indicated. (B) The relative (% total) and absolute [normalized intensity (QL)] amounts of each *collagen were determined as described in the Experimental section (n=3). *P<0.05; **P<0.01 (Student's t test).
The results show that pre-TGN processing at the procollagen C-propeptide is a feature common to both mouse and rat postnatal tail tendon, but which is not confined to postnatal development. The observed loss of pNα2, following BFA treatment after labelling, indicates that any pre-TGN processing of procollagen to collagen occurs via the pCcollagen intermediate (at least for the α2 chain) and that intracellular collagen generated in pre-TGN compartments results from the sequential action of the procollagen N-proteinase and C-proteinase enzymes.
Preventing the furin-mediated activation of procollagen N- and C-proteinases reduces, but does not abolish, procollagen processing in tendon explants
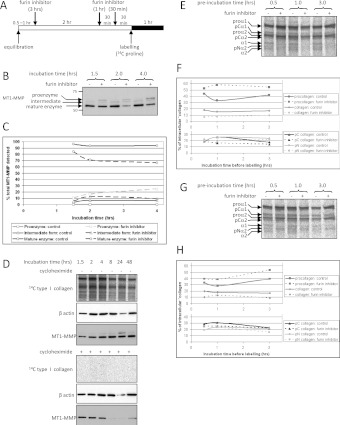
To determine the extent to which intracellular procollagen processing is mediated by newly activated procollagen proteinases, we inhibited the intracellular activation of the procollagen N- and C-proteinases using the furin inhibitor Dec-RVKR-CMK. Dec-RVKR-CMK inhibits all seven basic amino-acid-specific furin-like proprotein convertases [30–32]. However, it was not possible to detect BMP1/TLL metalloproteinases (the C-proteinases) or ADAMTS 2, 3 or 14 (the N-proteinases) directly because of the absence of specific antibodies. Therefore we chose to determine whether Dec-RVKR-CMK inhibited the furin-activation of MT1-MMP, for which specific antibodies are available. MT1-MMP is activated by furin-like proprotein convertases [33] and can be detected in intracellular embryonic chick tendon extracts by Western blotting (Figure 8). Treatment with the furin inhibitor caused a time-dependent accumulation of the pro-form of the protein, and a slow loss of the mature form of MT1-MMP (Figures 8B and 8C). To determine the time-scale for MT1-MMP turnover, embryonic chick metatarsal tendons were incubated with cycloheximide for 1.5–48 h, to inhibit new protein synthesis. MT1-MMP levels fell 4–8 h after cycloheximide addition (Figure 8D), indicating that MT1-MMP levels are stable during the 4-h time window. The results indicated that Dec-RVKR-CMK effectively inhibits proprotein convertases in tendon.
Figure 8. Effect of the furin inhibitor Dec-RVKR-CMK on intracellular procollagen processing in embryonic chick metatarsal and postnatal CD1 mouse tail tendon.
(A) Schematic diagram of the protocol used to assess the effect of pre-incubation with Dec-RVKR-CMK on the extent of intracellular procollagen processing. (B) Intracellular (N) extracts from tendons treated with Dec-RVKR-CMK were analysed by Western blotting, using an antibody directed against the catalytic domain of MT1-MMP. Furin inhibitor treatment resulted in the appearance of bands corresponding to the proenzyme and intermediate forms of MT1-MMP. (C) The relative amounts of the proenzyme, intermediate and mature forms of MT1-MMP were quantified by densitometry. Furin inhibitor resulted in a time-dependent decrease in the relative amount of the mature enzyme and an increase in the amount of the proenzyme. (D) To determine whether MT1-MMP would be completely turned over during 4 h of incubation, embryonic chick metatarsal tendons were treated with the protein synthesis inhibitor cycloheximide for various durations up to 48 h. Samples were labelled for 1 h before the end of each incubation. The intracellular extracts were analysed by electrophoresis and autoradiography, to confirm the effectiveness of cycloheximide, and also by Western blotting using antibodies directed against MT1-MMP and β-actin. (E and G) Embryonic chick metatarsal (E) or postnatal CD1 mouse tail (G) tendons were incubated according to the protocol shown in (A) were subjected to sequential salt and NP40 detergent extractions, and the extracts were analysed by electrophoresis and autoradiography as described in the Experimental section. The intracellular (N) extracts are shown for each treatment. Bands corresponding to each of the type I procollagen-processing intermediates are indicated. (F and H) The relative amount of procollagen, pCcollagen, pNcollagen and fully processed collagen in each of the intracellular NP40 detergent extracts from embryonic chick metatarsal (F) or postnatal CD1 mouse tail (H) tendon was quantified by densitometry.
Having shown that Dec-RVKR-CMK was effective at inhibiting furin-like proprotein convertases in cells in tendon explants, we added the inhibitor to embryonic chick metatarsal tendon explants for 30 min, 1 h and 3 h before labelling with [14C]proline (Figure 8). The intracellular extracts were analysed by Western blotting. Dec-RVKR-CMK treatment reduced, but did not ablate, intracellular procollagen processing in embryonic chick metatarsal tendon (Figure 8E). Quantification of the extent of processing at each time point indicated that the proportions of pCcollagen and pNcollagen remained relatively stable, but the relative amount of intracellular procollagen was consistently increased, and the relative amount of intracellular fully processed collagen consistently decreased (Figure 8F). Therefore intracellular processing in embryonic chick metatarsal tendon is mediated by newly synthesized procollagen N- and C-proteinases, as well as by proteinases with an intracellular half-life greater than 4 h. Postnatal CD1 mouse tail tendons were treated similarly with Dec-RVKR-CMK. Again, intracellular processing was reduced, but not ablated (Figure 8G), and the relative amounts of intracellular procollagen and collagen were increased and decreased respectively (Figure 8H). The results indicate that a fraction of the procollagen processed before secretion is cleaved by newly activated proteinases. A time-dependent decrease in processing was not observed, indicating that procollagen molecules are also processed by a longer-lived population of intracellular N- and C-proteinases.
Conclusions
The near constant proportion of pCcollagen in pulse–chase analyses and following extended BFA treatment indicate that pCcollagen is produced in the cis-Golgi. Despite acting early in the secretory pathway in tendon, N-proteinases are not restricted to the early secretory pathway and also act within the TGN: pNcollagen (particularly pNα2) is produced inside the cell, but is rarely detected in extracellular tissue extracts. N-proteinases are also active within the extracellular matrix because secreted procollagen is eventually converted into collagen, via pCcollagen [6]. Our results demonstrate a stepwise activation of procollagen to collagen within the secretory pathway of tendon fibroblasts in situ. As activation of the procollagen N- and C-proteinases probably occurs in the TGN, mediated by furin-like proprotein convertases, it is possible that there is a stable pool of procollagen proteinases elsewhere within the secretory pathway. For the procollagen N-proteinases, this pool appears to reside in the ER or the cis-Golgi, but in Golgi and pre-TGN compartments for the procollagen C-proteinases.
The procollagen C-proteinases (BMP1/tolloid family metalloproteinases) cleave numerous substrates in addition to type I procollagen. For example, BMP1 activates lysyl oxidase [34], a key enzyme in collagen and elastin cross-linking that has recently been implicated in tumour progression [35]. BMP1/tolloid family metalloproteinases also participate in essential developmental processes such as dorsal–ventral patterning, heart development, TGFβ (transforming growth factor β) signalling and neurogenesis [36,37]. Therefore elucidation of the subcellular site of action of BMP1-like metalloproteinases has implications for the regulation of other morphogenic proteins, including other fibrillar and non-fibrillar collagens, chordin, biglycan, decorin, myostatin, GDF11 (growth differentiation factor 11) and LTBP (latent TGFβ-binding protein) [7,8,36,38,39].
AUTHOR CONTRIBUTION
Elizabeth Canty-Laird performed all of the experiments except for the electron microscopy. Yinhui Lu carried out the electron microscopy. Karl Kadler raised the funding for the research. Elizabeth Canty-Laird analysed the data. Elizabeth Canty-Laird and Karl Kadler wrote the paper. All authors approved the paper.
FUNDING
This work was supported by the Wellcome Trust.
References
- 1.Chapman J. A., Tzaphlidou M., Meek K. M., Kadler K. E. The collagen fibril: a model system for studying the staining and fixation of a protein. Electron Microsc. Rev. 1990;3:143–182. doi: 10.1016/0892-0354(90)90018-n. [DOI] [PubMed] [Google Scholar]
- 2.Bellamy G., Bornstein P. Evidence for procollagen, a biosynthetic precursors of collagen. Proc. Natl. Acad. Sci. U.S.A. 1971;68:1138–1142. doi: 10.1073/pnas.68.6.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kadler K. E., Hojima Y., Prockop D. J. Assembly of collagen fibrils de novo by cleavage of the type I pC-collagen with procollagen C-proteinase: assay of critical concentration demonstrates that collagen self-assembly is a classical example of an entropy-driven process. J. Biol. Chem. 1987;262:15696–15701. [PubMed] [Google Scholar]
- 4.Bonfanti L., Mironov A. A., Martinez-Menarguez J. A., Martella O., Fusella A., Baldassarre M., Buccione R., Geuze H. J., Luini A. Procollagen traverses the Golgi stack without leaving the lumen of cisternae: evidence for cisternal maturation. Cell. 1998;95:993–1003. doi: 10.1016/s0092-8674(00)81723-7. [DOI] [PubMed] [Google Scholar]
- 5.Polishchuk E. V., Di Pentima A., Luini A., Polishchuk R. S. Mechanism of constitutive export from the golgi: bulk flow via the formation, protrusion, and en bloc cleavage of large trans-Golgi network tubular domains. Mol. Biol. Cell. 2003;14:4470–4485. doi: 10.1091/mbc.E03-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canty E. G., Lu Y., Meadows R. S., Shaw M. K., Holmes D. F., Kadler K. E. Coalignment of plasma membrane channels and protrusions (fibripositors) specifies the parallelism of tendon. J. Cell Biol. 2004;165:553–563. doi: 10.1083/jcb.200312071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pappano W. N., Steiglitz B. M., Scott I. C., Keene D. R., Greenspan D. S. Use of Bmp1/Tll1 doubly homozygous null mice and proteomics to identify and validate in vivo substrates of bone morphogenetic protein 1/tolloid-like metalloproteinases. Mol. Cell. Biol. 2003;23:4428–4438. doi: 10.1128/MCB.23.13.4428-4438.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott I. C., Blitz I. L., Pappano W. N., Imamura Y., Clark T. G., Steiglitz B. M., Thomas C. L., Maas S. A., Takahara K., Cho K. W., Greenspan D. S. Mammalian BMP-1/Tolloid-related metalloproteinases, including novel family member mammalian Tolloid-like 2, have differential enzymatic activities and distributions of expression relevant to patterning and skeletogenesis. Dev. Biol. 1999;213:283–300. doi: 10.1006/dbio.1999.9383. [DOI] [PubMed] [Google Scholar]
- 9.Colige A., Li S. W., Sieron A. L., Nusgens B. V., Prockop D. J., Lapiere C. M. cDNA cloning and expression of bovine procollagen I N-proteinase: a new member of the superfamily of zinc-metalloproteinases with binding sites for cells and other matrix components. Proc. Natl. Acad. Sci. U.S.A. 1997;94:2374–2379. doi: 10.1073/pnas.94.6.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colige A., Vandenberghe I., Thiry M., Lambert C. A., Van Beeumen J., Li S. W., Prockop D. J., Lapiere C. M., Nusgens B. V. Cloning and characterization of ADAMTS-14, a novel ADAMTS displaying high homology with ADAMTS-2 and ADAMTS-3. J. Biol. Chem. 2002;277:5756–5766. doi: 10.1074/jbc.M105601200. [DOI] [PubMed] [Google Scholar]
- 11.Wang W. M., Lee S., Steiglitz B. M., Scott I. C., Lebares C. C., Allen M. L., Brenner M. C., Takahara K., Greenspan D. S. Transforming growth factor-β induces secretion of activated ADAMTS-2: a procollagen III N-proteinase. J. Biol. Chem. 2003;278:19549–19557. doi: 10.1074/jbc.M300767200. [DOI] [PubMed] [Google Scholar]
- 12.Leighton M., Kadler K. E. Paired basic/furin-like proprotein convertase cleavage of Pro-BMP-1 in the trans-Golgi network. J. Biol. Chem. 2003;278:18478–18484. doi: 10.1074/jbc.M213021200. [DOI] [PubMed] [Google Scholar]
- 13.Scamuffa N., Calvo F., Chretien M., Seidah N. G., Khatib A.-M. Proprotein convertases: lessons from knockouts. FASEB J. 2006;20:1954–1963. doi: 10.1096/fj.05-5491rev. [DOI] [PubMed] [Google Scholar]
- 14.Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat. Rev. Mol. Cell Biol. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seidah N. G., Khatib A. M., Prat A. The proprotein convertases and their implication in sterol and/or lipid metabolism. Biol. Chem. 2006;387:871–877. doi: 10.1515/BC.2006.110. [DOI] [PubMed] [Google Scholar]
- 16.Humphries S. M., Lu Y., Canty E. G., Kadler K. E. Active negative control of collagen fibrillogenesis in vivo: intracellular cleavage of the type I procollagen propeptides in tendon fibroblasts without intracellular fibrils. J. Biol. Chem. 2008;283:12129–12135. doi: 10.1074/jbc.M708198200. [DOI] [PubMed] [Google Scholar]
- 17.Donaldson J. G., Cassel D., Kahn R. A., Klausner R. D. ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein β-COP to Golgi membranes. Proc. Natl. Acad. Sci. U.S.A. 1992;89:6408–6412. doi: 10.1073/pnas.89.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donaldson J. G., Finazzi D., Klausner R. D. Brefeldin A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature. 1992;360:350–352. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- 19.Helms J. B., Rothman J. E. Inhibition by brefeldin A of a Golgi membrane enzyme that catalyses exchange of guanine nucleotide bound to ARF. Nature. 1992;360:352–354. doi: 10.1038/360352a0. [DOI] [PubMed] [Google Scholar]
- 20.Klausner R. D., Donaldson J. G., Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J. Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sciaky N., Presley J., Smith C., Zaal K. J., Cole N., Moreira J. E., Terasaki M., Siggia E., Lippincott-Schwartz J. Golgi tubule traffic and the effects of brefeldin A visualized in living cells. J. Cell Biol. 1997;139:1137–1155. doi: 10.1083/jcb.139.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chege N., Pfeffer S. Compartmentation of the Golgi complex: brefeldin-A distinguishes trans-Golgi cisternae from the trans-Golgi network. J. Cell Biol. 1990;111:893–899. doi: 10.1083/jcb.111.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lippincott-Schwartz J., Yuan L., Tipper C., Amherdt M., Orci L., Klausner R. D. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991;67:601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- 24.Canty E. G., Starborg T., Lu Y., Humphries S. M., Holmes D. F., Meadows R. S., Huffman A., O'Toole E. T., Kadler K. E. Actin filaments are required for fibripositor-mediated collagen fibril alignment in tendon. J. Biol. Chem. 2006;281:38592–38598. doi: 10.1074/jbc.M607581200. [DOI] [PubMed] [Google Scholar]
- 25.Ripley C. R., Fant J., Bienkowski R. S. Brefeldin A inhibits degradation as well as production and secretion of collagen in human lung fibroblasts. J. Biol. Chem. 1993;268:3677–3682. [PubMed] [Google Scholar]
- 26.Birk D. E., Trelstad R. L. Extracellular compartments in tendon morphogenesis: collagen fibril, bundle, and macroaggregate formation. J. Cell Biol. 1986;103:231–240. doi: 10.1083/jcb.103.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trelstad R. L., Hayashi K. Tendon collagen fibrillogenesis: intracellular subassemblies and cell surface changes associated with fibril growth. Dev. Biol. 1979;71:228–242. doi: 10.1016/0012-1606(79)90166-0. [DOI] [PubMed] [Google Scholar]
- 28.Richardson S. H., Starborg T., Lu Y., Humphries S. M., Meadows R. S., Kadler K. E. Tendon development requires regulation of cell condensation and cell shape via cadherin-11-mediated cell–cell junctions. Mol. Cell. Biol. 2007;27:6218–6228. doi: 10.1128/MCB.00261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapacee Z., Richardson S. H., Lu Y., Starborg T., Holmes D. F., Baar K., Kadler K. E. Tension is required for fibripositor formation. Matrix Biol. 2008;27:371–375. doi: 10.1016/j.matbio.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Denault J. B., D'Orleans-Juste P., Masaki T., Leduc R. Inhibition of convertase-related processing of proendothelin-1. J. Cardiovasc. Pharmacol. 1995;26(Suppl. 3):S47–S50. [PubMed] [Google Scholar]
- 31.Jean F., Stella K., Thomas L., Liu G., Xiang Y., Reason A. J., Thomas G. α1-Antitrypsin Portland, a bioengineered serpin highly selective for furin: application as an antipathogenic agent. Proc. Natl. Acad. Sci. U.S.A. 1998;95:7293–7298. doi: 10.1073/pnas.95.13.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fugère M., Limperis P. C., Beaulieu-Audy V., Gagnon F., Lavigne P., Klarskov K., Leduc R., Day R. Inhibitory potency and specificity of subtilase-like pro-protein convertase (SPC) prodomains. J. Biol. Chem. 2002;277:7648–7656. doi: 10.1074/jbc.M107467200. [DOI] [PubMed] [Google Scholar]
- 33.Yana I., Weiss S. J. Regulation of membrane type-1 matrix metalloproteinase activation by proprotein convertases. Mol. Biol. Cell. 2000;11:2387–2401. doi: 10.1091/mbc.11.7.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uzel M. I., Scott I. C., Babakhanlou-Chase H., Palamakumbura A. H., Pappano W. N., Hong H. H., Greenspan D. S., Trackman P. C. Multiple bone morphogenetic protein 1-related mammalian metalloproteinases process pro-lysyl oxidase at the correct physiological site and control lysyl oxidase activation in mouse embryo fibroblast cultures. J. Biol. Chem. 2001;276:22537–22543. doi: 10.1074/jbc.M102352200. [DOI] [PubMed] [Google Scholar]
- 35.Ng M. R., Brugge J. S. A stiff blow from the stroma: collagen crosslinking drives tumor progression. Cancer Cell. 2009;16:455–457. doi: 10.1016/j.ccr.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 36.Hopkins D. R., Keles S., Greenspan D. S. The bone morphogenetic protein 1/Tolloid-like metalloproteinases. Matrix Biol. 2007;26:508–523. doi: 10.1016/j.matbio.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee H. X., Mendes F. A., Plouhinec J.-L., De Robertis E. M. Enzymatic regulation of pattern: BMP4 binds CUB domains of Tolloids and inhibits proteinase activity. Genes Dev. 2009;23:2551–2562. doi: 10.1101/gad.1839309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.von Marschall Z., Fisher L. W. Decorin is processed by three isoforms of bone morphogenetic protein-1 (BMP1) Biochem. Biophys. Res. Commun. 2010;391:1374–1378. doi: 10.1016/j.bbrc.2009.12.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee S.-J. Genetic analysis of the role of proteolysis in the activation of latent myostatin. PLoS ONE. 2008;3:e1628. doi: 10.1371/journal.pone.0001628. [DOI] [PMC free article] [PubMed] [Google Scholar]