Abstract
BACKGROUND:
Esophageal cancer is the 8th most common cancer and the 6th leading cause of cancer-related death, worldwide. In Iran, the high incidence rates of this type of cancer have been reported from the Caspian Sea region. This study aimed at assessing the factors affecting survival of patients with esophageal cancer in neighbor provinces around Caspian Sea using parametric and semi-parametric models with univariate gamma frailty model.
METHODS:
In this study, we performed a prospective review of 359 patients presenting with esophageal cancer from 1990 to 1991. The data were obtained using the Cancer Registry information existed in Babol research center in Iran. Study participants were followed-up until 2006 for a period of 15 years. Hazard ratio was used to interpret the risk of death. The Akaike Information Criterion (AIC) was considered as a criterion to select the best model(s).
RESULTS:
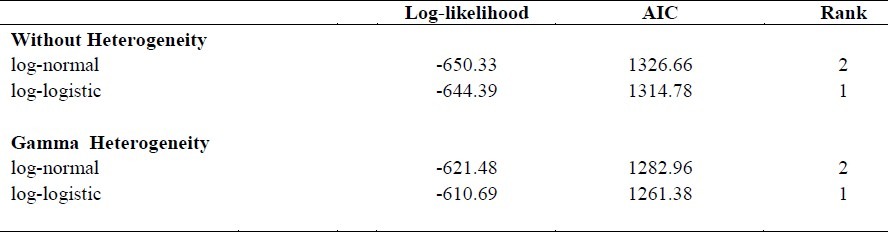
Of the 359 patients, 225 (62.7%) were male with a mean age of 60.0 years and 134 (37.3%) were female with a mean age of 55.3 at the time of diagnosis. 1- , 3- and 5-year survival rates after diagnosis were 23%, 15% and 13% , respectively. Comparison between Cox and parametric models of AIC showed that the overall fitting was improved under parametric models. Among parametric models, the log-logistic model with gamma frailty provided better performance than other models. Using this model, we found that gender (p=0.012) and family history of cancer (p= 0.003) were significant predictors.
CONCLUSIONS:
Since the proportionality assumption of the Cox model was not held (p = 0.01), the Cox regression model was not an appropriate choice for analyzing our data. According to our findings, log logistic model with gamma frailty could be considered as a useful statistical model in survival analysis of patients with esophageal cancer rather than Cox and log-normal models.
KEYWORDS: Esophageal Cancer, Survival Analysis, Univariate Gamma Frailty Model, Parametric Model
Cancer is one of the most important causes of death and disabilities worldwide.1,2 The disease is becoming increasingly popular and has been received a considerable amount of health care resources3 It has been estimated to become the leading cause of death in many developed and developing countries including Iran.1,4 Esophageal, stomach and colorectal cancers are the three most common cancers among Iranian people.5 Esophageal cancer is one of the ten most common malignancies worldwide. The five-year survival rate of people with this type of cancer is 3 to 10 percent.6,7 The results of several epidemiological studies showed that hot drinks, alcohol and tobacco are the main risk factors for esophageal cancer.8–13 Despite the fact that medical advances have increased survival rates, this disease is unique in desperation and deep fear that creates in person.14–16 There is no doubt that the diagnosis of life-threatening diseases such as cancer makes different effects on the patients quality of life.17–19 Geographical distribution is effective in esophageal cancer. Approximately 80% of the total cancer cases occur in developing countries. Esophageal cancer in the Western countries is relatively rare, but China, South Africa and North of Central Asia regions are considered as having the highest incidence of esophageal cancer.20,24 The northern regions of Iran have been observed to dominate malignancies of esophageal cancer.22,25 The highest incidence of esophageal cancer occurs in people aged 50-70 years, also the frequency of the disease is higher in men.4,23,26. Overall, gastrointestinal (GI) cancers account for approximately half (44.4%) of all cancer-related deaths in Iran.27,28 Unfortunately, patients suffering from esophageal cancer often refer to medical care when it is at advanced stages and so limited or no effective therapies are available to treat them.1,28 Theoretically, esophageal cancer cases may be treatable in their early stages, therefore, early detection is desirable.
Cox proportional hazards model is among the most common models in survival data analysis.29 In cases where the premise of proportional hazards is not met, however, this model will not be appropriate to use.29,30 One of the cases in which the Cox proportional hazards model should not be used is when the hazards increase initially but start to decrease after a period of time. In such cases, utilizing log-logistic and log-normal parametric models seem proper, due to having the non-monotonic hazards functions.31–33 In the present study, because of facing such models for the hazards function, the log-logistic and log-normal parametric models are used.
Based on asymptotic results, Efron and Oakes showed that under certain circumstances, parameter estimates in parametric models are led to become more efficient than Cox's model.34,35 Selected parametric models such as Weibull, log-logistic and log-normal models are alternatives to the Cox model.
In the Cox proportional hazard model and parametric models, it is assumed that if individuals have the same values of the covariates, they have the same survival function regardless of heterogeneities.36,37 But, extra-heterogeneities might exist, that we do not include in the model. We would collect data on all factors we think will influence survival, but there will always be others which have an additional effect that we miss.29,38–40
A model that is becoming increasingly popular for modeling association between individual survival times within subgroups is the use of a frailty model. A frailty is an unobservable random effect shared by subjects within a subgroup. Frailty models are also used in making adjustments for overdispersion in univariate survival studies. Here, the frailty represents the total effect on survival of the covariates not measured when collecting information on individual subjects. If these effects are ignored, the resulting survival estimates may be misleading. Corrections for this overdispersion allow for adjustments for other unmeasured important effects. The overdispersion in this case is indicated by an unobservable multiplicative effect on the hazard, or frailty.29 Since the hazard function cannot be negative, a positive distribution should be considered for frailty distribution. The frailty distributions most often applied are the gamma distribution, inverse Gaussian, log-normal, the positive stable distribution, Compound Poisson and a three-parameter distribution [Power Variance Function (PVF)].29 Also, because of over-parameterization and identifiability problem, and as frailty is considered as a random effect that indicates the effect of unknown variables, it is necessary to assume that the mean of frailty equals one.29,38–42. The aim of the present study was to assess the factors influencing the survival of patients with esophageal cancer using parametric models with univariate gamma frailty model and to compare the results with those obtained in Cox model.
Methods
This survey was a prospective study. 359 patients with developed esophageal cancer registered at the Babol Cancer Registration Center during 1990-1991 were included in the study. They, then, were followed up for 15 years until 2006. The socio-demographic and clinical data were obtained using questionnaire and the patients′ clinical records. The factors we considered in our study were age at diagnosis, gender, place of residence, province, type of cancer, method of cancer detection, family history of cancer, education, job, marital status, cigarette smoking, ethnicity, migration status, and drug use.
The coding of the samples was done under the direct supervision of pathology specialists based on the international classification of disease for oncology (ICD-O) coding43 The study was confirmed by the Ethics Committee of Tehran University of Medical Sciences. In this study, in order to compare the efficiency of parametric and Cox models the AIC44 (Akaike Information Criterion) was used. The AIC is a criterion that assesses goodness of fit of a statistical model, and the lower value of AIC suggests a better model.
In multiple analysis, RR (relative risk) and HR (hazard rate) were used to interpret the risk of death in parametric models and Cox model, respectively.29,37 For the statistical analysis, the statistical softwares SAS 9.1 (figures) and R (analysis) were used. In all cases, p < 0.05 was defined as the statistical significance.
One of the limitations of this study was the absence of clinical variables including type of esophageal cancer (adenocarcinoma, squamous), stage of disease, etc. The reason was absence of recorded clinical data in the Babol cancer registry and the lack of access to medical records of patients.
Univariate Gamma Frailty Model
The proportional hazards of univariate frailty model assumes that based on condition of the frailty variable U, the hazard function for the lifetime T is
hi(t / x, u)=h0(t)ui exp(βTX)
where h0(t) is the baseline hazard function with X = (X1, . . . , Xk) and β = (β1, . . . , βk) as covariates and regression parameters, respectively.29 Here, ui is considered a nonnegative random variable (frailty variable) for the ith (i=1, 2, …, n) patient. Consequently, a frailty model is a generalization of the well-known proportional hazards model. The proportional hazards model is obtained if the frailty distribution degenerates to U=1 for all individuals.29 Since the hazard function cannot be negative, a positive distribution should be considered for frailty distribution. The frailty distributions most often applied are the gamma distribution, inverse Gaussian, log-normal, the positive stable distribution, Compound Poisson and a three-parameter distribution (PVF). Also, because of over-parameterization and identifiability problem, and because frailty as a random effect indicates the effect of unknown variables, it is necessary to assume that the mean of frailty equals one.29 We considered the frailty model with observed covariates and focused on the main aspects of univariate frailty modeling.
The density of a gamma-distributed random variable (with mean one and variance σ2) [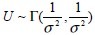 ] is given by
] is given by
[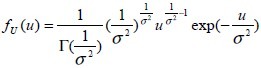 ]
]
Results
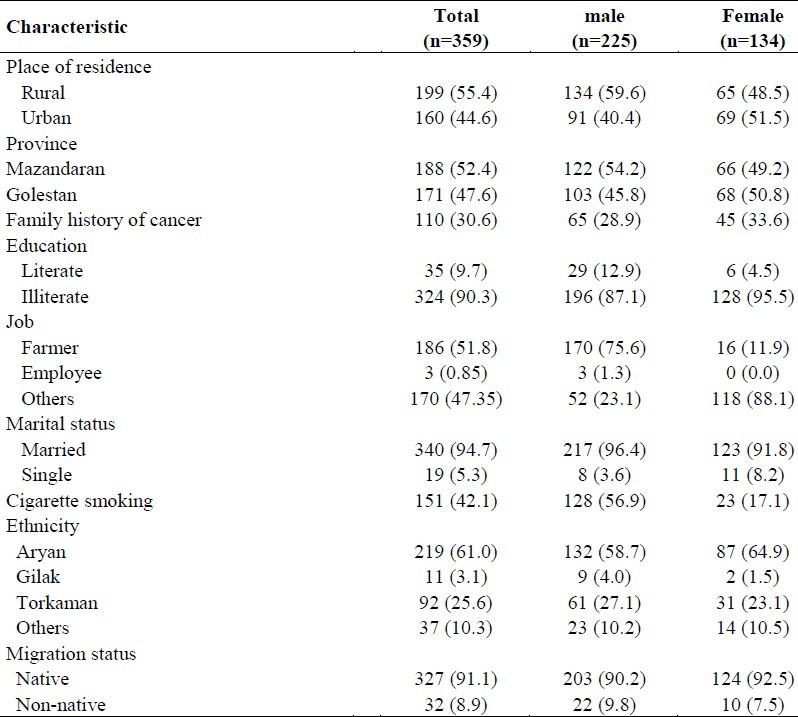
Of the 359 patients with esophageal cancer enrolled in this study, 225 (62.7%) were men and 134 (37.3%) were women. The mean (standard deviation) age at diagnosis was 55.23 (± 11.01) years (Table 1). The median survival time in this study reached about 9 months and 1-, 3- and 5-year survival rates following diagnosis were estimated 23%, 15% and 13%, respectively. A total of 310 (86.3%) deaths were observed (non-censored observations) during the follow-up period; among them 63.2% were men, 36.8% were women and 49 (13.6%) patients either survived or were lost to follow-up and were considered as right censored observations.
Table 1.
Characteristics of patients with esophageal cancer diagnosis
As the proportionality assumption of Cox model was not met in our data (p = 0.01), the Cox regression was not suitable. Even after adding frailty term (gamma) into Cox model, proportionality assumption was violated and there was no remedy in the violation of the PH assumption.
Table 2 shows the mean, median, standard deviation and confidence interval for survival time (month) of patients diagnosed with esophageal cancer, in different age groups. Based on Breslow estimator, probability value was obtained significant at 0.05 (p = 0.04 ); i.e., survival functions were different for different age groups (Figure 1).
Table 2.
Means, Medians and Confidence Interval for Survival Time (Month) of patients with esophageal cancer, by different age groups
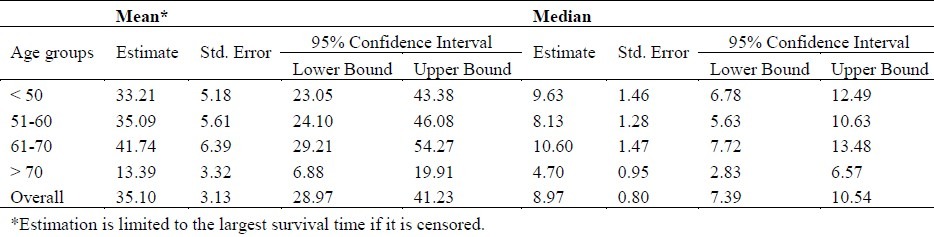
Figure 1.
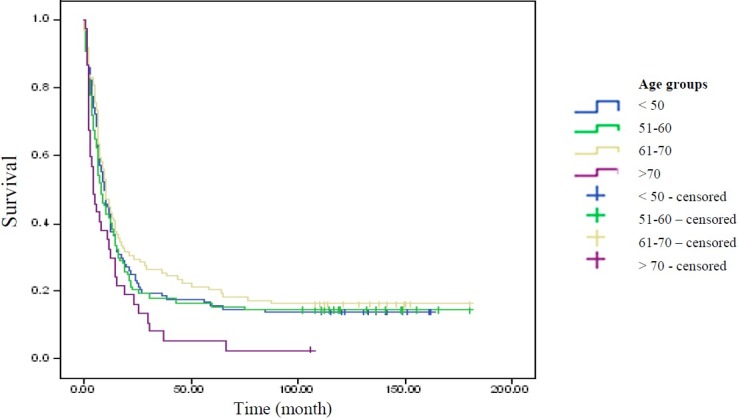
Comparison of survival functions of patients with esophageal cancer in different age groups
Review of residual plots (Cox-Snell residuals, deviance residuals) represented a better fit of parametric models related to Cox model. Among the parametric models, the log-logistic model with gamma frailty was more appropriate to fit the data (mean deviance residuals were 0.187, 0.248 and 0.672 for log-logistic, log-normal and Cox models, respectively, Figure 2).
Figure 2.
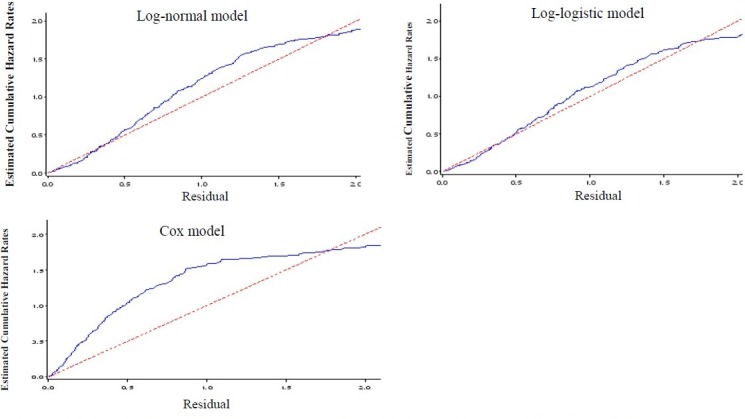
Cox-Snell residuals obtained from fitting various survival models to the esophageal cancer data.
The panels indicate the Cox-Snell residuals (together with their cumulative hazard function) obtained from fitting different parametric AFT models to the same data via maximum likelihood estimation. Obviously, the lines related to the Cox-Snell residuals of the log-normal and log-logistic models with gamma frailty are nearest to the line through the origin, indicating that these models fit the data best. In addition, the Cox model does not appear to fit the data well; it means that the proportional hazards assumption is violated. These results are consistent with our finding based on Akaike's Information Criterion
To enable the comparison, we calculated the AIC. Table 3 shows that according to this criterion, the log-logistic model scored best, followed by the log-normal model and the Cox model came third. According to the AIC, the log-logistic allowing for gamma heterogeneity is thus the preferred model, followed by the log-normal model. By comparing these findings, we came to the conclusion that the log-logistic model with gamma frailty is more efficient than the Cox model and log-normal model (with and without gamma frailty).
Table 3.
Overview of the Akaike's Information Criterion Scores
As expected, the effects of covariates were biased downwards in the Cox model when not corrected for unobserved heterogeneity in the study population (Table 4). Note that the standard deviation also increased in the gamma frailty model, and that the large standard deviation of the frailty variance (σ2) estimate did not exclude the possibility of no unobserved heterogeneity (σ2= 0).
Table 4.
Parameter estimates (s.e.) in the Cox, log-normal and log-logistic models and the gamma frailty model applied to esophageal cancer data.
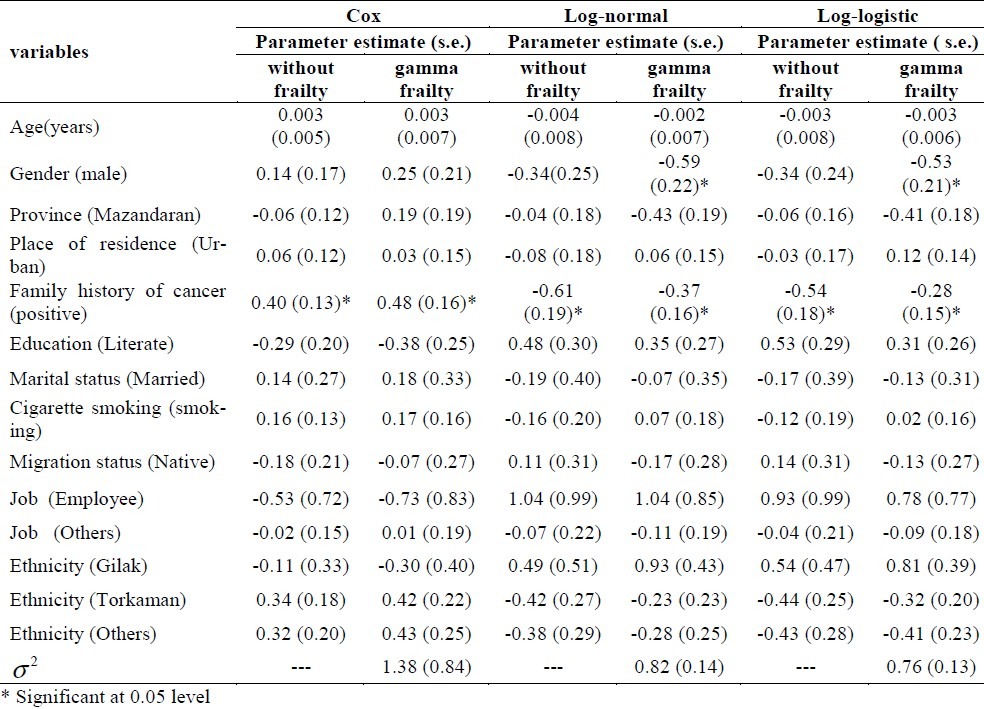
Also, Table 4 shows that the log-normal and log logistic models (with and without gamma frailty) have presented estimated parameters and standard error better than the Cox model. Between these two models, the log-logistic model with gamma frailty seemed more appropriate. These results were consistent with our finding based on Akaike's Information Criterion and residuals plots.
As expected, the log-logistic and log-normal analysis with gamma frailty model suggested that males faced a higher risk of the death caused by esophageal cancer than females.
Table 5 shows the results of multiple analyses for Cox models and parametric models (with and without frailty) based on hazard ratio (HR), relative risk (RR) and confidence interval for each variable. Results of multiple analyses showed a significant difference in patients who had a family history of cancer in all models.
Table 5.
Multiple analysis of parametric and Cox models with and without gamma frailty
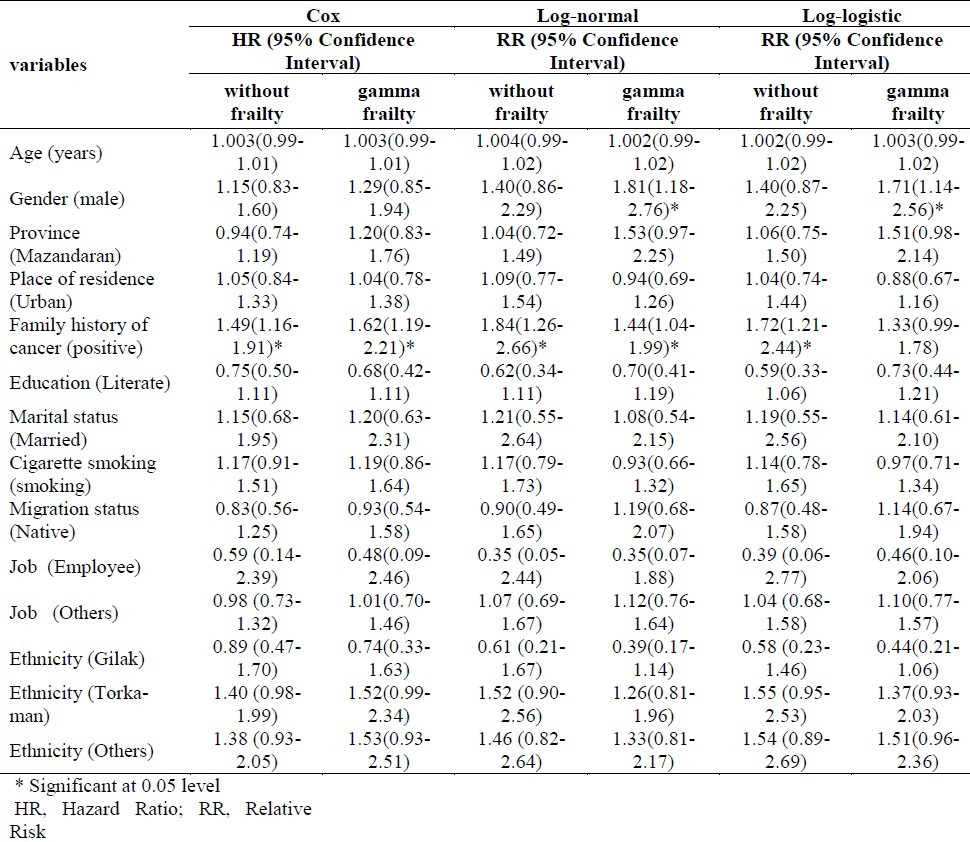
In the log-logistic with gamma frailty model (the selected model), the relative risk reported 1.33 for family history of cancer variable indicated that the desired event (death) occurred more often (33%) in patients with positive family history of cancer compared to those without such family history.
Gender was significant under the log-normal and log-logistic with gamma frailty model but not a significant factor under the other model. This indicated that the level of the death risk due to esophageal cancer was reduced significantly for the women in the study during the follow-up period. Also, the relative risk of 1.71 for gender variable (in selected model) indicated that the event (death) occurred more often (71%) in male than in female patients.
Neither parametric models nor Cox model showed age, place of residence, province, educational level, smoking, job, marital status, ethnicity and migration status as prognostic factors.
Discussion
Esophageal cancer is one of the most common cancers in Iran.27 The cancer is a particularly devastating form of cancer with a relatively low survival rate, and people generally will not live a long time after diagnosis. The five-year survival rate in this study was 13% which is lower than that of many other countries.45–48 This may be accounted for by the fact that Iranian patients generally seek medical advice and delayed diagnosis, when the disease has reached an advanced stage. Several factors are known in various studies as influencing prognosis factors and have been introduced.49–58
In the literature, there are many studies on the field of cancer, but researchers tend to examine the effects of covariates on patients survival using Cox regression model instead of parametric ones. A systematic study on cancer journals showed that only in 5% of cancer studies in which Cox regression model is used, the assumptions of the model have been investigated.59 If presumptions are not met, results of Cox model are seriously under question. As an alternative, parametric models such as log-normal, log logistic, Weibull and exponential can be employed. The only assumption of parametric models is that the variable time follows a specific distribution.30,37
This study assessed the relationship between survival of patients with esophageal cancer and numerous prognostic factors such as age at diagnosis, sex, family history of cancer, marital status, smoking status, ethnicity, medication status, education, place of residence and migration status.
Gender was a strong and independent prognostic factor, and our finding in multiple analyses was similar to the previous reports indicating better survival for female patients. This means that women getting affected by esophageal cancer have a slightly longer survival than men in Northern Iran; these findings are consistent with those obtained by European countries.1,60–62
In this study, family history of cancer is another important prognostic factor of esophageal cancer and many studies indicate that the survival depends on the presence of family history of cancer. These findings are aligned with the results of many studies in this field.63 Yuequan et al. showed that the five prognostic factors determined as significant by p value were tumor size, grade of differentiation, lymphadenopathy, stage of cancer, and family history of esophageal cancer after esophagectomy. Family history of esophageal cancer is an important prognostic factor that surgeons should take into consideration when selecting a treatment method.64
Statistical assessment of considered models using AIC criteria revealed the log-logistic model with gamma frailty to be the most powerful one in predicting survival of cancerous patients when compared to Cox and log-normal models.
There are preferences to use parametric models, because of good discrimination, provided censoring percentage not to exceed 40-50 percent;65 the condition our data was satisfied by censoring rate of close to 14 percent, so results of parametric models were considered acceptable.
In Nardi et al. study, Cox model and alternative parametric models in three clinical studies were compared.65 They used normal-deviate residuals for evaluation of parametric models assumptions66 Nardi et al. also studied Weibull model based on the estimated variation of parameter rate criteria and showed that it was better than the other models. In our study, the same result was obtained by log-logistic model with gamma frailty. Orbe et al. in a simulation study, compared Cox regression and accelerated failure time (AFT) models.67 They used the proposed method by Stute for fitting linear regression models with right-censored data.68 The results showed that if the proportional hazards assumption is violated or this assumption is met, log logistic, log-normal and Stute models are more efficient than the Cox model.
Bradburn et al. evaluated the adequacy type of parametric models and Cox proportional hazard model through residuals and AIC criterion.69 In their study performed on patients with ovarian and lung cancer, generalized gamma model compared to Cox and other parametric models reached a higher log-likelihood and lower AIC that make it more efficient model.
In Cox and parametric models, hazard function may depend on unknown or non-measurable factors which can cause the regression coefficients estimated from such models to be biased.38,70. In consequence, in order to overcome the problem and better model survival of patients, the frailty models were introduced. In fact, these models are used to explain the random variation of survival function due to unknown risk factors, such as genetic factors and numerous environmental factors.38,41,70–72
Vaupel et al. for the first time proposed frailty in order to describe the consequences of the existence of multiple variation source for univariate lifetime data.73
Random effects models are called frailty models in survival analysis. These models are relatively new in survival and widely studied in the 1990's and now are being used as the subject of many investigations. Technical problems in estimating the parameters have been caused the Cox model to be used less. Henderson and Oman in a theoretical method revealed that in case of non-use of frailty model when there is frailty effect, bias may occur in the estimates of regression coefficients.74 Schumacher et al. showed how to delete an important factor and reduce the relative risk estimates.75 Keiding et al. showed that removing one of the two explanatory variables might increase the hazard variance function and cause bias in estimating the other variable in the model.76 They also suggested that in order to account for the effect of unknown variables, in univariate survival data, it might be better to use the accelerated failure time (AFT) models.
Conclusion
In conclusion, the overall survival rates for patients with esophageal cancer in Northern Iran are relatively low. Since stage of cancer is a very important factor influencing survival of patients with esophageal cancer, these short survival rates can be due to the fact that patients with esophageal cancer in Northern Iran are, in general, referred to physicians at late stages of the disease.
Our study indicated that gender and family history of cancer were related factors to lifetime survival in patients with esophageal cancer.
According to our findings, the early recognition of family history of cancer and, in consequence, awareness of family members to consider the possibility of family screening may result in a decrease in death rate due to esophageal cancer. Steady public and professional education is required to increase awareness of hereditary esophageal cancer and the possibility of family screening, early diagnosis and therapy. Also, we recommend adding psychosocial support for these at-risk patients and their families as well as preventive lifestyle and dietary intervention. Furthermore, women experience reduced risk of death due to esophageal cancer than men. Also, comparing parametric models and Cox model showed that log-logistic with gamma frailty model (AIC and mean deviance residual has the lowest values compared to other models) can be a useful statistical model to find prognostic factors.
Authors’ Contributions
All the authors have carried out the study, participated in the design of the study and acquisition of data performed the statistical analysis and wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Authors are urged to express their gratitude to the National Institute of Health Research (NIHR) and Tehran University of Medical Sciences for their support in data collection, financial support and collaboration in this study.
Footnotes
Conflict of Interests Authors have no conflict of interests.
References
- 1.Yazdanbod A, Nasseri Moghadam S, Malekzadeh R. Upper gastrointestinal cancer in Ardabil, North West of Iran: A review. Arch Iranian Med. 2004;7(3):173–7. [Google Scholar]
- 2.Incidence and Mortality Figures [Online] 2011 Available from URL: http://www.qub.ac.uk/researchcentres/nicr/Data/OnlineStatistics/AllCancers . [Google Scholar]
- 3.Zali M. Indices related to gastric cancer in Tehran and seven city provinces in the years 1999 to 2002. J of Islamic Azad University Med. 2005;15(1):15–8. [Google Scholar]
- 4.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010 doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 5.Sadjadi A, Marjani H, Semnani S, Nasseri-Moghaddam S. Esophageal cancer in Iran: A review. Middle East Journal of Cancer. 2010;1(1):5–14. [Google Scholar]
- 6.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24(14):2137–50. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 7.Trivers KF, Sabatino SA, Stewart SL. Trends in esophageal cancer incidence by histology, United States, 1998-2003. Int J Cancer. 2008;123(6):1422–8. doi: 10.1002/ijc.23691. [DOI] [PubMed] [Google Scholar]
- 8.Bollschweiler E, Wolfgarten E, Nowroth T, Rosendahl U, Monig SP, Holscher AH. Vitamin intake and risk of subtypes of esophageal cancer in Germany. J Cancer Res Clin Oncol. 2002;128(10):575–80. doi: 10.1007/s00432-002-0380-z. [DOI] [PubMed] [Google Scholar]
- 9.Eloubeidi MA, Desmond R, Arguedas MR, Reed CE, Wilcox CM. Prognostic factors for the survival of patients with esophageal carcinoma in the U.S. The importance of tumor length and lymph node status. Cancer. 2002;95(7):1434–43. doi: 10.1002/cncr.10868. [DOI] [PubMed] [Google Scholar]
- 10.Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med. 2003;349(23):2241–52. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 11.Di FF, Lecleire S, Pop D, Rigal O, Hamidou H, Paillot B, et al. Baseline nutritional status is predictive of response to treatment and survival in patients treated by definitive chemoradiotherapy for a locally advanced esophageal cancer. Am J Gastroenterol. 2007;102(11):2557–63. doi: 10.1111/j.1572-0241.2007.01437.x. [DOI] [PubMed] [Google Scholar]
- 12.Siewert JR, Stein HJ, Feith M, Bruecher BL, Bartels H, Fink U. Histologic tumor type is an independent prognostic parameter in esophageal cancer: lessons from more than 1,000 consecutive resections at a single center in the Western world. Ann Surg. 2001;234(3):360–7. doi: 10.1097/00000658-200109000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsottles ND, Reedy AM. Esophageal cancer. In: Yarbro CH, Frogge MH, Goodman M, editors. Cancer Nursing : Principles and Practice. 6th ed. Boston: Jones and Bartlett; 2005. [Google Scholar]
- 14.Mohammad K, Atef V, Nasr-Esfahani M, Saberi Esfeedvajani M, Naji-Isfahani H, Shojaei MR, et al. Quality of life, religious attitude and cancer coping in a sample of Iranian patients with cancer. J Res Med Sci. 2011;16(7):928–37. [PMC free article] [PubMed] [Google Scholar]
- 15.Brunelli C, Mosconi P, Boeri P, Gangeri L, Pizzetti P, Cerrai F, et al. Evaluation of quality of life in patients with malignant dysphagia. Tumori. 2000;86(2):134–8. doi: 10.1177/030089160008600205. [DOI] [PubMed] [Google Scholar]
- 16.Gradauskas P, Rubikas R, Saferis V. Changes in quality of life after esophageal resections for carcinoma. Medicina (Kaunas) 2006;42(3):187–94. [PubMed] [Google Scholar]
- 17.Courrech Staal EF, van Sandick JW, van TH, Cats A, Aaronson NK. Health-related quality of life in long-term esophageal cancer survivors after potentially curative treatment. J Thorac Cardiovasc Surg. 2010;140(4):777–83. doi: 10.1016/j.jtcvs.2010.05.018. [DOI] [PubMed] [Google Scholar]
- 18.Kirby JD. Quality of life after oesophagectomy: the patients’ perspective. Dis Esophagus. 1999;12(3):168–71. doi: 10.1046/j.1442-2050.1999.00040.x. [DOI] [PubMed] [Google Scholar]
- 19.Watt E, Whyte F. The experience of dysphagia and its effect on the quality of life of patients with oesophageal cancer. Eur J Cancer Care (Engl) 2003;12(2):183–93. doi: 10.1046/j.1365-2354.2003.00376.x. [DOI] [PubMed] [Google Scholar]
- 20.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55(2):74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 21.Corley DA, Buffler PA. Oesophageal and gastric cardia adenocarcinomas: analysis of regional variation using the Cancer Incidence in Five Continents database. Int J Epidemiol. 2001;30(6):1415–25. doi: 10.1093/ije/30.6.1415. [DOI] [PubMed] [Google Scholar]
- 22.Mohebbi M, Mahmoodi M, Wolfe R, Nourijelyani K, Mohammad K, Zeraati H, et al. Geographical spread of gastrointestinal tract cancer incidence in the Caspian Sea region of Iran: spatial analysis of cancer registry data. BMC Cancer. 2008;8:137. doi: 10.1186/1471-2407-8-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koide N, Kitazawa M, Komatsu D, Suzuki A, Miyagawa S. Gender differences in clinicopathologic features and outcomes of esophageal cancer patients treated surgically. Esophagus. 2011;8(2):107–12. [Google Scholar]
- 24.Stein HJ, von Rahden BH, Siewert JR. Survival after oesophagectomy for cancer of the oesophagus. Langenbecks Arch Surg. 2005;390(4):280–5. doi: 10.1007/s00423-004-0504-9. [DOI] [PubMed] [Google Scholar]
- 25.Azizi F. The Epidemiology of Common Diseases in Iran. Tehran: Eshtiagh; 1999. [Google Scholar]
- 26.Steyerberg EW, Neville B, Weeks JC, Earle CC. Referral patterns, treatment choices, and outcomes in locoregional esophageal cancer: a population-based analysis of elderly patients. J Clin Oncol. 2007;25(17):2389–96. doi: 10.1200/JCO.2006.09.7931. [DOI] [PubMed] [Google Scholar]
- 27.Naghavi M. Iranian Annual of National Death Registration Report. Tehran: Ministry of Health and Medical Education; 2005. [Google Scholar]
- 28.Naghavi N. Death Report from 23 Provinces in Iran. Ministry of Health and Medical Education. 2004 [Google Scholar]
- 29.Hougaard P. Analysis of Multivariate Survival Data. New York: Springer; 2000. [Google Scholar]
- 30.Kleinbaum GD, Klein M. Survival Analysis- A Self-Learning Text. 2nd ed. New York: Springer; 2005. [Google Scholar]
- 31.Cox DR. Regression models and life tables(with Discussion) Journal of the Royal statistical society. 1972;34(2):187–220. [Google Scholar]
- 32.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. 2nd ed. New York: Wiely; 2002. [Google Scholar]
- 33.Lawless JF. Parametric Models in Survival Analysis. New York: Wiley; 1998. [Google Scholar]
- 34.Efron B. The efficiency of Cox's likelihood function for censored data. J Am Stat Assoc. 1977;72(359):557–65. [Google Scholar]
- 35.Oakes D. Comparison of models for survival data. Stat Med. 1983;2(2):305–11. doi: 10.1002/sim.4780020227. [DOI] [PubMed] [Google Scholar]
- 36.Andersen PK, Keiding N. Survival and Event History Analysis. Hoboken: John Wiley & Sons Ltd; 2006. [Google Scholar]
- 37.Klein JP, Moeschberger M. Survival Analysis: Techniques for Censored and Truncated Data (Statistics for Biology and Health) New York: Springer; 2003. [Google Scholar]
- 38.Duchateau L, Janssen P. The Frailty Model. New York: Springer; 2008. [Google Scholar]
- 39.Hougaard P. Modeling heterogeneity in survival data. J Appl Probab. 1991;28(3):695–701. [Google Scholar]
- 40.Gohari MR, Mahmoudi M, Mohammed K, Pasha E, Khodabakhshi R. Recurrence in breast cancer. Analysis with frailty model. Saudi Med J. 2006;27(8):1187–93. [PubMed] [Google Scholar]
- 41.Aalen OO. Effects of frailty in survival analysis. Stat Methods Med Res. 1994;3(3):227–43. doi: 10.1177/096228029400300303. [DOI] [PubMed] [Google Scholar]
- 42.O’Quigley J, Stare J. Proportional hazards models with frailties and random effects. Stat Med. 2002;21(21):3219–33. doi: 10.1002/sim.1259. [DOI] [PubMed] [Google Scholar]
- 43.Fritz PA, Percy C, Jack A, Shanmugaratnuers K, Sobin L, Parkin DM, et al. International Classification of Diseases for Oncology. 3rd ed. Geneva: International Agency for Research on Cancer; 2000. [Google Scholar]
- 44.Akaike H. A new look at the statistical model identification. Institute of Statistical Mathematics. 1974;19(6):716–23. [Google Scholar]
- 45.Hallas CN, Patel N, Oo A, Jackson M, Murphy P, Drakeley MJ, et al. Five-year survival following oesophageal cancer resection : psychosocial functioning and quality of life. Department of Psychology, University of Liverpool. 2001;6(1):85–94. [Google Scholar]
- 46.Samadi F, Babaei M, Yazdanbod A, Fallah M, Nouraie M, Nasrollahzadeh D, et al. Survival rate of gastric and esophageal cancers in Ardabil province, North-West of Iran. Arch Iran Med. 2007;10(1):32–7. [PubMed] [Google Scholar]
- 47.O’Rourke RW, Diggs BS, Spight DH, Robinson J, Elder KA, Andrus J, et al. Psychiatric illness delays diagnosis of esophageal cancer. Dis Esophagus. 2008;21(5):416–21. doi: 10.1111/j.1442-2050.2007.00790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spence R, Gavin A. Survival of Cancer Patients in Northern Ireland: 1993-2004. Northern Ireland Cancer Registry [Online] 2007. Available from: www.qub.ac.uk/nicr .
- 49.Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19(2):305–13. doi: 10.1200/JCO.2001.19.2.305. [DOI] [PubMed] [Google Scholar]
- 50.Chen HS, Sheen-Chen SM. Obstruction and perforation in colorectal adenocarcinoma: an analysis of prognosis and current trends. Surgery. 2000;127(4):370–6. doi: 10.1067/msy.2000.104674. [DOI] [PubMed] [Google Scholar]
- 51.Yoshida Y, Okamura T, Ezaki T, Kawahara H, Shirakusa T. [An evaluation of prognostic factors in patients with esophageal carcinoma] J UOEH. 1993;15(2):155–60. doi: 10.7888/juoeh.15.155. [DOI] [PubMed] [Google Scholar]
- 52.Holscher AH, Bollschweiler E, Schneider PM, Siewert JR. Prognosis of early esophageal cancer. Comparison between adeno- and squamous cell carcinoma. Cancer. 1995;76(2):178–86. doi: 10.1002/1097-0142(19950715)76:2<178::aid-cncr2820760204>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 53.Ikeda M, Furukawa H, Imamura H, Shimizu J, Ishida H, Masutani S, et al. Poor prognosis associated with thrombocytosis in patients with gastric cancer. Ann Surg Oncol. 2002;9(3):287–91. doi: 10.1007/BF02573067. [DOI] [PubMed] [Google Scholar]
- 54.Lerose R, Molinari R, Rocchi E, Manenti F, Villa E. Prognostic features and survival of hepatocellular carcinoma in Italy: impact of stage of disease. Eur J Cancer. 2001;37(2):239–45. doi: 10.1016/s0959-8049(00)00354-3. [DOI] [PubMed] [Google Scholar]
- 55.Monreal M, Fernandez-Llamazares J, Pinol M, Julian JF, Broggi M, Escola D, et al. Platelet count and survival in patients with colorectal cancer--a preliminary study. Thromb Haemost. 1998;79(5):916–8. [PubMed] [Google Scholar]
- 56.Alidina A, Gaffar A, Hussain F, Islam M, Vaziri I, Burney I, et al. Survival data and prognostic factors seen in Pakistani patients with esophageal cancer. Ann Oncol. 2004;15(1):118–22. doi: 10.1093/annonc/mdh014. [DOI] [PubMed] [Google Scholar]
- 57.Petrequin P, Huguier M, Lacaine F, Houry S. [Surgically treated esophageal cancers: predictive model of survival] Gastroenterol Clin Biol. 1997;21(1):12–6. [PubMed] [Google Scholar]
- 58.Gohari MR, Mahmoudi M, Mohammed K, Pasha Y, Khodabakhshi R. Disease-free survival and metastases pattern in breast cancer patients after mastectomy: An application of Stratified Markov Model. International Journal of Cancer Research. 2006;2(1):10–8. [Google Scholar]
- 59.Altman DG, De Stavola BL, Love SB, Stepniewska KA. Review of survival analyses published in cancer journals. Br J Cancer. 1995;72(2):511–8. doi: 10.1038/bjc.1995.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Curtis RE, Kennedy BJ, Myers MH, Hankey BF. Evaluation of AJC stomach cancer staging using the SEER population. Semin Oncol. 1985;12(1):21–31. [PubMed] [Google Scholar]
- 61.Cenitagoya GF, Bergh CK, Klinger-Roitman J. A prospective study of gastric cancer. ‘Real’ 5-year survival rates and mortality rates in a country with high incidence. Dig Surg. 1998;15(4):317–22. doi: 10.1159/000018645. [DOI] [PubMed] [Google Scholar]
- 62.Swisher SG, Deford L, Merriman KW, Walsh GL, Smythe R, Vaporicyan A, et al. Effect of operative volume on morbidity, mortality, and hospital use after esophagectomy for cancer. J Thorac Cardiovasc Surg. 2000;119(6):1126–32. doi: 10.1067/mtc.2000.105644. [DOI] [PubMed] [Google Scholar]
- 63.Munoz SE, Ferraroni M, La VC, Decarli A. Gastric cancer risk factors in subjects with family history. Cancer Epidemiol Biomarkers Prev. 1997;6(2):137–40. [PubMed] [Google Scholar]
- 64.Yuequan J, Shifeng C, Bing Z. Prognostic factors and family history for survival of esophageal squamous cell carcinoma patients after surgery. Ann Thorac Surg. 2010;90(3):908–13. doi: 10.1016/j.athoracsur.2010.05.060. [DOI] [PubMed] [Google Scholar]
- 65.Nardi A, Schemper M. Comparing Cox and parametric models in clinical studies. Stat Med. 2003;22(23):3597–610. doi: 10.1002/sim.1592. [DOI] [PubMed] [Google Scholar]
- 66.Nardi A, Schemper M. New residuals for Cox regression and their application to outlier screening. Biometrics. 1999;55(2):523–9. doi: 10.1111/j.0006-341x.1999.00523.x. [DOI] [PubMed] [Google Scholar]
- 67.Orbe J, Ferreira E, Nunez-Anton V. Comparing proportional hazards and accelerated failure time models for survival analysis. Stat Med. 2002;21(22):3493–510. doi: 10.1002/sim.1251. [DOI] [PubMed] [Google Scholar]
- 68.Stute W. Consistent estimation under random censorship when covariables are present. Journal of Multivariate Analysis. 1993;45:83–103. [Google Scholar]
- 69.Bradburn MJ, Clark TG, Love SB, Altman DG. Survival analysis Part III: multivariate data analysis choosing a model and assessing its adequacy and fit. Br J Cancer. 2003;89(4):605–11. doi: 10.1038/sj.bjc.6601120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Habil RN. Frailty Models in Survival Analysis. Wittenberg: Halle-Wittenberg; 2007. [Google Scholar]
- 71.Ghadimi R, Taheri H, Suzuki S, Kashifard M, Hosono A, Esfandiary I, et al. Host and environmental factors for gastric cancer in Babol, the Caspian Sea Coast, Iran. Eur J Cancer Prev. 2007;16(3):192–5. doi: 10.1097/01.cej.0000220639.61717.67. [DOI] [PubMed] [Google Scholar]
- 72.Boccia B. Genetic Determinants of Gastric Cancer. Rome: Erasmus university Rotterdam; 2009. [Google Scholar]
- 73.Vaupel JW, Manton KG, Stallard E. The impact of heterogeneity in individual frailty on the dynamics of mortality. Demography. 1979;16(3):439–54. [PubMed] [Google Scholar]
- 74.Henderson R, Oman P. Effect of frailty on marginal regression. J R Statist Soc B. 1999;61(2):367–79. [Google Scholar]
- 75.Schumacher M, Olschewski M, Schmoor C. The impact of heterogeneity on the comparison of survival times. Stat Med. 1987;6(7):773–84. doi: 10.1002/sim.4780060708. [DOI] [PubMed] [Google Scholar]
- 76.Keiding N, Andersen PK, Klein JP. The role of frailty models and accelerated failure time models in describing heterogeneity due to omitted covariates. Stat Med. 1997;16(1-3):215–24. doi: 10.1002/(sici)1097-0258(19970130)16:2<215::aid-sim481>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]


