Abstract
BACKGROUND:
Aluminium phosphide (AlP) is used as a fumigant. It produces phosphine gas which is a mitochondrial poison. Although this poisoning has been repeatedly reported in literature with a high mortality rate, there is no known antidote for AlP intoxication. In the present study, we studied the effects of hyperbaric oxygenation (HBO) on the survival time of AlP intoxicated rats.
METHODS:
Intoxicated rats with AlP (11.5 mg/kg, oral gavage) were placed in hyperbaric oxygenation with different concentrations of compressed air and oxygen.
RESULTS:
All the animals exposed to AlP died within 5 days. The mean survival times of rats exposed to AlP without any intervention, treated with hyperbaric condition by compressed air, and treated with hyperbaric condition by pure O2 were 91 ± 1, 262 ± 8, and 276 ± 6 minutes, respectively. In analysis of survival times, there was a significant difference between Group 2 which received AlP and the groups which underwent intervention (Groups 2 and 3, p < 0.001; Groups 2 and 4, p < 0.001).
CONCLUSIONS:
Hyperbaric oxygenation may probably improve the survival time of the intoxicated rats with aluminium phosphide, but it may not decrease the mortality rate.
KEYWORDS: Intoxication, Aluminium Phosphide, Hyperbaric Oxygen Therapy, Oxygen, Compressed Air
Aluminum phosphide (AlP) is a solid pesticide commonly applied for preserving rice and grains. In Iran, it is known as the rice tablet that is cheap and highly toxic.1 After exposure to moisture, it releases phosphine gas (PH3), the active pesticide component with garlic-like odor, which is immediately absorbed through inhalation, ingestion, or contact. With oral intake, the released phosphine gas is absorbed by the gastrointestinal tract by simple diffusion and is mainly excreted by the kidneys and lungs.1,2 PH3 is thought to have the central role in mechanism of AlP toxicity as it inhibits cytochrome c oxidase.3 Inhibition of plasma cholinesterase activity and methemoglobinemia have been reported from animal studies in acute AlP poisoning.4–8
AlP poisoning has been repeatedly reported in literature.9,10 Mortality rates from AlP toxicity published in the literature vary between 40-80%.11 Consumption of AlP is a common choice for suicide in India, Iran, and Jordan.12–15 In a retrospective study of AlP poisoning in Tehran, Iran, 471 patients were admitted to a referral hospital and the overall case fatality ratio was 31%.16
There is no antidote for phosphine or AlP poisoning and a great majority of patients die despite intensive care. Thus, supportive measures are all that can be offered.17 Although gastric acid diluted by sodium bicarbonate and potassium permanganate, to oxidize the phosphides, are recommended management strategies, their efficacy has not yet been verified in clinical trials.16 By diminution of oxidant stress caused by phosphine, trimetazidine is used for the treatment of cardiac toxicity.8 For the first time, Mittra et al. showed a 40% survival rate of animals exposed to AlP by using atropine and pralidoxine chloride.18 They concluded cholinesterase inhibition as one of the underlying mechanisms for AlP intoxication. Animal studies have suggested increased survival time with the use of N-acetylcystein.19
Rare cases of methemoglobinemia due to phosphine poisoning have been reported.20 Shadnia et al. confirmed methemoglobinemia as a potential complication of phosphine poisoning in 2 patients.21 Methemoglobinemia may complicate the course of phosphine poisoning that seems resistant to methylene blue and ascorbic acid. However, hyperbaric oxygen therapy might have beneficial effects on resolving this condition.21
Hyperbaric oxygen (HBO) therapy has been shown to be effective in the management of intoxication with CO, cyanide, hydrogen sulfide and carbon tetrachloride.8,22–24 Lack of effective treatment, high mortality rate of AlP toxicity, and similarities in mechanism between AlP poisoning and some of other poisonings in which HBO has been used successfully, necessitate designing a study to evaluate the effectiveness of HBO in AlP poisoning. In this study, the effectiveness of HBO (oxygen or compressed air) is assessed in the treatment of rats intoxicated by AlP.
Methods
Experimental Design
Wistar strain rats, with the weight of 150-250 grams, were acclimatized for one week under controlled conditions in a temporary animal house with access to food and water ad libitum. All the experiments performed in this study have been carried out according to the World Medical Association statement on animal use in biomedical research.25 The animals were then transported to the HBO unit at the time of operation and categorized into four groups: Group 1 (15 ml saline gavage, n = 10); Group 2 (11.5 mg/kg of powdered AlP26,27 in 15 ml saline gavage, n = 15); Group 3 (11.5 mg/kg of powdered AlP in 15ml saline gavage, n = 15, this group was also under hyperbaric condition by compressed air at 1.4-2 atmosphere absolute (ATA)); and Group 4 (11.5 mg/kg of powdered AlP in 15 ml saline gavage, n = 15, they also underwent hyperbaric condition by oxygen at 1.4-2 ATA). The chamber was pressurized to 1.4 ATA in 10 minutes and to 2 ATA in 15 minutes. An isobaric stage of 30 minutes was selected for all animals and the chamber was decompressed to surface pressure within 20-30 minutes. Survived animals were then transferred to another room. They were continuously observed for 5 days by two cameras that recorded all the events in the chamber. The survival time was determined by direct observation through the cameras. Group 1 was monitored and sham operated. They also took saline gavage. In all other animals (Groups 2-4), AlP dissolved in normal saline was administered intragastrically by using gavage syringe and oral devices. Since powdered Phostoxin was used as AlP source, we utilized an extra 5 ml of saline for the powder to dissolve better. A flush of pure normal saline was then administered to all rats. Although the rats were not permitted to be fed in the chamber, the survivors were fed with food and water ad libitum.
Drugs
AlP (Phostoxin, 3 mg tablet, AlP 56%), Alcan, Romania
Equipments
The chamber used in this study was a type B hyperbaric chamber with the characteristics as follows:
A cylindrical body made of stainless steel with polymethyl methacrylic acid (PMMA) viewports operating by compressed air or oxygen allowed either completely open or semi-closed modes using CO2 remover. The chamber was compressed to 1.4 ATA in 10 minutes. Considering the potential risk of phosphine gas especially in oxygen enriched environments, a second chamber made of polytetrafluoroethylene (PTFE) was designed. The exposure of animals to hyperbaric oxygen was done by placing the second chamber in the main chamber. The main chamber was injected by compressed air and the second chamber was ventilated by oxygen at a rate of 50 lpm. This chamber was sealed and ventilation was applicable by using 2 special rotameters. The time for changing the profile of HBO treatment occurred when the first rat in Group 2 died. At this point, the chamber was pressurized to 2 ATA in 15 minutes. Isobaric condition was sustained by ventilation of 100 lpm of compressed air in Groups 3 and 100 lpm of oxygen in Group 4.
This monoplace hyperbaric chamber was completely designed, fabricated and installed as a laboratory instrument by the researchers at Rasul-e-Akram Medical Center to be used in a series of studies. At the time of the study, the chamber was under examination of inspectors from Iranian Ministry of Health in search for its technical capabilities.
Statistical Analysis
The survival times of different groups were compared using log rank test of Kaplan-Meier analysis. The p value of less than 0.05 was considered significant. All the analyses were done using SPSS 18 (SPSS Inc., Chicago, Illinois, USA). Survival times of different groups were noted using estimated means (minutes) ± standard error (SEM).
Results
All the animals in Group 1 survived and all in the other three groups died within 5 days. The mean survival time of AlP poisoned rats without any intervention was 91 ± 1 minutes. Among the intoxicated rats which underwent hyperbaric condition with compressed air at 1.4-2 ATA (Group 3), the maximum survival time was 330 minutes. The mean survival time for rats in Group 3 was 262 ± 8 minutes. In Group 4, the group under hyperbaric oxygenation with oxygen, the mean survival time was 276 ± 6 minutes. The maximum survival time in poisoned rats of Group 4 was 332 minutes (Figure 1). In survival analysis, there was a significant difference between Group 2 which received AlP and the groups which underwent intervention (Groups 2 and 3, p < 0.001; Groups 2 and 4, p < 0.001). We also found that the difference between the mean survival time in animals under either hyperbaric oxygen or compressed air was not significant (p > 0.05).
Figure 1.
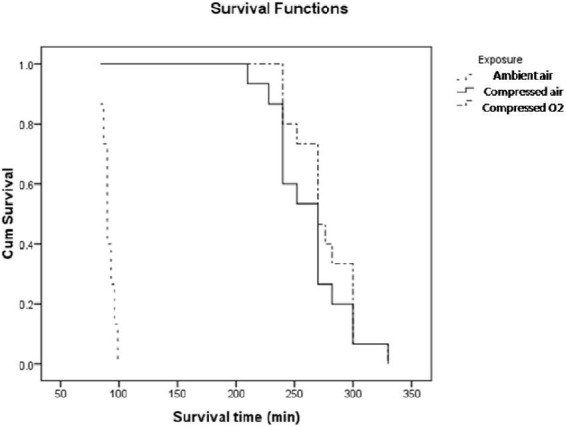
Survival time in different groups of rats.
Discussion
AlP poisoning is commonly used for suicidal attempts with a high mortality rate in Iran and other countries, especially in developing countries.28–32 The results of the present study showed that hyperbaric oxygen therapy may increase survival in AlP poisoned rats but there was no significant difference between pure O2 and compressed air.
Despite over a century of use in medical settings, hyperbaric oxygen remains a controversial therapy. The last 20 years have seen a clarification of the mechanism of action of hyperbaric therapy and a greater understanding of its potential benefit.33
There have been some reports about methemoglobinemia occurrence in acute AlP poisoned victims.16,21,26,34 Methemoglobinemia is a rare clinical presentation in acute AlP poisoning that may complicate the course of poisoning.21,26 Generalized cyanosis in the presence of normal arterial oxygen tension and failure of the cyanosis to resolve with oxygen therapy is an important diagnostic clue which almost always represents methemoglobinemia. PH3 and arsine are chemically very similar and thus the fact that methemoglobinemia may occur by both of them is not surprising.27 The other mechanism of action of PH3-induced methemoglobinemia is induction of free radicals.6,20
Mostafazadeh et al. found a significant association between blood level of methemoglobin and mortality in patients with AlP intoxication.26 Decreased capacity of methemoglobin to deliver enough oxygen to tissues may be another cause of multiple organ failure following AlP intoxication. Moreover, intravenous hemolysis has also been reported due to AlP intoxication which may have additional effects on impaired oxygen delivery to the target tissues.35 In other word, methemoglobinemia is a condition in which an abnormal proportion of the iron in heme moiety of the hemoglobin is oxidized to the ferric state leading to impaired oxygen transport and anemic hypoxia. In this situation, the oxygen dissociation curve of the unaffected hemoglobin shifts to the left.21
In previous studies, there have been some controversies about the effectiveness of methylene blue in treatment of methemoglobinemia in AlP poisoned victims.6,21,26 In one study, it was mentioned that hyperbaric oxygen therapy would be useful in management of AlP poisoning.21
Phosphine is thought to have the central role in mechanism of AlP toxicity as it inhibits cytochrome c oxidase.3 AlP toxicity can inhibit cytochrome C oxidase, and there are similarities between AlP and some other chemicals such as cyanide that binds to the enzyme cytochrome oxidase aa3 similar to carbon monoxide and blocks the mitochondrial respiration chain which in turn causes depletion of adenosine triphosphate. The mechanism of toxicity is disruption of the electron transport chain in mitochondria, resulting in intracellular hypoxia.36 This disturbance in oxygen delivery affects the human machine functionally and then structurally. Loss of consciousness and neurologic damage that occur by inhalation of 100% N2, high levels of H2S, HCN, or CO are due to binding to cytochrome a3 and stopping the oxidative flow of electrons and subsequent cellular hypoxia.22 Considering the unique mechanism of toxicity, there have been many studies showing usefulness of hyperbaric treatment in CO, hydrogen sulfide, H2S and CCL4 poisoning both in human and animal models. For moderate to severe CO poisoning, hyperbaric oxygen is the treatment of choice.37 Bitterman et al. found that in rats poisoned with LD75 hydrogen sulfide, pure oxygen at 1ATA was effective in preventing death.38 Gunn and Wong reported five patients with severe H2S poisoning who were treated successfully with HBO.39 Goldenberg et al. suggested HBO therapy to reduce mortality in H2S poisoned patients.36 Animal experimental studies on the effects of HBO in CCL4 poisoning showed lower mortality rates and less impairment of the liver function.40 Mininberg and Kvetnoi studied the effects of HBO on rats with hepatotoxicity induced by carbon tetrachloride. They found a reparative process, absence of necrosis, and increased activity of the liver chromaffin cells in the liver tissue of treated animals.23
Lawson et al. studied other mechanisms at work and suggested phosphine or its products to be likely to have some role by formation of organophosphines as a precursor for synthesis of organophosphates.41 Prolongation of the survival of the animals poisoned with phosphoorganic compounds was reported by Savateev et al. when they used HBO at 3 ATA for 2-4 hours.42
Conclusion
Although our findings showed HBO to be able to prolong survival time after intoxication, it will not decrease mortality rate in AlP toxicity in rat model. Even after HBO treatment process, a higher mortality rate in AlP toxicity than that of other intoxications indicates that the mechanism of action of AlP may be more complex and not just simply inhibiting cytochrome oxidase. In addition, such inhibition may be much more potent and eventually more lethal. To the best of our knowledge, no similar study has been done before. More studies incorporating gastro-intestinal decontamination methods and other drugs that are known to protect mitochondrial oxidative phosphorylation such as NAC19, hydroxycobalamine and vitamin C should be conducted.
Limitations
Lack of special devices such as type C hyperbaric chamber and advanced monitoring equipments, as well as unavailability of bio-enzyme assays (plasma cholinesterase level, myocardial malonyldialdehyde, catalase, glutathione peroxidase), were some of the limitations of this study that could be considered by researchers in future works. Another limitation was lack of a control group with normal saline and oxygen.
Financial Support
This research was financially supported by Research deputy of Tehran University of Medical Sciences, Tehran, Iran (Project No. 8760195209).
Authors’ Contributions
HS provided the idea of the study. FS documented the findings, collected evidence, and reviewed the literature. All authors contributed in proposal writing, performing the experiments, data analysis, and writing the final manuscript.
Acknowledgement
We wish to thank Mr Gholamreza Bayat for his technical assistance.
Footnotes
Conflict of Interests Authors have no conflict of interests.
References
- 1.Mehrpour O, Singh S. Rice tablet poisoning: a major concern in Iranian population. Hum Exp Toxicol. 2010;29(8):701–2. doi: 10.1177/0960327109359643. [DOI] [PubMed] [Google Scholar]
- 2.Sharma A, Gathwala G. Oral aluminium phosphide poisoning in Indian children. J Trop Med Hyg. 1992;95(3):221–2. [PubMed] [Google Scholar]
- 3.Dua R, Gill KD. Effect of aluminium phosphide exposure on kinetic properties of cytochrome oxidase and mitochondrial energy metabolism in rat brain. Biochim Biophys Acta. 2004;1674(1):4–11. doi: 10.1016/j.bbagen.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Siwach SB, Yadav DR, Arora B, Dalal S, Jagdish Acute aluminum phosphide poisoning--an epidemiological, clinical and histo-pathological study. J Assoc Physicians India. 1988;36(10):594–6. [PubMed] [Google Scholar]
- 5.Anger F, Paysant F, Brousse F, Le N I, Develay P, Gaillard Y, et al. Fatal aluminum phosphide poisoning. J Anal Toxicol. 2000;24(2):90–2. doi: 10.1093/jat/24.2.90. [DOI] [PubMed] [Google Scholar]
- 6.Lall SB, Peshin SS, Mitra S. Methemoglobinemia in aluminium phosphide poisoning in rats. Indian J Exp Biol. 2000;38(1):95–7. [PubMed] [Google Scholar]
- 7.Lall SB, Peshin SS, Seth SS. Acute poisoning: a ten years retrospective hospital based study. Ann Natl Acad Med Sci. 1994;30(1):35–44. [Google Scholar]
- 8.Duenas A, Perez-Castrillon JL, Cobos MA, Herreros V. Treatment of the cardiovascular manifestations of phosphine poisoning with trimetazidine, a new antiischemic drug. Am J Emerg Med. 1999;17(2):219–20. doi: 10.1016/s0735-6757(99)90075-x. [DOI] [PubMed] [Google Scholar]
- 9.Burkhart KK. Methyl Bromide and Other fumigants. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, editors. Goldfrank's Toxicologic Emergencies. 9th ed. New York: Mc Graw Hill; 2010. pp. 1557–63. [Google Scholar]
- 10.Nocera A, Levitin HW, Hilton JM. Dangerous bodies: a case of fatal aluminium phosphide poisoning. Med J Aust. 2000;173(3):133–5. doi: 10.5694/j.1326-5377.2002.tb04471.x. [DOI] [PubMed] [Google Scholar]
- 11.Chugh SN, Dushyant, Ram S, Arora B, Malhotra KC. Incidence & outcome of aluminium phosphide poisoning in a hospital study. Indian J Med Res. 1991;94:232–5. [PubMed] [Google Scholar]
- 12.Jayaraman KS. Death pills from pesticide. Nature. 1991;353(6343):377. doi: 10.1038/353377c0. [DOI] [PubMed] [Google Scholar]
- 13.Abder-Rahman HA, Battah AH, Ibraheem YM, Shomaf MS, el-Batainch N. Aluminum phosphide fatalities, new local experience. Med Sci Law. 2000;40(2):164–8. doi: 10.1177/002580240004000214. [DOI] [PubMed] [Google Scholar]
- 14.Moghadamnia AA, Abdollahi M. An epidemiological study of poisoning in northern Islamic Republic of Iran. East Mediterr Health J. 2002;8(1):88–94. [PubMed] [Google Scholar]
- 15.Shadnia S, Rahimi M, Pajoumand A, Rasouli MH, Abdollahi M. Successful treatment of acute aluminium phosphide poisoning: possible benefit of coconut oil. Hum Exp Toxicol. 2005;24(4):215–8. doi: 10.1191/0960327105ht513oa. [DOI] [PubMed] [Google Scholar]
- 16.Shadnia S, Sasanian G, Allami P, Hosseini A, Ranjbar A, Amini-Shirazi N, et al. A retrospective 7-years study of aluminum phosphide poisoning in Tehran: opportunities for prevention. Hum Exp Toxicol. 2009;28(4):209–13. doi: 10.1177/0960327108097194. [DOI] [PubMed] [Google Scholar]
- 17.Proudfoot AT. Aluminium and zinc phosphide poisoning. Clin Toxicol (Phila) 2009;47(2):89–100. doi: 10.1080/15563650802520675. [DOI] [PubMed] [Google Scholar]
- 18.Mittra S, Peshin SS, Lall SB. Cholinesterase inhibition by aluminium phosphide poisoning in rats and effects of atropine and pralidoxime chloride. Acta Pharmacol Sin. 2001;22(1):37–9. [PubMed] [Google Scholar]
- 19.Azad A, Lall SB, Mittra S. Effect of N-acetylcysteine and L-NAME on aluminium phosphide induced cardiovascular toxicity in rats. Acta Pharmacol Sin. 2001;22(4):298–304. [PubMed] [Google Scholar]
- 20.Lakshmi B. Methemoglobinemia with aluminum phosphide poisoning. Am J Emerg Med. 2002;20(2):130–2. doi: 10.1053/ajem.2002.31140. [DOI] [PubMed] [Google Scholar]
- 21.Shadnia S, Soltaninejad K, Hassanian-Moghadam H, Sadeghi A, Rahimzadeh H, Zamani N, et al. Methemoglobinemia in aluminum phosphide poisoning. Hum Exp Toxicol. 2011;30(3):250–3. doi: 10.1177/0960327110384287. [DOI] [PubMed] [Google Scholar]
- 22.Meter KV, Weiss L, Harch PG. HBO in emergency medicine. In: Jain KK, Richard A, Neubauer MD, Baydin SA, Bookspan J, editors. Textbook of Hyperbaric Medicine. 4th ed. Ashland: Horefe and Huber; 2004. pp. 422–49. [Google Scholar]
- 23.Mininberg ES, Kvetnoi IM. Use of hyperbaric oxygenation in the prevention and treatment of acute hepatic insufficiency. Anesteziol Reanimatol. 1979;(4):46–9. [PubMed] [Google Scholar]
- 24.Nakakita H, Katsumata Y, Ozawa T. The effect of phosphine on respiration of rat liver mitochondria. J Biochem. 1971;69(3):589–93. [PubMed] [Google Scholar]
- 25.World Medical Association. WMA Statement on Animal Use in Biomedical Research[Online] 2011. [cited 2011 May 12]. Available from URL: http://www.wma.net/en/30publications/10policies/a18/index.html .
- 26.Mostafazadeh B, Pajoumand A, Farzaneh E, Aghabiklooei A, Rasouli MR. Blood levels of methemoglobin in patients with aluminum phosphide poisoning and its correlation with patient's outcome. J Med Toxicol. 2011;7(1):40–3. doi: 10.1007/s13181-010-0121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curry SC. Hematologic syndromes: Hemolysis, methemoglobinemia, sulfhemoglobinemia. In: Brent J, Wallace KL, Burkhart KK, Phillips S, Donovan J, editors. Critical Care toxicology, Diagnosis and Management of the Critically Poisoned Patient. New York: Mosby; 2004. pp. 336–41. [Google Scholar]
- 28.Bumbrah GS, Krishan K, Kanchan T, Sharma M, Sodhi GS. Phosphide poisoning: A review of literature. Forensic Sci Int. 2011 doi: 10.1016/j.forsciint.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Bogle RG, Theron P, Brooks P, Dargan PI, Redhead J. Aluminium phosphide poisoning. Emerg Med J. 2006;23(1):e3. doi: 10.1136/emj.2004.015941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meel BL. Aluminium phosphide (tank pill) poisoning in the Transkei region of South Africa: a case report. Med Sci Law. 2011;51(2):116–8. doi: 10.1258/msl.2010.010205. [DOI] [PubMed] [Google Scholar]
- 31.Saidi H, Shojaie S. Effect of sweet almond oil on survival rate and plasma cholinesterase activity of aluminum phosphide-intoxicated rats. Hum Exp Toxicol. 2011 doi: 10.1177/0960327111407229. [DOI] [PubMed] [Google Scholar]
- 32.Soltaninejad K, Faryadi M, Sardari F. Acute pesticide poisoning related deaths in Tehran during the period 2003-2004. J Forensic Leg Med. 2007;14(6):352–4. doi: 10.1016/j.jflm.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 33.Grim PS, Gottlieb LJ, Boddie A, Batson E. Hyperbaric oxygen therapy. JAMA. 1990;263(16):2216–20. [PubMed] [Google Scholar]
- 34.Soltaninejad K, Nelson LS, Khodakarim N, Dadvar Z, Shadnia S. Unusual complication of aluminum phosphide poisoning: Development of hemolysis and methemoglobinemia and its successful treatment. Indian J Crit Care Med. 2011;15(2):117–9. doi: 10.4103/0972-5229.83021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aggarwal P, Handa R, Wig N, Biswas A, Saxena R, Wali JP. Intravascular hemolysis in aluminium phosphide poisoning. Am J Emerg Med. 1999;17(5):488–9. doi: 10.1016/s0735-6757(99)90255-3. [DOI] [PubMed] [Google Scholar]
- 36.Goldenberg I, Shoshani O, Mushkat Y, Bentur Y, Melamed Y, Shupak A. [Hyperbaric oxygen for hydrogen sulfide poisoning] (360).Harefuah. 1994;127(9):300–2. [PubMed] [Google Scholar]
- 37.Martindale LG. Carbon monoxide poisoning: the rest of the story. J Emerg Nurs. 1989;15(2(Pt 1)):101–4. [PubMed] [Google Scholar]
- 38.Bitterman N, Talmi Y, Lerman A, Melamed Y, Taitelman U. The effect of hyperbaric oxygen on acute experimental sulfide poisoning in the rat. Toxicol Appl Pharmacol. 1986;84(2):325–8. doi: 10.1016/0041-008x(86)90140-7. [DOI] [PubMed] [Google Scholar]
- 39.Gunn B, Wong R. Noxious gas exposure in the outback: two cases of hydrogen sulfide toxicity. Emerg Med (Fremantle) 2001;13(2):240–6. doi: 10.1046/j.1442-2026.2001.00220.x. [DOI] [PubMed] [Google Scholar]
- 40.Bernacchi A, Myers R, Trump BF, Marzella L. Protection of hepatocytes with hyperoxia against carbon tetrachloride-induced injury. Toxicol Pathol. 1984;12(4):315–23. doi: 10.1177/019262338401200403. [DOI] [PubMed] [Google Scholar]
- 41.Lawson MA, Lieske CN, Fox-Talbot MK, Meyer HG. Spontaneous reactivation of phosphinylated human erythrocyte acetylcholinesterase and human serum butyrylcholinesterase. Life Sci. 1985;36(18):1715–20. doi: 10.1016/0024-3205(85)90553-3. [DOI] [PubMed] [Google Scholar]
- 42.Savateev NV, Tonkopii VD, Brestkina LM, Gromov AE. [An analysis of the mechanism of the therapeutic effect of oxygen under pressure in phosphoorganic compound poisoning] Biull Eksp Biol Med. 1973;75(3):51–3. [PubMed] [Google Scholar]


