Abstract
BACKGROUND:
Midazolam has analgesic properties. The aim of the present study was to assess the analgesic effect of midazolam when added to lidocaine in intravenous regional anesthesia (IVRA).
METHODS:
Sixty patients undergoing hand surgery were randomly allocated into two groups to receive 3 mg/kg 2% lidocaine diluted with saline to a total volume of 40 mL in the control group (group lidocaine saline ~ LS, n=30) or 50 μg/kg midazolam plus 3 mg/kg 2% lidocaine diluted with saline to a total volume of 40 mL in the midazolam group (group lidocaine midazolam ~ LM, n=30). Before and after the tourniquet application, hemodynamic variables, tourniquet pain, sedation, and analgesic use were recorded.
RESULTS:
Shortened sensory and motor block onset time [4.20 (0.84) vs. 5.94 (0.83) min, p = 0.001 and 6.99 (0.72) vs. 9.07 (0.99) min, p = 0.001 in LM and LS groups, respectively], prolonged sensory and motor block recovery times [8.41 (0.94) vs. 5.68 (0.90) min, p = 0.001 and 11.85 (1.18) vs. 7.06 (0.82) min, p = 0.001 in LM and LS groups, respectively], shortened visual analog scale (VAS) scores of tourniquet pain (p < 0.05), and improved quality of anesthesia were found in group LM (p < 0.05). VAS scores were lower in group LM in the postoperative period (p = 0.001). Postoperative analgesic requirements were significantly smaller in group LM (p = 0.001).
CONCLUSIONS:
The addition of 50 μg/kg midazolam to lidocaine for IVRA shortens the onset of sensory and motor block, and improves quality of anesthesia and perioperative analgesia without causing side effects.
KEYWORDS: Anaesthetic Techniques, IV Regional Lidocaine, Postoperative, Analgesics, Midazolam, Tourniquet Pain
Intravenous regional anesthesia (IVRA) is a simple, reliable, and cost-effective technique which has success rates of 94 - 98%. It is ideal for short operative ambulatory procedures performed on the extremities.1,2
Local anesthetic (LA) toxicity, slow onset, poor muscle relaxation, tourniquet pain, and minimal postoperative pain relief are problems encountered with IVRA.1,3
The ideal solution for IVRA should have rapid onset, reduce dose of LA, reduce tourniquet pain, and prolong post-deflation analgesia. Right now, this is accomplished by the addition of adjuncts.1–2,4,5
Dickenson et al.6 showed that midazolam, a benzodiazepine (BDZ) agonist, has analgesic properties mediated via the γ-aminobutyric acid (GABA) A receptor in the spinal cord. Due to antinociceptic effect of midazolam, it was known to augment the effect of local anesthetics7–12 and opioids13 when given epidurally or intrathecally.
One previous case report14 showed that intravenous administration of midazolam was effective in relieving severe phantom limb pain during spinal anesthesia.
The above studies encouraged us to investigate whether midazolam had an analgesic effect via intravenous delivery as additive to IVRA.
To our knowledge, there are no published studies that compared the characteristics of IVRA block when midazolam was used as adjuvant to lidocaine. Therefore, we designed this study to investigate the sensory and motor block onset and recovery time, the quality of anesthesia, intraoperative and postoperative hemodynamic variables, intraoperative and postoperative pain, sedation, and side-effect profile of midazolam when added to lidocaine in IVRA.
Methods
Sixty American social anesthesia (ASA) physical status I-II15 patients, aged 20-50 years old, scheduled for elective hand or forearm surgery (i.e., carpal tunnel release and tendon release) gave written informed consent to participate in this randomized prospective double-blind study, which was approved by our institute Ethics Committee.
Patients with Reynaud disease, sickle cell anemia or history of allergy to any drug used were excluded from the study.
No premedication was given to the patients. In the preoperative period, a visual analog scale (VAS) consisting of a 10-cm line, in which 0 represented no pain and 10 represented the worst possible pain, was explained to all patients.
After arrival of patients to the operating room, mean arterial pressure (MAP), peripheral oxygen saturation (SpO2), and heart rate (HR) were monitored. Before beginning the anesthetic block, two intravenous cannula were inserted; one in a dorsal vein of the operative hand and the other in the opposite hand for infusion of crystalloid.
The operative arm was elevated for 2 minutes after that exsanguinated with an Esmarch bandage. A 10 cm pneumatic tourniquet was then placed around the upper arm and proximal cuff was inflated to 250 mm Hg. Circulatory isolation of the arm was confirmed by inspection, absence of radial pulse, and loss of pulse oximetry tracing in the ipsilateral index finger.
A randomization list was generated and identical syringes containing each drug were prepared by an anesthesiologist who blinded to the study. A resident of anesthesiology blinded to the group and drug allocation applied the concealed syringes and recorded all data.
IVRA was achieved with 3 mg/kg 2% lidocaine diluted with saline to a total volume of 40 mL, in the control group (group lidocaine saline ~LS, n=30) or with 50 μg/kg midazolam plus 3 mg/kg 2% lidocaine diluted with saline to a total volume of 40 mL in the midazolam group (group lidocaine midazolam ~LM, n=30). The solution was administered over 90 s by an anesthesiologist blinded to the group assignments.
The sensory block was assessed continuously at 30 s intervals by a pinprick performed with a 22 gauge short beveled needle.
Motor function was evaluated by asking the patient to flex and extend his/her wrist and fingers, and complete motor block was noted when voluntary movement was impossible. Onset of sensory block (defined as the time elapsed from injection of the study drug to sensory block achieved in all dermatomes), and onset of motor block (defined as the time elapsed from injection of study drug to complete motor block) were recorded.
After completion of sensory and motor block, the distal cuff was inflated to 250 mm Hg, and the proximal tourniquet was released.
After that the operation was started. MAP, HR, Spo2, visual analog scale (VAS) scores (0 = no pain and 10=worst pain imaginable) and degree of sedation (scale 1–5, 1 = completely awake, 2 = awake but drowsy, 3 = asleep but responsive to verbal commands, 4 = asleep but responsive to tactile stimulus, 5 = asleep and not responsive to any stimulus)16 were recorded before and after tourniquet inflation at 5, 10, 15, 20, 30, 40, 50 min after the injection of study drugs and at 15, 30,45,60 min after tourniquet release.
Hypotension (30% decrease from baseline value) was treated with IV ephedrine (5 to 10-mg bolus), bradycardia (30% decrease from the baseline value) was treated with IV atropine 0.5 mg, and arterial oxygen saturation less than 90% was treated with O2 supplementation via a face mask.
During intraoperative period, boluses of fentanyl 1 μg/kg were administered for tourniquet pain treatment when VAS was more than 4 and total fentanyl consumption was recorded. The time elapsed after tourniquet inflation to the first patient request for fentanyl was also recorded.
Data were recorded postoperatively at 6, 12, and 24 h. Postoperatively, when VAS was more than 4, boluses of morphine 0.05 mg/kg were administered and total morphine consumption was recorded. The time elapsed after tourniquet release to the first patient request for morphine was also recorded.
After the operation, qualification of operative condition such as disturbing movement of the arm and too much bleeding was done by the surgeon who did not know group allocation according to the following numeric scale: 0 = unsuccessful, 1 = poor, 2 = acceptable, and 3 = perfect.
At postoperative period, the anesthesiologist was asked to qualify the operative conditions according to the following numeric scale: 4 (excellent) = no complaint from patient, 3 (good) = minor complaint with no need for supplemental analgesics, 2 (moderate) = complaint that required supplemental analgesics, and 1 (unsuccessful) = patient given general anesthesia.17
The tourniquet was not deflated before 30 min and was not inflated for more than 1.5 h. At the end of surgery, the tourniquet deflation was completed by cyclic deflation technique.
Sensory recovery time (defined as the time elapsed after tourniquet deflation up to recovery of pain in all dermatomes determined by pinprick test) was recorded. Motor block recovery time (defined as the time elapsed after tourniquet deflation up to movement of fingers) was also recorded.
Throughout the study period, the patients were asked about any side effects (tinnitus, skin rash, gastric discomfort, nausea and other side effects). Measurements and data recording in all patients were performed by the same person.
The statistical analysis was done by SPSS 15 statistical software package. Based on a SD of 4 mm, a group size of 30 patients would be sufficient to detect a difference of 32 mm of the VAS at 30 min intraoperatively18 with 80% power and a type I error of 5%.
Evaluation of the quantitative data was statistically analyzed by Student t-test. Mann-Whitney U-test was used for statistical analysis of intraoperative-postoperative sedation scores and the quality of the anesthesia. Side effects and operation type were compared with Fischer's exact test. Data are presented as mean (SD). A p-value < 0.05 was assumed to be statistically significant.
Results
Sixty patients were enrolled in the study. No patient was excluded from the study due to technical failure. There was no significant difference between the two groups in demographic data (Table 1).
Table 1.
Patient characteristic, types of surgery, operation and tourniquet times in two groups.
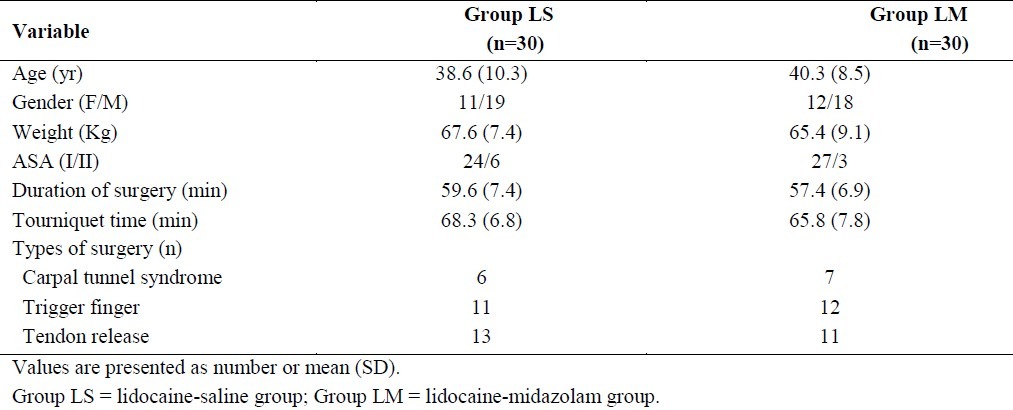
Types of surgical procedure, tourniquet time and duration of surgery were similar statistically between the two groups (Table 1). Mean heart rate, mean arterial pressure and SPO2 were not statistically different between the two groups at any time intervals during surgery and in the postoperative period.
Sensory and motor block onset times were significantly shorter in LM-group compared with LS-group (p < 0.05). Sensory and motor block recovery times were also statistically prolonged in LM-group (p < 0.05, Table 2).
Table 2.
Onset and recovery times of sensory and motor block, initial time of tourniquet pain, numbers of patient needed supplemental fentanyl, and the amount of intraoperative and postoperative analgesic requirements in two groups
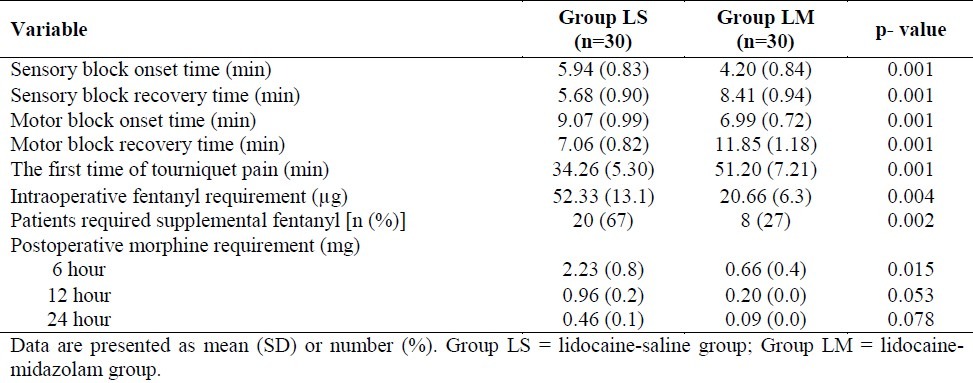
The initial time for beginning tourniquet pain was significantly longer in group LM (p = 0.001, Table 2). Intraoperatively, the numbers (%) of patient required supplemental fentanyl for tourniquet pain were significantly higher in LS-group compared with LM-group. The first fentanyl requirement time for tourniquet pain was also significantly prolonged in LM-group compared with LS-group. The total dosage of fentanyl administration for relieving tourniquet pain was significantly less in LM-group compared with LS-group (Table 2).
Median (range) sedation values at any intraoperative and postoperative period were not statistically different between the two groups [1 (1-2) in both LS and LM groups, respectively, p = 0.317].
VAS scores of tourniquet pain were significantly lower at 5, 10, 15, 20, 30, 40, and 50 minutes in LM-group compared with LS- group (Figure 1). VAS scores of tourniquet pain were significantly lower in LM-group compared with LS-group in the patients didn’t receive fentanyl during intraoperative periods (1.6 ± 0.50 vs. 2.6 ± 0.52 in LM and LS groups, respectively, p = 0).
Figure 1.
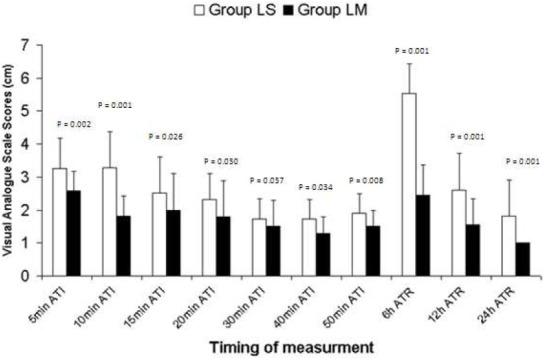
Intraoperative (tourniquet pain) and postoperative visual analogue scale scores. Data are presented as mean (SD). Group LS = lidocaine-saline group; Group LM = lidocaine-midazolam group. ATI = after tourniquet inflation; ATR = after tourniquet release
In the postoperative period, VAS scores were also significantly lower at 6, 12, and 24 hours in LM-group compared with LS-group (Figure 1). Postoperative morphine consumption was less in LM-group compared with LS-group at 6, 12, 24 h but it was statistically significant only at 6 h (Table 2).
Quality of anesthesia [median (range)] determined by the anesthesiologist was significantly better in LM-group compared with LS-group [4 (3-4) vs. 2 (2-3), respectively, p = 0.015]. Anesthesia quality [median (range)] determined by the surgeon was also significantly better in LM-group [3 (2-4) vs. 2 (2-3), p < 0.05].
No drop out was seen during the study period due to insufficient anesthesia. There were no cases of respiratory depression, apnea, hypoxia, bradycardia, hypotension or any other side effects throughout the study.
Discussion
Our study showed that the addition of 50 μg/kg midazolam to lidocaine for IVRA improved quality of anesthesia and intraoperative postoperative analgesia without causing side effects. This conclusion proposes that midazolam may possibly act at a peripheral site to yield analgesia.
There are different suggested sites for action of IVRA. Raj et al.19 described that the action of local anaesthetics is on major nerve trunks, perhaps reaching to the nerve trunk through small venules inside the nerve core, while Rosenberg20 afforded strong proof related to a peripheral site.
It is currently believed that together, the nerve endings and trunks, are affected.21 It was possible that analgesic effect of midazolam added to IVRA was due to its action on binding sites in periphery. This is confirmed by a significant decrease in VAS scores throughout intraoperative and twenty-four postoperative hours and by a prolonged delay between the IVRA administration and additional analgesic requirement.
Naguib7 and Nishiyama8 et al. showed that midazolam administrations at doses of 0.05 mg/kg epidurally or 0.03 mg/kg intrathecally produce significant analgesia in both animal and human studies.
Antinociceptive effects of neuraxial midazolam arise from agonism at the BDZ binding site on a subunit of pentameric GABA-A receptors, which operates paradoxically to reduce the transmitter release, a form of presynaptic inhibition.13,22
Consistent with this effect and from BDZ subunit expression in dorsal root ganglion and on spinal nerves, BDZ have a propensity to suppress afferent evoked excitation in substantia gelatinosa and motor horn22 leading to an antinociceptive effect.23,24
A variety of authors have also showed the existence of these GABA-A receptors in peripheral nerves.25
A comparable action of midazolam on GABA-A receptors in afferent nerve endings in the wrist and hand peripheral tissue may be responsible for its analgesic action throughout the intra- and postoperative period.
Besides GABA effects, spinal midazolam stimulates the opioid system through δ or κ receptors.26 In vitro studies have shown that midazolam displaced [3H]-deprenorphione binding from cloned human κ and δ receptors, and this effect of midazolam was inhibited by selective κ and δ agonists.27
Peripheral opioid receptors present in the peripheral wrist and hand tissue and their stimulation by midazolam can be responsible for IVRA analgesia.
Additionally, coexistent local tissue inflammation may perhaps lead to up-regulation or activation of peripheral opioid receptors.28 Stimulation of these opioid receptors by midazolam possibly will be responsible for its analgesic effect. The effect of midazolam on peripheral receptors has been confirmed by its effectiveness in augmenting the duration of analgesia when used in combination with bupivacaine for brachial plexus block.29
Batra et al.30 study showed that intraarticular administration of midazolam decreases postoperative pain after arthroscopic knee surgery, when compared with placebo. Their investigation also proposes that midazolam may act at a peripheral site in the joint, to produce analgesia.
Su et al.14 explained a case whose phantom pain of the amputated limb stump was twice induced by spinal anaesthesia during the two successive surgeries in the contralateral lower limb. It was showed that intravenous administration of 3 mg midazolam was successful in treating this rare phantom limb pain after spinal anesthesia.
Melzack and colleagues31 in clarifying the mechanism of phantom limb pain induced by spinal anaesthesia described that the occurrence of phantom pain after regional anesthesia could result from a decrease in the tonic inhibitory influence exerted by the brainstem reticular formation.
Complete loss of sensory input after subarachnoid block may possibly decrease the intensity of inhibition and may increase self-sustaining neural activity. Melzack31 explained that intravenous administration of 10 mg diazepam could alleviate recurring phantom pain successfully in two patients following spinal anesthesia.
In SU et al.14 case, midazolam was effective for relief of the pain. Benzodiazepines facilitate the inhibition of gamma aminobutyric acid binding sites in the central nervous system. The above described mechanisms may possibly explain the efficacy of intravenous midazolam in relieving intra- and postoperative pain when added to IVRA. This assumption needs further investigation before final conclusion.
It is possible that IVRA might not be a perfect model to differentiate peripheral versus central mechanisms of analgesia.
It was proposed that tourniquet pain evolve from ischaemia and oxidative stress.32 Oxygen free radicals (OFRs) are extremely reactive classes of molecules described by an unpaired electron in the outer electron ring. They are produced constantly in the organism following enzymatic reactions, or as metabolits of oxidation processes.33
Coderre and colleagues34 advocated that antioxidant therapy such as N-acetyl-L cysteine may decrease experimental ischaemic pain owing to oxidative damage. Additionally, antioxidants for pain treatment may reduce the dose of analgesics and inhibit the negative influence of reactive oxygen species on nociception.35
There are a number of researches describing the effects of midazolam on OFRs in vitro. Ischemic model have not been employed in these investigations and the findings are contradictory.
Davidson et al.36 showed that midazolam minimally affected OFRs production. Midazolam was shown to reduce superoxide anion production in doses that was higher than those used in clinical practice.37 It was without significant effect in a different study.38
As previous studies showed, the antioxidative effect of midazolam is controversial and needs further investigation before final conclusion. However, due to isolation of intravenous midazolam in arm circulation by tourniquet for 1-1.5 hours, it is possible that local administration of midazolam might attenuate tourniquet pain by antioxidative mechanism. More studies are necessary before we could have such assumption.
IVRA midazolam was also associated with faster onset of sensory and motor block, and prolonged sensory and motor block recovery times.
Chang et al.39 demonstrated that midazolam produces vasodilatation by endothelium-dependent and independent mechanisms. Endothelium-dependent vasodilatation produced by midazolam possibly is mediated through the release of nitrous oxide (NO) from endothelium. Endothelium independent vasodilatation appears to be related to inhibition of voltage-gated Ca2+ channels.
The beneficial effects of midazolam, which were showed in our study, probably will also depend on vasodilatory effect that promotes distribution of lidocaine to nerves. This would explain the rapid onset of sensory and motor block.
Reis Júnior40,41 showed that after tourniquet release in ischemic limb, the anaesthetic releases biphasically in systemic circulation and this release lasts 20 minutes or more. It was shown that only the amount of drug that stayed in the vascular bed (25% to 50%) leaves the limb quickly. This indicates that a substantial fraction of the drug remains in the area for a prolonged time.
It has been shown that plasma levels of local anaesthetics from the anesthetized limb are always higher than in the blood from the contralateral limb, even after 40 minutes or more.40–42
A clinical sign that confirms the staying of the anaesthetic in the operated limb is that it is feasible to reinstitute an excellent anaesthesia (continuous IVRB), with approximately half of the initial dose, 5 to 10 minutes after release of the tourniquet (“respiratory period”).43,41
It is possible that due to remaining midazolam and lidocaine for a prolonged time in the operating limb after tourniquet release, the sensory and motor block recovery times were significantly longer in LM group compared with LS group. Also, it is probable that vasodilatory effect of midazolam was not lasted till removal of tourniquet or at least, it was not similar to the initial periods of tourniquet inflation. These assumptions need further investigation before final conclusion can be elicited.
The substantial prolongation of motor blockade in the LM group compared with the LS group could also be described by benzodiazepine-induced attenuation of motor tonus at the ventral horn of the spinal cord after tourniquet release.23
While a variety of adjuvants have been recommended for improving intraoperative and postoperative analgesia and maintaining better operative conditions, these adjutants can cause side effects such as sedation, dizziness, nausea, vomiting, wound hematoma, skin rash, and hypotension.1,2,4,5,44,45
In our investigation, owing to the high lipophilicity, rapid clearance (6–11 mL/ kg/min), and short half-life (1.7–2.6 h) of midazolam28,46 there was no significant difference in side effects among groups. The tourniquet was not released before 30 min and the tourniquet deflation was performed by the cyclic deflation technique at the conclusion of surgery.
Multiple studies have demonstrated the potential for medications administered via IVRA to have sub-tourniquet leakage into the systemic circulation. However, Grice et al.47 used isotope scanning to show that the combination of distal extremity IV line, Esmarch exsanguination, injection over more than 90 seconds, and tourniquet pressure of 300 mm Hg had no IVRA solution leakage into the systemic circulation. Because our technique reflected his recommendations, we do not believe sub-tourniquet leakage into the systemic circulation was likely.
The degree of sedation was recorded at 15, 30, 45, and 60 min after tourniquet removal. At the time of patients’ discharge from post-anesthesia care unit (PACU), all patients had sedation level of 1. Also, there were no cases of apnea, hypoxia, respiratory depression, bradycardia, hypotension or any other side effects after tourniquet release till 24 hours after surgery. Therefore, if hospital discharge criteria were met for the outpatient surgery, it seems there were no problems concerning midazolam. But, the authors think, final decision for discharging the patients should be made with caution till further investigation carried out.
Our study was limited by the lack of a systemic midazolam control group and an active analgesic comparator (for example, IVRA opioid). Also, our study did not provide perfect substantiation of the comparative analgesic significance of central versus peripheral sites of action of midazolam.
Therefore, more studies comparing groups receiving IVRA midazolam with IV saline, IVRA saline with IV midazolam, IVRA saline with IV saline, and the addition of a standard analgesic are needed to establish the efficacy of IVRA midazolam.
In conclusion, addition of 50 μg/kg midazolam to lidocaine in IVRA shortens sensory and motor block onset times, prolongs sensory and motor block recovery times, and improves tourniquet pain while it prolongs first analgesic requirement time, and decreases total amount of analgesic without side effects. More studies must be carried out with experimental models and different doses to determine a relevant conclusion before midazolam routine use so its significance for the field of IVRA remains unclear.
Authors’ Contributions
PK has planned the study and finalized it; KM and HMH have planned the study and finalized it too; MRS and AH did the statistical analysis and prepared the first version of manuscript and revised final version for publish. All authors read and approved the final manuscript.
Acknowledgment
The authors wish to sincerely thank the support of all the colleagues in Alzahra Hospital Medical Center affiliated to Isfahan University of Medical Sciences in Isfahan, Iran. Furthermore, our special thanks go to the patients, who wholeheartedly and actively assisted us to carry out this research. No conflict of interest existed. This prospective randomized observational study was approved by the Ethics Committee of our university, (Isfahan University of Medical Sciences) and all patients gave written, informed consent.
Footnotes
Conflict of Interests Authors have no conflict of interests.
References
- 1.Choyce A, Peng P. A systematic review of adjuncts for intravenous regional anesthesia for surgical procedures. Can J Anaesth. 2002;49(1):32–45. doi: 10.1007/BF03020416. [DOI] [PubMed] [Google Scholar]
- 2.Turan A, Karamanlyoglu B, Memis D, Kaya G, Pamukcu Z. Intravenous regional anesthesia using prilocaine and neostigmine. Anesth Analg. 2002;95(5):1419–22. doi: 10.1097/00000539-200211000-00058. table. [DOI] [PubMed] [Google Scholar]
- 3.Brown EM, McGriff JT, Malinowski RW. Intravenous regional anaesthesia (Bier block): review of 20 years’ experience. Can J Anaesth. 1989;36(3 Pt 1):307–10. doi: 10.1007/BF03010770. [DOI] [PubMed] [Google Scholar]
- 4.Estebe JP, Gentili ME, Langlois G, Mouilleron P, Bernard F, Ecoffey C. Lidocaine priming reduces tourniquet pain during intravenous regional anesthesia: A preliminary study. Reg Anesth Pain Med. 2003;28(2):120–3. doi: 10.1053/rapm.2003.50123. [DOI] [PubMed] [Google Scholar]
- 5.Memis D, Turan A, Karamanlioglu B, Pamukcu Z, Kurt I. Adding dexmedetomidine to lidocaine for intravenous regional anesthesia. Anesth Analg. 2004;98(3):835–40. doi: 10.1213/01.ane.0000100680.77978.66. table. [DOI] [PubMed] [Google Scholar]
- 6.Dickenson AH, Chapman V, Green GM. The pharmacology of excitatory and inhibitory amino acid-mediated events in the transmission and modulation of pain in the spinal cord. Gen Pharmacol. 1997;28(5):633–8. doi: 10.1016/s0306-3623(96)00359-x. [DOI] [PubMed] [Google Scholar]
- 7.Naguib M, el GM, Elhattab YS, Seraj M. Midazolam for caudal analgesia in children: comparison with caudal bupivacaine. Can J Anaesth. 1995;42(9):758–64. doi: 10.1007/BF03011172. [DOI] [PubMed] [Google Scholar]
- 8.Nishiyama T, Hanaoka K. Effect of diluent volume on post-operative analgesia and sedation produced by epidurally administered midazolam. Eur J Anaesthesiol. 1998;15(3):275–9. doi: 10.1017/s0265021598000520. [DOI] [PubMed] [Google Scholar]
- 9.Shah FR, Halbe AR, Panchal ID, Goodchild CS. Improvement in postoperative pain relief by the addition of midazolam to an intrathecal injection of buprenorphine and bupivacaine. Eur J Anaesthesiol. 2003;20(11):904–10. doi: 10.1017/s0265021503001455. [DOI] [PubMed] [Google Scholar]
- 10.Kim MH, Lee YM. Intrathecal midazolam increases the analgesic effects of spinal blockade with bupivacaine in patients undergoing haemorrhoidectomy. Br J Anaesth. 2001;86(1):77–9. doi: 10.1093/bja/86.1.77. [DOI] [PubMed] [Google Scholar]
- 11.Kumar P, Rudra A, Pan AK, Acharya A. Caudal additives in pediatrics: a comparison among midazolam, ketamine, and neostigmine coadministered with bupivacaine. Anesth Analg. 2005;101(1):69–73. doi: 10.1213/01.ANE.0000153862.95153.2E. table. [DOI] [PubMed] [Google Scholar]
- 12.Valentine JM, Lyons G, Bellamy MC. The effect of intrathecal midazolam on post-operative pain. Eur J Anaesthesiol. 1996;13(6):589–93. doi: 10.1046/j.1365-2346.1996.00044.x. [DOI] [PubMed] [Google Scholar]
- 13.Tucker AP, Mezzatesta J, Nadeson R, Goodchild CS. Intrathecal midazolam II: combination with intrathecal fentanyl for labor pain. Anesth Analg. 2004;98(6):1521–7. doi: 10.1213/01.ANE.0000112434.68702.E4. table. [DOI] [PubMed] [Google Scholar]
- 14.Su CJ, Liu K, Wang YM. Midazolam as an effective drug for severe phantom limb pain in a patient after undergoing spinal anesthesia for two consecutive surgeries in the contralateral lower limb. Acta Anaesthesiol Taiwan. 2009;47(1):32–5. doi: 10.1016/S1875-4597(09)60018-7. [DOI] [PubMed] [Google Scholar]
- 15.Owens WD. American Society of Anesthesiologists Physical Status Classification System in not a risk classification system. Anesthesiology. 2001;94(2):378. doi: 10.1097/00000542-200102000-00042. [DOI] [PubMed] [Google Scholar]
- 16.Wilson E, David A, MacKenzie N, Grant IS. Sedation during spinal anaesthesia: comparison of propofol and midazolam. Br J Anaesth. 1990;64(1):48–52. doi: 10.1093/bja/64.1.48. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong P, Watters J, Whitfield A. Alkalinisation of prilocaine for intravenous regional anaesthesia.Suitability for clinical use. Anaesthesia. 1990;45(11):935–7. doi: 10.1111/j.1365-2044.1990.tb14622.x. [DOI] [PubMed] [Google Scholar]
- 18.Sen S, Ugur B, Aydin ON, Ogurlu M, Gezer E, Savk O. The analgesic effect of lornoxicam when added to lidocaine for intravenous regional anaesthesia. Br J Anaesth. 2006;97(3):408–13. doi: 10.1093/bja/ael170. [DOI] [PubMed] [Google Scholar]
- 19.Raj PP, Garcia CE, Burleson JW, Jenkins MT. The site of action of intravenous regional anesthesia. Anesth Analg. 1972;51(5):776–86. [PubMed] [Google Scholar]
- 20.Rosenberg PH. 1992 ASRA Lecture. Intravenous regional anesthesia: nerve block by multiple mechanisms. Reg Anesth. 1993;18(1):1–5. [PubMed] [Google Scholar]
- 21.Brill S, Middleton W, Brill G, Fisher A. Bier's block; 100 years old and still going strong! Acta Anaesthesiol Scand. 2004;48(1):117–22. doi: 10.1111/j.1399-6576.2004.00280.x. [DOI] [PubMed] [Google Scholar]
- 22.Yaksh TL, Allen JW. The use of intrathecal midazolam in humans: a case study of process. Anesth Analg. 2004;98(6):1536–45. doi: 10.1213/01.ANE.0000122638.41130.BF. table. [DOI] [PubMed] [Google Scholar]
- 23.Kohno T, Kumamoto E, Baba H, Ataka T, Okamoto M, Shimoji K, et al. Actions of midazolam on GABAergic transmission in substantia gelatinosa neurons of adult rat spinal cord slices. Anesthesiology. 2000;92(2):507–15. doi: 10.1097/00000542-200002000-00034. [DOI] [PubMed] [Google Scholar]
- 24.Edwards M, Serrao JM, Gent JP, Goodchild CS. On the mechanism by which midazolam causes spinally mediated analgesia. Anesthesiology. 1990;73(2):273–7. doi: 10.1097/00000542-199008000-00015. [DOI] [PubMed] [Google Scholar]
- 25.Bazzichi L, Betti L, Giannaccini G, Rossi A, Lucacchini A. Peripheral-type benzodiazepine receptors in human mononuclear cells of patients affected by osteoarthritis, rheumatoid arthritis or psoriasic arthritis. Clin Biochem. 2003;36(1):57–60. doi: 10.1016/s0009-9120(02)00408-3. [DOI] [PubMed] [Google Scholar]
- 26.Goodchild CS, Guo Z, Musgreave A, Gent JP. Antinociception by intrathecal midazolam involves endogenous neurotransmitters acting at spinal cord delta opioid receptors. Br J Anaesth. 1996;77(6):758–63. doi: 10.1093/bja/77.6.758. [DOI] [PubMed] [Google Scholar]
- 27.Cox RF, Collins MA. The effects of benzodiazepines on human opioid receptor binding and function. Anesth Analg. 2001;93(2):354–8. doi: 10.1097/00000539-200108000-00024. [DOI] [PubMed] [Google Scholar]
- 28.Stein C. Peripheral mechanisms of opioid analgesia. Anesth Analg. 1993;76(1):182–91. doi: 10.1213/00000539-199301000-00031. [DOI] [PubMed] [Google Scholar]
- 29.Jarbo K, Batra YK, Panda NB. Brachial plexus block with midazolam and bupivacaine improves analgesia. Can J Anaesth. 2005;52(8):822–6. doi: 10.1007/BF03021776. [DOI] [PubMed] [Google Scholar]
- 30.Batra YK, Mahajan R, Kumar S, Rajeev S, Singh DM. A dose-ranging study of intraarticular midazolam for pain relief after knee arthroscopy. Anesth Analg. 2008;107(2):669–72. doi: 10.1213/ane.0b013e3181770f95. [DOI] [PubMed] [Google Scholar]
- 31.Melzack R. Phantom limb pain: implications for treatment of pathologic pain. Anesthesiology. 1971;35(4):409–19. [PubMed] [Google Scholar]
- 32.Chabel C, Russell LC, Lee R. Tourniquet-induced limb ischemia: a neurophysiologic animal model. Anesthesiology. 1990;72(6):1038–44. doi: 10.1097/00000542-199006000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Bast A, Haenen GR, Doelman CJ. Oxidants and antioxidants: state of the art. Am J Med. 1991;91(3C):2S–13S. doi: 10.1016/0002-9343(91)90278-6. [DOI] [PubMed] [Google Scholar]
- 34.Coderre TJ, Xanthos DN, Francis L, Bennett GJ. Chronic post-ischemia pain (CPIP): a novel animal model of complex regional pain syndrome-type I (CRPS-I; reflex sympathetic dystrophy) produced by prolonged hindpaw ischemia and reperfusion in the rat. Pain. 2004;112(1-2):94–105. doi: 10.1016/j.pain.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Ben-David B, Katz E, Gaitini L, Goldik Z. Comparison of i.m. and local infiltration of ketorolac with and without local anaesthetic. Br J Anaesth. 1995;75(4):409–12. doi: 10.1093/bja/75.4.409. [DOI] [PubMed] [Google Scholar]
- 36.Davidson JA, Boom SJ, Pearsall FJ, Zhang P, Ramsay G. Comparison of the effects of four i.v. anaesthetic agents on polymorphonuclear leucocyte function. Br J Anaesth. 1995;74(3):315–8. doi: 10.1093/bja/74.3.315. [DOI] [PubMed] [Google Scholar]
- 37.Krumholz W, Demel C, Jung S, Meuthen G, Knecht J, Hempelmann G. The effects of thiopentone, etomidate, ketamine and midazolam on several bactericidal functions of polymorphonuclear leucocytes in vitro. Eur J Anaesthesiol. 1995;12(2):141–6. [PubMed] [Google Scholar]
- 38.Weiss M, Buhl R, Medve M, Schneider EM. Tumor necrosis factor-alpha modulates the selective interference of hypnotics and sedatives to suppress N-formyl-methionyl-leucyl-phenylalanine-induced oxidative burst formation in neutrophils. Crit Care Med. 1997;25(1):128–34. doi: 10.1097/00003246-199701000-00024. [DOI] [PubMed] [Google Scholar]
- 39.Chang KS, Feng MG, Davis RF. Midazolam produces vasodilation by mixed endothelium-dependent and -independent mechanisms. Anesth Analg. 1994;78(4):710–7. doi: 10.1213/00000539-199404000-00017. [DOI] [PubMed] [Google Scholar]
- 40.Reis A., Junior . Rio de Janeiro: Atheneu; 1996. Anestesia Regional Intravenosa. [Google Scholar]
- 41.Cangiani LM, Posso IP, Poterio GMB. Tratado de anestesiologia. In: Reis A Junior, editor. Anestesia Regional Intravenos. 6th ed. Sao Paulo: Atheneu; 2006. pp. 1295–315. [Google Scholar]
- 42.Simon MA, Vree TB, Gielen MJ, Booij LH. Comparison of the effects and disposition kinetics of articaine and lidocaine in 20 patients undergoing intravenous regional anaesthesia during day case surgery. Pharm World Sci. 1998;20(2):88–92. doi: 10.1023/a:1008622018161. [DOI] [PubMed] [Google Scholar]
- 43.Aldrete JA. Texto teorico-practico de anestesiologia. In: Reis A Junior, editor. Anestesia Regional Intravenosa. Mexico: Salvat; 1986. pp. 813–36. [Google Scholar]
- 44.Turan A, Memis D, Karamanlioglu B, Guler T, Pamukcu Z. Intravenous regional anesthesia using lidocaine and magnesium. Anesth Analg. 2005;100(4):1189–92. doi: 10.1213/01.ANE.0000145062.39112.C5. [DOI] [PubMed] [Google Scholar]
- 45.Honarmand A, Safavi MR. Comparison of prophylactic use of midazolam, ketamine, and ketamine plus midazolam for prevention of shivering during regional anaesthesia: a randomized double-blind placebo controlled trial. Br J Anaesth. 2008;101(4):557–62. doi: 10.1093/bja/aen205. [DOI] [PubMed] [Google Scholar]
- 46.Reves JG, Fragen RJ, Vinik HR, Greenblatt DJ. Midazolam: pharmacology and uses. Anesthesiology. 1985;62(3):310–24. [PubMed] [Google Scholar]
- 47.Grice SC, Morell RC, Balestrieri FJ, Stump DA, Howard G. Intravenous regional anesthesia: evaluation and prevention of leakage under the tourniquet. Anesthesiology. 1986;65(3):316–20. [PubMed] [Google Scholar]


