Abstract
BACKGROUND:
Despite the new developments in sepsis treatment, mortality rate is still high. In this study, we aimed to investigate endocrinologic changes and the effects of moderate dosage steroid treatment in patients with sepsis.
METHODS:
Fifty-five patients were included in the study. Basal hormonal evaluation and adrenocorticotropin hormone (ACTH) stimulation test were performed within 24 h in all patients. Both groups received standard treatment for sepsis. However, one group (steroid group) was also given intravenous prednisolone (20 mg/day). All-cause mortality was assessed during the first 28 days.
RESULTS:
Analysis of the findings revealed a 59.3% mortality rate in steroid group compared with a 53.6% mortality rate in placebo group (p = 0.787). Acute Physiology and Chronic Health Evaluation (APACHE) II and Sequential Organ Failure Assessment (SOFA) scores, and peak cortisol and ACTH levels were significant factors related to mortality. The incidence of adrenal insufficiency (AI) was 10.9% and relative adrenal insufficiency (RAI) 36.4%. It was also found that steroid treatment did not have any effects on the mortality of patients with AI and RAI (p = 0.075 and p = 0.999, respectively).
CONCLUSIONS:
Moderate-dose steroid therapy has no effect on mortality. Higher basal cortisol and peak cortisol levels were found more reliable mortality indicators compared to RAI. In addition, the study revealed that ACTH level was a significant indicator of mortality.
KEYWORDS: ACTH, Prednisolone, Cortisol, Adrenal Insufficiency, Sepsis
Sepsis is a major cause of mortality and morbidity worldwide, causing a mortality rate of 30 to 50%. Despite dramatic improvements in diagnostic and treatment procedures, mortality rates among patients with severe sepsis remained unchanged from the 1960s through to 2000s.1,2
During sepsis, there is a strong stimulation of the hypothalamic-pituitary-adrenal (HPA) axis which explains usually high plasma cortisol levels. The degree of activation is proportional to the severity of disease, and it has been suggested that the assessment of HPA axis may have prognostic value. Both high and low basal cortisol concentrations have been associated with an adverse outcome.3,4 High cortisol levels reflect more severe stress, while low levels indicate an inability of the HPA axis to respond sufficiently to stress, suggesting that a properly functioning HPA axis is essential for survival. Relative adrenal insufficiency (RAI) is conceptually defined by some investigators as an inadequate cortisol response to severe illness associated with rapid clinical and hemodynamic improvement after low dose corticosteroid therapy.5–7 High plasma cortisol levels and blunted cortisol responses to adrenocorticotropin hormone (ACTH) stimulation are linked to higher mortality in septic shock.6–8
Until recent years, available evidence did not support the use of corticosteroids in sepsis and septic shock.9,10 There has been a renewal of interest in corticosteroid therapy in septic shock through recent studies demonstrating hemodynamic improvement in patients depending on catecholamines with supraphysiological doses of hydrocortisone.11–14 Recent advances in our understanding of the role played by corticosteroid insufficiency in the pathogenesis of septic shock has resulted in international guidelines recommending the use of low dose corticosteroids for treatment of septic shock.15,16 While the Surviving Sepsis Campaign recommended the use of stress dose of corticosteroids for septic shock regardless of adrenal function,15 the American College of Critical Care Medicine Task Force suggested that stress dose of corticosteroids should be used only in refractory septic shock or in adrenal insufficient patients.16 In contrast, the Corticus study, which included close to 500 patients, recently showed that hydrocortisone did not improve survival or reversal of shock in patients with septic shock, either overall or in patients who did not respond to a corticotropin test.17 In a recent meta-analysis, neither the low-dose nor the high-dose cohort showed a significant steroid treatment effect on the mortality odds ratio (OR), although there was modest evidence of benefit in the low-dose cohort. The odds of mortality for both corticotrophin responders and non-responders were not significantly different compared with the control.18
Thus, we designed this placebo-controlled study to assess whether a replacement therapy with moderate-dose prednisolone (20 mg/day) could improve 28-day survival in patients with sepsis, with particular interest in patients with RAI. We also tried to assess the significance of basal and peak cortisol levels and other endocrinological changes as prognostic indicators in patients with sepsis.
Methods
Study Design
This prospective, randomized, double-blind, placebo-controlled trial was conducted between April 2005 and May 2008 in the Department of Medical ICU and the Department of Infectious Diseases of School of Medicine at Erciyes University (2003.03.15; ClinicalTrials.gov Identifier: NCT01275638). The study was approved by our Institutional Review Board and informed consents were obtained from patients’ relatives. The study did not alter the standard sepsis therapy (i.e. administration of antibiotics, fluid replacement, vasoactive drugs, and mechanical ventilatory support) and each patient's clinical care was determined by their own physician.15,16
Patients
Patients over 17 years old diagnosed with sepsis were consecutively included in the study. Diagnosis of sepsis and classification of disease severity were performed based on the definition of American College of Chest Physicians/Society of Critical Care Medicine (ACCP-SCCM).19 Patients with already known pre-existing adrenal disease or adrenalectomy, known malignancies, tuberculosis that might have involved the adrenal gland, and administration of steroids within the 3–month period before the admission were excluded. In addition, patients with burns and hemorrhagic shock, or those who had suffered myocardial infarction were not included.
Treatment Protocol
Soon after the presumptive diagnosis of sepsis, initial laboratory specimens were obtained within 2 hours, and the patients enrolled in the study were treated with standard therapy used in the treatment of sepsis and septic shock. This therapy could include administration of antibiotics, fluid replacement, vasoactive drugs, mechanical ventilatory support, and any other form of supportive therapy.15,16 The patients were randomized to be treated with prednisolone or placebo within 24 h of admission. The treatment groups were determined by a computer-generated randomization procedure (in a 1:1 ratio). The steroid group received prednisolone at a moderate-dose (20 mg/day).20 Prednisolone was given intravenously three times a day at 06.00 (10 mg), 14.00 (5 mg), and 22.00 (5 mg) for 10 days. The standard therapy group received a placebo infusion containing physiological saline solution in an identical manner. Patients and their primary physicians were blinded as to which therapy was administered.
Data Collection
Clinical data including demographics, underlying diagnosis, the disease severity as assessed by vital signs, Acute Physiology and Chronic Health Evaluation (APACHE) II and Sequential Organ Failure Assessment (SOFA) were recorded.21,22
Within 24 h of admission, morning blood samples were collected to determine basal cortisol, adrenocorticotropin hormone (ACTH), dehydroepiandrosterone sulfate (DHEAS), growth hormone (GH), prolactin (PRL), insulin-like growth factor-1 (IGF-1), free triiodothyronine (fT3), free thyroxine (fT4) and thyroid-stimulating hormone (TSH) levels. Subsequently, ACTH stimulation test was performed using 0.25 mg of synthetic corticotropin (Synacthen, Novartis, Germany) administered intravenously and serum samples were obtained for cortisol before corticotropin administration and 30 and 60 minutes following it. These measurements were performed before randomization for all patients. The cases with peak cortisol levels lower than 20 μg/dl were considered as adrenal insufficient (AI).23 Relative adrenal insufficiency (RAI) was diagnosed as a rise in serum cortisol ≤ 9 μg/dL after the administration of corticotropin (inadequate cortisol response).6,7
Assays
Hormonal variables were measured by radioimmunoassay (RIA) immunoradiometric assay (IRMA) or chemiluminescent immunometric methods using commercially available kits namely serum cortisol, RIA (Cortisolo-RIA-CT, Radim, Rome, Italy), DHEAS, RIA (DHEAS,RIA-CT, Radim, Rome, Italy), plasma ACTH, IRMA (ACTİVE-ACTH-IRMA, DSL-5100i, Texas, USA), GH, IRMA (Hgh-IRMA-CT, Radim, Rome, Italy), PRL, chemiluminescent immunometric (IMMULITE 2000 Prolactin, SIEMENS), plasma IGF-I, IRMA (ACTİVE IGF-1 with extraction DSL-5600, Texas, USA), and TSH IRMA (KIP-1891, KIP-1894, Biosource Europe S.A., Nivelles, Belgium). All samples for an individual subject were determined in a single assay.
Follow-up and End Points
During the 28-day period after randomization, data were collected daily regarding vital signs, use of vasopressor drugs, urine output, results from laboratory tests and cultures of specimens drawn from any new site of infection, and interventions that were performed. The outcomes were assessed during the first 28 days or until hospital discharge. Telephone follow-up was conducted after hospital discharge. The primary endpoint was 28-day mortality from all causes. The secondary endpoints were adverse occurrences including possible complications of drug therapy and morbid events such as the progression of initial infection and the development of secondary infection. All medications given to the patients, any complications, reversal of organ system failure the duration of hospitalization, the mortality rate, and causes of death were recorded and compared between the groups. We also compared average values of basal cortisol, peak cortisol and cortisol responses to ACTH between survivors and non-survivors on the first day.
Statistical Analysis
The Shapiro-Wilk test was used to test for normal distribution of the data. Values are expressed as means ± SD and percentages. Student's t-test or the Mann–Whitney U test were used to compare continuous variables, χ2 and Fisher's χ2 tests were used for proportions, and logistic regression was used for effects of factors on mortality. The Kruskal-Wallis test followed by Dunn's method was used to compare multiple unrelated non-normal samples. Comparisons within the groups were performed by Friedman and Wilcoxon test and, if significant (Friedman), further analysis was made with the Student-Newman-Keuls test for all possible pairwise comparisons. Logistic regression was used to calculate the odds ratios of risk factors and a log-rank test was used to evaluate factors associated with survival. The area under the receiver operating characteristic (ROC) curve was used to evaluate the ability to discriminate between patients who survived and those who died. An optimal cut off was obtained considering the combination of highest sensitivity and specificity. Statistical analyses were performed using SPSS 15.0 (SPSS, Chicago, IL) and differences were considered significant when p < 0.05.
Results
Fifty five patients (36 males and 19 females) with the presumptive diagnosis of sepsis were enrolled in this study. Clinical and demographic characteristics of the patients are given in Table 1 for the steroid and placebo groups. The median age of the patients was 70 years (range 20-91), and 31 patients (56.4%) died in the hospital from refractory shock and/or multiple organ failure within 28 days. Placebo patients were younger than steroid patients, and more males than females were tested in the steroid group. In all patients, the mean APACHE II and initial SOFA scores were 20.7 and 8.9, respectively. Community-acquired infection was present at 70.9% (39/55) of patients and nosocomial infection was present at 29.1% (16/55) of the patients. Twenty-two patients (40%) had documented bacteremia. The major sources of infection were urinary (46.4%) or pulmonary (42.8%). In addition, the most frequent infectious agent group was gram-negative bacilli (54.5%) and the most frequent infectious agent in gram-negative bacilli was E. coli (51%).
Table 1.
General characteristics of patients with sepsis on admission
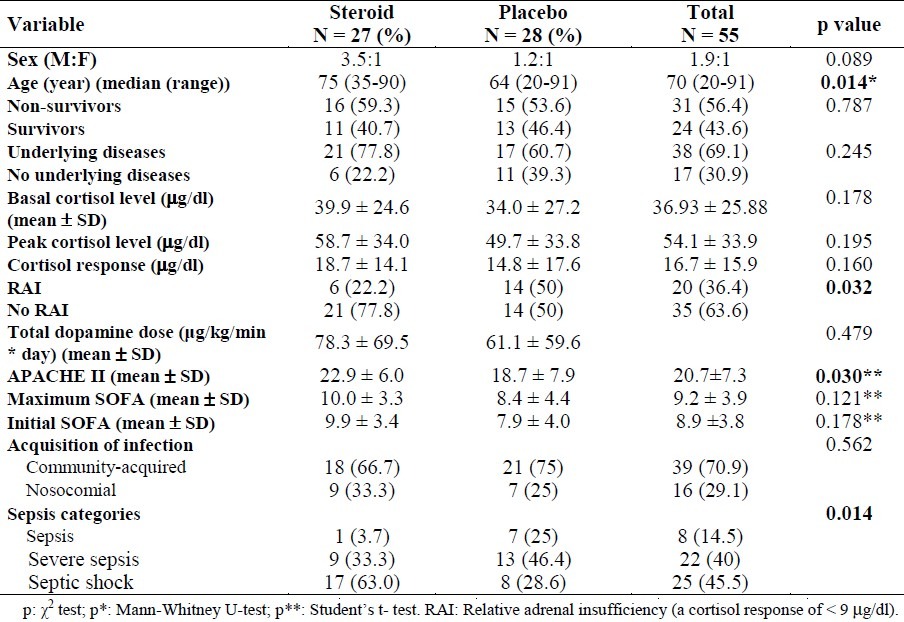
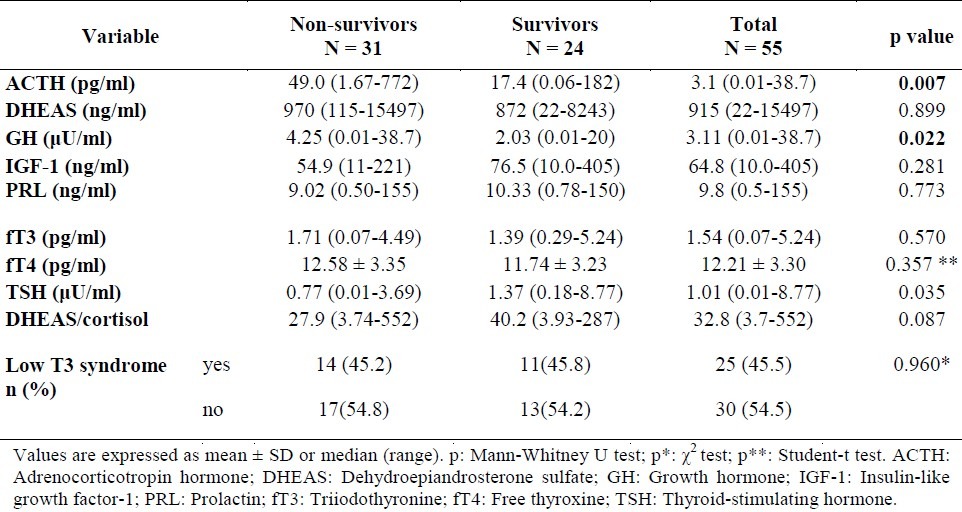
Mean basal cortisol level was 36.9 μg/dl (median: 29.9 and min-max: 5.6-114.6 μg/dl). Peak cortisol level was 54.1 μg/dl (median: 45.8 and min-max: 5.6-167.4 μg/dl) and cortisol response was 16.7 μg/dL (median: 12.1 μg/dl, min-max: -13.5-66.9 μg/dl) in all patients. There was no statistically significant difference in basal cortisol, peak cortisol and cortisol response levels according to the severity of the infection. The incidence of adrenal insufficiency in our study population was 10.9%, and the incidence of relative adrenal insufficiency was 36.4%. Median or mean hormone levels in survivors and non-survivors of sepsis are demonstrated in Table 2. Median ACTH and GH levels were significantly higher in non-survivors than in survivors (p = 0.007 and p = 0.022, respectively). The number of patients with low T3 syndrome, in which only fT3 is low while the values of TSH and fT4 are normal, was 45.5% (25/55). When the hormonal findings of the patients were compared according to severity of the infection, it was found that the rates of DHEAS and DHEAS/cortisol decreased in the process from sepsis to septic shock, in other words the production slid towards cortisol. In addition, while GH concentration did not change, the decrease in IGF-1 level supported GH resistance (data is not shown).
Table 2.
Basal hormone levels in survivors and non-survivors of sepsis
Factors related to survival
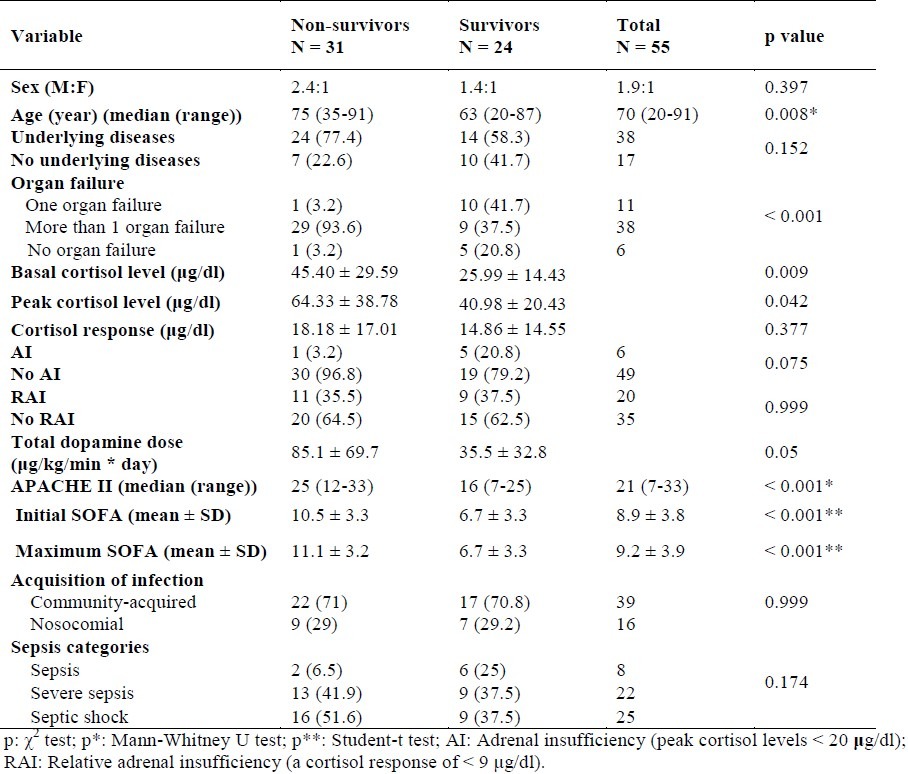
General characteristics of the 55 patients according to survivors and non-survivors in both groups are presented in Table 3. The median time to death was 6 days in the steroid group and 4 days in the placebo group. The reason for death in all patients was attributed to sepsis. The mean APACHE II, and initial and maximum SOFA scores for the 31 non-survivors were significantly higher than the mean scores for the 24 survivors (p < 0.001).
Table 3.
General characteristics of survivor and non-survivor patients with sepsis
Basal cortisol concentrations were also predictive of mortality. The basal cortisol level was 26.0 μg/dl in survivors and 45.4 μg/dl in non-survivors. The survivors group demonstrated significantly lower basal cortisol levels (p = 0.009). The area under the ROC curve for basal cortisol was 0.707 (95% CI, 0.569-0.822), showing that the model discriminated well between patients who lived and those who died (Figure 1). With an optimal calculated basal cortisol threshold of 21.61 μg/dl, sensitivity and specificity were 50.0 and 83.9% with positive and negative likelihood ratios of 3.10 and 0.60, respectively. With this cut off value, mortality was 29.4% for basal values < 21.61 μg/dl and 68.4% for values above this level (p = 0.009).
Figure 1.
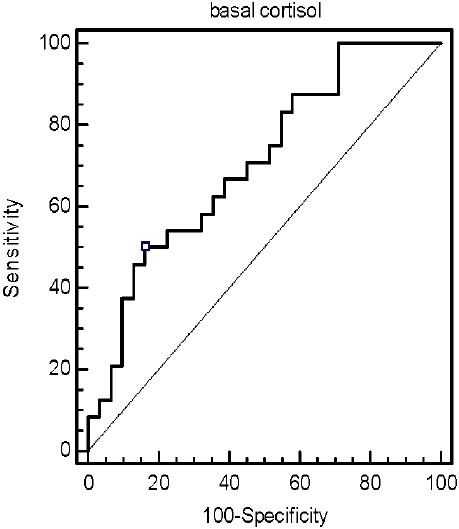
ROC curve analysis of basal cortisol for predicting survival from sepsis (The area under the ROC curve is 0.707)
In the evaluated subgroups, 2 patients (3.6%) had basal cortisol levels below 10 μg/dl, 12 patients (21.8%) had levels between 10 and 19 μg/dl, 33 patients (60%) had levels between 20 and 60 μg/dl, and 8 patients (14.5%) had levels above 60 μg/dl. Basal cortisol levels above 20 μg/dl were significantly associated with hospital mortality (65.9% (27/41) mortality in patients with basal cortisol above 20 μg/dl vs. 28.6% (4/14) mortality in patients with basal cortisol below 20 μg/dl; p = 0.027; Figure 2). Considering the occurrence of RAI, survivors (37.5%) and non-survivors (35.5%) had similar rates.
Figure 2.
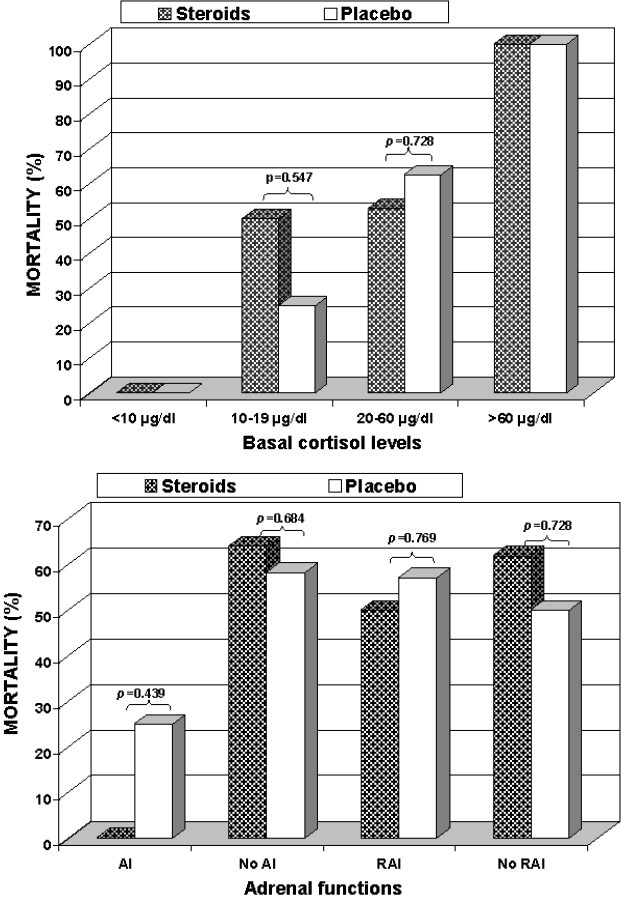
Mortality rates according to basal cortisol categories (Panel A) and adrenal functional status (Panel B) in both groups
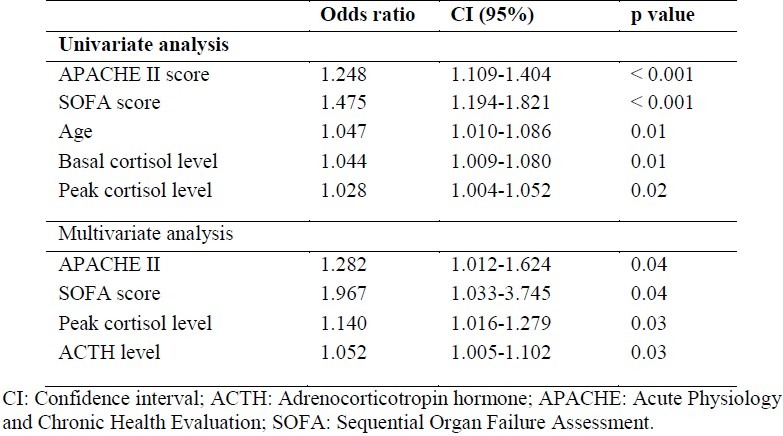
Based on univariate analysis, older age, high APACHE II and SOFA scores, high basal cortisol, peak cortisol and ACTH levels were determined as factors that affect mortality. When multivariate analysis was performed with these parameters, APACHE II and SOFA scores, peak cortisol and ACTH levels were factors affecting mortality. Logistic regression analysis of risk factors for hospital mortality is presented in Table 4.
Table 4.
Logistic regression analysis of risk factors for hospital mortality in sepsis
Efficacy of steroid treatment
Twenty-seven of 55 patients with sepsis in our study received prednisolone at a moderate-dose (20 mg/day) for 10 days. There were 16 deaths (59.3%) in the steroid group and 15 (53.6%) in the placebo group. No significant difference in mortality was observed between patients who received moderate-dose steroid treatment and those who did not (p = 0.787). It was also found that steroid treatment did not have any effects on the mortality of patients with RAI and AI (p = 0.999 and p = 0.075, respectively; Figure 2). The mortality rate among patients with a basal cortisol level below 20 mg/dl was 40% (2 of 5) in the steroid group and 22.2% (2 of 9) in the placebo group (p = 0.580). The mortality rate among those with a basal cortisol level above 40 μg/dl was 88.9% (8 of 9) in the steroid group and 71.4% (5 of 7) in the placebo group (p = 0.550).
Adverse Events
There were no serious local or systemic treatment-related adverse events, including nosocomial infections, gastrointestinal bleeding, or hyperglycemia in both the steroid and placebo groups.
Discussion
In accordance with previous observations,6,7,24,25 this study confirmed that serum plasma cortisol levels are increased in patients with sepsis. Although the majority of cortisol levels were between 5.6 and 114.6 μg/dl, there was a wide range of cortisol levels in our patient population. The higher cortisol levels generally seen in patients with septic shock can involve various mechanisms including activation of the HPA axis, loss of circadian rhythm and of negative feedback, decreased cortisol clearance, and peripheral resistance by decreased receptor affinity.26 Most investigations on mixed critically ill patients have focused on basal cortisol levels and have reported controversial results. Non-survivors may have higher or lower baseline cortisol concentrations than survivors.27 In our study, the mean basal plasma cortisol concentration was significantly lower in survivors compared with non-survivors with sepsis (26 ± 14.4 vs. 45.4 ± 29.6 μg/dl; p = 0.009). In addition, based on univariate analysis, older age, high ACTH, basal cortisol and peak cortisol levels, high APACHE II and SOFA scores were determined as factors that affect mortality (Table 4).
There is limited data regarding GH/IGF-I axis and DHEAS levels in critically ill patients. Consistent with some of the previous studies, we showed GH resistance and decreased DHEAS levels associated with increased severity of sepsis. Peripheral resistance to GH action (probably due to decreased GH-receptor expression), manifested by high basal GH concentrations with low IGF-I, has been reported in patients with critical illnesses including traumatic brain injury.28 This response seems to be an adaptive response because GH is an anabolic hormone and in the acute phase of critical illness catabolism is the initial response to serve fuel for vital organs. DHEA and DHEAS are the most abundant steroids secreted by the adrenal cortex. It is known that during any stressful condition when higher cortisol synthesis is required, DHEAS synthesis shifts to cortisol synthesis. Serum DHEAS concentration is low in patients with primary adrenal insufficiency, but limited data are present on DHEAS levels in critically ill patients. Extremely low DHEAS concentrations have been demonstrated in septic shock and in multiple trauma patients, frequently showing adrenal insufficiency, suggesting DHEAS as a prognostic marker and a sign of exhausted adrenal reserve in critical illness.29 However we could not demonstrate any significant association between GH or DHEAS levels and mortality in either univariate or multivariate analysis. One of the important findings in the present study was the association of high ACTH levels with mortality in multivariate analysis. Based on these data, it is tempting to speculate that the central activation of HPA axis increases proportionally with the severity of sepsis.30
Considering sepsis as a continuous stress situation, some authors proposed a basal plasma cortisol concentration > 20-25 μg/dl as appropriate for critically ill patients,24,26 whereas others have proposed a cutoff value of 15 μg/dl.25 In the present study, basal cortisol levels > 20 μg/dl were significantly associated with hospital mortality (65.9% mortality in patients with basal cortisol > 20 μg/dl vs. 28.6% mortality in patients with basal cortisol less than 20 μg/dl; p = 0.027). All patients with basal cortisol levels higher than 60 μg/dL have died from refractory shock and/or multiple organ failure in the two study groups (Figure 2). However, two patients (one in each group) with basal cortisol levels lower than 10 μg/dl survived to the end of the study. Some authors believe that the conflicting reports reflect a bimodal distribution of mortality related with random cortisol levels during sepsis, with the highest mortality rates observed in patients with extremely low cortisol levels (< 25 μg/dl) or very high levels (> 45 μg/dl).6–8,25,31 In our opinion, a high basal cortisol is enough evidence to show that adrenal is adequately responding to stress and excludes the need for exogenous stimulation. Moreover, high serum cortisol concentration on first day is an independent predictor of poor outcome in patients with sepsis.
The reported prevalence of adrenal insufficiency varies widely (from 10% to 71%) in critically ill patients, depending on the population of patients studied and the diagnostic criteria.3,6,7 Based on our present and previous studies,32,33 AI is probably not uncommon amongst patients with sepsis (10.9%, 16.3%, and 2.5%, respectively). The prevalence of AI in our studies were relatively low compared to other studies.8,31 The explanation for this seemingly contradictory finding may lie in the fact that most of the sepsis patients in this study had raised cortisol levels. Overall mortality rate in septic patients with AI in this study was low (1 of 6, 16.7%), but the small number of patients suggests this to be a topic for further study.
It is usually considered that a single plasma cortisol determination, with the exception of the very low (< 15 μg/dl) and high values (> 34 μg/dl), has no predictive value on patient outcome in septic shock and is not predictive of the adrenal ability to respond in stress.3,6–8,25 For this reason, evaluation of the activation of the HPA axis in critically ill patients is largely based on stimulation tests. In recent years, several authors have proposed a syndrome of RAI in sepsis in the presence of normal or even elevated serum cortisol concentrations.3,6,14 This pattern is encountered in approximately one-third of patients with sepsis.6 We report here that the overall incidence of RAI in patients with sepsis was 36.4% (55 patients) which was similar among survivors and non-survivors (37.5% and 35.5%, respectively; p = 0.999). RAI may be more relevant to the prognostic value of a low response to corticotropin. However, it has been challenged by reports suggesting that the corticotropin test is not reproducible in critically ill patients. In addition, most studies performed on patients with sepsis displayed no prognostic value of the test.25,34
Recent clinical trials on stress doses of hydrocortisone in patients with septic shock showed beneficial effects on hemodynamics12,13 and mortality.35 In the study by Bollaert et al., these effects were not related to RAI.12 On the other hand, the prognostic importance of a high serum cortisol level may be different in patients receiving corticosteroid replacement therapy due to the influence of the corticosteroid drug. In contrast to other studies, our study was performed on patients with sepsis, severe sepsis, and septic shock using moderate-dose prednisolone with or without vasopressor support. The administration of prednisolone did not affect the levels of cortisol in this study since it was given to the patients after ACTH stimulation test.
The main finding of the present study was that the administration of moderate-dose of prednisolone for 10 days had no significant effect on the rate of death in patients with sepsis at 28 days. These results are in marked contrast to those of the study by Annane et al. in which a 7-day treatment with low doses of hydrocortisone and fludrocortisone significantly reduced the risk of death in patients with septic shock and RAI.14 However, in our present and previous studies, prednisolone proved of no statistically significant benefit in patients who did not respond to corticotropin. At 28 days, there were 11 deaths in the 20 nonresponder patients given prednisolone compared with 20 deaths in the 35 responder patients given placebo (55% and 57.1%, respectively; p = 0.999). Sixteen of the 27 patients given prednisolone died as did 15 of the 28 patients who received placebo (59.3% and 53.6%, respectively; p = 0.787).
There were several limitations in the present study. First, in the steroid replacement group, mean age, APACHE II scores, and percentage of septic shock was significantly higher than the placebo group. This factor might be one possible cause of the ineffectiveness of steroid replacement therapy. Another limitation was lack of adequate number of patients in the subgroups.
Conclusion
Moderate-dose steroid treatment did not affect mortality. Higher basal cortisol and peak cortisol levels were found more estimable mortality indicators compared to RAI. In addition, the study revealed that ACTH was one of the best indicators of mortality. We are of the opinion that the borders regarding which patients require steroid treatment should be more clearly drawn, that more optimal methods should be tested for diagnosis of RAI, and that hormonal changes in sepsis should be evaluated in a larger group of patients.
Authors’ Contributions
OY and BA designed the study and directed its implementation, contributed to the analysis, interpretation of the data, and to the redaction of the manuscript. SŞ participated in the design and coordination of the study, carried out the data collection and interpretation of the data, performed the statistical analysis, drafted the manuscript, and contributed to the analysis. FT and FK participated in the statistical analysis and to the redaction of the manuscript. All authors have read and approved the final manuscript.
Footnotes
Conflict of Interests Authors have no conflict of interests.
References
- 1.Angus DC, Wax RS. Epidemiology of sepsis: an update. Crit Care Med. 2001;29(7 Suppl):S109–S116. doi: 10.1097/00003246-200107001-00035. [DOI] [PubMed] [Google Scholar]
- 2.Annane D, Bellissant E, Cavaillon JM. Septic shock. Lancet. 2005;365(9453):63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 3.Cooper MS, Stewart PM. Corticosteroid insufficiency in acutely ill patients. N Engl J Med. 2003;348(8):727–34. doi: 10.1056/NEJMra020529. [DOI] [PubMed] [Google Scholar]
- 4.Lipiner-Friedman D, Sprung CL, Laterre PF, Weiss Y, Goodman SV, Vogeser M, et al. Adrenal function in sepsis: the retrospective Corticus cohort study. Crit Care Med. 2007;35(4):1012–8. doi: 10.1097/01.CCM.0000259465.92018.6E. [DOI] [PubMed] [Google Scholar]
- 5.Zaloga GP. Sepsis-induced adrenal deficiency syndrome. Crit Care Med. 2001;29(3):688–90. doi: 10.1097/00003246-200103000-00051. [DOI] [PubMed] [Google Scholar]
- 6.Annane D, Sebille V, Troche G, Raphael JC, Gajdos P, Bellissant E. A 3-level prognostic classification in septic shock based on cortisol levels and cortisol response to corticotropin. JAMA. 2000;283(8):1038–45. doi: 10.1001/jama.283.8.1038. [DOI] [PubMed] [Google Scholar]
- 7.Rothwell PM, Udwadia ZF, Lawler PG. Cortisol response to corticotropin and survival in septic shock. Lancet. 1991;337(8741):582–3. doi: 10.1016/0140-6736(91)91641-7. [DOI] [PubMed] [Google Scholar]
- 8.Briegel J, Schelling G, Haller M, Mraz W, Forst H, Peter K. A comparison of the adrenocortical response during septic shock and after complete recovery. Intensive Care Med. 1996;22(9):894–9. doi: 10.1007/BF02044113. [DOI] [PubMed] [Google Scholar]
- 9.Lefering R, Neugebauer EA. Steroid controversy in sepsis and septic shock: a meta-analysis. Crit Care Med. 1995;23(7):1294–303. doi: 10.1097/00003246-199507000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Annane D, Cariou A, Maxime V, Azoulay E, D’honneur G, Timsit JF, et al. Corticosteroid treatment and intensive insulin therapy for septic shock in adults: a randomized controlled trial. JAMA. 2010;303(4):341–8. doi: 10.1001/jama.2010.2. [DOI] [PubMed] [Google Scholar]
- 11.Arabi YM, Aljumah A, Dabbagh O, Tamim HM, Rishu AH, Al-Abdulkareem A, et al. Low-dose hydrocortisone in patients with cirrhosis and septic shock: a randomized controlled trial. CMAJ. 2010;182(18):1971–7. doi: 10.1503/cmaj.090707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bollaert PE, Charpentier C, Levy B, Debouverie M, Audibert G, Larcan A. Reversal of late septic shock with supraphysiologic doses of hydrocortisone. Crit Care Med. 1998;26(4):645–50. doi: 10.1097/00003246-199804000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Briegel J, Forst H, Haller M, Schelling G, Kilger E, Kuprat G, et al. Stress doses of hydrocortisone reverse hyperdynamic septic shock: a prospective, randomized, double-blind, single-center study. Crit Care Med. 1999;27(4):723–32. doi: 10.1097/00003246-199904000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Annane D, Sebille V, Charpentier C, Bollaert PE, Francois B, Korach JM, et al. Effect of treatment with low doses of hydrocortisone and fludrocortisone on mortality in patients with septic shock. JAMA. 2002;288(7):862–71. doi: 10.1001/jama.288.7.862. [DOI] [PubMed] [Google Scholar]
- 15.Dellinger RP, Carlet JM, Masur H, Gerlach H, Calandra T, Cohen J, et al. Surviving Sepsis Campaign guidelines for management of severe sepsis and septic shock. Crit Care Med. 2004;32(3):858–73. doi: 10.1097/01.ccm.0000117317.18092.e4. [DOI] [PubMed] [Google Scholar]
- 16.Hollenberg SM, Ahrens TS, Annane D, Astiz ME, Chalfin DB, Dasta JF, et al. Practice parameters for hemodynamic support of sepsis in adult patients: 2004 update. Crit Care Med. 2004;32(9):1928–48. doi: 10.1097/01.ccm.0000139761.05492.d6. [DOI] [PubMed] [Google Scholar]
- 17.Sprung CL, Annane D, Keh D, Moreno R, Singer M, Freivogel K, et al. Hydrocortisone therapy for patients with septic shock. N Engl J Med. 2008;358(2):111–24. doi: 10.1056/NEJMoa071366. [DOI] [PubMed] [Google Scholar]
- 18.Moran JL, Graham PL, Rockliff S, Bersten AD. Updating the evidence for the role of corticosteroids in severe sepsis and septic shock: a Bayesian meta-analytic perspective. Crit Care. 2010;14(4):R134. doi: 10.1186/cc9182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20(6):864–74. [PubMed] [Google Scholar]
- 20.Edsbacker S, Nilsson M, Larsson P. A cortisol suppression dose-response comparison of budesonide in controlled ileal release capsules with prednisolone. Aliment Pharmacol Ther. 1999;13(2):219–24. doi: 10.1046/j.1365-2036.1999.00472.x. [DOI] [PubMed] [Google Scholar]
- 21.Ho KM, Lee KY, Williams T, Finn J, Knuiman M, Webb SA. Comparison of Acute Physiology and Chronic Health Evaluation (APACHE) II score with organ failure scores to predict hospital mortality. Anaesthesia. 2007;62(5):466–73. doi: 10.1111/j.1365-2044.2007.04999.x. [DOI] [PubMed] [Google Scholar]
- 22.Kajdacsy-Balla Amaral AC, Andrade FM, Moreno R, Artigas A, Cantraine F, Vincent JL. Use of the sequential organ failure assessment score as a severity score. Intensive Care Med. 2005;31(2):243–9. doi: 10.1007/s00134-004-2528-6. [DOI] [PubMed] [Google Scholar]
- 23.Oelkers W. Adrenal insufficiency. N Engl J Med. 1996;335(16):1206–12. doi: 10.1056/NEJM199610173351607. [DOI] [PubMed] [Google Scholar]
- 24.Schein RM, Sprung CL, Marcial E, Napolitano L, Chernow B. Plasma cortisol levels in patients with septic shock. Crit Care Med. 1990;18(3):259–63. doi: 10.1097/00003246-199003000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Bouachour G, Tirot P, Gouello JP, Mathieu E, Vincent JF, Alquier P. Adrenocortical function during septic shock. Intensive Care Med. 1995;21(1):57–62. doi: 10.1007/BF02425155. [DOI] [PubMed] [Google Scholar]
- 26.Zaloga GP, Marik P. Hypothalamic-pituitary-adrenal insufficiency. Crit Care Clin. 2001;17(1):25–41. doi: 10.1016/s0749-0704(05)70150-0. [DOI] [PubMed] [Google Scholar]
- 27.Ray DC, Macduff A, Drummond GB, Wilkinson E, Adams B, Beckett GJ. Endocrine measurements in survivors and non-survivors from critical illness. Intensive Care Med. 2002;28(9):1301–8. doi: 10.1007/s00134-002-1427-y. [DOI] [PubMed] [Google Scholar]
- 28.Tanriverdi F, Senyurek H, Unluhizarci K, Selcuklu A, Casanueva FF, Kelestimur F. High risk of hypopituitarism after traumatic brain injury: a prospective investigation of anterior pituitary function in the acute phase and 12 months after trauma. J Clin Endocrinol Metab. 2006;91(6):2105–11. doi: 10.1210/jc.2005-2476. [DOI] [PubMed] [Google Scholar]
- 29.Beishuizen A, Thijs LG, Vermes I. Decreased levels of dehydroepiandrosterone sulphate in severe critical illness: a sign of exhausted adrenal reserve? Crit Care. 2002;6(5):434–8. doi: 10.1186/cc1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bondanelli M, Zatelli MC, Ambrosio MR, degli Uberti EC. Systemic illness. Pituitary. 2008;11(2):187–207. doi: 10.1007/s11102-008-0112-8. [DOI] [PubMed] [Google Scholar]
- 31.Marik PE, Zaloga GP. Adrenal insufficiency during septic shock. Crit Care Med. 2003;31(1):141–5. doi: 10.1097/00003246-200301000-00022. [DOI] [PubMed] [Google Scholar]
- 32.Aygen B, Inan M, Doganay M, Kelestimur F. Adrenal functions in patients with sepsis. Exp Clin Endocrinol Diabetes. 1997;105(3):182–6. doi: 10.1055/s-0029-1211749. [DOI] [PubMed] [Google Scholar]
- 33.Yildiz O, Doganay M, Aygen B, Guven M, Kelestimur F, Tutuu A. Physiological-dose steroid therapy in sepsis [ISRCTN36253388] Crit Care. 2002;6(3):251–9. doi: 10.1186/cc1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schroeder S, Wichers M, Klingmuller D, Hofer M, Lehmann LE, von ST, et al. The hypothalamic-pituitary-adrenal axis of patients with severe sepsis: altered response to corticotropin-releasing hormone. Crit Care Med. 2001;29(2):310–6. doi: 10.1097/00003246-200102000-00017. [DOI] [PubMed] [Google Scholar]
- 35.Annane D. Effects of the combination of hydto- cottisone and fludtocottisone on mottality in septic shock. Ctit Cate Med. 2000;28:A63. [Google Scholar]


