Abstract
BACKGROUND:
Cognitive impairment associated with temporal lobe epilepsy (TLE) has been recognized in multiple studies. We designed this study to find a specific cognitive profile in patients with TLE who were candidates for epilepsy surgery. We also sought to find if neuropsychological assessment could differentiate left TLE, right TLE and normal subjects.
METHODS:
The sample of this study consisted of 29 patients with right TLE, 31 with left TLE, and 32 subjects without history of seizure as the control group. For all recruited patients and controls, demographic questionnaire, Wechsler Memory Scale-III (WMS-III) and Wechsler Adult Intelligence Scale-R (WAIS-R) were administered. Multivariate analysis of variance was carried out to reveal differences in memory and intelligence performance between the three groups.
RESULTS:
All of the mean scores of the WMS-III indexes were significantly higher in the control group in comparison with the right or the left TLE groups (p < 0.001). There were not any significant differences between mean scores of WMS-III indexes of the right and the left. The WAIS-R also showed significantly better mean scores of full scale intelligence quotient (FSIQ) and performance intelligence quotient (PIQ) in the control groups than both of the right and left TLE patients (p < 0.001). Although the verbal intelligence quotient (VIQ) mean scores were significantly different between the left TLE and the control group (p = 0.037), there were not any significant differences between the right TLE patients and the control group.
CONCLUSIONS:
These findings indicated that WMS-III and WAIS-R can differentiate patients with refractory temporal lobe epilepsy from normal subjects. However, the obtained cognitive profile could not differentiate between the right and the left TLE.
KEYWORDS: Temporal Lobe Epilepsy, Cognitive Impairment, Memory, Wechsler Memory Scale
Temporal lobe epilepsy (TLE) is the most prevalent form of complex partial seizures (CPS) with specific temporal lobe related symptoms.1–2 Some studies showed that recurrent seizures affect all aspects of cognitive functioning including attention, language, praxis, executive function intelligence, judgment, insight, and problem solving.3–5 However, the most important cognitive deficit in TLE is memory impairment.6–7 Damage to the mesial structure of the temporal lobe, particularly the amygdale and hippocampus, has the main role in these memory difficulties.8–11 Another factors, including the long-term administration of antiepileptic drugs and seizure-related factors, i.e. age of onset, duration of the epilepsy, type of seizure, and psychosocial effects may also contribute to the cognitive decline over years.12–13
Approximately 30% to 45% of patients with TLE are refractory to antiepileptic pharmacotherapy.14–15 In this condition, surgical removal of the epileptogenic tissue would be considered. Resection of brain tissue would be limited to the epileptogenic zone to prevent disruption of normal brain functions.15–16 A variety of diagnostic and assessment techniques are used to find location of epileptogenic focus and prediction of epilepsy surgery consequences.15,17–18 Long term monitoring (LTM) of patients with prolonged electroencephalography (EEG) and video recording is utilized to find the specific source of discharges. Magnetic resonance imaging (MRI) is used to reveal structural abnormalities that may be related to seizures.15,19–20 Neuropsychological assessments provide current cognitive profile, which help for lateralization and localization of the damages and prediction of post-surgical outcome.2,5,21 For example, if there is significantly lower score of performance intelligence quotient (PIQ) than verbal one, the neuropsychologist may conclude lateralization of the epileptogenic zone in non-dominant brain hemisphere.7,15,21
Temporal lobe, especially its mesial region, is crucial for processing of memory. Studies following lesions of this region have provided material-specific lateralization of information, which the dominant mesial temporal region is specific for verbal and non-dominant for visual memory.15,21–22
Wechsler Memory Scale-Third Edition (WMS-III) is the measure that has been used widely for memory assessment of adults.23 Vast majorities of epilepsy surgery centers use it as a component of presurgical neuropsychological evaluations.21,24 This instrument tries to evaluate verbal and visual domains separately. Several researchers showed that left temporal lobe epilepsy patients had significantly lower scores on the auditory subscale than visual one, while right-sided temporal dysfunction may produce deficits in nonverbal memory who obtain higher scores on verbal than nonverbal indexes.23,25–30 However, there were multiple studies that could not reveal any differences between auditory and visual scores in one-sided mesial temporal epileptogenic focus.8,31–32 The most replicable finding in assessment of memory of TLE patients showed significant lower scores of the WMS-III in comparison with normal group.30,32
This study was designed for patients with refractory TLE for two purposes. The first purpose was to evaluate cognitive state of patients who were candidates for epilepsy surgery. We sought to find if there was a specific cognitive profile in TLE patients. The second aim was to determine ability of WMS-III to differentiate left TLE, right TLE, and normal subjects with Persian language.
Methods
Participants
From May 2007 to February 2009, all of 132 patients with refractory epilepsy who were referred to Ayatollah Kashani Comprehensive Epilepsy Program, Isfahan University of Medical Sciences (Isfahan, Iran), were evaluated to establish TLE based on EEG and MRI findings. Sixty patients with established TLE were recruited. The inclusion criteria were age between 15 and 40, full scale intelligence quotient (FSIQ) more than 70, at least elementary school education and absence of major mental or neurological disorders except for epilepsy. The exclusion criteria were informed consent withdrawal and exacerbation of seizures that led to invalid neuropsychological test performance.
Control subjects who were matched for age and education, were selected from the patients’ accompanying persons. They met inclusion criteria and did not have history of epilepsy. Finally, 29 right TLE, 31 left TLE, and 32 control subjects were recruited in this study.
Measures
A demographic checklist was completed for each patient. This checklist included questions about age, education, seizure duration, handedness and marital status. The WMS-III was used to assess auditory and visual declarative memory and working abilities in adults and adolescents. It includes 11 subtests, 6 of which are considered primary and 5 optional. Primary subtests must be given to obtain index scores and optional subtests can be given to obtain supplementary information. In this study, primary subtests were used including logical memory I and II, face I and II, verbal paired associates I and II, family picture I and II, letter-number sequencing and spatial span. The index scores are obtained by summing these primary subtests.33 One study in Iran revealed internal consistency of 0.65 to 0.85 for WMS-III subtests and 0.76 to 0.83 for WMS-III indexes by Cronbach's alpha coefficient.34 WMS-III was administered by a student of PhD in psychology.
Measures of full scale, verbal, and performance intelligence quotient (IQ) were obtained using Wechsler Adult Intelligence Scale-R (WAIS-R). In one Iranian study, reliability and validity of WAIS-R were studied.35 The WAIS-R subscales showed reliability from 0.69 to 0.87 on test–retest stability and their internal consistency was 0.77 to 0.88 with Split-half coefficient.36 WAIS-R was administered by a student of PhD in psychology.
Statistical Analysis
All data were compared between patients (right TLE and left TLE) and controls. Discrete variables were analyzed by the chi-square test. Analysis of Variance (ANOVA) was employed for continuous variables. The Multivariate Analyses of Variance (MANOVA) was used to analyze the data of WMS-III and WAIS-R between groups. The results were analyzed by SPSS version 14.
Results
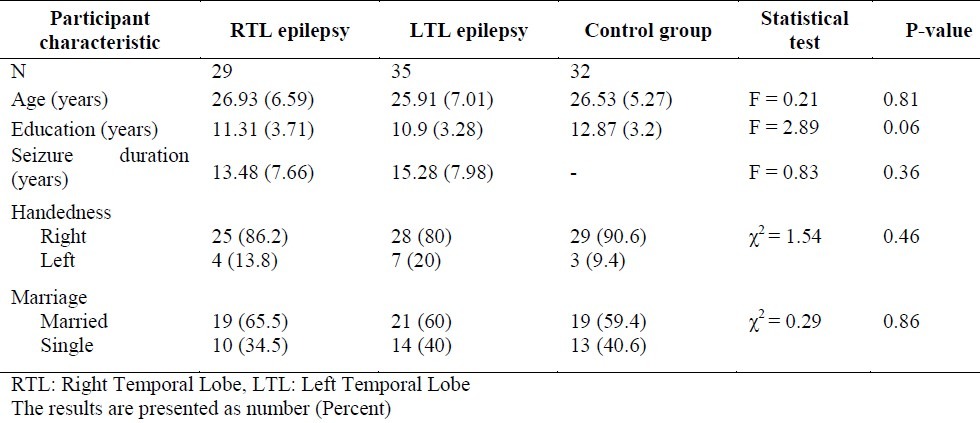
The demographic characteristics of right TLE patients, left TLE patients and the control subjects were summarized in table 1. Homogeneity of variance between the groups for each of the dependent variable was checked. Various measures of memory were analyzed by MANOVA. The results of MANOVA revealed a significant difference between groups [Pillai's Trace F (12,170) = 6.57, p < 0.001].
Table 1.
Demographic characteristics by group
As shown in table 2, results of MANOVA revealed significant differences between the groups for mean scores on PIQ subscale of WAIS-R (p < 0.001), FSIQ subscale of WAIS-R (p < 0.002) and all primary indexes of WMS-III (p < 0.001). There were no significant differences between right and left TLE regarding mean scores of WAIS-R and WMS-III.
Table 2.
Wechsler Adult Intelligence Scale-R and Wechsler Memory Scale-III primary indexes and their subscale scores and comparisons by group
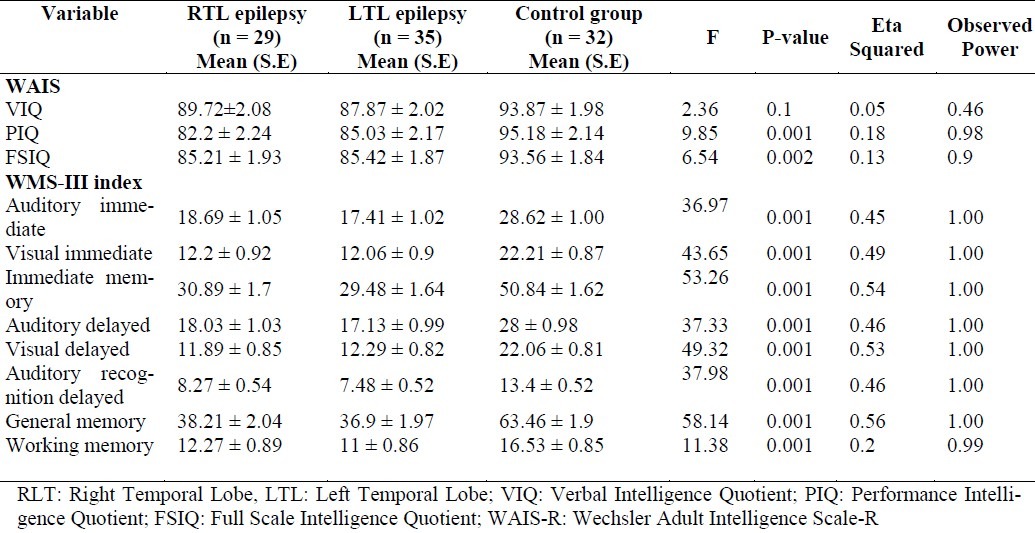
Although the verbal intelligence quotient (VIQ) mean scores were significantly different between the left TLE and the control group (p = 0.037), there were not any significant differences between the right and the control group.
Discussion
Many investigators have reported specific cognitive deficits that differentiate TLE from the other types of epilepsy. Problems in memory have been the most shared cognitive deficit in patients with TLE.37 Early neuropsychological studies indicated that resection of the left temporal lobe may impair the ability to learn verbal materials while right temporal resection can produce a deficit in learning of nonverbal and visuospatial information.24,38 These phenomena were also found in patients with unilateral temporal lobe seizures with less power.39–40 However, there were other studies that failed to show material specific of memory impairment to lateralize right or left TLE.41–42
In our study, patients with TLE as a single group, had significant lower scores in all of the memory indexes and in the most subscales in comparison with control subject. In addition, a comparison of the IQ scores between patients with epilepsy and subjects in control group showed significantly higher scores in FSIQ and PIQ scores. This findings were consistent with those studies that showed cognitive dysfunction in temporal lobe epilepsy.30,32
In patients with the right TLE or the left TLE as two different groups, there were no significant differences between the two groups regarding scores of WAIS-R and WMS-III indexes and subscales. These results were not consistent with findings of Doss et al.23 and Wilde et al.29 that showed material-specific dichotomized deficits in TLE patients who were undergone anterior temporal lobectomy. However, some researchers have reported non-specific memory deficits in right or left TLE. Baker et al.32 and Vannucci31 found that there were no significant disparities between auditory and visual scores of patients with left temporal focal epilepsy group. Bachtler and Dodrill showed that no significant group differences were found for visual immediate or delayed or auditory immediate indexes.43 These results were consistent with our finding.
We can suggest three possible explanations for these results. First, most of the findings that revealed material-specific memory problems were based on patients who were undergone anterior temporal lobectomy. Our results derived from pre-surgical evaluation of the TLE patients. Second, wilde et al. reported that the ability of the WMS-III to predict lateralization was particularly weak for those with left temporal dysfunction.29 Finally, although many of the neuropsychological tests possessed face validity, their genuine capabilities to assess what was prepared for them were in doubt. In WMS-III, the visual memory items invite verbal encoding during inspection, thus contralateral temporal lobe may have alternative or supplementary strategy for encoding of seemingly visual items in non-dominant hemisphere temporal lobe epilepsy. The results of this study showed ability of the WMS-III to find cognitive decline in patients with TLE. However, it has limitation in lateralizing epileptogenic zone.
This study had several limitations. We could not discontinue antiepileptic drugs because of medical ethics. Although the sample size had enough power to reveal difference between patients with TLE and control group, it was not able to differentiate right versus left epileptogenic zone. The WMS-III has multiple subscales but it would have been better if more cognitive assessment tools had been used to raise validity of the findings.
Authors’ Contributions
This study has been derived from Ph.D. thesis of MT. All neuropsychological assessments, data gathering, and analysis were curried out by MT. MB was the main designer of this study and involved in all clinical and neuropsychological evaluations. HTN and HM were Ph.D. supervisors. RKN were advisors of Ph.D. thesis. AM was consultant of WMS-III administration and its psychometric properties. JM and MZ selected patients and localized the site of epileptogenic focus. All authors have read and approved the content of the manuscript.
Acknowledgments
Thanks are due to the staffs of Ayatatollah Kashani Comprehensive Epilepsy Program, Isfahan University of Medical, Isfahan, Iran.
Footnotes
Conflict of Interests Authors have no conflict of interests.
References
- 1.Leijten FS, Alpherts WC, Van Huffelen AC, Vermeulen J, Van Rijen PC. The effects on cognitive performance of tailored resection in surgery for nonlesional mesiotemporal lobe epilepsy. Epilepsia. 2005;46(3):431–9. doi: 10.1111/j.0013-9580.2005.33604.x. [DOI] [PubMed] [Google Scholar]
- 2.Akanuma N, Alarcon G, Lum F, Kissani N, Koutroumanidis M, Adachi N, et al. Lateralising value of neuropsychological protocols for presurgical assessment of temporal lobe epilepsy. Epilepsia. 2003;44(3):408–18. doi: 10.1046/j.1528-1157.2003.24502.x. [DOI] [PubMed] [Google Scholar]
- 3.Hermann BP, Seidenberg M, Dow C, Jones J, Rutecki P, Bhattacharya A, et al. Cognitive prognosis in chronic temporal lobe epilepsy. Ann Neurol. 2006;60(1):80–7. doi: 10.1002/ana.20872. [DOI] [PubMed] [Google Scholar]
- 4.Hermann B, Seidenberg M, Lee EJ, Chan F, Rutecki P. Cognitive phenotypes in temporal lobe epilepsy. J Int Neuropsychol Soc. 2007;13(1):12–20. doi: 10.1017/S135561770707004X. [DOI] [PubMed] [Google Scholar]
- 5.Keary TA, Frazier TW, Busch RM, Kubu CS, Iampietro M. Multivariate neuropsychological prediction of seizure lateralization in temporal epilepsy surgical cases. Epilepsia. 2007;48(8):1438–46. doi: 10.1111/j.1528-1167.2007.01098.x. [DOI] [PubMed] [Google Scholar]
- 6.Lee TM, Yip JT, Jones-Gotman M. Memory deficits after resection from left or right anterior temporal lobe in humans: a meta-analytic review. Epilepsia. 2002;43(3):283–91. doi: 10.1046/j.1528-1157.2002.09901.x. [DOI] [PubMed] [Google Scholar]
- 7.Hermann B, Seidenberg M. Neuropsychology and temporal lobe epilepsy. CNS Spectr. 2002;7(5):343–8. doi: 10.1017/s1092852900017806. [DOI] [PubMed] [Google Scholar]
- 8.Dobbins IG, Kroll NE, Tulving E, Knight RT, Gazzaniga MS. Unilateral medial temporal lobe memory impairment: type deficit, function deficit, or both? Neuropsychologia. 1998;36(2):115–27. doi: 10.1016/s0028-3932(97)00094-8. [DOI] [PubMed] [Google Scholar]
- 9.Gleissner U, Helmstaedter C, Elger CE. Memory reorganization in adult brain: observations in three patients with temporal lobe epilepsy. Epilepsy Res. 2002;48(3):229–34. doi: 10.1016/s0920-1211(01)00333-3. [DOI] [PubMed] [Google Scholar]
- 10.Bell BD, Giovagnoli AR. Recent innovative studies of memory in temporal lobe epilepsy. Neuropsychol Rev. 2007;17(4):455–76. doi: 10.1007/s11065-007-9049-3. [DOI] [PubMed] [Google Scholar]
- 11.Kennepohl S, Sziklas V, Garver KE, Wagner DD, Jones-Gotman M. Memory and the medial temporal lobe: hemispheric specialization reconsidered. Neuroimage. 2007;36(3):969–78. doi: 10.1016/j.neuroimage.2007.03.049. [DOI] [PubMed] [Google Scholar]
- 12.Moore PM, Baker GA. The neuropsychological and emotional consequences of living with intractable temporal lobe epilepsy: implications for clinical management. Seizure. 2002;11(4):224–30. doi: 10.1053/seiz.2001.0668. [DOI] [PubMed] [Google Scholar]
- 13.Sayin U, Sutula TP, Stafstrom CE. Seizures in the developing brain cause adverse long-term effects on spatial learning and anxiety. Epilepsia. 2004;45(12):1539–48. doi: 10.1111/j.0013-9580.2004.54903.x. [DOI] [PubMed] [Google Scholar]
- 14.Mendez MF. Neropsychiatric aspects of epilepsy. In: Sadock BJ, Sadock VA, Ruiz P, editors. Kaplan and Sadock's Comprehensive Textbook of Psychiatry. 9th ed. New York: Lippincott Williams & Wilkins; 2009. pp. 377–8. [Google Scholar]
- 15.Martin AC, Bortz JJ, Snyder P. Epilepsy and nonepileptic seizure disorders. In: Snyder PJ, ussbaum PD, editors. Clinical Neuropsychology: A Pocket Handbook for Assessment. 2nd ed. Washington, DC: American Psychological Association; 2006. pp. 320–4. [Google Scholar]
- 16.Meneses RF, Pais-Ribeiro JL, da Silva AM, Giovagnoli AR. Neuropsychological predictors of quality of life in focal epilepsy. Seizure. 2009;18(5):313–9. doi: 10.1016/j.seizure.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Dodrill CB. Neuropsychological effects of seizures. Epilepsy Behav. 2004;5(Suppl 1):S21–S24. doi: 10.1016/j.yebeh.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 19.Hwang DY, Golby AJ. The brain basis for episodic memory: insights from functional MRI, intracranial EEG, and patients with epilepsy. Epilepsy Behav. 2006;8(1):115–26. doi: 10.1016/j.yebeh.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C. Hippocampal system and declarative (relational) memory: summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9(1):83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 21.Jones-Gotman M, Smith ML, Risse GL, Westerveld M, Swanson SJ, Giovagnoli AR, et al. The contribution of neuropsychology to diagnostic assessment in epilepsy. Epilepsy Behav. 2010;18(1-2):3–12. doi: 10.1016/j.yebeh.2010.02.019. [DOI] [PubMed] [Google Scholar]
- 22.Raspall T, Donate M, Boget T, Carreno M, Donaire A, Agudo R, et al. Neuropsychological tests with lateralizing value in patients with temporal lobe epilepsy: reconsidering material-specific theory. Seizure. 2005;14(8):569–76. doi: 10.1016/j.seizure.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Doss RC, Chelune GJ, Naugle RI. WMS-III performance in epilepsy patients following temporal lobectomy. J Int Neuropsychol Soc. 2004;10(2):173–9. doi: 10.1017/S1355617704102026. [DOI] [PubMed] [Google Scholar]
- 24.Jones-Gotman M, Harnadek MC, Kubu CS. Neuropsychological assessment for temporal lobe epilepsy surgery. Can J Neurol Sci. 2000;27(Suppl 1):S39–S43. doi: 10.1017/s0317167100000639. [DOI] [PubMed] [Google Scholar]
- 25.Hermann BP, Seidenberg M, Haltiner A, Wyler AR. Adequacy of language function and verbal memory performance in unilateral temporal lobe epilepsy. Cortex. 1992;28(3):423–33. doi: 10.1016/s0010-9452(13)80152-9. [DOI] [PubMed] [Google Scholar]
- 26.Hawkins KA. Indicators of brain dysfunction derived from graphic representation of the WAIS-III/WMS-III technical manual clinical samples data: A preliminary approach to clinical utility. The Clinical Neuropsychologist. 1998;12:535–55. [Google Scholar]
- 27.Martin R, Sawrie S, Gilliam F, Mackey M, Faught E, Knowlton R, et al. Determining reliable cognitive change after epilepsy surgery: development of reliable change indices and standardized regression-based change norms for the WMS-III and WAIS-III. Epilepsia. 2002;43(12):1551–8. doi: 10.1046/j.1528-1157.2002.23602.x. [DOI] [PubMed] [Google Scholar]
- 28.Doss RC, Risse GL, Gate JR. Predicting mesial temporal sclerosis in epilepsy patients using the WMS-III and traditional measures of learning and memory. Epilepsia. 2000;41(suppl 7):158–67. [Google Scholar]
- 29.Wilde N, Strauss E, Chelune GJ, Loring DW, Martin RC, Hermann BP, et al. WMS-III performance in patients with temporal lobe epilepsy: group differences and individual classification. J Int Neuropsychol Soc. 2001;7(7):881–91. [PubMed] [Google Scholar]
- 30.Bell BD, Fine J, Dow C, Seidenberg M, Hermann BP. Temporal lobe epilepsy and the selective reminding test: the conventional 30-minute delay suffices. Psychol Assess. 2005;17(1):103–9. doi: 10.1037/1040-3590.17.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vannucci M. Visual memory deficits in temporal lobe epilepsy: toward a multifactorial approach. Clin EEG Neurosci. 2007;38(1):18–24. doi: 10.1177/155005940703800107. [DOI] [PubMed] [Google Scholar]
- 32.Baker GA, Austin NA, Downes JJ. Validation of the Wechsler Memory Scale-III in a population of people with intractable temporal lobe epilepsy. Epilepsy Res. 2003;53(3):201–6. doi: 10.1016/s0920-1211(03)00023-8. [DOI] [PubMed] [Google Scholar]
- 33.Strauss E, Sherman EMS, Spreen O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. 3rd ed. New York: Oxford University Pre; 2006. [Google Scholar]
- 34.Saed A. Standardization of Wechsler Memory Scale III (WMS-III) Tehran: Department of Education and Psychology, Shahed University; 2006. [Google Scholar]
- 35.Groth-Marnat G, Gallagher RE, Hale JB, Kaplan E. Neuropsychological Assessment in Clinical Practice: A Guide to Test Interpretation and Integration. New York: John Wiley & Sons; 2000. [Google Scholar]
- 36.Abedi MR. Standardization of Wechsler Adult Intelligence Scale-R (WAIS-R) Tehran: Tehran Psychiatric Institute, Iran University of Medical Sciences; 1994. [Google Scholar]
- 37.Jones-Gotman M, Zatorre RJ, Olivier A, Andermann F, Cendes F, Staunton H, et al. Learning and retention of words and designs following excision from medial or lateral temporal-lobe structures. Neuropsychologia. 1997;35(7):963–73. doi: 10.1016/s0028-3932(97)00024-9. [DOI] [PubMed] [Google Scholar]
- 38.Kimura D. Right temporal-lobe damage. Perception of unfamiliar stimuli after damage. Arch Neurol. 1963;8:264–71. doi: 10.1001/archneur.1963.00460030048004. [DOI] [PubMed] [Google Scholar]
- 39.Lee GP, Loring DW, Thompson JL. Construct validity of material-specific memory measures following unilateral temporal lobectomy. Psychol Assess. 1989;1(3):192–7. [Google Scholar]
- 40.Milner B. Psychological aspects of focal epilepsy and its neurosurgical management. Adv Neurol. 1975;8:299–321. [PubMed] [Google Scholar]
- 41.Barr WB. Examining the right temporal lobe's role in nonverbal memory. Brain Cogn. 1997;35(1):26–41. doi: 10.1006/brcg.1997.0925. [DOI] [PubMed] [Google Scholar]
- 42.Naugle RI, Chelune GJ, Cheek R, Luders H, Awad IA. Detection of changes in material-specific memory following temporal lobectomy using the Wechsler Memory Scale-Revised. Arch Clin Neuropsychol. 1993;8(5):381–95. [PubMed] [Google Scholar]
- 43.Bachtler S, Dodrill CB. Wechsler Memory Scale III (WMS-III) auditory and visual memory scores and lesion laterality. Epilepsia. 2001;42(Suppl 7):234–42. [Google Scholar]


