Abstract
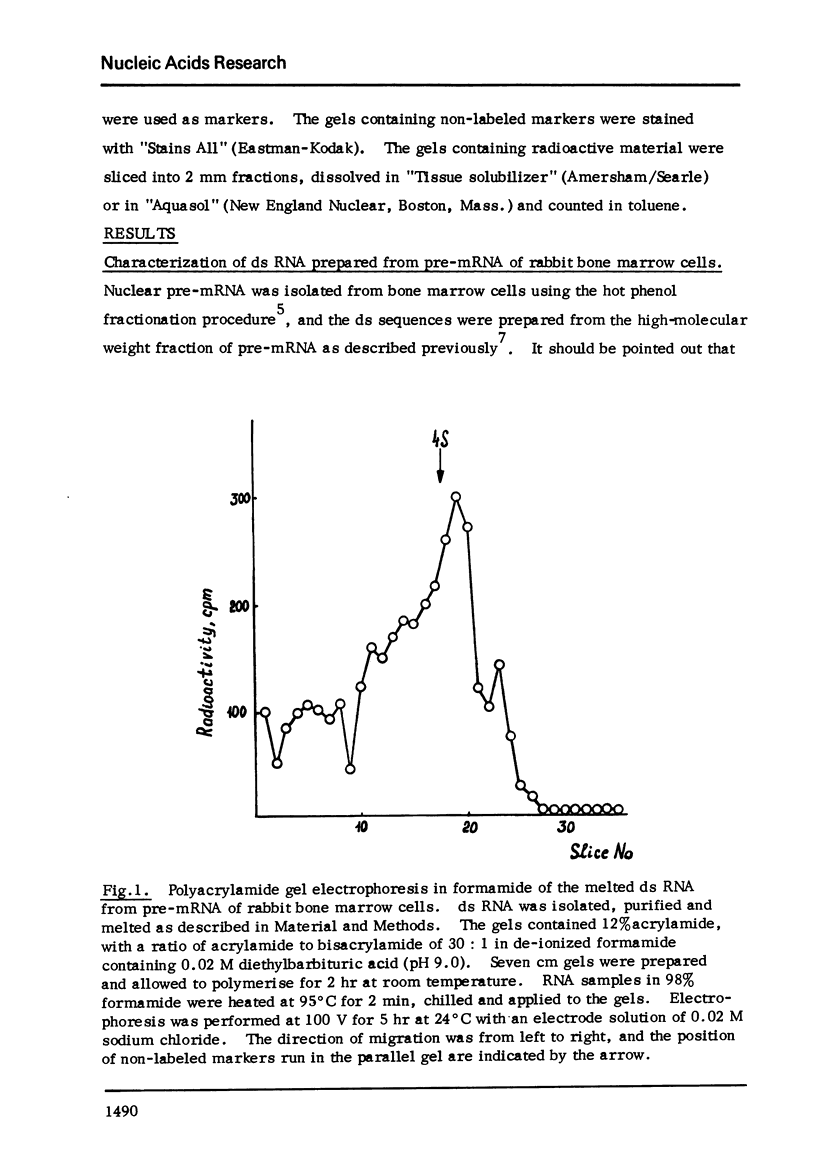
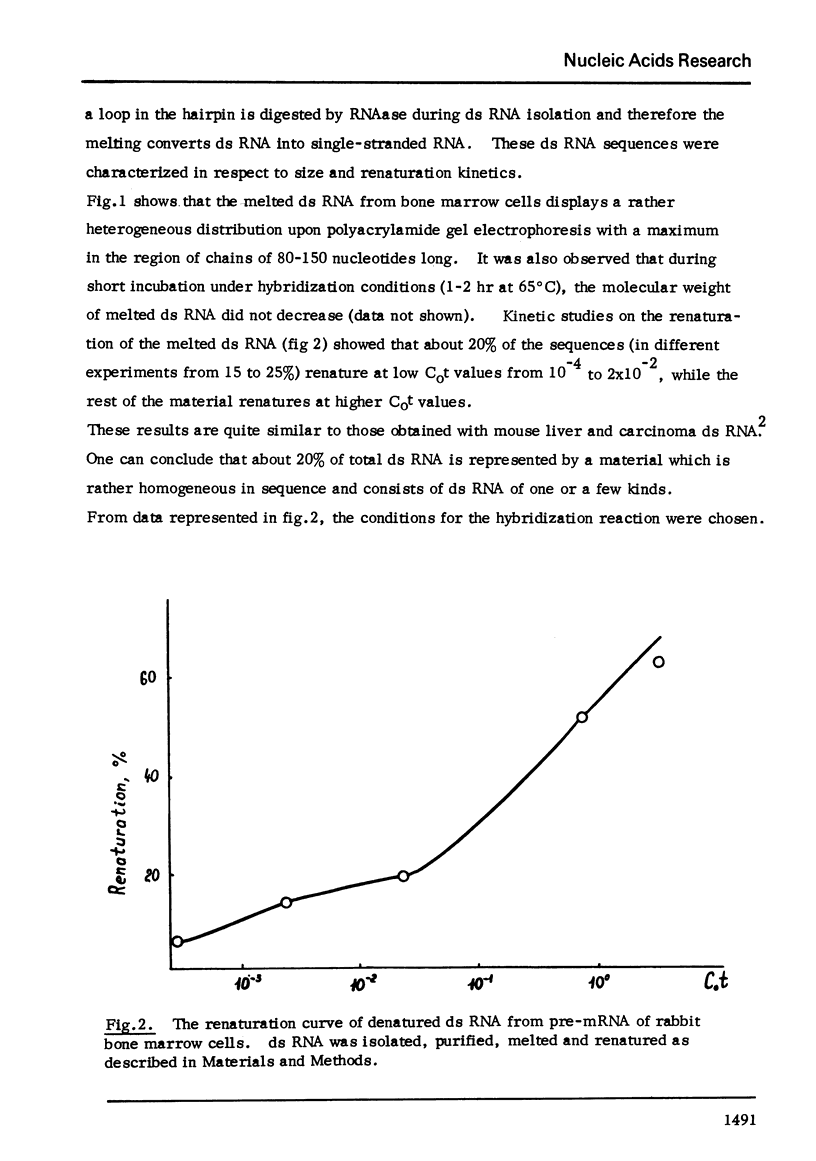
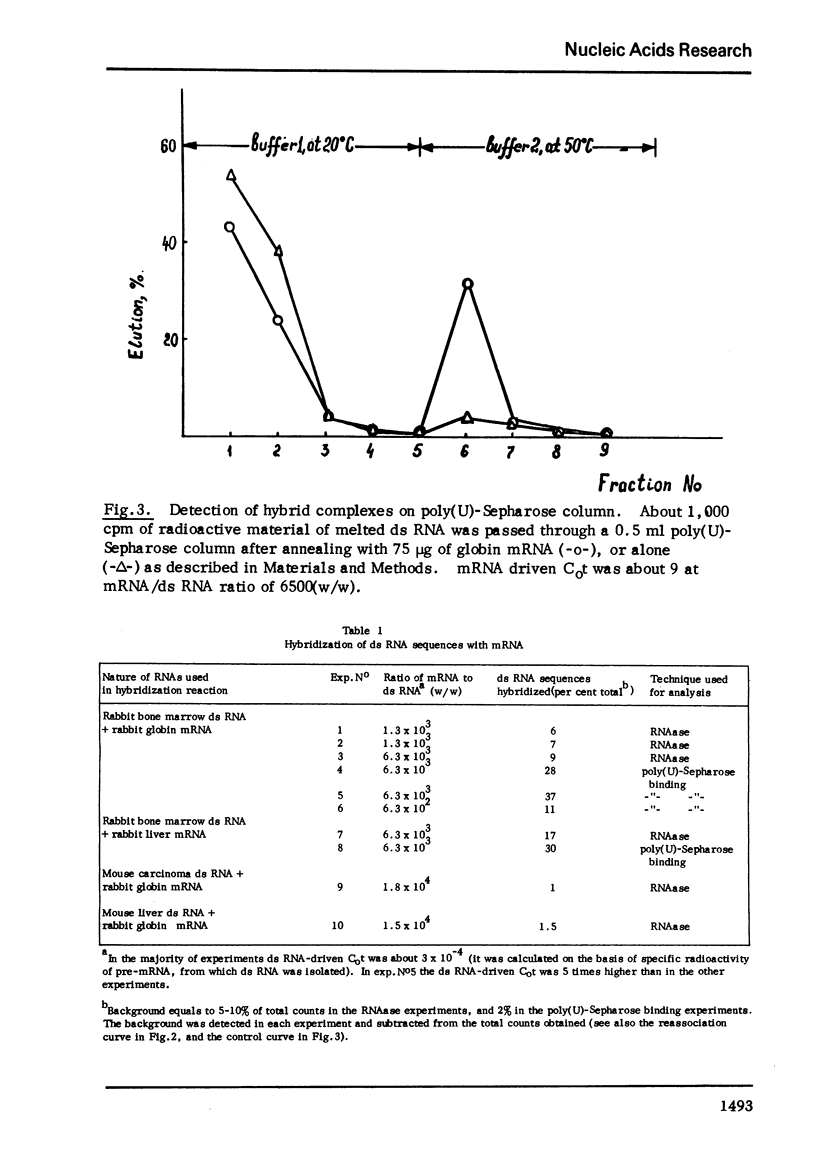
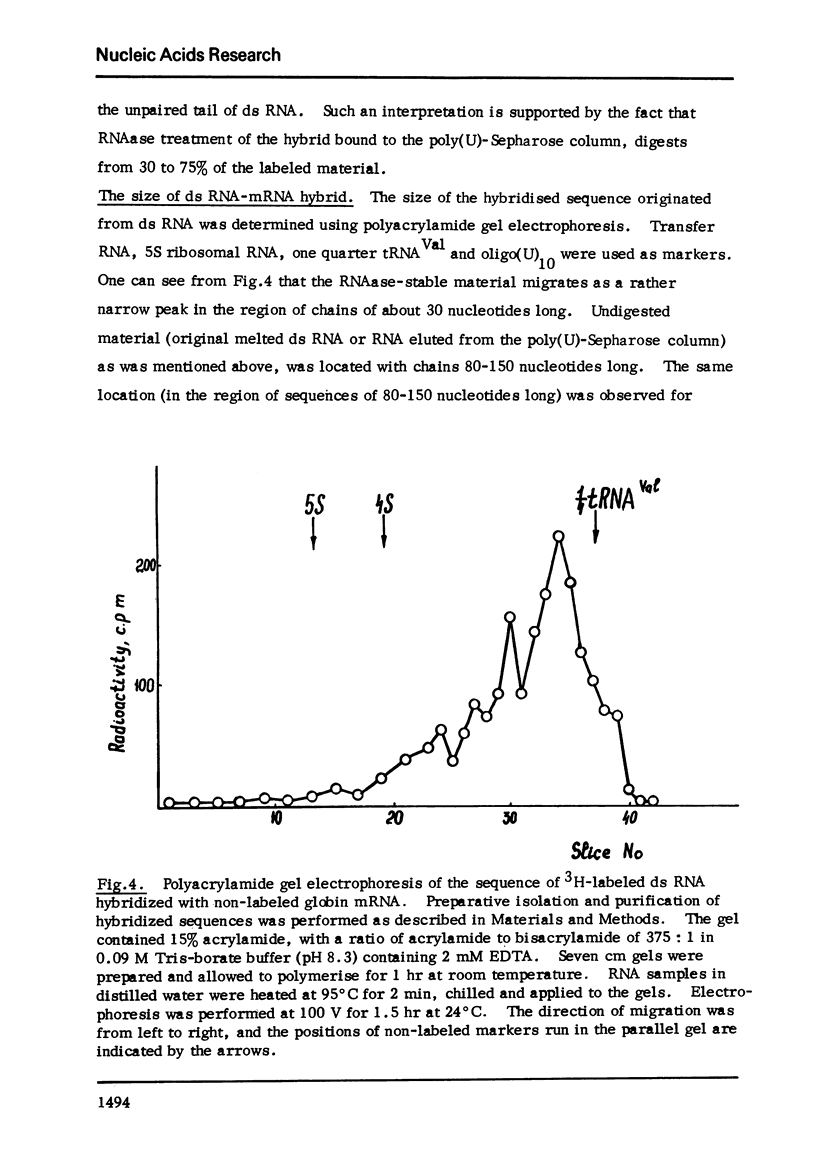
Melted ds RNA isolated from rabbit bone marrow pre-mRNA was hybridized with excess of globin mRNA which was prepared from rabbit reticulocytes. 7-9% of ds sequences became RNAase-stable and about 30% of the sequences could be bound to poly(U)-Sepharose through poly (A) of mRNA. The size of RNAase-stable hybrid is about 30 nucleotides, that is one fourth of the length of one strand of the ds RNA.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brykina E. V., Podobed O. V., Chernovskaya T. V., Lerman M. I. Lack of correlation between the lifetimes of mRNA and the initial lengths of their poly A segments. Mol Biol Rep. 1974 Sep;1(7):417–422. doi: 10.1007/BF00385675. [DOI] [PubMed] [Google Scholar]
- Campo M. S., Bishop J. O. Two classes of messenger RNA in cultured rat cells: repetitive sequence transcripts and unique sequence transcripts. J Mol Biol. 1974 Dec 25;90(4):649–663. doi: 10.1016/0022-2836(74)90530-0. [DOI] [PubMed] [Google Scholar]
- Crippa M., Meza I., Dina D. Sequence arrangement in mRNA: presence of poly(A) and identification of a repetitive fragment at the 5' end. Cold Spring Harb Symp Quant Biol. 1974;38:933–942. doi: 10.1101/sqb.1974.038.01.095. [DOI] [PubMed] [Google Scholar]
- Dunn J. J., Studier F. W. T7 early RNAs and Escherichia coli ribosomal RNAs are cut from large precursor RNAs in vivo by ribonuclease 3. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3296–3300. doi: 10.1073/pnas.70.12.3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. J., Lingrel J. B. Hemoglobin messenger ribonucleic acid. Synthesis of 9S and ribosomal ribonucleic acid during erythroid cell development. Biochemistry. 1969 Jul;8(7):3000–3005. doi: 10.1021/bi00835a048. [DOI] [PubMed] [Google Scholar]
- Georgiev G. P., Ryskov A. P., Coutelle C., Mantieva V. L., Avakyan E. R. On the structure of transcriptional unit in mammalian cells. Biochim Biophys Acta. 1972 Jan 31;259(2):259–283. doi: 10.1016/0005-2787(72)90066-4. [DOI] [PubMed] [Google Scholar]
- Georgiev G. P., Varshavsky A. J., Ryskov A. P., Church R. B. On the structural organization of the transcriptional unit in animal chromosomes. Cold Spring Harb Symp Quant Biol. 1974;38:869–884. doi: 10.1101/sqb.1974.038.01.089. [DOI] [PubMed] [Google Scholar]
- Hercules K., Schweiger M., Sauerbier W. Cleavage by RNase 3 converts T3 and T7 early precursor RNA into translatable message. Proc Natl Acad Sci U S A. 1974 Mar;71(3):840–844. doi: 10.1073/pnas.71.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Darnell J. E. Double-stranded regions in heterogeneous nuclear RNA from Hela cells. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2537–2541. doi: 10.1073/pnas.69.9.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladhoff A. M., Thiele B. J., Coutelle C. Precursor mRNA from erythroid-enriched bone-marrow cells of the rabbit. Electron microscope investigation of precursor mRNA molecules, molecular weight about 1.7 X 10(7), containing mRNA-like structures at one end. Eur J Biochem. 1975 Oct 15;58(2):431–438. doi: 10.1111/j.1432-1033.1975.tb02390.x. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naora H., Whitelam J. M. Presence of sequences hybridisable to dsRNA in cytoplasmic mRNA molecules. Nature. 1975 Aug 28;256(5520):756–759. doi: 10.1038/256756a0. [DOI] [PubMed] [Google Scholar]
- Nikolaev N., Silengo L., Schlessinger D. Synthesis of a large precursor to ribosomal RNA in a mutant of Escherichia coli. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3361–3365. doi: 10.1073/pnas.70.12.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinder J. C., Staynov D. Z., Gratzer W. B. Electrophoresis of RNA in formamide. Biochemistry. 1974 Dec 17;13(26):5373–5378. doi: 10.1021/bi00723a019. [DOI] [PubMed] [Google Scholar]
- Ryskov A. P., Kramerov D. A., Limborskaia S. A., Georgiev G. P. Struktura iadernoi pro-mRNK. VII. Gibridizatsionnye i renaturatsionye svoistva dvuspiral'nykh uchastkov pro-mRNK. Mol Biol (Mosk) 1975 Jan-Feb;9(1):6–18. [PubMed] [Google Scholar]
- Ryskov A. P., Saunders G. F., Farashyan V. R., Georgiev G. P. Double-helical regions in nuclear precursor of mRNA (pre-mRNA). Biochim Biophys Acta. 1973 Jun 8;312(1):152–164. doi: 10.1016/0005-2787(73)90060-9. [DOI] [PubMed] [Google Scholar]


