Abstract
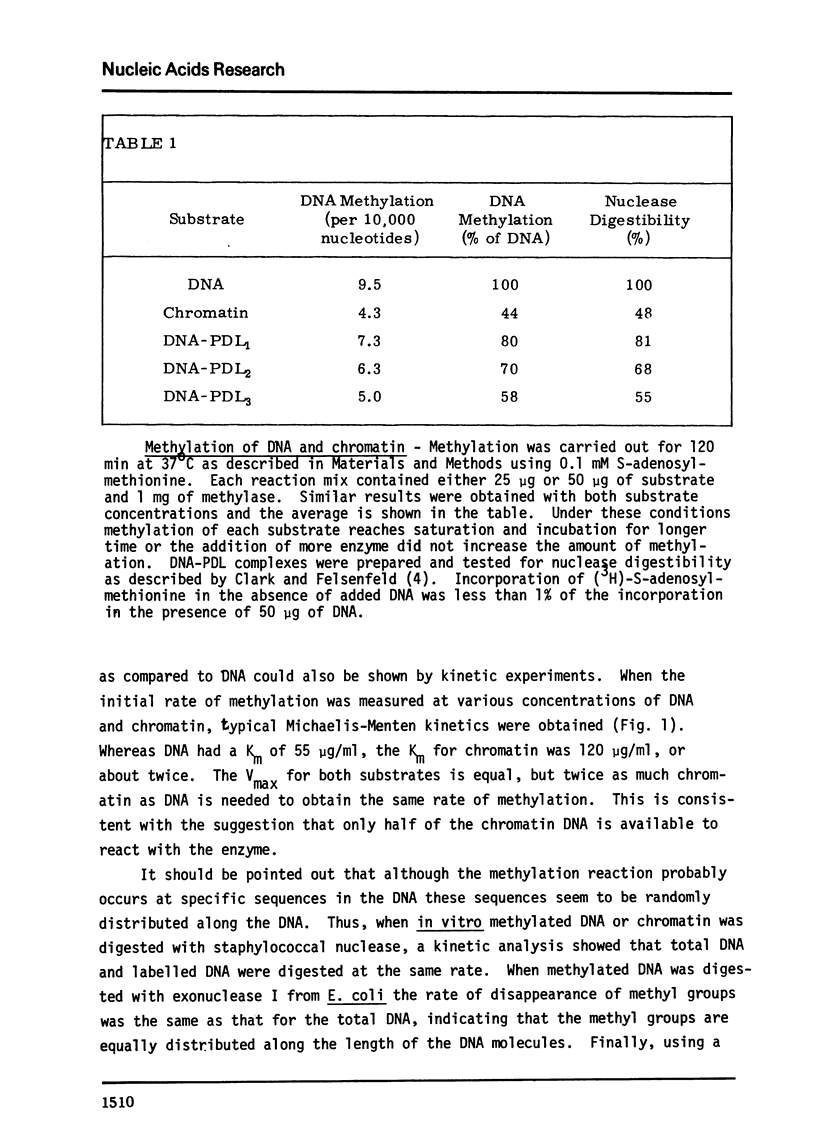
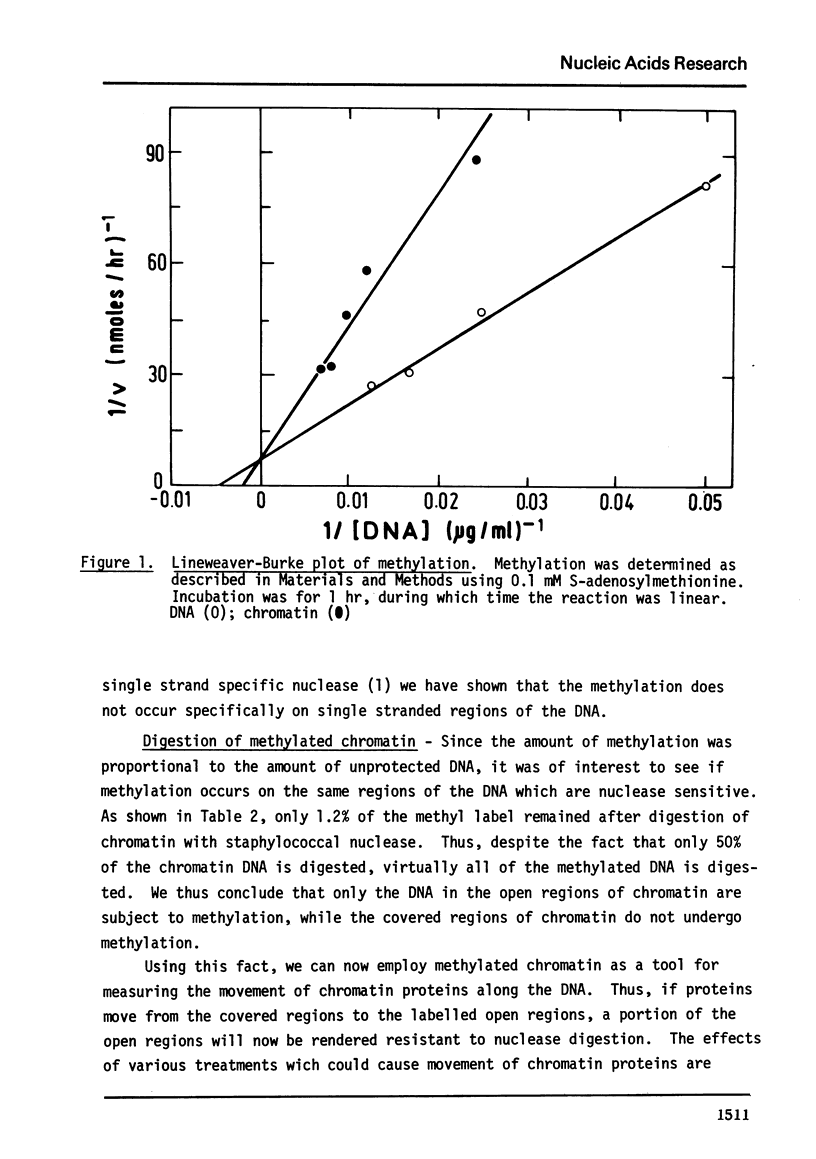
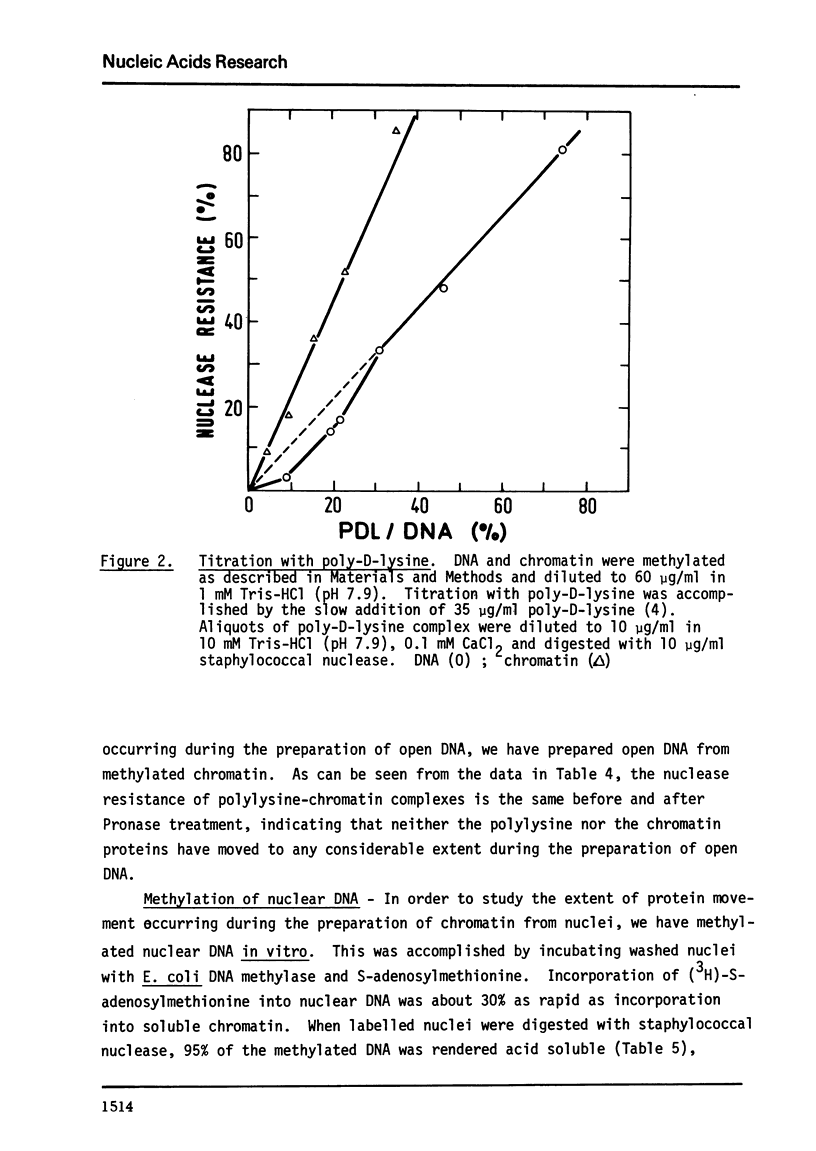
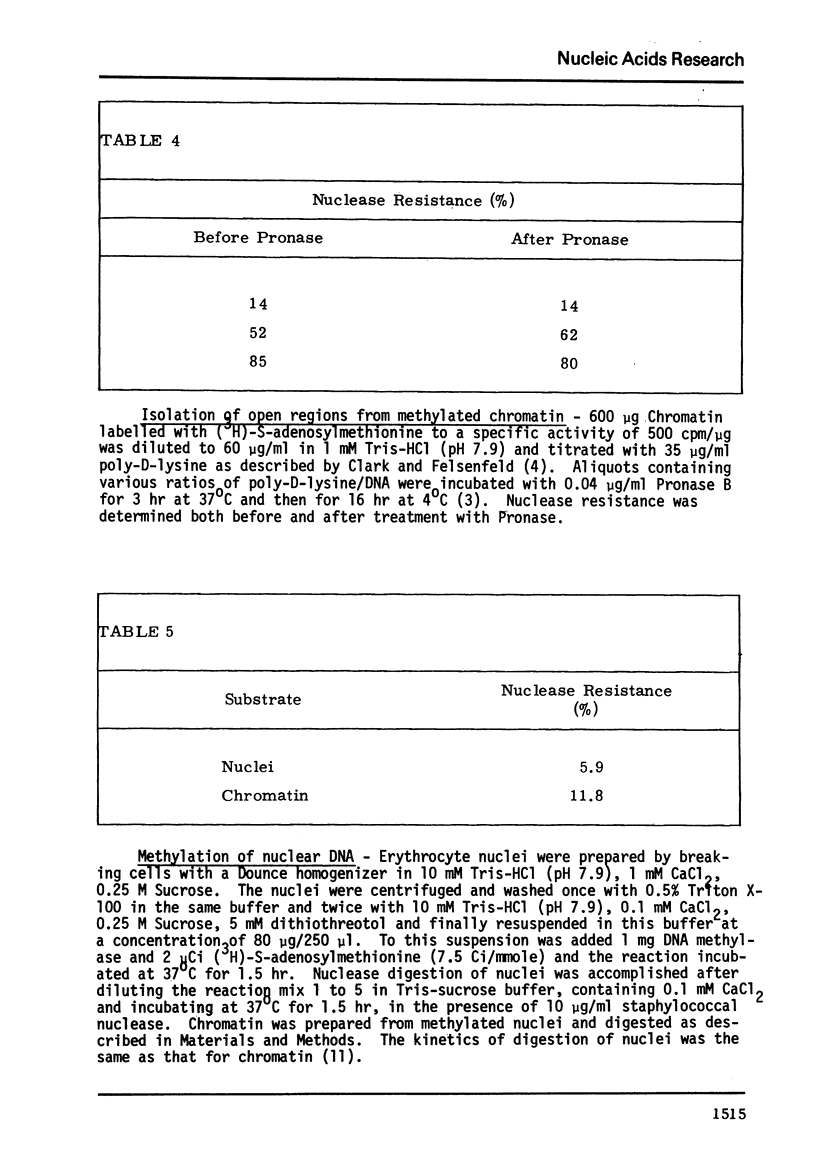
E. coli DNA methylase has been used to methylate chromatin DNA in vitro. At saturation only 50% of the chromatin DNA becomes methylated. The methylated regions of chromatin correspond to that fraction of the chromatin which is sensitive to staphylococcal nuclease. Using in vitro methylated chromatin followed by nuclease digestion movement of chromatin proteins along the DNA can be detected. By this criterion, sonication of chromatin or precipitation with MnCl2 causes 10% of the previously uncovered methylated regions to become covered by protein. Reconstitution of methylated chromatin results in the randomization of the chromatin proteins. Using nuclei which were methylated in vitro we have demonstrated that a small degree of protein sliding does occur during the preparation of chromatin from nuclei. Finally, we have prepared open region DNA by polylysine titration. This procedure does not cause displacement of chromatin proteins.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axel R., Cedar H., Felsenfeld G. Synthesis of globin ribonucleic acid from duck-reticulocyte chromatin in vitro. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2029–2032. doi: 10.1073/pnas.70.7.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axel R., Cedar H., Felsenfield G. The structure of the globin genes in chromatin. Biochemistry. 1975 Jun 3;14(11):2489–2495. doi: 10.1021/bi00682a031. [DOI] [PubMed] [Google Scholar]
- Axel R. Cleavage of DNA in nuclei and chromatin with staphylococcal nuclease. Biochemistry. 1975 Jul;14(13):2921–2925. doi: 10.1021/bi00684a020. [DOI] [PubMed] [Google Scholar]
- Axel R., Melchior W., Jr, Sollner-Webb B., Felsenfeld G. Specific sites of interaction between histones and DNA in chromatin. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4101–4105. doi: 10.1073/pnas.71.10.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T., Maryanka D., Hamlyn P. H., Gould H. J. Nonhistone proteins control gene expression in reconstituted chromatin. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5057–5061. doi: 10.1073/pnas.71.12.5057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. J., Felsenfeld G. Chemical probes of chromatin structure. Biochemistry. 1974 Aug 13;13(17):3622–3628. doi: 10.1021/bi00714a034. [DOI] [PubMed] [Google Scholar]
- Clark R. J., Felsenfeld G. Structure of chromatin. Nat New Biol. 1971 Jan 27;229(4):101–106. doi: 10.1038/newbio229101a0. [DOI] [PubMed] [Google Scholar]
- Gilmour R. S., Paul J. Tissue-specific transcription of the globin gene in isolated chromatin. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3440–3442. doi: 10.1073/pnas.70.12.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. Interphase chromosomal deoxyribonucleoprotein isolated as a discrete structure from cultured cells. J Mol Biol. 1974 Jul 5;86(3):649–663. doi: 10.1016/0022-2836(74)90187-9. [DOI] [PubMed] [Google Scholar]
- Marinus M. G., Morris N. R. Isolation of deoxyribonucleic acid methylase mutants of Escherichia coli K-12. J Bacteriol. 1973 Jun;114(3):1143–1150. doi: 10.1128/jb.114.3.1143-1150.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll M., Thomas J. O., Kornberg R. D. Preparation of native chromatin and damage caused by shearing. Science. 1975 Mar 28;187(4182):1203–1206. doi: 10.1126/science.187.4182.1203. [DOI] [PubMed] [Google Scholar]
- Paul J., Gilmour R. S., Affara N., Birnie G., Harrison P., Hell A., Humphries S., Windass J., Young B. The globin gene: structure and expression. Cold Spring Harb Symp Quant Biol. 1974;38:885–890. doi: 10.1101/sqb.1974.038.01.090. [DOI] [PubMed] [Google Scholar]
- Razin A., Sedat J. W., Sinsheimer R. L. Structure of the DNA of bacteriophage phiX174. VII. Methylation. J Mol Biol. 1970 Oct 28;53(2):251–259. doi: 10.1016/0022-2836(70)90298-6. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. J., Georgiev G. P. Clustered arrangement of histones F2al and F3 along DNA in chromosomal deoxyribonucleoproteins. Biochim Biophys Acta. 1972 Nov 9;281(4):669–674. doi: 10.1016/0005-2787(72)90166-9. [DOI] [PubMed] [Google Scholar]
- Zimmerman S. B., Levin C. J. DNA ligase activity in chromatin and its analogs. Rejoining of DNA strands in polylysine-DNA complexes and in reconstituted chromatins. Biochemistry. 1975 Apr 22;14(8):1671–1677. doi: 10.1021/bi00679a019. [DOI] [PubMed] [Google Scholar]


