Abstract
Background
The estrogen receptor (ER), progesterone receptor (PR), and HER2 profile of a primary breast carcinoma plays a significant role in patient management and treatment. Because of the increasing utilization of neoadjuvant chemotherapy or hormone therapy, surgically-resected carcinomas often show marked treatment effect. The aim of this study was to compare immunohistochemical (IHC) profiles (ER, PR, HER2, HER2 FISH) of primary breast carcinomas before and after neoadjuvant chemotherapy to assess the subsequent effects on hormone receptor status.
Design
Primary breast carcinomas from 38 female patients treated with neoadjuvant therapy after needle core biopsy or fine needle aspiration diagnosis were included. Histologic data was collected for each case, including site, type, grade, tumor size (cm), pre- and post- neoadjuvant treatment IHC panel (ER, PR, HER2), and fluorescence in-situ hybridization (FISH) for HER2.
Results
Of the 38 carcinomas studied, 45 % were positive for ER by IHC both pre- and post- neoadjuvant treatment (P=1.00). IHC studies for PR in these 38 patients showed 37% positivity for PR pre-neoadjuvant therapy and 21% positivity post-treatment (p=0.03). For 37 patients with HER2 IHC, 32% were positive pre-treatment, and 22% were positive post-treatment (P = 0.20). For 7 patients, HER2 FISH was positive in 71% pre-therapy and in 57% post-treatment (P=0.32).
Conclusions
Profiles for ER, HER2 IHC, and HER2 FISH were not significantly different in primary breast carcinomas before and after neoadjuvant chemotherapy. Further investigation is warranted to assess reproducibility of technique and investigate clinical implications of significant loss of PR status in treated patients.
Keywords: ER, PR, HER2, breast, neoadjuvant, immunohistochemistry
Introduction
The estrogen receptor (ER), progesterone receptor (PR), and proto-oncogene HER2/neu (HER2) profile of a female primary breast carcinoma plays a significant role as a predictive marker in patient management. In addition to factors such as age, tumor size, lymph node involvement, histologic type, and tumor grade, the hormone receptor and HER2 status at the time of initial diagnosis has been established as a clinically useful, standard-of-care parameter in determining treatment options and subsequent patient response. Current therapeutic strategies for management of primary breast carcinomas rely on the accurate immunohistochemical (IHC) determination of hormone receptor status in order to determine the clinical utility of hormone-directed therapies such as selective estrogen receptor modulators (SERMs). For example, multiple major clinical trials conducted by the National Surgical Adjuvant Breast and Bowel Project have shown that addition of Tamoxifen, a SERM, to conventional chemotherapeutic and surgical treatment protocols consistently improves disease free survival in women with hormone receptor-positive tumors [1].
In addition to hormone receptors, HER2 has emerged in recent years as an important independent predictive marker in primary breast carcinoma. Approximately 15-20% of breast cancers have amplification of the HER2 gene or overexpression of its protein product. In a hallmark study carried out by Slamon et al in 1987, HER2 amplification was verified as a significant independent negative predictor of overall survival and time to relapse [2]. Since then, HER2 status, as determined by either fluorescence in-situ hybridization (FISH) or IHC, has become important for prognostic implication and to assess potential response of patients to treatment with the monoclonal antibody trastuzumab (Herceptin). Several large, randomized clinical trials sponsored by the National Cancer Institute in 2005 (NSABP-B-31 and NCCTG-N9831) have demonstrated that patients with early-stage HER2-positive breast cancer treated with Herceptin and chemotherapy had a significantly decreased risk of recurrence compared with patients who received chemotherapy alone [3].
Preoperative (neoadjuvant) chemotherapy with agents such as doxorubicin and cyclophosphamide had historically been offered to patients with locally advanced disease with a goal of reducing tumor size to enable surgical resection. In recent years, neoadjuvant chemotherapy has additionally become an option for patients with operable tumors who desire breast conservation therapy. Several randomized clinical trials have demonstrated no statistically significant difference in disease-free survival or overall survival in the patients receiving preoperative therapy as opposed to those receiving postoperative chemotherapy [4].
Few studies to date have examined the effect of neoadjuvant chemotherapy on the hormone receptor status of primary breast carcinomas with somewhat conflicting results. Arens et al in 2005 found no significant differences in expression patterns of ER, PR, and HER2 from diagnostic core biopsies prior to chemotherapy as compared to final resection specimens after neoadjuvant therapy in thirty patients [5]. In contrast, Adams et al in 2008 in a study of forty patients found an increase in the proportion of tumors with HER2 overexpression following neoadjuvant chemotherapy but no change in hormone receptor status [6]. As neoadjuvant chemotherapy has become standard-of-care in some clinical settings and because of the important clinical utility of IHC markers, any alteration in the IHC profile of breast carcinomas from chemotherapeutic agents could affect the post-surgical utility of hormone-directed or HER2-specific therapy.
The aim of this study was to compare IHC profiles (ER, PR, HER2) and HER2 FISH of primary breast carcinomas before and after neoadjuvant chemotherapy to assess the effects of these treatment modalities on hormone receptor and HER2 status.
Materials and methods
Approval for use of human subjects was obtained by permission of the Emory University Institutional Review Board. Fifty-five female patients with primary breast carcinoma treated with neoadjuvant chemotherapy, who were diagnosed from 2004 to 2008 by either fine needle aspiration or needle core biopsy at Emory University Hospital, were identified through retrospective review of surgical pathology report databases and medical chart review. Patients without both pre- and post- neoadjuvant chemotherapy surgical pathology reports and tumors without complete corresponding pre- and post-neoadjuvant chemotherapy hormone receptor and HER2 expression profiles were excluded. Neoadjuvant chemotherapeutic regimens for treated patients included combination anthracyclines, taxanes, and alkylating agents.
From each surgical pathology report for both pre- and post- treatment specimens, histological data was collected, including tumor size (if surgical resection specimen), histologic grade, site, and type (ductal, lobular). Tumors were graded on a three-point scale according to the extent of tubule formation (T), the amount of nuclear pleomorphism (N), and the degree of mitotic activity (M), with 1 being well-differentiated, and 3 being poorly differentiated. The TNM scores for each tumor were then summed to give a total histologic grade; a combined score of 3 to 5 = well-differentiated or grade I, 6 to 7= moderately differentiated or grade II, and 8 to 9 = poorly differentiated or grade III. All surgical specimens had been evaluated by faculty surgical pathologists at Emory University Hospital. Through review of surgical pathology reports and oncologic records, clinical information including patient age, tumor size, treatment type, recurrence, lymphovascular involvement, and presence or absence of metastases was obtained for each patient.
For included patients, the hormone receptor and HER2 IHC profile as well as HER2 FISH results were available for both the pretreatment core/fine needle biopses and post- treatment segmental or total mastectomy specimens. All IHC and HER2 FISH for pre- and post- treatment specimens were performed on formalin-fixed, paraffin-embedded tissue sections. IHC was performed, following optimized epitope retrieval, with a polymer-based detection system (Envision-plus, DAKO; Carpinteria, CA) using mouse monoclonal antibodies: ER (1D5) (1:50), and PR (PgR 636) (1:400) (DAKO). Polyclonal HER2 antibody in the Herceptin kit (HercepTest, DAKO) was used according to the manufacturer’s instructions.
For performance of the IHC staining for ER and PR, antigen retrieval was performed as follows: 5 micron sections are deparaffinized and rehydrated to deionized water. They are heated in citrate buffer (pH 6.0) using an electric pressure cooker for 3 minutes at 12-15 pounds per square inch (PSI) at approximately 120 degrees Celsius. They are then cooled for 10 minutes prior to immunostaining. All slides were loaded onto an automated system (DAKO Autostainer plus, DAKO) and exposed to 3% hydrogen peroxide for 5 minutes, incubated with primary antibody for 30 minutes, with labeled polymer (Envision® + dual link) for 30 minutes, 3, 3’-diaminobenzidine (DAB) as chromogen for 5 minutes, and then with hematoxylin as counterstain for 5 minutes. These incubations are performed at room temperature; between incubations sections are washed with Tris-buffered saline (TBS). Coverslipping was performed using the Tissue-Tek SCA (Sakura Finetek USA, Inc, Torrance, CA) Coverslipper. Positive controls of known positive tissues (endometrium and breast) and negative controls with primary antibody replaced with TBS were run with the patient slides.
Antigen retrieval for HER2 using HercepTest is performed by immersing the slides in 10 mmol/L citrate buffer in a calibrated water bath (required temperature is 95-99 degrees Celsius). The slides are then incubated for 40 minutes at 95-99 degrees Celsius. After decanting the epitope retrieval solution, the sections are rinsed in the wash buffer and later soaked in the buffer for 5-20 minutes prior to staining. The slides are loaded onto the autostainer using the HercepTest® program™ as described in the manufacturers’ insert. In the autostainer, the slides are rinsed, followed by 200 uL Peroxidase-Blocking Reagent for 5 minutes followed by rinsing, placed in 200 uL primary Anti-HER2 Protein (or Negative Control Reagent) for 30 minutes, rinsed twice, and finally immersed in 200 uL substrate-chromogen solution (DAB) for 10 minutes. The slides are then removed from the autostainer, counterstained in hematoxylin, and finally coverslipped.
The stained slides are quantitated visually by light microscopy by a single pathologist. ER and PR are scored using <10% of tumor staining as the negative cutoff. HER2 results are determined based on the maximum staining intensity and distribution as follows: 0= no staining; 1+ = weak and incomplete membranous staining in invasive tumor cells; 2+ = moderate, circumferential membranous staining in at least 10% of invasive tumor cells or intense, complete membrane staining in 30% or less of tumor cells; 3+ = strong, circumferential membranous staining in more than 30% of invasive carcinoma cells. For HER2 IHC, tumors with 0 and 1+ staining are considered negative, cases scored as 2+ are called equivocal, and 3+ positive. HER2 FISH was performed on all HER2 IHC equivocal and positive cases.
For HER2 FISH analysis (PathVysion HER2 DNA probe kit, Abbott Molecular Inc., IL), the slides with breast carcinoma were deparaffinized by immersion in CitriSolv for 10 minutes, followed by dehydration in 100% ethanol at room temperature, and finally air-dried in a slide warmer at 45-50° Celsius. The slides were then pretreated by immersion in 0.2 N HCl for 20 minutes, followed by purified water for 3 minutes, wash buffer for 3 minutes, pretreatment solution at 80° Celsius for 30 minutes, purified water for 1 minute, and wash buffer for 5 minutes. The slides then underwent protease treatment by immersing them in protease solution for 10 minutes at 37 °C, followed by wash buffer for 5 minutes, and finally air-dried on a slide warmer. The slides were then subjected to denaturation by immersing them in denaturing solution at 72 ± 1°C for 5 minutes, followed by 70% ethanol for 1 minute, 85% ethanol for 1 minute, 100% ethanol for 1 minute, and finally air dried on a slide warmer. The slides then underwent hybridization by applying 10 mL of probe mixture to the target area of the slide. Next, a 22 x 22 mm glass cover slip was placed over the probe to allow even spreading, and the edges of the cover slip were sealed with rubber cement. The slides were then placed into a prewarmed humidified hybridization chamber and then incubated at 37 °C overnight for 14-18 hours. After removing the cover slips and rubber cement, the slides were immersed in 2XSSC/0.3% NP-40 (100 ml 20XSCC (pH 5.3) + 847 ml purified water + 3 ml NP-40; pH adjusted to 7.0-7.5 with 1N NaOH) at 72 ± 1°C for 2 minutes. The slides were then air-dried in the dark in an upright position. Then, 10 μl of DAPI counterstain was applied to the target areas of the slide which was coverslipped. For HER2 FISH amplification, the PathVysion DNA probe kit (model 35-161060; Vysis) uses a dual-color probe for determining the number of copies of HER2 (orange) and chromosome 17 centromeres (green). A minimum of 60 nuclei were scored by 2 observers using an Olympus BX 41 fluorescent microscope with a Chroma filter set (DAPI/spectrum orange/spectrum green triple bandpass). Areas scored were limited to regions of invasive disease as compared with a companion hematoxylin and eosin-stained section. The ratio of HER2 signals (orange) to chromosome 17 centromere signals (CEP-17, green) was calculated. The HER2 gene was considered amplified if the signal ratio of HER2/CEP-17 was greater than 2.2, equivocal if the ratio ranged from 1.8 to 2.2, and negative if the ratio was less than 1.8.
Overall agreement as well as score confidence intervals (CI) between the pre- and post- chemotherapy ER, PR, HER2, and HER2 FISH results were calculated. In addition, simple kappa coefficients were calculated to describe agreement. Percent positivity for each receptor both pre- and post- chemotherapy were calculated and compared using McNemar’s test. Significance was defined at p< 0.05.
Results
The final sample size after exclusion of patients was 38. All patients were female, with mean age of 53 years (range: 30– 84 years). Twenty-five of these 38 patients were treated with a neoadjuvant chemotherapeutic regimen of adriamycin and cyclophosphamide (4 cycles) (AC), followed by paclitaxel or docetaxel after initial diagnostic biopsy. Four patients received AC neoadjuvant therapy alone, 3 with neoadjuvant paclitaxel or docetaxel only, two with neoadjuvant cyclophosphamide and docetaxel only, one with neoadjuvant docetaxel and capecytabine only, and one with neoadjuvant paclitaxel and gemcytabine only. Neoadjuvant chemotherapy regimen data was unavailable for two patients. Initial carcinoma diagnoses were obtained by either core biopsies (33 patients) or cytologic specimens (5 patients).
Of the 38 carcinomas studied, 92% (35/38) were of ductal histologic type, and 8% (3/38) were lobular. The mean size of carcinomas was 3.9 cm (range 0.1-12cm). In terms of histologic grade, 58% of studied tumors prior to neoadjuvant chemotherapy were grade III (22/38), 37% were grade II (14/38), 3% were grade I (1/38), and 3% were not assigned a grade (1/38). Fifty percent (19/38) of tumors had metastasized to axillary lymph nodes, with 45% of patients (17/38) free of axillary involvement. One of the 38 patients had lymphovascular invasion in peritumoral tissue identified on biopsy but no definitive axillary involvement, and data was not available for one patient. Local recurrence was seen in 4 patients, with two of these presenting with additional supraclavicular lymph node metastasis. One patient without local recurrence had metastasis to the contralateral neck and brain, and another exhibited isolated ovarian metastasis.
Immunohistochemical profiles of these 38 carcinomas examined in pre- and post- neoadjuvant chemotherapy specimens showed no difference in ER or HER2 expression (Table 1). Seven tumors had HER2 FISH performed subsequent to equivocal or positive HER2 IHC. Five of these seven tumors (71%) were initially positive for HER2 amplification, but only 4 were positive after neoadjuvant chemotherapy (p=0.32).
Table 1.
ER, PR, and HER2 Immunohistochemical Data and HER2 FISH Results in Pre- and Post- Neoadjuvant Chemotherapy Specimens
| Marker (n) | Number of Positive Pre- (%) | Number of Positive Post- (%) | P value |
|---|---|---|---|
| ER (38) | 17 (45) | 17 (45) | 1 |
| PR (38) | 17 (37) | 8 (21) | 0.03 |
| HER2 (37) | 12 (32) | 8 (22) | 0.2 |
| HER2FISH (7) | 5 (71) | 4 (57) | 0.32 |
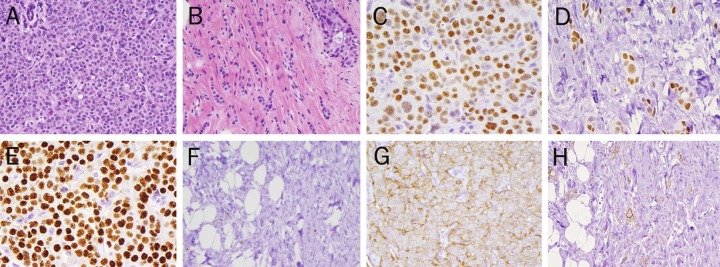
In contrast, a statistically significant loss of PR expression following neoadjuvant chemotherapy was identified (p=0.03). Overall concordance for ER, PR, HER2, and HER2 FISH ranged from 73.0 % to 89.5%. Representative pre- and postcarcinoma morphology and immunohistochemical staining is depicted in Figure 1 (A-H).
Figure 1.
ER, PR, and HER2 IHC Pre- and Post- Neoadjuvant Chemotherapy. A. Pre-treatment core biopsy, infiltrating ductal carcinoma, Grade II, with lobular features (40x, H&E) B. Post-neoadjuvant chemotherapy, residual infiltrating tubulolobular carcinoma, Grade I (40x, H&E) C. Pre-treatment Estrogen Receptor (ER) IHC: Positive (3+, 90%) (40x) D. Post-treatment Estrogen Receptor (ER) IHC: Positive (3+, 90%) (40x) E. Pre-treatment Progesterone Receptor (PR) IHC: Positive (3+, 90%) (40x) F. Post-treatment Progesterone Receptor (PR) IHC: Negative (<10%) (40x) G. Pre- treatment Her2 IHC: Positive (3+, 80%) (40x) H. Post-treatment Her2 IHC: Positive (3+, 50%) (40x).
Discussion
In addition to such parameters as tumor size, lymph node status, histologic type, and grade, the hormone receptor and HER2 status of a primary breast carcinoma carry clinical utility in determining patient treatment options and overall prognosis. Because of the increasing use of neoadjuvant chemotherapy for patients with primary breast carcinoma and subsequent breast conservation, the effect of these agents on receptor status has been questioned. Few studies to date have examined the effect of neoadjuvant chemotherapy on hormone receptor (ER and PR) IHC profiles with somewhat conflicting results. Arens et al. in a study of 25 patients, reported no significant differences in expression patterns of ER or PR following neoadjuvant chemotherapy when compared with matched control patients who had not received neoadjuvant treatment. In both the treated and control groups, there were rare cases in which hormone receptor expression changed in the final surgical specimen when compared with the initial core biopsy; these changes did not meet statistical significance [5]. A similar trend was reported by Adams et al in a study of 26 patients who underwent neoadjuvant chemotherapy prior to surgical management of primary breast carcinoma. Although there was an overall decrease in both ER and PR expression following neoadjuvant therapy, this change in hormone receptor status was not significant [6].
In contrast, statistically significant changes in hormone receptor status of primary breast carcinomas following neoadjuvant chemotherapy have been reported. In a series by Taucher et al of 214 patients treated with chemotherapy prior to surgery, 14% demonstrated a statistically significant loss of expression of ER in the posttreatment final surgical specimen, with 51.7% showing a significant loss of PR expression when compared with matched controls [7]. Additionally, the untreated control group in this study showed a non-significant decrease in ER and/or PR expression. Overall, the onset of menopause induced by preoperative chemotherapy was hypothesized to explain this decrease in ER but not PR expression [7]. Furthermore, Kasami et al. found a statistically significant negative change in PR status in 28.8% of patients (n=173) and no significant change in ER following neoadjuvant chemotherapy, which is consistent with the results of this investigation [8]. From the cases examined, this study shows that the ER status of primary breast carcinomas are stable following neoadjuvant chemotherapy, while PR positivity decreases (p=0.03). This reduction in PR positivity may correspond to a decrease in PR expression, which could be explained by differential tumor sampling between the core biopsy and the final surgical specimen, chemotherapy-selective cytotoxicity of PR-expressing cells, small patient sample size, or inherent variability of PR immunohistochemistry. Furthermore, interobserver variability with regard to stain interpretation could have contributed to observed differences in PR expression following neoadjuvant treatment; however, the vast majority of both pre- and post-therapy immunohistochemical stains were interpreted by a single pathologist at our institution, thus minimizing this risk. The alteration of PR expression without change in ER status observed after neoadjuvant chemotherapy is of undetermined clinical significance, as ER is generally considered a stronger predictor of response to hormone- directed therapy. Overall, determination of both a pre-treatment and post-treatment PR IHC profile is merited due to the possible change in PR expression following neoadjuvant chemotherapy. Further research is needed in order to examine the biological interaction of cytotoxic agents with steroid hormone receptors, the reproducibility of results with matched controls, and the clinical ramifications of loss of PR expression in these patients.
For HER2 IHC and HER2 FISH, no significant difference in expression was found in this study in primary breast carcinomas between pre- and post- neoadjuvant treatment specimens. The stability of HER2 expression is relevant to the use of Herceptin as second-line therapy in that this treatment option in HER2/neu-overexpressing tumors is retained after neoadjuvant chemotherapy. Similar to the body of literature on steroid hormone receptor expression, several studies have shown conflicting data on HER2 expression following neoadjuvant chemotherapy. Adams et al in 2005 found a statistically significant increase in HER2/neu expression by IHC following neoadjuvant chemotherapy with doxorubicin, paclitaxel, and cyclophosphamide or docetaxel and doxorubicin [6]. Of the 26 patients studied, twelve patients (46%) had positive HER2 IHC staining pre-therapy, and 18 (69%) had positive HER2 IHC post- therapy, representing a statistically significant increase in expression by IHC (p=0.027). HER2 FISH was not examined. The observed increase in HER2 expression in the Adams et al study was attributed to possible chemoresistance of HER2 expressing clones, sample size, interobserver variability, or staining technique.
Alternatively, other studies have demonstrated no statistically significant changes in oncoprotein expression by both HER2 IHC and FISH following neoadjuvant chemotherapy, similar to the results of this current study [5,8]. Varga et al reported similar results in that both HER2 IHC and HER2 FISH in 23 patients were not significantly different following neoadjuvant chemotherapy [9]. Based on the stability of HER2 expression following neoadjuvant chemotherapy, determination of IHC and/or FISH status in the pretreatment specimen alone may be sufficient for directing subsequent management and would reduce overall laboratory testing costs to the patient.
In summary, neoadjuvant chemotherapy in the treatment of primary breast carcinomas has been increasingly used to reduce tumor size prior to surgical breast conservation therapy. Determination of steroid hormone receptor (ER and PR) and HER2 status in these patients both before and after neoadjuvant treatment has been central in directing patient management and potential response to hormonal and biological agents. This study demonstrates no significant difference in ER or HER2 expression by IHC or HER2 FISH in pretreatment and posttreatment primary breast carcinomas. However, there was a statistically significant decrease in PR expression by IHC following neoadjuvant chemotherapy, the cause and clinical significance of which require further investigation.
Acknowledgments
All authors, Drs. Kinsella, Nassar, Siddiqui, and Cohen, have contributed to, read, and approved this final manuscript for submission.
Conflicts of interest statement
No editorial or financial conflicts of interest exist for this submission.
References
- 1.Mamounas EP. NSABP breast cancer clinical trials: recent results and future directions. Clin Med Res. 2003;4:309–326. doi: 10.3121/cmr.1.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 3.Baselga J, Perez EA, Pienkowski T, Bell R. Adjuvant trastuzumab: a milestone in the treatment of HER-2-positive early breast cancer. Oncologist. 2006;11:4–12. doi: 10.1634/theoncologist.11-90001-4. [DOI] [PubMed] [Google Scholar]
- 4.Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, Margolese RG, Hoehn JL, Vogel VG, Dakhil SR, Tamkus D, King KM, Pajon ER, Wright MJ, Robert J, Paik S, Mamounas EP, Wolmark N. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J. Clin. Oncol. 2008;26:778–785. doi: 10.1200/JCO.2007.15.0235. [DOI] [PubMed] [Google Scholar]
- 5.Arens N, Bleyl U, Hildenbrand R. Her2Neu, p53, Ki67, and hormone receptors do not change during neoadjuvant chemotherapy in breast cancer. Virch Arch. 2005;446:489–496. doi: 10.1007/s00428-005-1244-0. [DOI] [PubMed] [Google Scholar]
- 6.Adams AL, Eltoum I, Krontiras H, Wang W, Chhieng DC. The effect of neoadjuvant chemotherapy on histologic grade, hormone receptor status, and Her2/neu status in breast carcinoma. Breast J. 2008;14:141–146. doi: 10.1111/j.1524-4741.2007.00544.x. [DOI] [PubMed] [Google Scholar]
- 7.Taucher S, Rudas M, Gnant M, Thomanek K, Dubsky P, Roka S, Bachleitner T, Kandioler D, Wenzel C, Steger G, Mittlböck M, Jakesz R. Sequential steroid hormone receptor measurements in primary breast cancer with and without intervening primary chemotherapy. Endocr Relat Cancer. 2003;10:91–98. doi: 10.1677/erc.0.0100091. [DOI] [PubMed] [Google Scholar]
- 8.Kasami M, Uematsu T, Honda M. Yabuzaki T, Sanuki J, Uchida Y, Sugimura H. Comparison of estrogen receptor, progesterone receptor, and Her-2 status in breast cancer pre- and post- neoadjuvant chemotherapy. Breast. 2008;17:523–527. doi: 10.1016/j.breast.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Varga Z, Caduff R, Pestalozzi B. Stability of the HER2 gene after primary chemotherapy in advanced breast cancer. Virchows Arch. 2005;446:136–141. doi: 10.1007/s00428-004-1164-4. [DOI] [PubMed] [Google Scholar]