Abstract
Primary small cell carcinoma of the urinary bladder is very rare; only several studies have been reported in the English literature. A 62-year-old woman was admitted to our hospital because of hematuria and dysuria. Bladder endoscopy revealed a large polypoid tumor at the bladder base. Transurethral bladder tumorectomy (TUR-BT) was performed. Many TUR-BT specimens were obtained. Histologically, the bladder tumor was pure small cell carcinoma. Immunohistochemically, the tumor cells were positive for cytokeratin (CK) AE1/3, CK CAM5.2, CK8, CK18, neurone-specific enolase, chromogranin, NCAM (CD56), synaptophysin, Ki-67 (labeling=100%), p53, KIT (CD117), and platelet-derived growth factor receptor-α (PDGFRA). The tumor cells were negative for CK5/6, CK 34BE12, CK7, CK14, CK19, CK20, p63, CD45, and TTF-1. A molecular genetic analysis using PCR-direct sequencing showed no mutations of KIT (exons 9, 11, 13 and 17) and PDGFRA (exons 12 and 18) genes. No metastases were found by various imaging techniques. The patient is now treated by cisplatin-based chemotherapy.
Keywords: Small cell carcinoma, urinary bladder, immunohistochemistry, KIT, PDGFRA
Introduction
Primary small cell carcinoma of the urinary bladder is very rare; only a few studies have been reported in the English literature [1-5]. Like small cell lung carcinoma (SCLC), small cell carcinoma of the urinary bladder shows aggressive biological behavior and the prognosis is poor [1-5]. However, extensive immunohistochemical studies have not been performed in small cell carcinoma of the urinary bladder. KIT and platelet-derived growth factor receptor-α (PDGFRA) have been investigated only once [5] in small cell carcinoma of the urinary bladder.
Recent studies of SCLC showed that KIT protein is expressed in a significant percentage of SCLC [6-15]. In addition, one report showed that KIT gene mutations are present in SCLC [13], but others not [12,14,15]. PDGFRA protein in SCLC has not been reported. PDGFRA gene in SCLC has been investigated in one study [15], which showed no mutations.
KIT and PDGFRA, both mapped to 4q12, encode receptor tyrosine kinase oncoproteins called KIT (CD117) and PDGFRA, respectively [16-21]. Both molecules are transmembranous oncoproteins involved in tumorigenesis, in particular in gastrointestinal stromal tumor [16-21].
The author reports herein an autopsy case of small cell carcinoma of the urinary bladder with an emphasis on immunohistochemistry and on KIT and PDGFRA.
Case report
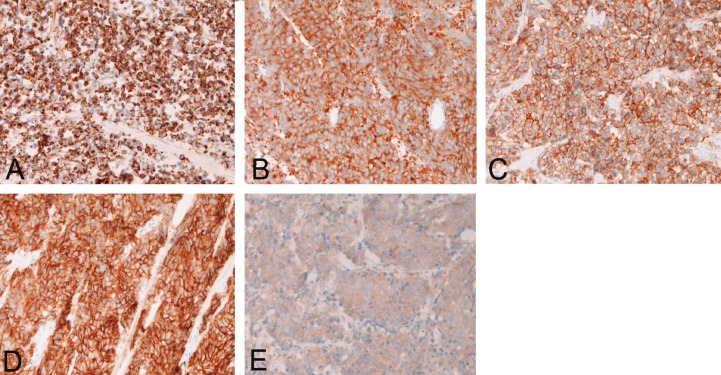
A 62-year-old woman was admitted to our hospital because of hematuria and dysuria. Bladder endoscopy revealed a large polypoid tumor at the bladder base. Transurethral bladder tumorectomy (TUR-BT) were performed. Many TUR-BT specimens were obtained. Histologically, the bladder tumor is pure small cell carcinoma consisting of small cells with hyperchromatic nuclei, inconspicuous nucleoli, molded nuclei, scant cytoplasm, and increased nucleo-cytoplasmic ration (Figure 1A and 1B). An immunohistochemical study was performed with the used of Dako EnVision method as previously described [22-24]. Immunohistochemically, the tumor cells were positive for cytokeratin (CK) AE1/3 (Figure 2A), CK CAM5.2, CK8, CK18, neurone-specific enolase, chromogranin, NCAM (CD56) (Figure 2B), synaptophysin (Figure 2C), Ki-67 (labeling=100%), p53, KIT (CD117) (Figure 2D), and PDGFRA (Figure 2E). The tumor cells were negative for CK5/6, CK 34BE12, CK7, CK14, CK19, CK20, p63, CD45, and TTF-1.
Figure 1.
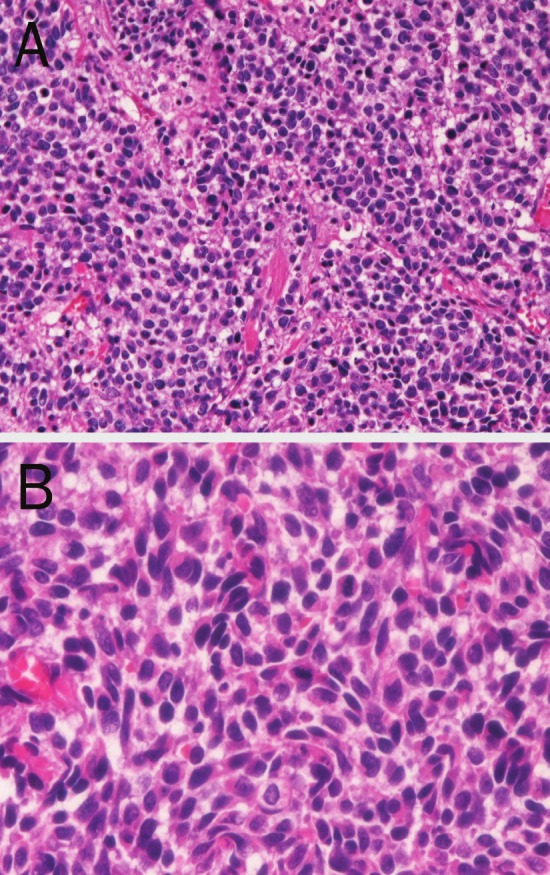
A: Low power view of small cell carcinoma of the bladder. The tumor cells are medullary and consist of small cell with hyperchromatic nuclei. No differentiation is noted. Transurethral tumorectomy specimen, HE, x40. B: High power view of small cell carcinoma of the bladder. The tumor cells are small and show hyperchromatic nuclei, inconspicuous nucleoli, molded nuclei, scant cytoplasm and increased nucleo-cytoplasmic rationb. Transurethral tumorectomy specimen, HE, x200.
Figure 2.
Immunohistochemistry. The tumor cells are positive for cytokeratin AE1/3 (A), NCAM (CD56) (B), synaptophysin (C), KIT (D), and PDGFRA (E). Immunostaining, x200.
A molecular genetic analysis for KIT (exons 9, 11, 13 and 17) and PDGFRA (exons 12 and 18) genes was performed, in paraffin microdissection specimens, by the PCR-direct sequencing method, as previously described [25-29]. The exons of both genes were selected because they are frequent mutation sites [16]. The primers are shown in Table 1. In brief, genomic DNA was extracted from paraffin blocks with proteinase K digestion and phenol/chloroform extraction, and subjected to PCR for 40 cycles (94°C for one minute, 52°C for one minute, 72°C for one minute), using a thermal cycler (GeneAmp PCR system 9700, Applied Biosystems, ABI, CA). The annealing temperature was 53°C. PCR products were extracted, and subjected to a computed automatic DNA sequencer (ABI PRISM 3100 Genetic Analyzer, Applied Biosystems, ABI, CA). These techniques revealed that there were no mutations of the KIT gene (exons 9, 11, 13, and 17) and PDGFRA gene (exons 12 and 18) in this tumor. The patient is now treated by cisplatin-based chemotherapy.
Table 1.
Primer sequence
| Forward | Reverse |
|---|---|
| KIT exon 9 | |
| 5’-TCC TAG AGT AAG CCA GGG CTT-3’ | 5’-TGG TAG ACA GAG CCT AAA CAT CC-3’ |
| KIT exon11 | |
| 5’-GAT CTA TTT TTC CCT TTC TC-3’ | 5’AGC CCC TGT TTC ATA CTG AC-3’ |
| KIT exon 13 | |
| 5’-GCT TGA CAT CAG TTT GCC AG -3’ | 5’-AAA GGC AGC TTG GAC ACG GCT TTA-3’ |
| KIT exon 17 | |
| 5’-CTC CTC CAA CCT AAT AGT GT-3’ | 5’-GTC AAG CAG AGA ATG GGT AC-3’ |
| PDGFRA exon12 | |
| 5’-TTG GAT ATT CAC CAG TTA CCT GTC-3’ | 5’-CAA GGG AAA AGC TCT TGG-3’ |
| PDGFRA exon 18 | |
| 5’-ACC ATG GAT CAG CCA GTC TT-3’ | 5’-TGA AGG AGG ATG AGC CTG ACC-3’ |
Discussion
The present urinary bladder tumor was apparent primary small cell carcinoma histologically. Immunohistochemical demonstration of neuron-specific enolase, chromogranin, synaptophysin, NCAM (CD56) and KIT supports the diagnosis. Small cell carcinoma can occur in any organ, though the vast majority occurs in the lung. Small cell carcinoma of the urinary bladder is extremely rare. In general, small cell carcinoma is very aggressive tumor with poor prognosis.
There have been no comprehensive immunohistochemical studies in small cell carcinoma of the urinary bladder. The present case showed the immunoprofile. CK immunoprofile of bladder small cell carcinoma have not been reported. In the present case, the CK profile was CK AE1/3 +, CK CAM5.2 +, CK8 +, CK 18+, CK5/6 -, CK 34BE12 -, CK7 -, CK14 -, CK19 -, and CK20 -. The CK7-/CK20- pattern is compatible with primary bladder small cell carcinoma. Neuroendocrine antigens (chromogranin, synaptophysin, NCAM, neuron specific enolase) were all positive, indicating that the present tumor is small cell neuroendocrine carcinoma. p63 was negative, indicating that the present tumor is not urothelial carcinoma. Ki-67 labeling was 100%, indicating very high proliferative activity of this tumor. p53 was positive, suggesting p53 gene mutation. CD45 was negative, indicating that the tumor cells are not hematopoietic cells. TTF-1 was negative, suggesting that the present tumor is not metastasis from SCLC.
KIT and PDGFRA gene mutational status has been reported only once in small cell carcinoma of the urinary bladder [5]. The report showed no mutations of KIT and PDGFRA genes. KIT is expressed in various tumors including gastrointestinal stromal tumor (GIST), mast cell neoplasm, melanoma, germ cell tumor, hematopoietic malignancies and SCLC [16,21]. The KIT protein expression in SCLC varies among researchers [6-15]; it is reported to be 100% [6], 73% [7], 37% [8], 60% [9], 78% [10], 53% [11], 40% [13], 64% [14], and 30% [14]. KIT expression without KIT gene mutations is thought to be due to KIT gene amplification [15]. The prognostic implications of positive KIT protein in SCLC is controversial and no definite conclusions were obtained [7-10,13]. If activating KIT mutations are present, treatment of imatinib methylate may be effective [15,21].
KIT mutations are frequent in GIST, acute myeloid leukemia and mast cell neoplasms [21]. With regard to SCLC, one report showed KIT mutations [13], while others indicated no KIT mutations in SCLC [10,12,14]. Boldrini et al. [13] reported two mutations in exon 9 and three mutations of exon 11 in KIT gene were found in 60 SCLC. In contrast, Sihto et al. [15] showed no KIT mutations in 31 SCLC. More studies of KIT mutations remain to be performed in small cell carcinoma.
To the best of my knowledge, there is only one study of PDGFRA mutations in small cell carcinoma. Sihto et al. [15] found no PDGFRA mutations in 31 SCLC. The present case also showed no PDGFRA mutations. PDGFRA protein expression has not been reported in small cell carcinoma. The present case showed weak expression of PDGFRA, suggesting that a small amount of PDGFRA is present in small cell carcinoma. Much more studies remain to be done in PDGFRA expression and PDGFRA gene mutations in small cell carcinoma.
In conclusion, the author reported a case of primary small cell carcinoma of the urinary bladder with immunohistocheimcal studies and molecular genetic analysis of KIT and PDGFRA.
Conflict of interest statement
The author declares that he has no conflict of interest.
References
- 1.Shahab N. Extrapulmonary small cell carcinoma of the bladder. Semin Oncol. 2007;34:15–21. doi: 10.1053/j.seminoncol.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 2.Jones TD, Kernek KM, Yang XJ, Lopez-Beltran A, MacLennan GT, Eble JN, Lin H, Pan CX, Tretiakova M, Baldridge LA, Cheng L. Thyroid transcriptional factor 1 expression in small cell carcinoma of the urinary bladder: an immunohistochemical prolifile of 44 cases. Hum Pathol. 2005;36:718–723. doi: 10.1016/j.humpath.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 3.Kurosa N, Hayashi Y, Nishida Y, Itoh H. Combined small and transitional cell carcinoma of the urinary bladder with CA19-9 production. Pathol Int. 1999;49:462–467. doi: 10.1046/j.1440-1827.1999.00880.x. [DOI] [PubMed] [Google Scholar]
- 4.Cheng C, Nicholson A, Lowe DG, Kirby RS. Oat cell carcinoma of urinary bladder. Urology. 1992;39:504–507. doi: 10.1016/0090-4295(92)90002-e. [DOI] [PubMed] [Google Scholar]
- 5.Terada T. Autopsy case of primary small cell carcinoma of the urinary bladder: KIT and PDGFRA expression and mutations. Pathol Int. 2009;59:247–250. doi: 10.1111/j.1440-1827.2009.02358.x. [DOI] [PubMed] [Google Scholar]
- 6.LaPoint RJ, Bourne PA, Wang HL, Xu H. Coexpression of c-kit and bcl-2 in small cell carcinoma and large cell neuroendocrine carcinoma of the lung. Appl Immunohistochem Mol Morphol. 2007;15:401–406. doi: 10.1097/01.pai.0000213153.41440.7d. [DOI] [PubMed] [Google Scholar]
- 7.López-Martin A, Ballestín C, Garcia-Carbonero R, Castaño A, Lopez-Ríos F, López-Encuentra A, Sánchez-Cespedes M, Castellano D, Bartolomé A, Cortés-Funes H, Paz-Ares L. Prognostic value of KIT expression in small cell lung carcinoma. Lung Cancer. 2007;56:405–413. doi: 10.1016/j.lungcan.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 8.Micke P, Basrai M, Faldum A, Bittinger F, Rönnstrand L, Blaukat A, Beeh KM, Oesch F, Fischer B, Buhl R, Hengstler JG. Characterization of c-kit expression in small cell lung carcinoma: prognostic and therapeutic implications. Clin Cancer Res. 2003;9:188–194. [PubMed] [Google Scholar]
- 9.Camps C, Sirera R, Bremnes RM, Garde J, Safont MJ, Blasco A, Berrocal A, Sánchez JJ, Calabuig C, Martorell M. Analysis of c-kit expression in small cell lung carcinoma: prevalence and prognostic implications. Lung Cancer. 2006;52:343–347. doi: 10.1016/j.lungcan.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Rossi G, Cavazza A, Marchioni A, Migaldi M, Bavieri M, Facciolongo N, Petruzzelli S, Longo L, Tamberi S, Crinò L. KIT expression in small cell carcinoma of the lung: effects of chemotherapy. Mod Pathol. 2003;16:1041–1047. doi: 10.1097/01.MP.0000089780.30006.DE. [DOI] [PubMed] [Google Scholar]
- 11.Naeem M, Dahiya M, Clark JI, Creech SD, Alkin S. Analysis of c-kit protein expression in small-cell lung carcinoma and its implications for prognosis. Hum Pathol. 2002;33:1182–1187. doi: 10.1053/hupa.2002.129199. [DOI] [PubMed] [Google Scholar]
- 12.Mojica WD, Saxena R, Starostik P, Cheney RT. CD117 + small cell lung cancer lacks the asp 816 val point mutation in exon 17. Histopathology. 2005;47:517–522. doi: 10.1111/j.1365-2559.2005.02259.x. [DOI] [PubMed] [Google Scholar]
- 13.Boldrini L, Ursino S, Gisfredi S, Faviana P, Donati V, Camacci T, Lucchi M, Mussi A, Basolo F, Pingitore R, Fontanini G. Expression and mutational status of c-kit in small-cell lung cancer: prognostic relevance. Clin Cancer Res. 2004;15:4101–4108. doi: 10.1158/1078-0432.CCR-03-0664. [DOI] [PubMed] [Google Scholar]
- 14.Burger H, Den Bakker MA, Stoter G, Verweij J, Nooter K. Lack of c-kit exon 11 activating mutations in c-kit/CD117-positive SCLC tumor specimens. Eur J Cancer. 2003;39:793–799. doi: 10.1016/s0959-8049(03)00026-1. [DOI] [PubMed] [Google Scholar]
- 15.Sihto H, Sarlomo-Rikala M, Tynninen O, Tanner M, Andersson LC, Franssila K, Nupponen NN, Joensuu H. KIT and platelet-derived growth factor receptor alpha tyrosine kinase gene mutations and KIT amplifications in human solid tumors. J Clin Oncol. 2005;23:49–57. doi: 10.1200/JCO.2005.02.093. [DOI] [PubMed] [Google Scholar]
- 16.Hirota S, Isozaki K. Pathology of gastrointestinal stromal tumor. Pathol Int. 2006;56:1–9. doi: 10.1111/j.1440-1827.2006.01924.x. [DOI] [PubMed] [Google Scholar]
- 17.Losota J, Miettinen M. KIT and PDGFRA mutations in gastrointestinal stromal tumors (GISTs) Semin Diang Pathol. 2006;23:91–101. doi: 10.1053/j.semdp.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Miettinen M, Lasota J. Gastrointestinal stromal tumor: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130:1466–1478. doi: 10.5858/2006-130-1466-GSTROM. [DOI] [PubMed] [Google Scholar]
- 19.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumor. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 20.Hirota S, Ohashi A, Nishida T, Isozaki K, Kinoshita K, Shinomura Y, Kitamura Y. Gain-of-function mutations of platelet-derived growth factor receptor alpha gene in gastrointestinal stromal tumor. Gastroenterology. 2003;125:660–667. doi: 10.1016/s0016-5085(03)01046-1. [DOI] [PubMed] [Google Scholar]
- 21.Miettinen M, Lasota J. KIT (CD117): a review on expression in normal and neoplastic tissue, and mutations and their clinicopathologic correlation. Appl Immunohistochem Mol Morphol. 2005;13:205–220. doi: 10.1097/01.pai.0000173054.83414.22. [DOI] [PubMed] [Google Scholar]
- 22.Terada T, Kawaguchi M, Furukawa K, Sekido Y, Osamura Y. Minute mixed ductal-endocrine carcinoma of the pancreas with predominant intraductal growth. Pathol Int. 2002;52:740–746. doi: 10.1046/j.1440-1827.2002.01416.x. [DOI] [PubMed] [Google Scholar]
- 23.Terada T, Kawaguchi M. Primary clear cell adenocarcinoma of the peritoneum. Tohoku J Exp Med. 2005;271:271–275. doi: 10.1620/tjem.206.271. [DOI] [PubMed] [Google Scholar]
- 24.Terada T. Ductal adenoma of the breast: Immunohistochemistry of two cases. Pathol Int. 2008;58:801–805. doi: 10.1111/j.1440-1827.2008.02315.x. [DOI] [PubMed] [Google Scholar]
- 25.Terada T. Gastrointestinal stromal tumor of the uterus: a case report with genetic analyses of c-kit and PDGFRA genes. Int J Gynecol Pathol. 2009;28:29–34. doi: 10.1097/PGP.0b013e3181808000. [DOI] [PubMed] [Google Scholar]
- 26.Terada T. Primary extragastrointestinal stromal tumors of the transverse mesocolon without c-Kit mutations but with PDGFRA mutations. Med Oncol. 2009;26:233–237. doi: 10.1007/s12032-008-9092-9. [DOI] [PubMed] [Google Scholar]
- 27.Terada T. Primary multiple extragastrointestinal stromal tumors of the omentum with different mutations of c-kit gene. World J Gastroenterol. 2008;14:7256–7259. doi: 10.3748/wjg.14.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terada T. Mediastinal seminoma with multiple KIT gene mutations. Pathology. 2009;41:695–697. doi: 10.3109/00313020903305852. [DOI] [PubMed] [Google Scholar]
- 29.Terada T. Low incidence of KIT gene mutations and no PDGFRA gene mutations in primary cutaneous melanoma: an immunohistochemical and molecular genetic study of Japanese cases. Int J Clin Oncol. 2010;15:453–456. doi: 10.1007/s10147-010-0087-0. [DOI] [PubMed] [Google Scholar]
- 30.Terada T. Mutations and protein expression of KIT and PDGFRA genes in ipsilateral testicular seminomas: an immunohistochemical and molecular genetic study. Appl Immunohistochem Mol Morphol. 2011;19:450–453. doi: 10.1097/PAI.0b013e31820d2872. [DOI] [PubMed] [Google Scholar]