Abstract
The skeletal muscles play an essential role in life, providing the mechanical basis for respiration and movement. Skeletal muscle dysfunction is prevalent in all stages of chronic obstructive pulmonary disease (COPD), and significantly influences symptoms, functional capacity, health related quality of life, health resource usage and even mortality. Furthermore, in contrast to the lungs, the skeletal muscles are potentially remedial with existing therapy, namely exercise-training. This review summarizes clinical and laboratory observations of the respiratory and peripheral skeletal muscles (in particular the diaphragm and quadriceps), and current understanding of the underlying etiological processes. As further progress is made in the elucidation of the molecular mechanisms of skeletal muscle dysfunction, new pharmacological therapies are likely to emerge to treat this important extra-pulmonary manifestation of COPD.
Keywords: skeletal muscle, pulmonary rehabilitation, exercise, quadriceps, diaphragm
Introduction
Chronic obstructive pulmonary disease (COPD) is major health problem. By 2020, COPD is predicted to be the third leading cause of death and fifth leading cause of chronic disability worldwide.1 Although a disease of the lungs, extra-pulmonary features of COPD are increasingly recognized as important contributors to morbidity and mortality.2 Skeletal muscle dysfunction is of particular interest, as it directly influences exercise performance,3 is associated with poor health status,4 and is an independent predictor of health care utilization5 and mortality.6 Furthermore, respiratory muscle function plays a key role in the pathogenesis of breathlessness7 and maximum inspiratory pressure is an independent predictor of survival in severe disease.8
The most commonly studied skeletal muscles are the quadriceps and the diaphragm. Cross-sectional studies, with careful matching of patients with controls, have revealed the complexity of muscle dysfunction in COPD. In turn, these have provided insight into the possible etiological factors and pathophysiological processes. This review describes the distribution and nature of changes to skeletal muscle function in COPD and how this relates to lung function. Possible etiological factors and mechanisms underpinning COPD muscle dysfunction will be discussed and how this may inform emerging non-pharmacological and pharmacological treatment approaches in this field.
The peripheral muscles
Compared with healthy controls matched for age and gender, isometric quadriceps strength – whether assessed by volitional9,10 or non-volitional measures11 – is reduced by about 20%–30% in patients with COPD. A marked increase in susceptibility to fatigue is also observed, with a more rapid decline in performance during continuous12,13 or repeated bouts of exercise.14,15
The reduction in strength can largely be explained by a comparable reduction in quadriceps cross-sectional area (CSA) and mass, the latter being assessed by magnetic resonance imaging16 and the former by ultrasound17 or computed tomography.9,18 Microscopically, atrophy of single muscle fibers has been observed with a predilection for type IIX fibers.19 Quadriceps endurance is more likely to be related to the relative loss of fatigue-resistant type I fibers20,21 and subsequent reduction in oxidative capacity. ***Matched case-control pairs of vastus lateralis samples reveal a shift in fiber type expression away from type I and toward type II/IIX fibers.20 Concurrent structural changes include a reduction in capillary density,22 number of capillary-muscle fiber contacts21 and levels of oxidative enzyme activity.23 Samples from patients with COPD consistently demonstrate reduced levels of aerobic enzyme activity – for example, citrate synthase and 3-hydroxyacyl CoA dehydrogenase23,24 – together with lower concentrations of adenosine-5′-triphosphate and creatine phosphate.25 The resultant impaired capacity for oxidative phosphorylation leads to a greater reliance on glycolysis during exercise and early accumulation of lactate that becomes limiting.26 Indeed, the oxidative:glycolytic enzyme activity ratio correlates moderately with quadriceps endurance.27
Where other peripheral muscles have been studied, a preferential distribution of muscle wasting and weakness to the lower limbs is observed. Mathur et al found reduced volumes and CSAs in the hamstrings and adductors (21% and 30%, respectively) of patients with moderate to severe disease.16 In contrast, relatively preserved or maintained strength has been found in upper-limb muscles, such as the adductor pollicis11 and elbow flexors,28 and grip strength.29 Structural and histochemical studies support this pattern of dysfunction. Biopsies of the biceps30 and deltoid muscles29 reveal no differences between patients and controls in fiber type profile, nor single-fiber CSA. Gea et al29 explored the metabolism in the deltoid muscle, with findings suggesting a preserved oxidative capacity with raised lactate dehydrogenase and citrate synthase activity and no differences in levels of phosphofructokinase or creatine kinase. In summary, distribution of peripheral muscle dysfunction is not uniform in COPD. Changes are most marked in the lower limbs, which are necessary for locomotion, providing evidence that local factors such as disuse/immobility may be more influential than any systemic process.
The respiratory muscles
Although the capacity of the diaphragm to generate transdiaphragmatic pressure is reduced in COPD, this is largely the product of hyperinflation, which places the muscle at a mechanical disadvantage. Indeed, when corrected for lung volume, the contractile strength of the diaphragm in COPD is not reduced compared with controls11 and may even be enhanced in some cases.31 The maintenance of strength in this muscle is probably due to persistent involuntary training secondary to the increased work of breathing. As a result, the diaphragm adapts by remodeling its fiber type profile toward a fatigue-resistant phenotype with a relative increase the proportion of type I fibers. Relative to controls, samples reveal increases 20%–50% in the overall proportion of type I fibers,32,33 matched by reductions in type IIX fibers.34
Less is known about change in single diaphragm fibers and debate exists as to whether their CSA or force-generating capacity is altered. Some studies report no change in fiber size,35 while others observe selective atrophy of type I fibers.36 Similarly, lower isometric force-generating capacity (normalized for CSA) has been reported among patient fibers tested in vitro,36,37 while others have found no difference between patient and control fibers.38 More established is the intrinsic resistance to fatigue that occurs via an increased concentration of mitochondria,38 capillary density,34 and capacity to generate adenosine-5′-triphosphate through oxidative pathways, marked by an increased succinate dehydrogenase activity.39 In COPD patients, no fatigue of the diaphragm is seen with maximum voluntary ventilation or exhaustive treadmill exercise.40,41 Diaphragm fibers from patients are also more efficient than those from controls, with a lower adenosine-5′-triphosphate cost to maintain a similar isometric force.36 The reduced energy cost may be accounted for by the number of cross-bridge formations within each fiber, with COPD diaphragm muscle fibers having fewer active cross-bridges and each exerting a greater force than in control muscle.35,36
Where other accessory respiratory muscles have been studied, these appear to adapt in the same manner in response to the increased work of breathing. The shift in fiber type from II to I observed in the diaphragm is also seen in the parasternal intercostal muscles of patients with severe disease.42 A contrasting shift in fiber type expression has been observed in the external intercostal muscles, which may reflect their postural role in this group.43 Functionally, pectoralis major and latissimus dorsi strength are preserved relative to the quadriceps,9 as is abdominal strength, presumably due to the additional activity of expiratory muscles in COPD.10
In summary, the changes seen in the respiratory muscles are in stark contrast to those in quadriceps muscle in COPD. Whereas the quadriceps muscle is characterized by a reduced mass and loss of fatigue-resistant type I fibers and oxidative capacity, which impairs strength and endurance, the diaphragm remodels toward a fatigue-resistant profile, with a relative increase in type I fibers and resultant increase in oxidative capacity (Table 1), a pattern reflected in other accessory muscles of respiration. These observations support muscle disuse being a major etiological factor for the differential adaptation of peripheral and respiratory muscles in COPD.
Table 1.
Quadriceps and diaphragm structure and function in patients with COPD compared with controls
| Quadriceps | Diaphragm | |
|---|---|---|
| Strength | Reduced | Unchanged |
| Endurance | Reduced | Increased |
| Overall CSA | Reduced | Unchanged |
| Single-fiber CSA | Reduced in type IIX | Reduced in type I |
| Fiber type shift | Type I to II | Type II to I |
| Capillary and mitochondrial density | Reduced | Increased |
| Metabolism – oxidative: glycolytic ratio | Reduced | Increased |
Abbreviation: CSA, cross-sectional area.
Muscle function and COPD severity
The traditional paradigm is that skeletal muscle dysfunction is a feature of severe or end-stage disease. Certainly, Bernard et al demonstrated a significant relationship between quadriceps strength and forced expiratory volume in 1 second (FEV1) percentage of predicted value, with the more flow-limited patients being weaker9 and the prevalence of quadriceps weakness rises with increasing Global initiative for chronic Obstructive Lung Disease (GOLD) stage.44 Others have also shown a moderate relationship between FEV1 and quadriceps endurance;45 whereas, in the diaphragm, muscle-fiber proportion shift advances with increasing disease severity. 37 However, the literature is far from being unequivocal and several studies have not found any correlations between airflow obstruction and muscle dysfunction.46,47 Very recent data supports the presence of muscle dysfunction, even in the early stages of the disease. Seymour and colleagues showed that quadriceps weakness is common across all disease stages with a mean (95% confidence interval [CI]) prevalence of 31% (25%–38%) in GOLD stage I/II, rising (though not sufficiently to achieve statistical significance) to 38% (31%–46%) in GOLD stage IV.44 Endurance is compromised even in patients with mild disease performing low-intensity tasks,15 while invasive evaluation of diaphragm contractile function, structure, and biochemistry demonstrated that cellular and molecular alterations occur, even in GOLD I/II patients.48 These data suggest that the relationship between airway obstruction and muscle dysfunction in COPD is modest at best and, certainly in some patients, muscle abnormalities may occur before any drop in FEV1 is detected.49 This could be attributed to potential etiological factors such as smoking49 or reductions in physical activity.50,51
Muscle function and physical inactivity in COPD
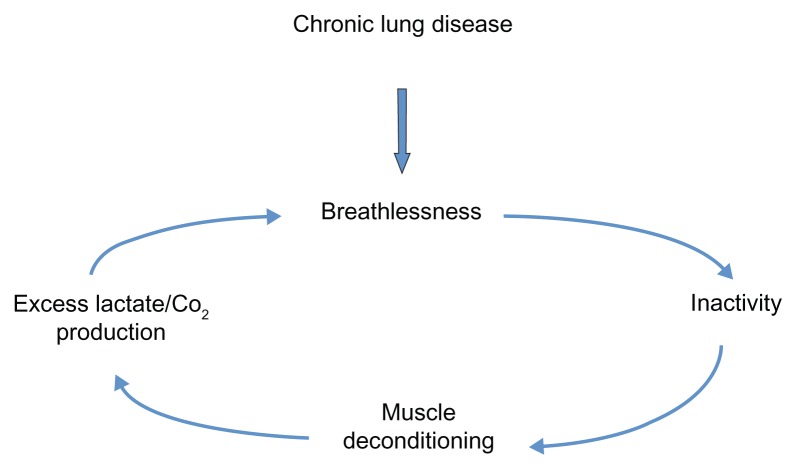
COPD patients often adopt sedentary lifestyles due to breathlessness and, in some, this can precipitate a downward spiral of disease (Figure 1).52 Anaerobic quadriceps metabolism results in lactate and carbon dioxide production, which stimulates ventilation and worsens breathlessness. Daily activity has been documented to be lower in COPD patients than in healthy controls and lower than recommended international guidelines for physical health maintenance.53 Recently, Watz et al50 demonstrated that physical activity and steps per day were reduced even in those with less severe disease. Individuals with early COPD were predominately (although not significantly) more sedentary than the control group of patients with “chronic bronchitis.”
Figure 1.
The downward spiral of disease.
Several lines of evidence support the important etiological role of physical inactivity in the development of COPD skeletal muscle dysfunction. With advancing airway obstruction severity, physical activity declines and matches the loss of muscle mass observed in COPD patients,54 which correlates with muscle force.55 Abnormalities in the quadriceps muscle in COPD patients are similarly observed in patients with other chronic diseases, such as heart failure,56 suggesting a common etiological factor like physical inactivity and resultant deconditioning. Furthermore, as previously discussed, muscle dysfunction is most marked in the lower limb muscles of locomotion, again supporting the role of disuse. During hospitalization for an exacerbation of COPD, physical inactivity is marked and there is a corresponding reduction in quadriceps strength.57 Exercise interventions, during or shortly after an exacerbation requiring hospitalization, result in significant improvements in quadriceps muscle strength.58,59
Large prospective population-based studies also support the relationships between physical inactivity and important clinical end-points in COPD. Physical activity is protective against hospital admissions60 and all-cause and respiratory mortality61 in COPD; furthermore, physical activity can also modify the smoking-related decline in lung function, therefore reducing the risk of COPD in those individuals.62
Other etiological factors
Low-grade systemic inflammation is thought to be reflected by higher levels of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α); interleukin (IL)-6, -8, -18; and acute-phase proteins in COPD patients. These are postulated to originate either from the peripheral lung or the respiratory muscles. Quadriceps strength has been related to IL-6 and TNF-α in a stable cohort of aged individuals63 and IL-8 in those with COPD during an exacerbation.57 Elevated IL-6 levels are also associated with radiological evidence of quadriceps wasting in COPD64 and reduced lean body mass.65 A similar association was suggested in early studies for TNF-α; however, a possible confounding factor includes changes in assays used to quantify cytokine levels. 66 Moreover, quadriceps biopsy findings have not shown increased muscle levels of pro-inflammatory cytokines, including TNF-α, IL-6, IL-8, interferon-gamma, and transforming growth factor-beta, in COPD,67–69 and further work is required to determine the contribution of local inflammation to muscle dysfunction.
Hypoxemia and inflammation are thought to be the up-stream mediators of oxidative stress.70 An increase in reactive oxygen species or reactive nitrogen species, and/or a reduction in antioxidant capacity, leads to local oxidative stress damaging cellular components. This can adversely affect muscle-fiber function via its contractile property71 and mitochondria respiration. Furthermore, oxidative stress can alter protein catabolism and anabolism and induce cell death.72 Peroxidation products from reactive oxygen speciesinduced lipid membrane damage can be detected peripherally and have been shown to be elevated in COPD patients at rest and during an exacerbation.73 Studies of antioxidant capacity in COPD are less consistent. Some investigators have reported elevated antioxidant enzymes in patients with severe COPD with muscle wasting,74,75 while others have shown no differences in levels in the quadriceps muscle between COPD patients and controls.72,76,77 Antioxidant enzyme function may be inadequate in the muscles of COPD patients and unable to respond to the increased oxidant stress after exercise.76 However, given that exercise generally improves muscle function in COPD, the observation that whole-body and localized-limb exercise induces increased oxidative stress in COPD patients76,78 questions whether oxidative stress is indeed pathological or simply a physiological reflection of the muscle repair cycle.
As more than 50% of very severe COPD patients have preserved quadriceps strength,44 studies have sought to demonstrate a genetic predisposition to either loss of muscle mass or muscle resistance to the effects of long-term physical inactivity. The deletion (D) rather than the insertion (I) polymorphic variant of the angiotensin-converting enzyme (ACE) gene is associated with preserved quadriceps strength in COPD.79 This is associated with higher tissue ACE and angiotensin II activity, which may affect muscle growth, and lower bradykinin levels. To establish whether the effects were mediated via increased ACE-related D allele kinin degradation, bradykinin receptor polymorphisms were later studied.80 The +9/+9 (base pair repeat present) receptor polymorphism, which is associated with reduced gene transcription and lower mRNA, was more prevalent in COPD patients with low fat-free mass (FFM) index. However, this did not explain the previously identified ACE gene findings, as there was no interaction between the two genotypes on strength.80 Polymorphisms of the vitamin D receptor are associated with reduced (FokI polymorphism) or greater quadriceps muscle strength (BsmI polymorphism) in COPD patients.81 However, this association was not seen in healthy controls, suggesting a gene–environment interaction.
Cachexia-associated polymorphisms of inflammatory cytokines such as TNF-α and IL-6 remain to be discovered. A -511 polymorphism of the IL-1β gene (the CC variant) has been shown to be associated with cachexia82 but functional implications are unknown. Cachexia or loss of muscle mass is well described in COPD, even in the presence of retained weight and fat mass.83 Whether this process can be attributed directly to nutritional insufficiency or is secondary to a systemic inflammatory process remains unclear. Certainly, nutritional supplementation alone does not appear to improve measurements of FFM, lung function, or exercise capacity.84 However, in combination with other anabolic stimuli, it can maintain or improve muscle mass but with undetermined effects on function.85
Imbalance between anabolic and catabolic hormones has also been suggested as contributing to muscle dysfunction in COPD. Reduced circulating anabolic hormones such as testosterone and insulin-like growth factor-1 (IGF-1) have been reported in COPD patients.64 Trials of testosterone have shown variable results; some have not been able to demonstrate an improvement in exercise capacity,86,87 but others report an increase in muscle mass and strength with a combination of testosterone and resistance training in male individuals with low baseline testosterone levels.88 Insulin resistance and changes in glucose metabolism have also been found in COPD patients, but this remains poorly studied in the COPD population therefore the relationship with skeletal muscle dysfunction remains inconclusive.89 Similarly, although the effect of long-term, low-dose systemic corticosteroids on the proximal muscles is well described,90 short-term use of higher doses (prednisolone 30 mg for 2 weeks) does not cause significant skeletal muscle dysfunction nor alter metabolic parameters during exercise.91
Potential molecular mechanisms and pathways
Cardinal molecular features of quadriceps muscle dysfunction include muscle-fiber atrophy and muscle-fiber shift. Muscle atrophy results in loss of muscle mass and can result from an imbalance between muscle protein synthesis (MPS) and muscle protein breakdown (MPB), and/or individual fiber loss and gain. Muscle-fiber shift has been less extensively studied, but the loss of aerobic type I fibers classically results in a decrease in oxidative capacity, a reduction in mitochondria, and reduced muscle endurance. It is unclear at present whether these processes occur independently or are linked.
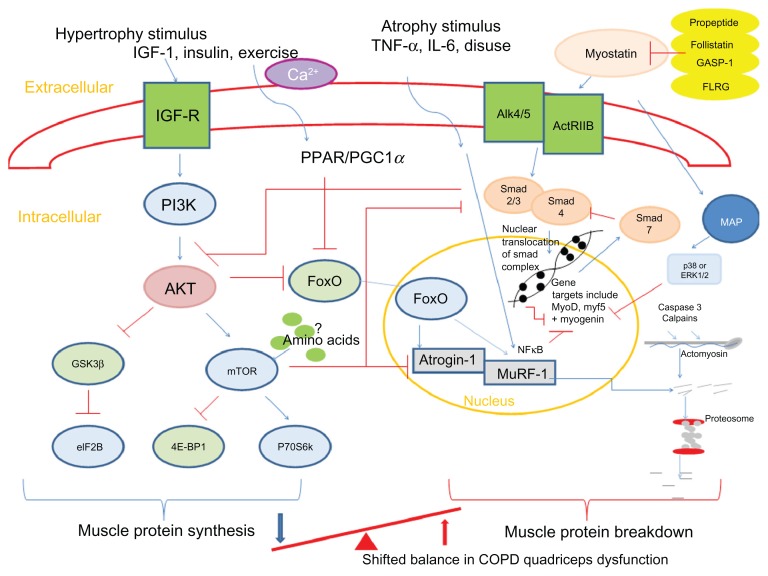
Animal models have identified key mediators in atrophy and hypertrophy signaling and therefore the control of muscle growth.92 These are summarized in Figure 2. The anabolic hormone and growth factor IGF-1 stimulates the phosphoinositide 3-kinase/Akt (protein kinase B) pathway in skeletal muscle cells. Upon phosphorylation, Akt is able to activate mammalian target of rapamycin (mTOR), which, in turn, activates 70-kD ribosomal S6 protein kinase93 and inhibits eukaryotic translation initiation factor 4E binding protein-1/PHAS-1;94 these processes stimulate MPS. Another downstream regulator of anabolism, which is mTOR independent, is the phosphorylation of glycogen synthase kinase-3β (GSK-3β) by phosphorylated Akt.95 This leads to the release of eukaryotic initiation factor 2B (eIF2B) which upregulates MPS. Phosphorylated Akt also plays a role in MPB pathways. It downregulates two muscle-specific E3 ligases, atrogin-1 (muscle atrophy F-box or MAFbx), and muscle-specific RING finger protein (MuRF)-1 via inactivation of the forkhead box class O (FoxO) family of transcription factors.96 The muscle-specific ubiquitin ligases contribute to protein degradation via the ubiquitin-proteasome pathway.97
Figure 2.
Summary of pathways controlling muscle protein synthesis (MPS) and muscle protein breakdown (MPB). The role of myostatin in MPS and MPB has also been included. Myostatin is held in an inactive state by its pro-peptide, follistatin, and inhibitory binding proteins – growth and differentiation factor-associated serum protein-1 (GASP-1) and follistatin-like related gene, (FLRG) as shown. Upon activation, it binds to its transmembrane receptor activin receptor type IIB (ActRIIB), which then forms homodimers with activin receptor-like kinase 4 or 5 (Alk 4/5). The SMAD signaling pathway is then activated and translocation of this transcription factor complex to the nucleus occurs, where MyoD production and therefore myoblast proliferation and fusion are blocked. Myostatin is also proposed to increase proteosomal activity in a FoxO-dependent manner. Activation of MAP kinase is mediated via myostatin either via p38 or ERK1/2, which leads to the blocking of genes involved in myogenesis.
Notes: → denotes stimulation; ⊣ indicates inhibition.
Abbreviations: 4E-BP1, eukaryotic translation initiation factor 4E binding protein-1; Akt, protein kinase B; ERK, extracellular signal-regulated kinase; eIF2B, eukaryotic initiation factor 2B; FoxO, forkhead box class O; GSK-3β, glycogen synthase kinase-3β; IGF-1, insulin-like growth factor-1; IL-6, interleukin-6; MAP, mitogen-activated protein; mTOR, mammalian target of rapamycin; MuRF, muscle-specific RING finger protein; p70S6k, 70-kD ribosomal S6 protein kinase; PGC1α, peroxisome proliferator-activated receptor gamma co-activator 1-alpha; PI3K, phosphoinositide 3-kinase; PPAR, peroxisome proliferator-activated receptor; SMAD,; TNF-α, tumor necrosis factor-alpha; GASP-1, growth and differentiation factor-associated serum protein-1; IGF-R, insulin-like growth factor-1 receptor; Ca2+, calcium ion; NFκB, nuclear factor κB.
Ubiquitin-mediated protein degradation may play an important role in COPD skeletal muscle dysfunction. Two small studies found elevated atrogin-198,99 and MuRF-198 in quadriceps from COPD patients, but, in one of these, it was alongside elevated hypertrophy signaling. Although larger studies are required to confirm these observations, this may suggest COPD patients actually fail to restore muscle mass and therefore have some form of synthetic resistance. Indeed, tracer studies from immobilized individuals would support this theory of resistance to MPS to protein nutrition (termed “anabolic resistance”).100 MPS is greatly suppressed in the immobilized post-absorptive state,101 while the contribution from MPB is minimal. Therefore, the relative contribution of MPB in the process of disuse-induced muscle atrophy remains under question.102
Myostatin, or growth differentiation factor-8, is a member of the transforming growth factor-β (TGF-β) super-family and is a potent negative regulator of muscle mass, as demonstrated by naturally occurring mutations occurring in mice, cattle, and humans.103–105 Myostatin can influence muscle wasting by affecting the number and size of muscle cells and by inducing muscle atrophy pathways. Myostatin upregulates p21 (cyclin-dependent kinase inhibitor), which negatively affects activated satellite cell/myoblast proliferation,106 and downregulates myogenic differentiation factors (MyoD, myf5, and myogenin), which inhibit myoblast differentiation.107 It can also exert an effect on muscle catabolism by activating the ubiquitin proteolytic system in a FoxO1-dependent manner108 and may possibly inactivate Akt, affecting MPS.108 Finally, myostatin has also been shown to inhibit satellite cell activation and self-renewal in a Pax7-dependent way.109
Several lines of evidence implicate a role for myostatin in COPD quadriceps dysfunction. Myostatin mRNA was elevated in weak COPD patients.99 Following an in-patient resistance training program, hospitalized COPD patients had reduced myostatin transcripts, with a trend toward an increase in MyoD and myogenin.59 Following exercise training, non- cachectic COPD patients demonstrated a reduction in myostatin protein, with a reduction in MuRF-1 and atrogin-1,110 while a modest reduction in myostatin was found following resistance training with or without testosterone.111 Our group have also demonstrated a negative association between quadriceps muscle myostatin mRNA expression and quadriceps muscle strength in COPD patients.112
Animal studies have shown that skeletal muscle-fiber phenotype appears to be regulated by several independent signaling pathways. Fiber type switching in mice can be induced by changes in nerve activity from differing electrical stimulations,113 enabling the study of pathways that affect myosin gene expression and metabolic profiles.
Myofiber gene activation occurs via calcium signaling through calcineurin (Cn) and various kinases – for example, Ca2+/calmodulin-dependent protein kinases II. Cn is a calcium/calmodulin-regulated protein phosphatase that acts on transcription factors of the nuclear factor of activated T cells (NFAT) family. Ca2+/calmodulin-dependent protein kinases II regulate myocyte enhancer factor 2, via histone deacetylase (HDAC), and has been suggested to interact with NFAT.114 Studies of transgenic mice115 and those treated with Cn inhibitor116 have implicated Cn signaling in activity-dependent maintenance of the slow gene program.113
Cn-NFAT signaling may also upregulate the transcription factor peroxisome proliferator-activated receptor (PPAR)-β/-γ and the transcriptional co-activator PPAR-γ (PGC-1α),117 both of which have been implicated in the muscle dysfunction of COPD patients. PPAR signaling can affect oxidative signaling and fiber type composition,118 in addition to having inflammatory properties via effects on the nuclear factor kappa-light-chain-enhancer of activated B cells pathway.119 The three isoforms of PPARS (α, β/δ, and γ) are all present in skeletal muscle. PPAR-δ regulates fatty acid utilization and energy homeostasis120 and higher levels are expressed in type I muscle fibers121 and can be induced by acute exercise in healthy young men.122 A transgenic “marathon mouse” model, in which PPAR-δ expression is increased, shows fiber shift opposite to that seen in COPD.121 PGC-1α is a co-activator of PPAR-δ that can interact with transcription factors and basal transcriptional machinery123 and can stimulate mitochondrial and oxidative enzymes.113 In one small study of 14 COPD patients and nine control subjects, PPAR-α and -δ protein levels and PGC-1α mRNA were significantly lower in the quadriceps of moderate/severe COPD patients than in controls with a similar smoking history124 and PPAR-α mRNA expression was lower still in cachectic patients. These findings suggest that PPAR-γ or -α content and/or function may in some way be involved in the change in oxidative gene program and mitochondrial dysfunction.125 However, as type I fibers have higher expression of PPARs, these results may simply represent an association rather causation.
The mitogen-activated protein kinase (MAPK) pathway may have an influence on fiber shift via extracellular signal-regulated kinase (ERK) signaling. In muscle cell lines, a type I phenotype is induced when the ERK pathway is inhibited and a shift toward type I/IIa from IIx myosin heavy chain (MHC) results from MAPK phosphatase-1.126 Recent data from COPD patients have been conflicting. Lemire and colleagues showed elevated ratios of phosphorylated to total level of p38 MAPK and ERK 1/2 in the quadriceps muscle compared with controls.127 These ratios were negatively associated with mid-thigh muscle CSA, supporting the hypothesis that MAPK may contribute to the development of skeletal muscle dysfunction in COPD.127 In contrast, data from a much larger cross-sectional study failed to show a role for p38 MAPK signaling.128
Emerging data suggest that microRNAs (miRNAs), small polynucleotides that can decrease mRNA translation or directly destabilize mRNA, may also be implicated in the control of skeletal muscle phenotype.129 Muscle-specific miRNAs include those which affect myocyte proliferation and differentiation, for example, miR-1 and miR-206,130 and others, such as miR-208b and miR-499,130,131 that modulate the expression of slow MHC genes through regulation of transcriptional repressors.132,133 In COPD, we have recently shown that the miRNA profile of the quadriceps muscle in COPD patients differs from that of controls, with a downregulation in the myocardin-related transcription-serum response factor axis and reduced expression of muscle-specific miRNAs, particularly miR-1.134 Reduction in miR-1 has been reported in other models of inactivity resulting from denervation, nerve entrapment, or space flight and targets include myostatin135 and IGF-1.136 We found IGF-1 was elevated in the COPD group consistent with previous reports of the overexpression of muscle hypertrophy pathways.98 MiR-1 may also contribute toward reduction in MHC I and fiber shift, via an increase of HDAC4. HDAC4 inhibits serum response factor, an important regulator of MHC1 expression, and the expression of follistatin,137 which may activate the myostatin pathway.
Non-pharmacological treatments for muscle dysfunction in COPD
Exercise training remains the only known intervention to reverse some of the underlying skeletal muscle abnormalities seen in COPD, further supporting the notion that reduced daily physical activity is the major etiological factor. Exercise training, in the form of pulmonary rehabilitation (PR), has emerged as the most effective non-pharmacological intervention in improving exercise capacity, dyspnea, and health status in COPD patients, as evidenced by numerous randomized controlled trials and meta-analyses.138 Given that PR does not directly improve lung mechanics or gas exchange,139 it is likely that the main area of improvement with exercise lies in the skeletal muscle. Dysfunction of the locomotor muscles may limit exercise performance because of leg discomfort,13 but also because early anaerobic metabolism leads to lactic acid production. Lactic acid, buffered by bicarbonate, causes production of carbon dioxide and an increased ventilatory stimulus. As expiratory flow limitation is commonly present in COPD, increased ventilation can exacerbate dynamic hyperinflation and promote premature exercise termination and dyspnea.140
Quadriceps strength, endurance, and fatigability all improve significantly following exercise training.58,141,142 Even in the acute setting, resistance training during an exacerbation can prevent muscle function deterioration,59 while PR shortly following hospital discharge can significantly accelerate recovery of quadriceps muscle strength.58 Debate remains as to the most effective mode of exercise to induce not only different skeletal muscle adaptations but also long-term improvements in clinically relevant health outcomes. Typically, chronic endurance training enhances the fatigue resistance of skeletal muscle by promoting a muscle-fiber type shift from fast-twitch fatigable type II fibers to slow-twitch fatigue-resistant type I fibers, increasing mitochondrial content and activity and improving skeletal muscle glucose transportation. However, resistance training reduces sarcopenia and promotes hypertrophy of muscle fibers, especially of type IIx.143
Intensity of exercise training is an important determinant of the physiological training effect.144 However, in patients with severe COPD, intolerable sensations of breathlessness may prevent sufficiently long periods of high-intensity training levels.145 Strategies to augment exercise tolerance by reducing dyspnea sensation or ventilatory limitation have included noninvasive mechanical ventilation,146 oxygen,147 and/or heliox supplementation,148 all of which have been demonstrated to increase exercise tolerance in the laboratory setting. However, these are rarely systematically used as part of clinical PR programs. An alternative approach, which may be particularly suitable for patients with more severe COPD, is interval training, which allows patients to complete short periods of high-intensity exercise not possible with classical aerobic exercise training.149
Although the emphasis has so far been on the muscles of the lower limbs, there have been studies examining the effects of training the upper limbs or the respiratory muscles in COPD. A systematic review of upper-limb exercise-training studies in COPD showed improvements in arm exercise capacity, but the effects on symptoms, overall exercise capacity, and health-related quality of life were inconsistent. 150 Similarly, debate continues with regard to the role of inspiratory muscle training in the context of PR. Although most studies have demonstrated a positive effect on voluntary inspiratory muscle strength,151 it remains unclear whether this is as a result of a genuine physiological improvement in the inspiratory muscles or a learning effect in performing the voluntary maneuver. Furthermore, the added benefit of inspiratory muscle training over a general exercise-training program seems relatively limited.151
In patients unable or unwilling to adhere to existing forms of exercise, neuromuscular electrical stimulation (NMES) may offer an alternative way of enhancing leg muscle strength.152 NMES uses a battery-powered stimulator unit to produce a controlled contraction of the muscles via skin electrodes. A typical program consists of 30–60 minutes of quadriceps stimulation, 3–5 times weekly for 4–6 weeks. NMES can lead to improvements in muscle strength and exercise performance, with pooled data revealing mean between-group differences in peak quadriceps torque and 6-minute walking distance of 9.7 Nm (95% CI 1.2, 18.1) and 48 m (95% CI 9, 86), respectively.153 Recent studies have also demonstrated favorable changes in markers of anabolism/catabolism154 and the quadriceps fiber type profile following NMES.155 However, studies remain small, follow-up data are lacking, and the patient phenotypes most likely to benefit have yet to be identified.
Pharmacological treatments for muscle dysfunction in COPD
Despite the many benefits of PR, there are limitations. Firstly, exercise training does not fully reverse all of the abnormalities observed in the quadriceps muscle. Secondly, a proportion of patients either has limited accessibility to PR or has issues with uptake and completion. Thirdly, improvements following PR decline toward baseline level within 12–18 months.156 Hence, there is interest in pharmacologically augmenting (or even replacing) exercise training to bring about structural and functional improvements in the skeletal muscles. However, as well as the technical problems involved in creating a drug that specifically benefits the muscles, there are regulatory hurdles to be overcome before such a compound can reach the market.157
As previously discussed, systemic inflammation, oxidative stress, and anabolic/catabolic hormone imbalance have been postulated as etiological factors for muscle dysfunction in COPD. An early trial of infliximab, an anti-TNF-α therapy, found that it was largely ineffective in improving lung function, exercise capacity, or health-related quality of life,158 although there did appear to be a trend for benefit in cachectic patients. Furthermore, antioxidant therapy with N-acetylcysteine led to a 25% increase in quadriceps endurance compared with placebo.70 However, this was a very small study of nine COPD patients in a controlled laboratory setting. Studies of anabolic hormones have had mixed results. Anabolic steroids increase body weight and FFM in COPD, either alone159 or in conjunction with exercise training,87 but not muscle strength or exercise capacity. However, the addition of testosterone to resistance training in hypogonadal COPD patients promotes anabolic pathways that can result in improved quadriceps strength and endurance.88 Recombinant growth hormone (GH) improves FFM compared with placebo but does not improve muscle strength or exercise capacity.86 Ghrelin is a novel GH-releasing peptide that induces a positive energy balance by decreasing fat utility and stimulating feeding through GH-independent mechanisms. In a small open-label study, ghrelin increased FFM, muscle strength, and 6-minute walk distance in cachectic COPD patients.160 Systemic side effects with hormonal drugs are a concern, hence there is current interest in the development of anabolic drugs without the unwanted side effects. An example is the selective androgen-receptor modulator class of drugs that have the benefits of anabolic/androgenic steroids with a hypothetically reduced risk of prostate cancer in men and virilizing effects in women.161
Another therapeutic approach is to use existing drugs for new indications. As previously discussed, common variations in the gene for the vitamin D receptor81 and deletion of the allele of the ACE79 have been demonstrated to influence muscle strength. Vitamin D supplementation or the administration of ACE inhibitors may have a future role in treating muscle dysfunction in COPD, or at least augmenting the benefits of exercise training. Certainly, this approach has been used with positive results in elderly people with functional impairment. 162 Similarly, levosimendan, a calcium sensitizer used as a cardiac inotrope, has recently been shown to improve neuro-mechanical efficiency and contractile function of the human diaphragm in healthy subjects,163 and conceivably it may also improve skeletal muscle dysfunction in COPD. However, with increasing understanding of the underlying molecular mechanisms, there is also hope that a number of novel pharmacological agents that address cachexia and skeletal muscle dysfunction in COPD will become available for clinical use. Already, prototype inhibitors of ubiquitin ligases and neutralizing antibodies to myostatin have been developed for cancer-related cachexia and muscle dystrophies. 164 PPAR-δ agonists have recently been shown to mimic and enhance exercise training and AICAR, an 5′ adenosine monophosphate-activated-kinase agonist, was sufficient to improve exercise endurance in mice alone.165 Thus, there is much future promise that novel therapeutic agents will become available to address important extra-pulmonary manifestations of COPD such as skeletal muscle dysfunction.
Future directions
A greater understanding of the etiology and basic mechanisms of skeletal muscle dysfunction should continue to underpin developments in the field, informing the identification and testing of new pharmacological agents and strategies to augment or optimize PR across community and in-patient settings. Phenotyping of patients according to skeletal muscle dysfunction will also enhance the identification of those most likely to response to specific treatments, thus should be embraced in clinical trial design and practice, where possible.
Acknowledgments
This work is funded in part by the National Institute for Health Research (NIHR) Respiratory Biomedical Research Unit, Royal Brompton and Harefield Foundation Trust and Imperial College. AVD and MIP are either fully or partly supported by the Biomedical Research Unit. MM is funded by an NIHR postdoctoral fellowship. WD-CM is supported by an NIHR Clinician Scientist Award and a Medical Research Council New Investigator Award. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the NIHR, nor the Department of Health.
Footnotes
Disclosure
The authors declare no conflicts of interest in this work.
References
- 1.Lopez AD, Murray CC. The global burden of disease, 1990–2020. Nat Med. 1998;4(11):1241–1243. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- 2.Man WD, Kemp P, Moxham J, Polkey MI. Skeletal muscle dysfunction in COPD: clinical and laboratory observations. Clin Sci (Lond) 2009;117(7):251–264. doi: 10.1042/CS20080659. [DOI] [PubMed] [Google Scholar]
- 3.Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med. 1996;153(3):976–980. doi: 10.1164/ajrccm.153.3.8630582. [DOI] [PubMed] [Google Scholar]
- 4.Simpson K, Killian K, McCartney N, Stubbing DG, Jones NL. Randomised controlled trial of weightlifting exercise in patients with chronic airflow limitation. Thorax. 1992;47(2):70–75. doi: 10.1136/thx.47.2.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Decramer M, Gosselink R, Troosters T, Verschueren M, Evers G. Muscle weakness is related to utilization of health care resources in COPD patients. Eur Respir J. 1997;10(2):417–423. doi: 10.1183/09031936.97.10020417. [DOI] [PubMed] [Google Scholar]
- 6.Swallow EB, Reyes D, Hopkinson NS, et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax. 2007;62(2):115–120. doi: 10.1136/thx.2006.062026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Man W, Mustfa N, Nikoletou D, et al. Effect of salmeterol on respiratory muscle activity during exercise in poorly reversible COPD. Thorax. 2004;59(6):471–476. doi: 10.1136/thx.2003.019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray-Donald K, Gibbons L, Shapiro SH, Macklem PT, Martin JG. Nutritional status and mortality in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1996;153(3):961–966. doi: 10.1164/ajrccm.153.3.8630580. [DOI] [PubMed] [Google Scholar]
- 9.Bernard S, LeBlanc P, Whittom F, et al. Peripheral muscle weakness in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158(2):629–634. doi: 10.1164/ajrccm.158.2.9711023. [DOI] [PubMed] [Google Scholar]
- 10.Man WD, Hopkinson NS, Harraf F, Nikoletou D, Polkey MI, Moxham J. Abdominal muscle and quadriceps strength in chronic obstructive pulmonary disease. Thorax. 2005;60(9):718–722. doi: 10.1136/thx.2005.040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Man W, Soliman M, Nikoletou D, et al. Non-volitional assessment of skeletal muscle strength in patients with chronic obstructive pulmonary disease. Thorax. 2003;58(8):665–669. doi: 10.1136/thorax.58.8.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allaire J, Maltais F, Doyon JF, et al. Peripheral muscle endurance and the oxidative profile of the quadriceps in patients with COPD. Thorax. 2004;59(8):673–678. doi: 10.1136/thx.2003.020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Man WD, Soliman MG, Gearing J, et al. Symptoms and quadriceps fatigability after walking and cycling in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168(5):562–567. doi: 10.1164/rccm.200302-162OC. [DOI] [PubMed] [Google Scholar]
- 14.Mador MJ, Deniz O, Aggarwal A, Kufel TJ. Quadriceps fatigability after single muscle exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168(1):102–108. doi: 10.1164/rccm.200202-080OC. [DOI] [PubMed] [Google Scholar]
- 15.Coronell C, Orozco-Levi M, Mendez R, Ramirez-Sarmiento A, Gáldiz JB, Gea J. Relevance of assessing quadriceps endurance in patients with COPD. Eur Respir J. 2004;24(1):129–136. doi: 10.1183/09031936.04.00079603. [DOI] [PubMed] [Google Scholar]
- 16.Mathur S, Takai KP, Macintyre DL, Reid D. Estimation of thigh muscle mass with magnetic resonance imaging in older adults and people with chronic obstructive pulmonary disease. Phys Ther. 2008;88(2):219–230. doi: 10.2522/ptj.20070052. [DOI] [PubMed] [Google Scholar]
- 17.Seymour JM, Ward K, Sidhu PS, et al. Ultrasound measurement of rectus femoris cross-sectional area and the relationship with quadriceps strength in COPD. Thorax. 2009;64(5):418–423. doi: 10.1136/thx.2008.103986. [DOI] [PubMed] [Google Scholar]
- 18.Marquis K, Debigaré R, Lacasse Y, et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2002;166(6):809–813. doi: 10.1164/rccm.2107031. [DOI] [PubMed] [Google Scholar]
- 19.Gosker HR, Engelen MP, van Mameren H, et al. Muscle fiber type IIX atrophy is involved in the loss of fat-free mass in chronic obstructive pulmonary disease. Am J Clin Nutr. 2002;76(1):113–119. doi: 10.1093/ajcn/76.1.113. [DOI] [PubMed] [Google Scholar]
- 20.Gosker HR, Zeegers MP, Wouters EF, Schols AM. Muscle fibre type shifting in the vastus lateralis of patients with COPD is associated with disease severity: a systematic review and meta-analysis. Thorax. 2007;62(11):944–949. doi: 10.1136/thx.2007.078980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whittom F, Jobin J, Simard PM, et al. Histochemical and morphological characteristics of the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Med Sci Sports Exerc. 1998;30(10):1467–1474. doi: 10.1097/00005768-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Jobin J, Maltais F, Doyon JF, et al. Chronic obstructive pulmonary disease: capillarity and fiber type characteristics of skeletal muscle. J Cardiopulm Rehabil. 1998;18(6):432–437. doi: 10.1097/00008483-199811000-00005. [DOI] [PubMed] [Google Scholar]
- 23.Maltais F, Simard AA, Simard C, Jobin J, Desgagnés P, LeBlanc P. Oxidative capacity of the skeletal muscle and lactic acid kinetics during exercise in normal subjects and in patients with COPD. Am J Respir Crit Care Med. 1996;153(1):288–293. doi: 10.1164/ajrccm.153.1.8542131. [DOI] [PubMed] [Google Scholar]
- 24.Jakobsson P, Jorfeldt L, Henriksson J. Metabolic enzyme activity in the quadriceps femoris muscle in patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1995;151(2 Pt 1):374–377. doi: 10.1164/ajrccm.151.2.7842194. [DOI] [PubMed] [Google Scholar]
- 25.Kutsuzawa T, Shioya S, Kurita D, Haida M, Ohta Y, Yamabayashi H. Muscle energy metabolism and nutritional status in patients with chronic obstructive pulmonary disease. A 31P magnetic resonance study. Am J Respir Crit Care Med. 1995;152(2):647–652. doi: 10.1164/ajrccm.152.2.7633721. [DOI] [PubMed] [Google Scholar]
- 26.Maltais F, Jobin J, Sullivan MJ, et al. Metabolic and hemodynamic responses of lower limb during exercise in patients with COPD. J Appl Physiol. 1998;84(5):1573–1580. doi: 10.1152/jappl.1998.84.5.1573. [DOI] [PubMed] [Google Scholar]
- 27.Swallow EB, Gosker HR, Ward KA, et al. A novel technique for nonvolitional assessment of quadriceps muscle endurance in humans. J Appl Physiol. 2007;103(3):739–746. doi: 10.1152/japplphysiol.00025.2007. [DOI] [PubMed] [Google Scholar]
- 28.Newell SZ, McKenzie DK, Gandevia SC. Inspiratory and skeletal muscle strength and endurance and diaphragmatic activation in patients with chronic airflow limitation. Thorax. 1989;44(11):903–912. doi: 10.1136/thx.44.11.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gea JG, Pasto M, Carmona MA, Orozco-Levi M, Palomeque J, Broquetas J. Metabolic characteristics of the deltoid muscle in patients with chronic obstructive pulmonary disease. Eur Respir J. 2001;17(5):939–945. doi: 10.1183/09031936.01.17509390. [DOI] [PubMed] [Google Scholar]
- 30.Sato Y, Asoh T, Honda Y, Fujimatsu Y, Higuchi I, Oizumi K. Morphologic and histochemical evaluation of muscle in patients with chronic pulmonary emphysema manifesting generalized emaciation. Eur Neurol. 1997;37(2):116–121. doi: 10.1159/000117421. [DOI] [PubMed] [Google Scholar]
- 31.Similowski T, Yan S, Gauthier AP, Macklem PT, Bellemare F. Contractile properties of the human diaphragm during chronic hyperinflation. N Engl J Med. 1991;325(13):917–923. doi: 10.1056/NEJM199109263251304. [DOI] [PubMed] [Google Scholar]
- 32.Levine S, Kaiser L, Leferovich J, Tikunov B. Cellular adaptations in the diaphragm in chronic obstructive pulmonary disease. N Engl J Med. 1997;337(25):1799–1806. doi: 10.1056/NEJM199712183372503. [DOI] [PubMed] [Google Scholar]
- 33.Mercadier JJ, Schwartz K, Schiaffino S, et al. Myosin heavy chain gene expression changes in the diaphragm of patients with chronic lung hyperinflation. Am J Physiol. 1998;274(4 Pt 1):L527–534. doi: 10.1152/ajplung.1998.274.4.L527. [DOI] [PubMed] [Google Scholar]
- 34.Doucet M, Debigaré R, Joanisse DR, et al. Adaptation of the diaphragm and the vastus lateralis in mild-to-moderate COPD. Eur Respir J. 2004;24(6):971–979. doi: 10.1183/09031936.04.00020204. [DOI] [PubMed] [Google Scholar]
- 35.Ottenheijm CA, Heunks LM, Sieck GC, et al. Diaphragm dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172(2):200–205. doi: 10.1164/rccm.200502-262OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stubbings AK, Moore AJ, Dusmet M, et al. Physiological properties of human diaphragm muscle fibres and the effect of chronic obstructive pulmonary disease. J Physiol. 2008;586(Pt 10):2637–2650. doi: 10.1113/jphysiol.2007.149799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levine S, Nguyen T, Kaiser LR, et al. Human diaphragm remodeling associated with chronic obstructive pulmonary disease: clinical implications. Am J Respir Crit Care Med. 2003;168(6):706–713. doi: 10.1164/rccm.200209-1070OC. [DOI] [PubMed] [Google Scholar]
- 38.Orozco-Levi M, Gea J, Lloreta JL, et al. Subcellular adaptation of the human diaphragm in chronic obstructive pulmonary disease. Eur Respir J. 1999;13(2):371–378. doi: 10.1183/09031936.99.13237199. [DOI] [PubMed] [Google Scholar]
- 39.Levine S, Gregory C, Nguyen T, et al. Bioenergetic adaptation of individual human diaphragmatic myofibers to severe COPD. J Appl Physiol. 2002;92(3):1205–1213. doi: 10.1152/japplphysiol.00116.2001. [DOI] [PubMed] [Google Scholar]
- 40.Polkey MI, Kyroussis D, Hamnegard CH, et al. Diaphragm performance during maximal voluntary ventilation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;155(2):642–648. doi: 10.1164/ajrccm.155.2.9032207. [DOI] [PubMed] [Google Scholar]
- 41.Polkey MI, Kyroussis D, Keilty SE, et al. Exhaustive treadmill exercise does not reduce twitch transdiaphragmatic pressure in patients with COPD. Am J Respir Crit Care Med. 1995;152(3):959–964. doi: 10.1164/ajrccm.152.3.7663810. [DOI] [PubMed] [Google Scholar]
- 42.Levine S, Nguyen T, Friscia M, et al. Parasternal intercostal muscle remodeling in severe chronic obstructive pulmonary disease. J Appl Physiol. 2006;101(5):1297–1302. doi: 10.1152/japplphysiol.01607.2005. [DOI] [PubMed] [Google Scholar]
- 43.Gea J, Orozco-Levi M, Aguar MC, et al. Adaptive changes concerning the types of fibres and isoforms of myosin in the external intercostal muscle of COPD patients. Eur Respir J. 1996;9:160S. [Google Scholar]
- 44.Seymour JM, Spruit MA, Hopkinson NS, et al. The prevalence of quadriceps weakness in COPD and the relationship with disease severity. Eur Respir J. 2010;36(1):81–88. doi: 10.1183/09031936.00104909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serres I, Gautier V, Varray A, Préfaut C. Impaired skeletal muscle endurance related to physical inactivity and altered lung function in COPD patients. Chest. 1998;113(4):900–905. doi: 10.1378/chest.113.4.900. [DOI] [PubMed] [Google Scholar]
- 46.Gosker HR, Kubat B, Schaart G, van der Vusse GJ, Wouters EF, Schols AM. Myopathological features in skeletal muscle of patients with chronic obstructive pulmonary disease. Eur Respir J. 2003;22(2):280–285. doi: 10.1183/09031936.03.00012803. [DOI] [PubMed] [Google Scholar]
- 47.Degens H, Sanchez Horneros JM, Heijdra YF, Dekhuijzen PN, Hopman MT. Skeletal muscle contractility is preserved in COPD patients with normal fat-free mass. Acta Physiol Scand. 2005;184(3):235–242. doi: 10.1111/j.1365-201X.2005.01447.x. [DOI] [PubMed] [Google Scholar]
- 48.Ottenheijm CA, Heunks LM, Dekhuijzen RP. Diaphragm adaptations in patients with COPD. Respir Res. 2008;9(1):12. doi: 10.1186/1465-9921-9-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van den Borst B, Koster A, Yu B, et al. Is age-related decline in lean mass and physical function accelerated by obstructive lung disease or smoking? Thorax. 2011;66(11):961–969. doi: 10.1136/thoraxjnl-2011-200010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watz H, Waschki B, Meyer T, Magnussen H. Physical activity in patients with COPD. Eur Respir J. 2009;33(2):262–272. doi: 10.1183/09031936.00024608. [DOI] [PubMed] [Google Scholar]
- 51.Kon SS, Man WD. Muscle mass and strength in obstructive lung disease: a smoking gun? Thorax. 2011;66(11):933–935. doi: 10.1136/thoraxjnl-2011-200774. [DOI] [PubMed] [Google Scholar]
- 52.Polkey MI, Moxham J. Attacking the disease spiral in chronic obstructive pulmonary disease. Clin Med. 2006;6(2):190–196. doi: 10.7861/clinmedicine.6-2-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bossenbroek L, de Greef MH, Wempe JB, Krijnen WP, Ten Hacken NH. Daily physical activity in patients with chronic obstructive pulmonary disease: a systematic review. COPD. 2011;8(4):306–319. doi: 10.3109/15412555.2011.578601. [DOI] [PubMed] [Google Scholar]
- 54.Decramer M, Rennard S, Troosters T, et al. COPD as a lung disease with systemic consequences – clinical impact, mechanisms, and potential for early intervention. COPD. 2008;5(4):235–256. doi: 10.1080/15412550802237531. [DOI] [PubMed] [Google Scholar]
- 55.Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(9):972–977. doi: 10.1164/rccm.200407-855OC. [DOI] [PubMed] [Google Scholar]
- 56.Gosker HR, Lencer NH, Franssen FM, van der Vusse GJ, Wouters EF, Schols AM. Striking similarities in systemic factors contributing to decreased exercise capacity in patients with severe chronic heart failure or COPD. Chest. 2003;123(5):1416–1424. doi: 10.1378/chest.123.5.1416. [DOI] [PubMed] [Google Scholar]
- 57.Spruit M, Gosselink R, Troosters T, et al. Muscle force during an acute exacerbation in hospitalised patients with COPD and its relationship with CXCL8 and IGF-I. Thorax. 2003;58(9):752–756. doi: 10.1136/thorax.58.9.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seymour JM, Moore L, Jolley CJ, et al. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax. 2010;65(5):423–428. doi: 10.1136/thx.2009.124164. [DOI] [PubMed] [Google Scholar]
- 59.Troosters T, Probst VS, Crul T, et al. Resistance training prevents deterioration in quadriceps muscle function during acute exacerbations of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;181(10):1072–1077. doi: 10.1164/rccm.200908-1203OC. [DOI] [PubMed] [Google Scholar]
- 60.Garcia-Aymerich J, Farrero E, Félez MA, Izquierdo J, Marrades RM, Antó JM Estudi del Factors de Risc d’Agudització de la MPOC investigators. Risk factors of readmission to hospital for a COPD exacerbation: a prospective study. Thorax. 2003;58(2):100–105. doi: 10.1136/thorax.58.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Anto JM. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61(9):772–778. doi: 10.1136/thx.2006.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garcia-Aymerich J, Lange P, Benet M, Schnohr P, Antó JM. Regular physical activity modifies smoking-related lung function decline and reduces risk of chronic obstructive pulmonary disease: a population-based cohort study. Am J Respir Crit Care Med. 2007;175(5):458–463. doi: 10.1164/rccm.200607-896OC. [DOI] [PubMed] [Google Scholar]
- 63.Yende S, Waterer GW, Tolley EA, et al. Inflammatory markers are associated with ventilatory limitation and muscle dysfunction in obstructive lung disease in well functioning elderly subjects. Thorax. 2006;61(1):10–16. doi: 10.1136/thx.2004.034181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Debigaré R, Marquis K, Côté CH, et al. Catabolic/anabolic balance and muscle wasting in patients with COPD. Chest. 2003;124(1):83–89. doi: 10.1378/chest.124.1.83. [DOI] [PubMed] [Google Scholar]
- 65.Eid AA, Ionescu AA, Nixon LS, et al. Inflammatory response and body composition in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1414–1418. doi: 10.1164/ajrccm.164.8.2008109. [DOI] [PubMed] [Google Scholar]
- 66.Wagner PD. Possible mechanisms underlying the development of cachexia in COPD. Eur Respir J. 2008;31(3):492–501. doi: 10.1183/09031936.00074807. [DOI] [PubMed] [Google Scholar]
- 67.Barreiro E, Schols AM, Polkey MI, et al. ENIGMA in COPD project. Cytokine profile in quadriceps muscles of patients with severe COPD. Thorax. 2008;63(2):100–107. doi: 10.1136/thx.2007.078030. [DOI] [PubMed] [Google Scholar]
- 68.Petersen AM, Penkowa M, Iversen M, et al. Elevated levels of IL-18 in plasma and skeletal muscle in chronic obstructive pulmonary disease. Lung. 2007;185(3):161–171. doi: 10.1007/s00408-007-9000-7. [DOI] [PubMed] [Google Scholar]
- 69.Crul T, Spruit MA, Gayan-Ramirez G, et al. Markers of inflammation and disuse in vastus lateralis of chronic obstructive pulmonary disease patients. Eur J Clin Invest. 2007;37(11):897–904. doi: 10.1111/j.1365-2362.2007.01867.x. [DOI] [PubMed] [Google Scholar]
- 70.Koechlin C, Couillard A, Simar D, et al. Does oxidative stress alter quadriceps endurance in chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2004;169(9):1022–1027. doi: 10.1164/rccm.200310-1465OC. [DOI] [PubMed] [Google Scholar]
- 71.Reid MB. Nitric oxide, reactive oxygen species, and skeletal muscle contraction. Med Sci Sports Exerc. 2001;33(3):371–376. doi: 10.1097/00005768-200103000-00006. [DOI] [PubMed] [Google Scholar]
- 72.Barreiro E, de la Puente B, Minguella J, et al. Oxidative stress and respiratory muscle dysfunction in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171(10):1116–1124. doi: 10.1164/rccm.200407-887OC. [DOI] [PubMed] [Google Scholar]
- 73.Supinski GS, Callahan LA. Free radical-mediated skeletal muscle dysfunction in inflammatory conditions. J Appl Physiol. 2007;102(5):2056–2063. doi: 10.1152/japplphysiol.01138.2006. [DOI] [PubMed] [Google Scholar]
- 74.Barreiro E, Rabinovich R, Marin-Corral J, Barberà JA, Gea J, Roca J. Chronic endurance exercise induces quadriceps nitrosative stress in patients with severe COPD. Thorax. 2009;64(1):13–19. doi: 10.1136/thx.2008.105163. [DOI] [PubMed] [Google Scholar]
- 75.Gosker HR, Bast A, Haenen GR, et al. Altered antioxidant status in peripheral skeletal muscle of patients with COPD. Respir Med. 2005;99(1):118–125. doi: 10.1016/j.rmed.2004.05.018. [DOI] [PubMed] [Google Scholar]
- 76.Couillard A, Maltais F, Saey D, et al. Exercise-induced quadriceps oxidative stress and peripheral muscle dysfunction in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;167(12):1664–1669. doi: 10.1164/rccm.200209-1028OC. [DOI] [PubMed] [Google Scholar]
- 77.Barreiro E, Gea J, Corominas JM, Hussain SN. Nitric oxide synthases and protein oxidation in the quadriceps femoris of patients with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2003;29(6):771–778. doi: 10.1165/rcmb.2003-0138OC. [DOI] [PubMed] [Google Scholar]
- 78.Couillard A, Koechlin C, Cristol JP, Varray A, Prefaut C. Evidence of local exercise-induced systemic oxidative stress in chronic obstructive pulmonary disease patients. Eur Respir J. 2002;20(5):1123–1129. doi: 10.1183/09031936.02.00014302. [DOI] [PubMed] [Google Scholar]
- 79.Hopkinson NS, Nickol AH, Payne J, et al. Angiotensin converting enzyme genotype and strength in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170(4):395–399. doi: 10.1164/rccm.200304-578OC. [DOI] [PubMed] [Google Scholar]
- 80.Hopkinson NS, Eleftheriou KI, Payne J, et al. +9/+9 Homozygosity of the bradykinin receptor gene polymorphism is associated with reduced fat-free mass in chronic obstructive pulmonary disease. Am J Clin Nutr. 2006;83(4):912–917. doi: 10.1093/ajcn/83.4.912. [DOI] [PubMed] [Google Scholar]
- 81.Hopkinson NS, Li KW, Kehoe A, et al. Vitamin D receptor genotypes influence quadriceps strength in chronic obstructive pulmonary disease. Am J Clin Nutr. 2008;87(2):385–390. doi: 10.1093/ajcn/87.2.385. [DOI] [PubMed] [Google Scholar]
- 82.Broekhuizen R, Grimble RF, Howell WM, et al. Pulmonary cachexia, systemic inflammatory profile, and the interleukin 1beta-511 single nucleotide polymorphism. Am J Clin Nutr. 2005;82(5):1059–1064. doi: 10.1093/ajcn/82.5.1059. [DOI] [PubMed] [Google Scholar]
- 83.Engelen MP, Schols AM, Baken WC, Wesseling GJ, Wouters EF. Nutritional depletion in relation to respiratory and peripheral skeletal muscle function in out-patients with COPD. Eur Respir J. 1994;7(10):1793–1797. doi: 10.1183/09031936.94.07101793. [DOI] [PubMed] [Google Scholar]
- 84.Ferreira IM, Brooks D, Lacasse Y, Goldstein RS. Nutritional support for individuals with COPD: a meta-analysis. Chest. 2000;117(3):672–678. doi: 10.1378/chest.117.3.672. [DOI] [PubMed] [Google Scholar]
- 85.Op den Kamp CM, Langen RC, Haegens A, Schols AM. Muscle atrophy in cachexia: can dietary protein tip the balance? Curr Opin Clin Nutr Metab Care. 2009;12(6):611–616. doi: 10.1097/MCO.0b013e3283319399. [DOI] [PubMed] [Google Scholar]
- 86.Burdet L, de Muralt B, Schutz Y, Pichard C, Fitting JW. Administration of growth hormone to underweight patients with chronic obstructive pulmonary disease. A prospective, randomized, controlled study. Am J Respir Crit Care Med. 1997;156(6):1800–1806. doi: 10.1164/ajrccm.156.6.9704142. [DOI] [PubMed] [Google Scholar]
- 87.Creutzberg EC, Wouters EF, Mostert R, Pluymers RJ, Schols AM. A role for anabolic steroids in the rehabilitation of patients with COPD? A double-blind, placebo-controlled, randomized trial. Chest. 2003;124(5):1733–1742. doi: 10.1378/chest.124.5.1733. [DOI] [PubMed] [Google Scholar]
- 88.Casaburi R, Bhasin S, Cosentino L, et al. Effects of testosterone and resistance training in men with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2004;170(8):870–878. doi: 10.1164/rccm.200305-617OC. [DOI] [PubMed] [Google Scholar]
- 89.Schols AM. Pulmonary cachexia. Int J Cardiol. 2002;85(1):101–110. doi: 10.1016/s0167-5273(02)00238-3. [DOI] [PubMed] [Google Scholar]
- 90.Decramer M, Lacquet LM, Fagard R, Rogiers P. Corticosteroids contribute to muscle weakness in chronic airflow obstruction. Am J Respir Crit Care Med. 1994;150(1):11–16. doi: 10.1164/ajrccm.150.1.8025735. [DOI] [PubMed] [Google Scholar]
- 91.Hopkinson NS, Man WD, Dayer MJ, et al. Acute effect of oral steroids on muscle function in chronic obstructive pulmonary disease. Eur Respir J. 2004;24(1):137–142. doi: 10.1183/09031936.04.00139003. [DOI] [PubMed] [Google Scholar]
- 92.Nader GA. Molecular determinants of skeletal muscle mass: getting the “AKT” together. Int J Biochem Cell Biol. 2005;37(10):1985–1996. doi: 10.1016/j.biocel.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 93.Rommel C, Bodine SC, Clarke BA, et al. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3(11):1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 94.Hara K, Yonezawa K, Kozlowski MT, et al. Regulation of eIF-4E BP1 phosphorylation by mTOR. J Biol Chem. 1997;272(42):26457–26463. doi: 10.1074/jbc.272.42.26457. [DOI] [PubMed] [Google Scholar]
- 95.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378(6559):785–789. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 96.Sandri M, Sandri C, Gilbert A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117(3):399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bodine SC, Latres E, Baumhueter S, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294(5547):1704–1708. doi: 10.1126/science.1065874. [DOI] [PubMed] [Google Scholar]
- 98.Doucet M, Russell AP, Léger B, et al. Muscle atrophy and hypertrophy signaling in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176(3):261–269. doi: 10.1164/rccm.200605-704OC. [DOI] [PubMed] [Google Scholar]
- 99.Plant PJ, Brooks D, Faughnan M, et al. Cellular markers of muscle atrophy in chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2010;42(4):461–471. doi: 10.1165/rcmb.2008-0382OC. [DOI] [PubMed] [Google Scholar]
- 100.Rennie MJ. Anabolic resistance: the effects of aging, sexual dimorphism, and immobilization on human muscle protein turnover. Appl Physiol Nutr Metab. 2009;34(3):377–381. doi: 10.1139/H09-012. [DOI] [PubMed] [Google Scholar]
- 101.de Boer MD, Selby A, Atherton P, et al. The temporal responses of protein synthesis, gene expression and cell signalling in human quadriceps muscle and patellar tendon to disuse. J Physiol. 2007;585(Pt 1):241–251. doi: 10.1113/jphysiol.2007.142828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marimuthu K, Murton AJ, Greenhaff PL. Mechanisms regulating muscle mass during disuse atrophy and rehabilitation in humans. J Appl Physiol. 2011;110(2):555–560. doi: 10.1152/japplphysiol.00962.2010. [DOI] [PubMed] [Google Scholar]
- 103.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature. 1997;387(6628):83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 104.McPherron AC, Lee SJ. Double muscling in cattle due to mutations in the myostatin gene. Proc Natl Acad Sci U S A. 1997;94(23):12457–12461. doi: 10.1073/pnas.94.23.12457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Schuelke M, Wagner KR, Stolz LE, et al. Myostatin mutation associated with gross muscle hypertrophy in a child. N Engl J Med. 2004;350(26):2682–2688. doi: 10.1056/NEJMoa040933. [DOI] [PubMed] [Google Scholar]
- 106.Thomas M, Langley B, Berry C, et al. Myostatin, a negative regulator of muscle growth, functions by inhibiting myoblast proliferation. J Biol Chem. 2000;275(51):40235–40243. doi: 10.1074/jbc.M004356200. [DOI] [PubMed] [Google Scholar]
- 107.Langley B, Thomas M, Bishop A, Sharma M, Gilmour S, Kambadur R. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J Biol Chem. 2002;277(51):49831–49840. doi: 10.1074/jbc.M204291200. [DOI] [PubMed] [Google Scholar]
- 108.McFarlane C, Plummer E, Thomas M, et al. Myostatin induces cachexia by activating the ubiquitin proteolytic system through an NF-kappaB- independent, FoxO1-dependent mechanism. J Cell Physiol. 2006;209(2):501–514. doi: 10.1002/jcp.20757. [DOI] [PubMed] [Google Scholar]
- 109.McFarlane C, Hennebry A, Thomas M, et al. Myostatin signals through Pax7 to regulate satellite cell self-renewal. Exp Cell Res. 2008;314(2):317–329. doi: 10.1016/j.yexcr.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 110.Vogiatzis I, Simoes DC, Stratakos G, et al. Effect of pulmonary rehabilitation on muscle remodelling in cachectic patients with COPD. Eur Respir J. 2010;36(2):301–310. doi: 10.1183/09031936.00112909. [DOI] [PubMed] [Google Scholar]
- 111.Lewis MI, Fournier M, Storer TW, et al. Skeletal muscle adaptations to testosterone and resistance training in men with COPD. J Appl Physiol. 2007;103(4):1299–1310. doi: 10.1152/japplphysiol.00150.2007. [DOI] [PubMed] [Google Scholar]
- 112.D-C Man W, Natanek SA, Riddoch-Contreras J, et al. Quadriceps myostatin expression in COPD. Eur Respir J. 2010;36(3):686–688. doi: 10.1183/09031936.00032510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schiaffino S, Sandri M, Murgia M. Activity-dependent signaling pathways controlling muscle diversity and plasticity. Physiology (Bethesda) 2007;22:269–278. doi: 10.1152/physiol.00009.2007. [DOI] [PubMed] [Google Scholar]
- 114.Wu H, Naya FJ, McKinsey TA, et al. MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type. EMBO J. 2000;19(9):1963–1973. doi: 10.1093/emboj/19.9.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Naya FJ, Mercer B, Shelton J, Richardson JA, Williams RS, Olson EN. Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J Biol Chem. 2000;275(7):4545–4548. doi: 10.1074/jbc.275.7.4545. [DOI] [PubMed] [Google Scholar]
- 116.Chin ER, Olson EN, Richardson JA, et al. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 1998;12(16):2499–2509. doi: 10.1101/gad.12.16.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Long YC, Glund S, Garcia-Roves PM, Zierath JR. Calcineurin regulates skeletal muscle metabolism via coordinated changes in gene expression. J Biol Chem. 2007;282(3):1607–1614. doi: 10.1074/jbc.M609208200. [DOI] [PubMed] [Google Scholar]
- 118.Luquet S, Lopez-Soriano J, Holst D, et al. Peroxisome proliferator-activated receptor delta controls muscle development and oxidative capability. FASEB J. 2003;17(15):2299–2301. doi: 10.1096/fj.03-0269fje. [DOI] [PubMed] [Google Scholar]
- 119.Becker J, Delayre-Orthez C, Frossard N, Pons F. Regulation of inflammation by PPARs: a future approach to treat lung inflammatory diseases? Fundam Clin Pharmacol. 2006;20(5):429–447. doi: 10.1111/j.1472-8206.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- 120.Luquet S, Gaudel C, Holst D, et al. Roles of PPAR delta in lipid absorption and metabolism: a new target for the treatment of type 2 diabetes. Biochim Biophys Acta. 2005;1740(2):313–317. doi: 10.1016/j.bbadis.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 121.Wang YX, Zhang CL, Yu RT, et al. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol. 2004;2(10):e294. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mahoney DJ, Parise G, Melov S, Safdar A, Tarnopolsky MA. Analysis of global mRNA expression in human skeletal muscle during recovery from endurance exercise. FASEB J. 2005;19(11):1498–1500. doi: 10.1096/fj.04-3149fje. [DOI] [PubMed] [Google Scholar]
- 123.Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24(1):78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 124.Remels AH, Schrauwen P, Broekhuizen R, et al. Peroxisome proliferator- activated receptor expression is reduced in skeletal muscle in COPD. Eur Respir J. 2007;30(2):245–252. doi: 10.1183/09031936.00144106. [DOI] [PubMed] [Google Scholar]
- 125.Remels AH, Gosker HR, Schrauwen P, Langen RC, Schols AM. Peroxisome proliferator-activated receptors: a therapeutic target in COPD? Eur Respir J. 2008;31(3):502–508. doi: 10.1183/09031936.00068207. [DOI] [PubMed] [Google Scholar]
- 126.Shi H, Scheffler JM, Pleitner JM, et al. Modulation of skeletal muscle fiber type by mitogen-activated protein kinase signaling. FASEB J. 2008;22(8):2990–3000. doi: 10.1096/fj.07-097600. [DOI] [PubMed] [Google Scholar]
- 127.Lemire BB, Debigare R, Dubé A, Thériault ME, Cote CH, Maltais F. MAPK signalling in the quadriceps of patients with chronic obstructive pulmonary disease. J Appl Physiol. Epub April. 2012;19 doi: 10.1152/japplphysiol.01518.2011. [DOI] [PubMed] [Google Scholar]
- 128.Riddoch-Contreras J, George T, Natanek SA, et al. p38 mitogen-activated protein kinase is not activated in the quadriceps of patients with stable chronic obstructive pulmonary disease. COPD. 2012;9(2):142–150. doi: 10.3109/15412555.2011.644359. [DOI] [PubMed] [Google Scholar]
- 129.Williams AH, Liu N, van Rooij E, Olson EN. MicroRNA control of muscle development and disease. Curr Opin Cell Biol. 2009;21(3):461–469. doi: 10.1016/j.ceb.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen JF, Mandel EM, Thomson JM, et al. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38(2):228–233. doi: 10.1038/ng1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Güller I, Russell AP. MicroRNAs in skeletal muscle: their role and regulation in development, disease and function. J Physiol. 2010;588(Pt 21):4075–4087. doi: 10.1113/jphysiol.2010.194175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.van Rooij E, Quiat D, Johnson BA, et al. A family of microRNAs encoded by myosin genes governs myosin expression and muscle performance. Dev Cell. 2009;17(5):662–673. doi: 10.1016/j.devcel.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.McCarthy JJ, Esser KA, Peterson CA, Dupont-Versteegden EE. Evidence of MyomiR network regulation of beta-myosin heavy chain gene expression during skeletal muscle atrophy. Physiol Genomics. 2009;39(3):219–226. doi: 10.1152/physiolgenomics.00042.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lewis A, Riddoch-Contreras J, Natanek SA, et al. Downregulation of the serum response factor/miR-1 axis in the quadriceps of patients with COPD. Thorax. 2012;67(1):26–34. doi: 10.1136/thoraxjnl-2011-200309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Clop A, Marcq F, Takeda H, et al. A mutation creating a potential illegitimate microRNA target site in the myostatin gene affects muscularity in sheep. Nat Genet. 2006;38(7):813–818. doi: 10.1038/ng1810. [DOI] [PubMed] [Google Scholar]
- 136.Elia L, Contu R, Quintavalle M, et al. Reciprocal regulation of microRNA-1 and insulin-like growth factor-1 signal transduction cascade in cardiac and skeletal muscle in physiological and pathological conditions. Circulation. 2009;120(23):2377–2385. doi: 10.1161/CIRCULATIONAHA.109.879429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sun Y, Ge Y, Drnevich J, Zhao Y, Band M, Chen J. Mammalian target of rapamycin regulates miRNA-1 and follistatin in skeletal myogenesis. J Cell Biol. 2010;189(7):1157–1169. doi: 10.1083/jcb.200912093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006;4:CD003793. doi: 10.1002/14651858.CD003793.pub2. [DOI] [PubMed] [Google Scholar]
- 139.Casaburi R, ZuWallack R. Pulmonary rehabilitation for management of chronic obstructive pulmonary disease. N Engl J Med. 2009;360(13):1329–1335. doi: 10.1056/NEJMct0804632. [DOI] [PubMed] [Google Scholar]
- 140.O’Donnell DE, Lam M, Webb KA. Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;158(5 Pt 1):1557–1565. doi: 10.1164/ajrccm.158.5.9804004. [DOI] [PubMed] [Google Scholar]
- 141.Mador MJ, Kufel TJ, Pineda LA, et al. Effect of pulmonary rehabilitation on quadriceps fatiguability during exercise. Am J Respir Crit Care Med. 2001;163(4):930–935. doi: 10.1164/ajrccm.163.4.2006125. [DOI] [PubMed] [Google Scholar]
- 142.O’Donnell DE, McGuire M, Samis L, Webb KA. General exercise training improves ventilatory and peripheral muscle strength and endurance in chronic airflow limitation. Am J Respir Crit Care Med. 1998;157(5 Pt 1):1489–1497. doi: 10.1164/ajrccm.157.5.9708010. [DOI] [PubMed] [Google Scholar]
- 143.Man WD, Kemp P, Moxham J, Polkey MI. Exercise and muscle dysfunction in COPD: implications for pulmonary rehabilitation. Clin Sci (Lond) 2009;117(8):281–291. doi: 10.1042/CS20080660. [DOI] [PubMed] [Google Scholar]
- 144.Casaburi R, Patessio A, Ioli F, Zanaboni S, Donner CF, Wasserman K. Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease. Am Rev Respir Dis. 1991;143(1):9–18. doi: 10.1164/ajrccm/143.1.9. [DOI] [PubMed] [Google Scholar]
- 145.Maltais F, LeBlanc P, Jobin J, et al. Intensity of training and physiologic adaptation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;155(2):555–561. doi: 10.1164/ajrccm.155.2.9032194. [DOI] [PubMed] [Google Scholar]
- 146.van’t Hul A, Gosselink R, Hollander P, Postmus P, Kwakkel G. Training with inspiratory pressure support in patients with severe COPD. Eur Respir J. 2006;27(1):65–72. doi: 10.1183/09031936.06.00036505. [DOI] [PubMed] [Google Scholar]
- 147.Somfay A, Pórszász J, Lee SM, Casaburi R. Effect of hyperoxia on gas exchange and lactate kinetics following exercise onset in nonhypoxemic COPD patients. Chest. 2002;121(2):393–400. doi: 10.1378/chest.121.2.393. [DOI] [PubMed] [Google Scholar]
- 148.Palange P, Valli G, Onorati P, et al. Effect of heliox on lung dynamic hyperinflation, dyspnea, and exercise endurance capacity in COPD patients. J Appl Physiol. 2004;97(5):1637–1642. doi: 10.1152/japplphysiol.01207.2003. [DOI] [PubMed] [Google Scholar]
- 149.Vogiatzis I. Strategies of muscle training in very severe COPD patients. Eur Respir J. 2011;38(4):971–975. doi: 10.1183/09031936.00075011. [DOI] [PubMed] [Google Scholar]
- 150.Janaudis-Ferreira T, Hill K, Goldstein R, Wadell K, Brooks D. Arm exercise training in patients with chronic obstructive pulmonary disease: a systematic review. J Cardiopulm Rehabil Prev. 2009;29(5):277–283. doi: 10.1097/HCR.0b013e3181b4c8d0. [DOI] [PubMed] [Google Scholar]
- 151.Gosselink R, De Vos J, van den Heuvel SP, Segers J, Decramer M, Kwakkel G. Impact of inspiratory muscle training in patients with COPD: what is the evidence? Eur Respir J. 2011;37(2):416–425. doi: 10.1183/09031936.00031810. [DOI] [PubMed] [Google Scholar]
- 152.Maddocks M, Murton AJ, Wilcock A. Improving muscle mass and function in cachexia: non-drug approaches. Curr Opin Support Palliat Care. 2011;5(4):361–364. doi: 10.1097/SPC.0b013e32834bdde3. [DOI] [PubMed] [Google Scholar]
- 153.Roig M, Reid WD. Electrical stimulation and peripheral muscle function in COPD: a systematic review. Respir Med. 2009;103(4):485–495. doi: 10.1016/j.rmed.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 154.Vivodtzev I, Debigaré R, Gagnon P, et al. Functional and muscular effects of neuromuscular electrical stimulation in patients with severe COPD: a randomized clinical trial. Chest. 2012;141(3):716–725. doi: 10.1378/chest.11-0839. [DOI] [PubMed] [Google Scholar]
- 155.Abdellaoui A, Préfaut C, Gouzi F, et al. Skeletal muscle effects of electrostimulation after COPD exacerbation: a pilot study. Eur Respir J. 2011;38(4):781–788. doi: 10.1183/09031936.00167110. [DOI] [PubMed] [Google Scholar]
- 156.Griffiths TL, Burr ML, Campbell IA, et al. Results at 1 year of outpatient multidisciplinary pulmonary rehabilitation: a randomised controlled trial. Lancet. 2000;355(9201):362–368. doi: 10.1016/s0140-6736(99)07042-7. [DOI] [PubMed] [Google Scholar]
- 157.Steiner MC, Roubenoff R, Tal-Singer R, Polkey MI. Prospects for the development of effective pharmacotherapy targeted at the skeletal muscles in chronic obstructive pulmonary disease: a translational review. Thorax. Epub May. 2012;5 doi: 10.1136/thoraxjnl-2012-201765. [DOI] [PubMed] [Google Scholar]
- 158.Rennard SI, Fogarty C, Kelsen S, et al. COPD Investigators. The safety and efficacy of infliximab in moderate to severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;175(9):926–934. doi: 10.1164/rccm.200607-995OC. [DOI] [PubMed] [Google Scholar]
- 159.Yeh SS, DeGuzman B, Kramer T M012 Study Group. Reversal of COPD-associated weight loss using the anabolic agent oxandrolone. Chest. 2002;122(2):421–428. doi: 10.1378/chest.122.2.421. [DOI] [PubMed] [Google Scholar]
- 160.Nagaya N, Itoh T, Murakami S, et al. Treatment of cachexia with ghrelin in patients with COPD. Chest. 2005;128(3):1187–1193. doi: 10.1378/chest.128.3.1187. [DOI] [PubMed] [Google Scholar]
- 161.Narayanan R, Mohler ML, Bohl CE, Miller DD, Dalton JT. Selective androgen receptor modulators in preclinical and clinical development. Nucl Recept Signal. 2008;6:e010. doi: 10.1621/nrs.06010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sumukadas D, Witham MD, Struthers AD, McMurdo ME. Effect of perindopril on physical function in elderly people with functional impairment: a randomized controlled trial. CMAJ. 2007;177(8):867–874. doi: 10.1503/cmaj.061339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Doorduin J, Sinderby CA, Beck J, et al. The calcium sensitizer levosimendan improves human diaphragm function. Am J Respir Crit Care Med. 2012;185(1):90–95. doi: 10.1164/rccm.201107-1268OC. [DOI] [PubMed] [Google Scholar]
- 164.Ande SR, Chen J, Maddika S. The ubiquitin pathway: an emerging drug target in cancer therapy. Eur J Pharmacol. 2009;625(1–3):199–205. doi: 10.1016/j.ejphar.2009.08.042. [DOI] [PubMed] [Google Scholar]
- 165.Narkar VA, Downes M, Yu RT, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134(3):405–415. doi: 10.1016/j.cell.2008.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]