Abstract
Introduction
Exercise limitation, dynamic hyperinflation, and exertional dyspnea are key features of symptomatic chronic obstructive pulmonary disease (COPD). We assessed the effects of glycopyrronium bromide (NVA237), a once-daily, long-acting muscarinic antagonist, on exercise tolerance in patients with moderate to severe COPD.
Methods
Patients were randomized to a cross-over design of once-daily NVA237 50 μg or placebo for 3 weeks, with a 14-day washout. Exercise endurance, inspiratory capacity (IC) during exercise, IC and expiratory volumes from spirometry, plethysmographic lung volumes, leg discomfort and dyspnea under exercise (Borg scales), and transition dyspnea index were measured on Days 1 and 21 of treatment. The primary endpoint was endurance time during a submaximal constant-load cycle ergometry test on Day 21.
Results
A total of 108 patients were randomized to different treatment groups (mean age, 60.5 years; mean post-bronchodilator, forced expiratory volume in 1 second [FEV1] 57.1% predicted). Ninety-five patients completed the study. On Day 21, a 21% difference in endurance time was observed between patients treated with NVA237 and those treated with placebo (P < 0.001); the effect was also significant from Day 1, with an increase of 10%. Dynamic IC at exercise isotime and trough FEV1 showed significant and clinically relevant improvements from Day 1 of treatment that were maintained throughout the study. This was accompanied by inverse decreases in residual volume and functional residual capacity. NVA237 was superior to placebo (P < 0.05) in decreasing leg discomfort (Borg CR10 scale) on Day 21 and exertional dyspnea on Days 1 and 21 (transition dyspnea index and Borg CR10 scale at isotime). The safety profile of NVA237 was similar to that of the placebo.
Conclusion
NVA237 50 μg once daily produced immediate and significant improvement in exercise tolerance from Day 1. This was accompanied by sustained reductions in lung hyperinflation (indicated by sustained and significant improvements in IC at isotime), and meaningful improvements in trough FEV1 and dyspnea. Improvements in exercise endurance increased over time, suggesting that mechanisms beyond improved lung function may be involved in enhanced exercise tolerance. (ClinicalTrials.gov Identifier: NCT01154127).
Keywords: COPD, dyspnea, FEV1, exercise tolerance, LAMA, NVA237
Introduction
Chronic obstructive pulmonary disease (COPD) is characterized by exertional dyspnea and reduced exercise tolerance.1–3 One of the major factors in exercise tolerance limitation in COPD is dynamic hyperinflation during exercise, which results in a disparity between inspiratory effort and ventilatory output, and is likely to be important in individuals with moderate-to-severe COPD.2,4 Lung hyperinflation causes a decrease in inspiratory capacity (IC) and an increase in end-expiratory lung volume, particularly during exercise, which results in dyspnea and exercise intolerance.5–7
Limitation in exercise capacity is a key feature of COPD and is one of the main factors to negatively impact patients’ quality of life.8 Improving exercise tolerance, lung hyperinflation, and dyspnea are therefore important goals in the therapeutic management of COPD as it will help patients be more active during the day. Inhaled muscarinic antagonists, which include ipratropium and tiotropium bromide, are one of the principal classes of bronchodilators used for the treatment of COPD. Short-acting ipratropium9,10 and long-acting tiotropium11,12 are known to improve lung function and reduce COPD symptoms. Both agents are generally well-tolerated in patients with COPD. Furthermore, long-acting muscarinic antagonists (LAMAs) have been shown to increase IC both at rest (prior to exercise) and during exercise in patients with COPD by reducing lung hyperinflation, with a resulting improvement in exercise capacity.13–15 Short-acting bronchodilators have not consistently been shown to improve exercise tolerance or dynamic hyperinflation.16
Glycopyrronium bromide (NVA237) is a novel, once-daily LAMA under development for the treatment of COPD. Preliminary studies in patients with moderate to severe COPD have shown that once-daily NVA237 provides sustained 24-hour bronchodilation, has, like short-acting bronchodilators, a rapid onset of action, and is well-tolerated, with a good safety profile.15,17,18 Results from the recently completed GLOW1 trial, a Phase III, randomized, placebo-controlled study lasting 26 weeks, support the findings of preliminary studies and confirm the efficacy and safety of NVA237 50 μg in patients with moderate to severe COPD.19
The principal objective of the Phase III GLycopyrronium bromide in COPD airWays clinical study 3 (GLOW3) was to evaluate the effect of once-daily NVA237 50 μg on exercise tolerance in patients with moderate to severe COPD.
Methods
Patients
Men and women with moderate to severe COPD (as defined in the 2008 Global Initiative for Chronic Obstructive Lung Disease [GOLD] guidelines) were eligible for enrolment in the GLOW3 trial if they were aged ≥40 years, had a smoking history of at least 10 pack-years, post-bronchodilator forced expiratory volume in 1 second (FEV1) of <80% and ≥40% of predicted normal, and post-bronchodilator FEV1/forced vital capacity (FVC) of <70%.
Patients were not eligible for the study if they fulfilled any of the following criteria: lower respiratory tract infection within the previous 6 weeks, required oxygen for chronic hypoxemia, concomitant pulmonary disease, history of asthma, history of malignancy within the previous 5 years (except localized basal cell carcinoma of the skin), history of long QT syndrome, QTc >450 ms for males or >470 ms for females, symptomatic prostatic hyperplasia, bladder-neck obstruction, moderate/severe renal impairment, urinary retention, narrow-angle glaucoma, or history of alpha-1 antitrypsin deficiency. Additionally, patients were excluded who had experienced adverse reactions to inhaled anticholinergic agents, long- and short-acting β2-agonists or sympathomimetic amines, were unable to use any of the study devices or perform spirometry procedures, were involved in the active phase of a supervised pulmonary rehabilitation program, women of child-bearing potential not using an accepted form of contraception, pregnant women, and nursing mothers. The maximal workload (Wmax) for each patient was determined at screening using the incremental cycle endurance test. This test consisted of 3 minutes resting, then 3 minutes of unloaded pedaling. Following this, at 60 revolutions/minute, a 10 W workload was added that was increased by 10 W/minute until exhaustion. Finally, patients undertook a 2-minute unloaded pedaling recovery period. Wmax was defined as the greatest workload maintained for a continuous 30-second period, at ≥40 revolutions/minute. Patients with a Wmax value <20 W, and patients whose exercise endurance time at sub-maximal workload was >25 minutes at baseline, were excluded.
Patients using fixed combinations of β2-agonists and inhaled corticosteroids prior to the study were transferred to the dose of inhaled corticosteroid contained in the combination product, with a washout period of ≥48 h prior to screening. Patients were provided with a salbutamol inhaler to use as rescue medication throughout the study. The use of long-acting anticholinergics, short-acting anticholinergics, long-acting and short-acting β2-agonists other than rescue medication used in the study, xanthines, parenteral or oral corticosteroids, intramuscular depot corticosteroids, roflumilast, cromoglycate, nedocromil, leukotriene antagonists, ketotifen, or systemic anticholinergics were not allowed during the study.
Study design and treatments
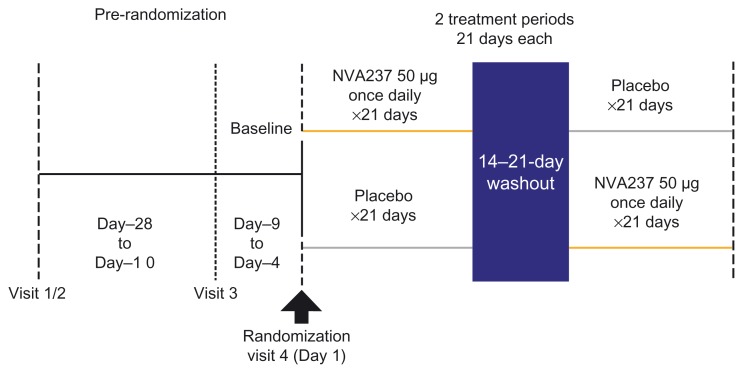
In this multicenter trial, patients were randomized within a cross-over design into two treatment arms: once-daily NVA237 50 μg followed by placebo or placebo followed by once-daily NVA237 50 μg, for 3 weeks, with a 14-day washout (Figure 1). At visit 1 of 2, patient eligibility was determined, COPD treatment was adjusted if required, and incremental exercise and bronchial reversibility tests were performed. At visit 3, baseline measurements of spirometry, body plethysmography, and a submaximal constant-load cycle ergometry test (SMET; described below) were taken. Study drugs were administered via a low-resistance, single-dose, dry-powder inhaler (Breezhaler®; Novartis Pharmaceuticals UK Ltd, Camberley, UK) in the morning between 08:00–11:00 hours.
Figure 1.
GLOW3 study design.
At visit 1 of 2, all patients gave written, informed consent to participate in the study, which was conducted according to the principles of Good Clinical Practice and the Declaration of Helsinki. The protocol was reviewed and approved by institutional review boards and ethics committees at each study center.
Efficacy assessments
The primary outcome measure was the effect of NVA237 50 μg on exercise tolerance versus placebo after 3 weeks of treatment (Day 21). Days 1 and 21, one hour after drug administration, exercise tolerance was measured by exercise endurance time during a SMET. Submaximal exercise testing was performed as described by the European Respiratory Society (ERS) Task Force on Standardization of Clinical Exercise Testing20 at 80% of maximum work capacity evaluated at screening (during the incremental cycle endurance test). Briefly, 3 minutes of rest on the bicycle was followed by 1 minute of unloaded pedaling. Patients then pedaled at 80% of Wmax until exhaustion, and finally pedaled unloaded for ≥2 minutes (recovery period). Exercise endurance was time from the commencement of loaded pedaling to stopping the exercise.
Spirometrically measured IC at isotime (measurements were taken within the final 30 seconds of each 2-minute interval of exercise; isotime refers to the last time point in the SMET at which the patient had a valid test result for both treatment periods) was a key secondary variable. Other secondary variables included IC at rest (post-dose, pre-SMET) and at peak during SMET (immediately prior to patient stopping exercise),21 peak and trough FEV1 and FVC measured by spirometry, functional residual capacity (FRC), residual volume (RV), total lung capacity (TLC), and specific airway conductance (sGAW) measured using plethysmography, exertional dyspnea (Borg CR10 Scale®) during SMET, and leg discomfort (Borg CR10 Scale®) during SMET after 3 weeks of treatment. Dyspnea and leg discomfort measurements were taken at the same time as IC measurements. Heart rate and blood pressure were measured directly prior to starting SMET and at the end of the preloaded exercise; heart rate and blood pressure over the corresponding time interval were then compared between treatments. All endpoints, with the exception of the Transition Dyspnea Index (TDI), were also assessed on treatment Day 1.
Quality assurance methods were put in place to ensure the quality of exercise endurance tests and data collection of data; where possible, the same spirometry equipment was used for all assessments performed by a patient during the study. Spirometers were calibrated for each study visit. All investigators received detailed training on the exercise endurance test methodology (with the training including practice runs of the exercise endurance tests). At site initiation visits, practice runs were conducted for pulmonary function and exercise tests to ensure that site staff were familiar with and competent in the tests; a limited number of staff, as designated by the investigator, evaluated all patients at all visits throughout the entire trial (where possible, the same technician performed all maneuvers for an individual patient to ensure consistency).
Safety assessments
The safety analysis population included all patients who received at least one dose of the study drug. Safety was assessed by recording of treatment-emergent adverse events (AEs) and monitoring of vital signs, electrocardiograms (ECGs), and laboratory analyses. Daily patient symptoms (respiratory symptoms, breathless feeling, sputum production, sputum color, cough, and wheeze) were assessed from screening until the last study visit in the morning and in the evening. AEs were recorded for both treatment periods for each patient. AEs were coded using the Medical Dictionary of Regulatory Activities (MedDRA) and summarized according to body system, severity, and preferred term for each treatment.
Statistics
We calculated that a sample size of 80 patients should have 80% power to detect a 90-second improvement in exercise endurance between NVA237 and the placebo, under the assumption that the variability in the measurement, in terms of within-patient standard deviation, was approximately 200 seconds. A blinded, interim sample size re-estimation was performed after 24 patients completed the study, ie, when 24 patients had completed both treatment periods. Because the blinded estimate of the standard deviation was less than 200 seconds, no change was made to the planned sample size of 80 patients. However, to allow for patients dropping out of the study, recruitment into the study continued during the interim analysis, resulting in a total of 108 patients randomized into the study. Analyses included data from both cross-over groups across both treatment periods for each patient included.
All pharmacodynamic (PD) endpoints, including exercise tolerance and spirometry endpoints, were analyzed using an analysis of covariance mixed model with sequence, period, baseline covariate, and treatment as a fixed effect and patient as a random effect. Patients were included in the PD analysis set if they completed at least one treatment period, had an evaluable PD assessment on Day 21 of this period, and had no major protocol deviation impacting primary PD assessment. Treatment least-squares means (LSM), LSM difference (NVA237–placebo), and corresponding 95% confidence intervals (CI) were estimated.
Results
Patient disposition and baseline characteristics
A total of 108 patients were randomized into the study. Ninety-five patients (88%) completed the study, with 3.7% patients completing only Period 1. Reasons for discontinuation included AEs (n = 9; 8.3%), protocol deviation (n = 1; 0.9%), abnormal test results (n = 1; 0.9%), and withdrawal of consent (n = 2; 1.9%). Mean age was 60.5 years, 58% of participants were male, and most were Caucasian (96%). Other baseline demographic and clinical characteristics are shown in Table 1. Mean Wmax for the incremental work test at screening was 87.8 W (standard deviation [SD] 28.8); submaximal workload averaged 70.2 W (SD 23.05).
Table 1.
Disposition and baseline demographic and clinical characteristics
| NVA237 50 μg/placebo (n = 55) | Placebo/NVA237 50 μg (n = 53) | Total (n = 108) | |
|---|---|---|---|
| Mean (SD) age, years | 61.3 (8.5) | 59.7 (8.78) | 60.5 (8.64) |
| Range | 42–80 | 41–76 | 41–80 |
| Male gender, n (%) | 30 (55) | 33 (62) | 63 (58) |
| Mean (SD) body mass index, kg/m2 | 26.4 (3.79) | 26.7 (4.23) | 26.6 (4.00) |
| Smoking history, n (%) | |||
| Ex-smoker | 27 (49) | 16 (30) | 43 (40) |
| Current smoker | 28 (51) | 37 (70) | 65 (60) |
| Mean (SD) duration of smoking, pack years | 41.4 (18.65) | 51.0 (22.70) | 46.1 (21.20) |
| Prior ICS use*, n (%) | 19 (35) | 18 (34) | 37 (34.3) |
| Mean (SD) post-bronchodilator FEV1, L | 1.6 (0.43) | 1.7 (0.46) | 1.7 (0.45) |
| Mean (SD) post-bronchodilator FVC, L | 3.4 (1.08) | 3.6 (0.96) | 3.5 (1.02) |
| Mean (SD) post-bronchodilator FEV1 percentage predicted, % | 57.3 (8.25) | 57.0 (8.86) | 57.1 (8.52) |
| Mean (SD) post-bronchodilator FEV1 reversibility, % | 19.8 (12.6) | 18.6 (10.2) | 19.2 (11.43) |
| Mean (SD) post-bronchodilator FEV1/FVC, % | 0.5 (0.1) | 0.5 (0.08) | 0.5 (0.09) |
Note:
Inhaled corticosteroids considered were budesonide (with/without formoterol fumarate), fluticasone propionate, and fluticasone proprionate with salmeterol.
Abbreviations: FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ICS, inhaled corticosteroid; SD, standard deviation.
Efficacy
Exercise testing
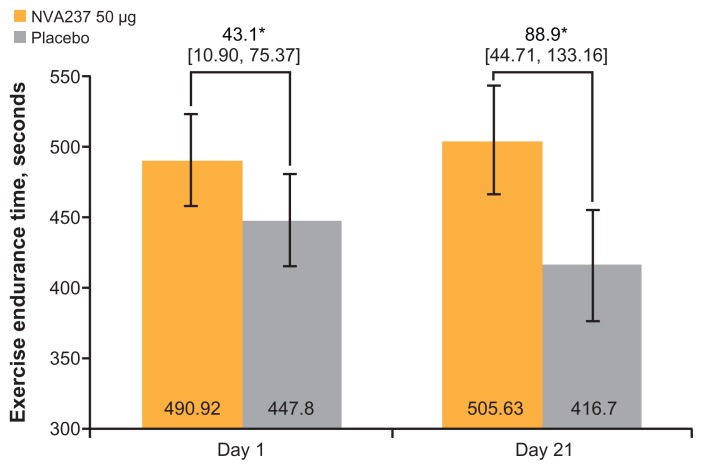
NVA237 treatment was statistically significantly superior to placebo with respect to exercise endurance time after 3 weeks (on Day 21) of treatment. The LSM treatment difference on Day 21 between treatment groups was 88.9 seconds, corresponding to an approximately 21% difference (P < 0.001; Figure 2). On Day 1, the LSM treatment difference between groups was 43.1 seconds, corresponding to an approximately 10% difference (P < 0.001; Figure 2).
Figure 2.
Exercise endurance time on Days 1 and 21.
Notes: Values are LSM [95% CI]. *P < 0.001.
Spirometry
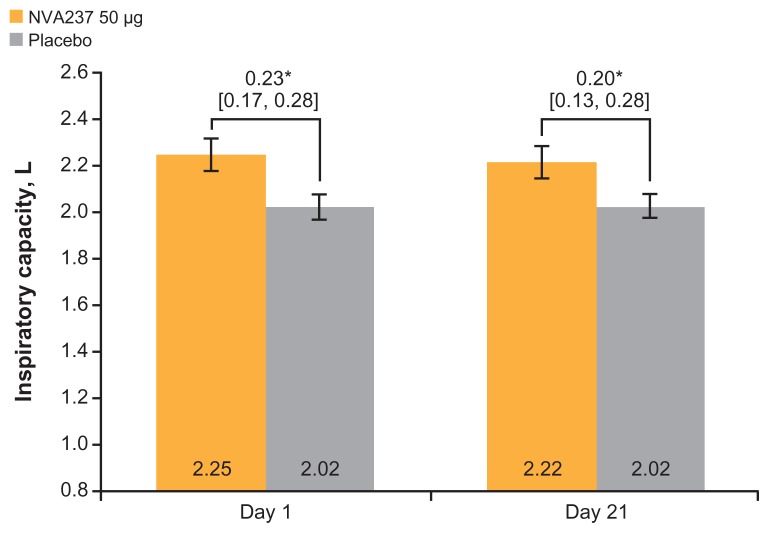
NVA237 produced a statistically significant treatment difference in IC at isotime on Day 1 versus placebo (P < 0.001) and on Day 21 versus placebo (P < 0.001; Figure 3). IC at rest (prior to exercise) and at peak exercise was consistently higher for NVA237 versus the placebo on Days 1 and 21 (Table 2). Treatment differences for IC at rest (prior to exercise) and at peak exercise between NVA237 and placebo were statistically significant (P < 0.05).
Figure 3.
Inspiratory capacity at isotime on Days 1 and 21.
Notes: Values are LSM (95% CI). *P < 0.001.
Table 2.
IC at rest (prior to exercise) and peak exercise on Days 1 and 21
| NVA237 (LSM, 95% CI) | Placebo (LSM, 95% CI) | NVA237–placebo (LSM difference, 95% CI) | |
|---|---|---|---|
| IC at peak exercise, L | |||
| Day 1 | 2.23 (2.18, 2.29) | 2.02 (1.96, 2.07) | 0.22 (0.16, 0.27) |
| Day 21 | 2.22 (2.16, 2.28) | 2.03 (1.97, 2.10) | 0.19 (0.12, 0.25) |
| IC at rest (Bodybox)*, L | |||
| Day 1 | 2.53 (2.46, 2.61) | 2.24 (2.16, 2.32) | 0.29 (0.19, 0.40) |
| Day 21 | 2.49 (2.40, 2.59) | 2.26 (2.17, 2.35) | 0.23 (0.14, 0.33) |
| IC at rest (Spirometry), L | |||
| Day 1 | 2.44 (2.38, 2.50) | 2.18 (2.12, 2.24) | 0.26 (0.18, 0.33) |
| Day 21 | 2.39 (2.33, 2.45) | 2.17 (2.10, 2.23) | 0.22 (0.15, 0.30) |
Note:
Whole body plethysmography.
Abbreviations: CI, confidence interval; IC, inspiratory capacity; LSM, least square mean.
Mean trough FEV1 on both Days 1 and 21 was significantly higher in patients receiving NVA237 (P < 0.05; Table 3). Peak FEV1 on Days 1 and 21 was also superior for NVA237. Additionally, patients receiving NVA237 showed significantly improved FRC, RV, sGAW, and TLC values versus the placebo on Day 21 (P < 0.05) (Table 3).
Table 3.
Trough and peak FEV1 and body plethysmography parameters on Days 1 and 21
| NVA237 (LSM, 95% CI) | Placebo (LSM, 95% CI) | NVA237–placebo (LSM difference, 95% CI) | P-value | |
|---|---|---|---|---|
| Trough FEV1, L | ||||
| Day 1 | 1.46 (1.43, 1.49) | 1.35 (1.31, 1.38) | 0.11 (0.06, 0.16) | |
| Day 21 | 1.44 (1.40, 1.48) | 1.33 (1.29, 1.37) | 0.11 (0.06, 0.16) | <0.05 |
| Peak FEV1, L | ||||
| Day 1 | 1.59 (1.56, 1.62) | 1.37 (1.34, 1.40) | 0.22 (0.18, 0.26) | |
| Day 21 | 1.60 (1.56, 1.64) | 1.35 (1.31, 1.39) | 0.25 (0.19, 0.30) | <0.05 |
| Functional residual capacity, L | ||||
| Day 1 | 4.41 (4.32, 4.51) | 4.77 (4.67, 4.86) | −0.36 (−0.49, −0.22) | |
| Day 21 | 4.32 (4.22, 4.42) | 4.78 (4.68, 4.87) | −0.46 (−0.58, −0.33) | <0.05 |
| Residual volume, L | ||||
| Day 1 | 3.49 (3.38, 3.59) | 3.92 (3.82, 4.02) | −0.44 (−0.58, −0.29) | |
| Day 21 | 3.46 (3.36, 3.55) | 3.95 (3.86, 4.05) | −0.50 (−0.63, −0.36) | <0.05 |
| sGAW, s−1 · kPa−1 | ||||
| Day 1 | 0.68 (0.65, 0.71) | 0.41 (0.38, 0.45) | 0.26 (0.22, 0.30) | |
| Day 21 | 0.66 (0.63, 0.70) | 0.42 (0.39, 0.46) | 0.24 (0.19, 0.29) | <0.05 |
| Total lung capacity, L | ||||
| Day 1 | 7.01 (6.90, 7.12) | 7.08 (6.97, 7.19) | −0.07 (−0.22, 0.08) | |
| Day 21 | 6.86 (6.75, 6.97) | 7.10 (6.99, 7.21) | −0.25 (−0.39, −0.10) | <0.05 |
Abbreviations: CI, confidence interval; FEV1, forced expiratory volume in 1 second; LSM, least square mean; sGAW, specific airway conductance.
Symptoms
Daily symptoms of patients showed a trend towards a higher number of patients with no or mild symptoms following NVA237 treatment compared with symptoms resulting from placebo administration on most treatment days.
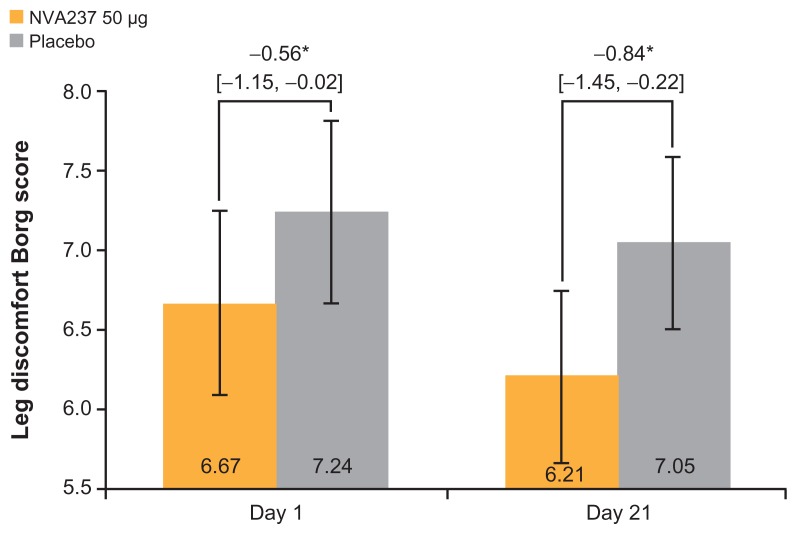
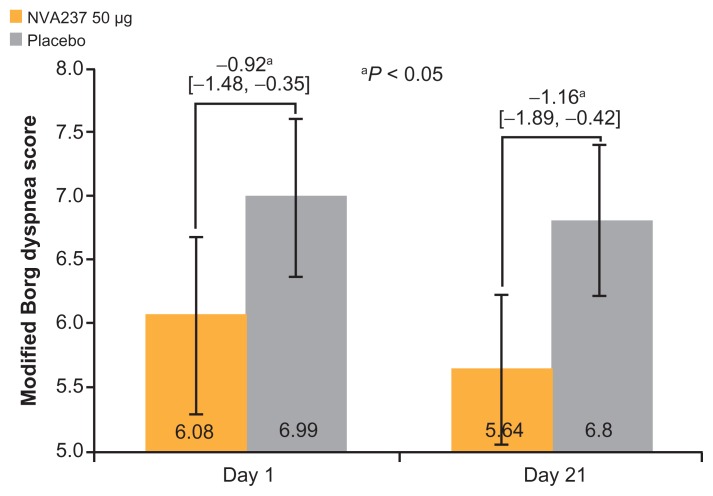
NVA237 was found to be superior to the placebo in decreasing leg discomfort on Day 21 and exertional dyspnea on Days 1 and 21 at isotime. On Day 21, the leg discomfort Borg score was significantly reduced in patients receiving NVA237 compared with those receiving the placebo (P < 0.05; Figure 4). A numerical trend in favor of NVA237 on Day 1 was also observed for leg discomfort. On Day 21, Modified Borg Dyspnea Score improved by 20% with NVA237 treatment versus placebo treatment (treatment difference: −1.16; P < 0.05; Figure 5); on Day 1, the treatment difference was −0.92 (P < 0.05).
Figure 4.
Leg discomfort Borg score at isotime on Days 1 and 21.
Notes: Values are LSM [95% CI]. *P < 0.05.
Figure 5.
Modified Borg dyspnea score at isotime on Days 1 and 21.
Note: Values are LSM (95% CI).
On Day 21, the focal score for TDI was higher in NVA237- (2.78) versus placebo-treated patients (0.49), with a treatment difference of 2.29, which exceeded the 1-point treatment difference considered as clinically important.22 The number of puffs of rescue medication per day was lower in patients receiving NVA237, with 14 NVA237-treated patients (14.1%) taking no rescue medication compared with 9 (9.4%) placebo recipients. The total number of rescue mediations taken between Days 1 and 21 was lower in patients receiving NVA237 (LSM 42.0) than in those receiving the placebo (49.8), with an LSM difference of −7.8.
Blood pressure
No effects of NVA237 versus the placebo at Days 1 or 21 were observed on systolic and diastolic blood pressure and heart rate at isotime during the exercise test. On Day 1, LSM (95% CI) diastolic blood pressure was 88.0 (85.3, 90.6) in patients receiving NVA237 and 88.2 (85.6, 90.9) in patients receiving the placebo, systolic blood pressure was 169.1 (163.6, 174.6) and 168.6 (163.1, 174.2), respectively, and heart rate was 124.7 (122.2, 127.2) and 127.8 (125.4, 130.3), respectively. On Day 21, LSM (95% CI) diastolic blood pressure was 87.6 (85.1, 90.2) in patients receiving NVA237 and 87.5 (85.0, 90.0) in placebo recipients, systolic blood pressure was 170.2 (165.5, 174.9) and 171.2 (166.5, 175.8), respectively, and heart rate was 126.0 (122.6, 129.4) and 125.9 (122.5, 129.3), respectively.
Safety
The proportion of patients experiencing at least one AE was similar in patients receiving NVA237 compared to those receiving the placebo (Table 4). Most AEs were mildly or moderately severe and not considered to be related to the study drug. Discontinuations due to AEs were low for patients treated with NVA237 and the placebo (Table 4). No death occurred during the study. The only serious AE reported during the study was a herniated disc diagnosed in a patient while receiving NVA237, which was not suspected to be drug-related. This patient was discontinued from the study. No clinically relevant changes were observed in vital signs, ECGs, and hematology or biochemistry parameters during the study.
Table 4.
Frequency of AEs occurring in ≥2 patients overall, serious AEs and discontinuations due to AEs (safety population)
| NVA237 50 μg (n = 102) | Placebo (n = 102) | |
|---|---|---|
| Patients with AEs, n (%) | 30 (29.4) | 25 (24.5) |
| Nasopharyngitis | 5 (4.9) | 4 (3.9) |
| Headache | 3 (2.9) | 4 (3.9) |
| COPD worsening | 3 (2.9) | 3 (2.9) |
| Back pain | 3 (2.9) | 2 (2.0) |
| Cough | 3 (2.9) | 1 (1.0) |
| Arthralgia | 1 (1.0) | 1 (1.0) |
| Joint swelling | 0 (0) | 2 (2.0) |
| Oropharyngeal pain | 2 (2.0) | 0 (0) |
| Rhinorrhea | 2 (2.0) | 0 (0) |
| Toothache | 1 (1.0) | 1 (1.0) |
| Patients with serious AEs, n (%) | 1 (1.0) | 0 (0) |
| Intervertebral disc protrusion | 1 (1.0) | 0 (0) |
| Discontinuations due to AEs, n (%) | 5 (9.1) | 4 (7.5) |
Abbreviations: AE, adverse event; COPD, chronic obstructive pulmonary disease.
Discussion
The results from the GLOW3 study showed that treatment with once-daily NVA237 50 μg for 3 weeks improves exercise tolerance in patients with moderate to severe COPD. Furthermore, meaningful improvements were observed in trough FEV1, accompanied by sustained reductions of lung hyperinflation at rest, signified by sustained and significant improvements in resting IC, RV, and FRC, and during exercise, signified by sustained and significant improvements in IC at isotime during exercise. A significant improvement in exercise endurance (21% vs placebo after 3-weeks of treatment; mean treatment difference 88.9 seconds) was reported by patients receiving NVA237, an effect that is perceptible by patients and is in the upper range of suggested thresholds for the clinically meaningful difference in exercise tolerance tests.23,24 Importantly, improvement in exercise endurance in NVA237-treated patients was visible from the first dose. Effects of NVA237 on exercise endurance are in the range of those observed in a previous double-blind, placebo-controlled study involving administration of once-daily LAMA, tiotropium, in which a statistically significant difference in endurance time was reported between tiotropium and placebo after 21 days (13.6%, P = 0.039) and after 42 days of treatment (21.4%, P = 0.0098).14
The magnitude of the effect increased during the 3-week treatment period, which may have been due to further adaptive responses following improved ventilatory mechanics. It is thus possible that treatment periods longer than 3 weeks may yield larger treatment improvements in exercise endurance.13,14 Additionally, reduced airway obstruction may, over time, result in increased activity with a training effect and lead to further improvements in exercise tolerance. Such improvements in exercise capacity have been noted in studies examining pulmonary rehabilitation.25
Improvement in exercise capacity is recognized as an important goal in the management of COPD.5 Dyspnea is known to adversely affect patient’s health-related quality of life.26 Additionally, reduced exercise capacity and dyspnea are associated with increased mortality.27,28
It has been suggested that the most important factor limiting exercise endurance in patients with COPD is dynamic hyperinflation,7 which typically results in decrease inspiratory capacity during exercise.29 The immediate functional improvement in exercise endurance afforded by NVA237 (10% vs placebo on Day 1) is therefore potentially significant. Increased IC with NVA237 may have facilitated greater expansion of tidal volume, contributing to significant improvements in dyspnea and exercise endurance.14 The magnitude of the effect of NVA237 on IC was the same on Day 1 and Day 21, indicating that NVA237 reduced dynamic hyperinflation after the first dose and maintained the effect over the 3-week treatment period. This reflects the full bronchodilator activity of NVA237 from the first dose onwards.19 NVA237 produced clinically meaningful improvements in trough FEV1 versus treatment with the placebo (0.11 L increase on Day 21). The magnitude of effect observed is consistent with the results of previous studies examining the use of NVA237.15,17,19 A comprehensive set of spirometric and body plethysmographic endpoints were investigated under resting conditions in the GLOW3 study. In addition to improvements in IC, NVA237 consistently decreased plethysmographic lung volumes, FRC, RV, and TLC, confirming that the observed effects on IC were reflective of decreased lung hyperinflation. Measuring sGAW using plethysmography can provide information regarding bronchodilator-induced changes in airway caliber, as an increase in airway diameter improves airway conductance. This measurement has been used as a sensitive method for assessing bronchodilator effects in clinical trials.30,31 NVA237 caused a large improvement in sGAW on Day 1, which was sustained through Day 21, confirming the time course of the changes in lung function observed using spirometry.
Breathlessness has been reported as one of the most frequent activity-limiting symptoms in patients with COPD.32 Thus, two measures of dyspnea (Borg CR10 and TDI) were included in this study. NVA237 significantly improved exertional dyspnea on Days 1 and 21 at isotime. NVA237 also resulted in statistically significant increases in TDI score that exceeded the 1-point difference considered clinically important. 22 Reduction in hyperinflation with NVA237 treatment, demonstrated by an increase in IC on Days 1 and 21 in the current study, is an important factor that likely contributed to the improvement in dyspnea and exercise capacity. Exercise-limiting leg discomfort has also been frequently reported in COPD patients.29,33 NVA237 significantly improved the Borg CR10 score on Day 21 versus placebo administration. Notably, leg discomfort tended to be lower in NVA237- versus placebo-treated patients on Day 1. From a mechanistic perspective, better oxygenation of the leg muscles is expected to lead to some improvement in muscular function. However, this finding has not been consistently found in other trials with bronchodilators. For example, treatment with the short acting bronchodilator ipratropium were not associated with a significant improvement in leg discomfort score.34 In the present study, a significant improvement was observed at isotime during exercise, whereas no differences were observed at peak exercise. Given the fact, that the exercise test used is symptom-limited, this finding at peak is not surprising since it reflects the cessation of exercise by patients due to intolerable dyspnea or leg discomfort. In contrast, the observed differences in the level of leg discomfort at isotime may have been the result of improved ventilation with NVA237, leading to improved oxygenation and peripheral muscle function during a given workload, and flattening of the slope of leg discomfort as a function of endurance time.
Importantly, consistency between improved lung function and body plethysmographic measures were observed as well as improved dyspnea measures at rest and exercise in this study. This may underscore the relevance of bronchodilatory therapy for improving exercise capacity.
In terms of safety, NVA237 50 μg once daily was well-tolerated and showed an acceptable safety profile. Safety results are consistent with those from previous studies.15,17–19
Limitations of this study include short time period and that it was not coupled with another intervention, such as rehabilitation, to augment the response to therapy. Both a longer duration of the trial beyond 3 weeks of treatment and combining therapy with a pulmonary rehabilitation program may lead to further clinically meaningful effects. Additionally, cycle ergometry is more artificial than other methods such as a walking test; some patients may not be used to this type of exercise. It has been shown that stopping exercise due to leg discomfort occurs more frequently when cycling than during walking tests.35 Thus, the effects of treatment on cycling endurance may be more difficult to translate into changes for COPD patients’ everyday activities than a walking test. Nevertheless, cycling ergometry has been shown to be reliable and safe.36 Furthermore, cycle ergometry offers a relatively easy and standardized method for measuring dynamic IC, in contrast to a walking test, which requires portable systems for measuring respiratory parameters throughout the exercise, thereby making obtaining reliable measures of IC more challenging.37
In conclusion, once-daily treatment with NVA237 results in significant improvement in exercise tolerance beginning with the first dose. Additionally, NVA237 reduces lung hyperinflation and offers clinically meaningful improvements in trough FEV1 and breathlessness.
Acknowledgments
The authors were assisted in preparing the manuscript by Melanie Stephens, a professional medical writer contracted to CircleScience (Macclesfield, UK), and Mark Fedele ( Novartis). Writing support was funded by the study sponsor.
Footnotes
Authors’ contributions
All authors were involved in drafting the manuscript or critical revisions for important intellectual content and gave final approval of the version to be published.
Kai M Beeh participated in the design of the study, provided study-specific exercise, spirometry, and plethysmography training and certification, and was the lead and coordinating investigator of the trial. Lilla Di Scala performed biostatistics calculations.
Anton Drollmann is an employee of the sponsor Novartis and was involved in designing the study, authoring the medical aspects of the study protocol, and supervising the conduct, analysis, and publication of the study medically and scientifically. Dave Singh was an investigator during the study and was involved in recruiting patients and collecting data.
Disclosure
This study was sponsored by Novartis Pharma AG. Lilla Di Scala and Anton Drollmann are employees of Novartis. Kai M Beeh has received compensation for serving on advisory boards for Boehringer Ingelheim, Pfizer, Novartis, and other pharmaceutical companies. He has participated as a speaker in scientific meetings or courses organized and financed by pharmaceutical companies (AstraZeneca, Boehringer Ingelheim, Novartis, Pfizer, and Takeda) from 2006–2011. The institution where Kai M Beeh is currently employed has received compensations for design and performance, or participation in single or multicenter clinical trials in 2006–2011 from multiple companies (Almirall, Altana, AstraZeneca, Boehringer Ingelheim, Cytos, Fujisawa, GSK, Medapharma, Merck Sharp and Dohme, Novartis, Pfizer, and Revotar Biopharmaceuticals). Dave Singh has received lecture fees, research grants, consultancy fees, and support for conference attendance from various pharmaceutical companies including AstraZeneca, Almirall, Boehringer Ingleheim, Chiesi, Cipla, GlaxoSmithKline, Merck, Novartis, Nycomed, and Roche.
References
- 1.Eisner MD, Iribarren C, Yelin EH, et al. Pulmonary function and the risk of functional limitation in chronic obstructive pulmonary disease. Am J Epidemiol. 2008;167:1090–1101. doi: 10.1093/aje/kwn025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;164:770–777. doi: 10.1164/ajrccm.164.5.2012122. [DOI] [PubMed] [Google Scholar]
- 3.O’Donnell DE, Laveneziana P. The clinical importance of dynamic lung hyperinflation in COPD. COPD. 2006;3:219–232. doi: 10.1080/15412550600977478. [DOI] [PubMed] [Google Scholar]
- 4.O’Donnell DE, Bertley JC, Chau LK, Webb KA. Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiologic mechanisms. Am J Respir Crit Care Med. 1997;155:109–115. doi: 10.1164/ajrccm.155.1.9001298. [DOI] [PubMed] [Google Scholar]
- 5.GOLD. Global strategy for diagnosis, management and prevention of COPD. 2011. [Accessed July 19, 2012]. Available from: http://www.goldcopd.org/guidelines-gold-summary-2011.html.
- 6.O’Donnell DE, Lam M, Webb KA. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:542–549. doi: 10.1164/ajrccm.160.2.9901038. [DOI] [PubMed] [Google Scholar]
- 7.O’Donnell DE, Webb KA. The major limitation to exercise performance in COPD is dynamic hyperinflation. J Appl Physiol. 2008;105:753–755. doi: 10.1152/japplphysiol.90336.2008b. [DOI] [PubMed] [Google Scholar]
- 8.Esteban C, Quintana JM, Aburto M, et al. Impact of changes in physical activity on health-related quality of life among patients with COPD. Eur Respir J. 2010;36:292–300. doi: 10.1183/09031936.00021409. [DOI] [PubMed] [Google Scholar]
- 9.Taylor T, Kotch A, Rice K, et al. Ipratropium bromide hydrofluoroalkane inhalation aerosol is safe and effective in patients with COPD. Chest. 2001;120:1253–1261. doi: 10.1378/chest.120.4.1253. [DOI] [PubMed] [Google Scholar]
- 10.Cuvelier A, Muir JF, Benhamou D, et al. Dry powder ipratropium bromide is as safe and effective as metered-dose inhaler formulation: a cumulative dose-response study in chronic obstructive pulmonary disease patients. Respir Care. 2002;47:159–166. [PubMed] [Google Scholar]
- 11.Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217–224. doi: 10.1183/09031936.02.00269802. [DOI] [PubMed] [Google Scholar]
- 12.van Noord JA, Bantje TA, Eland ME, Korducki L, Cornelissen PJ. A randomised controlled comparison of tiotropium and ipratropium in the treatment of chronic obstructive pulmonary disease. The Dutch Tiotropium Study Group. Thorax. 2000;55:289–294. doi: 10.1136/thorax.55.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maltais F, Hamilton A, Marciniuk D, et al. Improvements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPD. Chest. 2005;128:1168–1178. doi: 10.1378/chest.128.3.1168. [DOI] [PubMed] [Google Scholar]
- 14.O’Donnell DE, Fluge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23:832–840. doi: 10.1183/09031936.04.00116004. [DOI] [PubMed] [Google Scholar]
- 15.Verkindre C, Fukuchi Y, Flemale A, et al. Sustained 24-h efficacy of NVA237, a once-daily long-acting muscarinic antagonist, in COPD patients. Respir Med. 2010;104:1482–1489. doi: 10.1016/j.rmed.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Vagaggini B, Nieri D, Malagrinò, et al. Acute administration of bronchodilators on exercise tolerance in treated COPD patients. Pulm Pharmacol Ther. 2011;24:49–54. doi: 10.1016/j.pupt.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Fogarty C, Hattersley H, Di SL, Drollmann A. Bronchodilatory effects of NVA237, a once daily long-acting muscarinic antagonist, in COPD patients. Respir Med. 2011;105:337–342. doi: 10.1016/j.rmed.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 18.Vogelmeier C, Verkindre C, Cheung D, et al. Safety and tolerability of NVA237, a once-daily long-acting muscarinic antagonist, in COPD patients. Pulm Pharmacol Ther. 2010;23:438–444. doi: 10.1016/j.pupt.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 19.D’Urzo A, Ferguson GT, van Noord JA, et al. Efficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trial. Respir Res. 2011;12:156. doi: 10.1186/1465-9921-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.European Respiratory Society. Clinical exercise testing with reference to lung diseases: indications, standardization and interpretation strategies. ERS Task Force on Standardization of Clinical Exercise Testing. Eur Respir J. 1997;10:2662–2689. doi: 10.1183/09031936.97.10112662. [DOI] [PubMed] [Google Scholar]
- 21.Beeh KM, Wagner F, Khindri S, Drollmann AF. Effect of indacaterol on dynamic lung hyperinflation and breathlessness in hyperinflated patients with COPD. COPD. 2011;8:340–345. doi: 10.3109/15412555.2011.594464. [DOI] [PubMed] [Google Scholar]
- 22.Witek TJ, Jr, Mahler DA. Minimal important difference of the transition dyspnoea index in a multinational clinical trial. Eur Respir J. 2003;21:267–272. doi: 10.1183/09031936.03.00068503a. [DOI] [PubMed] [Google Scholar]
- 23.Puente-Maestu L, Villar F, de Miguel J, et al. Clinical relevance of constant power exercise duration changes in COPD. Eur Respir J. 2009;34:340–345. doi: 10.1183/09031936.00078308. [DOI] [PubMed] [Google Scholar]
- 24.Cazzola M, MacNee W, Martinez FJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J. 2008;31:416–469. doi: 10.1183/09031936.00099306. [DOI] [PubMed] [Google Scholar]
- 25.Nici L, Zuwallack R. Scope, background and definition of pulmonary rehabilitation. Eur J Phys Rehabil Med. 2011;47:465–474. [PubMed] [Google Scholar]
- 26.Jacobsen R, Frølich A, Godtfredsen NS. Impact of exercise capacity on dyspnea and health-related quality of life in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil Prev. 2012;32:92–100. doi: 10.1097/HCR.0b013e31823be107. [DOI] [PubMed] [Google Scholar]
- 27.Ozgür ES, Nayci SA, Ozge C, Tasdelen B. An integrated index combined by dynamic hyperinflation and exercise capacity in the prediction of morbidity and mortality in COPD. Respir Care. 2012 Feb 17; doi: 10.4187/respcare.01440. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121:1434–1440. doi: 10.1378/chest.121.5.1434. [DOI] [PubMed] [Google Scholar]
- 29.Guenette JA, Webb KA, O’Donnell DE. Does dynamic hyperinflation contribute to dyspnoea during exercise in patients with COPD? Eur Respir J. 2011 Dec 19; doi: 10.1183/09031936.00157711. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 30.Borrill ZL, Houghton CM, Tal-Singer R, et al. The use of plethysmography and oscillometry to compare long acting bronchodilators in patients with COPD. Br J Clin Pharmacol. 2008;65:244–252. doi: 10.1111/j.1365-2125.2007.03013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh SD, Brooks J, Hagan G, Cahn A, O’Connor B. ‘Triple’ therapy with salmeterol/fluticasone propionate and tiotropium bromide versus individual components in moderate to severe COPD. Thorax. 2008;63:592–598. doi: 10.1136/thx.2007.087213. [DOI] [PubMed] [Google Scholar]
- 32.Vogiatzis I. Strategies of muscle training in very severe COPD patients. Eur Respir J. 2011;38:971–975. doi: 10.1183/09031936.00075011. [DOI] [PubMed] [Google Scholar]
- 33.Debigaré R, Maltais F. The major limitation to exercise performance in COPD is lower limb muscle dysfunction. J Appl Physiol. 2008;105:751–753. doi: 10.1152/japplphysiol.90336.2008a. [DOI] [PubMed] [Google Scholar]
- 34.Saey D, Debigare R, LeBlanc P, et al. Contractile leg fatigue after cycle exercise: a factor limiting exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2003;168:425–430. doi: 10.1164/rccm.200208-856OC. [DOI] [PubMed] [Google Scholar]
- 35.Pepin V, Saey D, Whittom F, et al. Walking versus cycling: sensitivity to bronchodilation in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;172:1517–1522. doi: 10.1164/rccm.200507-1037OC. [DOI] [PubMed] [Google Scholar]
- 36.American Thoracic Society and American College of Chest Physicians. ATS/ACCP statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:211–277. doi: 10.1164/rccm.167.2.211. [DOI] [PubMed] [Google Scholar]
- 37.Brouillard C, Pepin V, Milot J, Lacasse Y, Maltais F. Endurance shuttle walking test: responsiveness to salmeterol in COPD. Eur Respir J. 2008;31:579–584. doi: 10.1183/09031936.00119007. [DOI] [PubMed] [Google Scholar]