Abstract
We describe how room temperature storage of a 1,120 member compound library prepared in either DMSO or in a hydrated DMSO/water (67/33) mixture affects the reproducibility of potency values as monitored using cytochrome P450 1A2 and 2D6 isozyme assays. The bioluminescent assays showed Z′-factors of 0.71 and 0.62, with 18% and 32% of the library found as active against the CYP 1A2 and 2D6 isozymes respectively. We tested the library using quantitative high-throughput screening to generate potency values for every library member which was measured at seven time intervals spanning 37 weeks. We calculated the minimum significant ratio (MSR) from these potency values at each time interval and we found that for the library stored in DMSO, the CYP 1A2 and 2D6 assay MSRs progressed from approximately 2.0 to 5.0. The hydrated conditions showed similar performance in both MSR progression and analytical QC results. Based on this study we recommend that DMSO samples be stored in 1,536-well plates for < 4 months at room temperature. Further, the study shows the magnitude of potency changes that can occur in a robust bioassay due to compound sample storage.
Keywords: HTS, compound storage, DMSO, quantitative HTS
Introduction
Compound library storage conditions must be amenable to both maintaining compound integrity and availability to screening operations. Studies of library storage conditions have focused on analytical chemistry methods, namely liquid chromatography coupled to mass spectrometry (LC-MS), to characterize the integrity of chemical libraries stored at industrial high-throughput screening (HTS) facilities.1–7 An overview of compound management by Chan and Hueso-Rodríguez,8 and an historical perspective by Archer,9 stated that storage of compound libraries in DMSO, in practice, falls into one of four strategies: ambient, +4 °C in a desiccated inert atmosphere to control water content and assure liquid solution storage, +4 °C with humidity control to encourage frozen solution storage, and −20 °C in a desiccated atmosphere to assure frozen solution storage and prevent ice build-up. In an experimental survey of compound storage issues, Cheng et al.2 monitored a collection of 644 compounds in DMSO under different temperature, water content, atmospheric conditions, and examined effects of freeze/thaw cycles. In this study, no significant loss of compound was detected after multiple freeze-thaw cycles when performed in a dry nitrogen environment, but samples stored in the presence of 5% water were less stable. A pair of papers from Procter and Gamble found that the probability of observing a compound by LC-MS decreased 48% after one year of storage in DMSO under ambient conditions, and the amount of compound detected reduced linearly with increased freeze/thaw cycles.6, 7 These studies have pointed to compound precipitation, augmented by both DMSO water content and freeze/thaw cycles, as one of the most important factors in determining compound library quality during storage.10
Measurements of purity within compound libraries have also been examined. Bowes et al.1 conducted a three-year stability study of representative compounds from the Biogen Idec library, which demonstrated greater stability of lyophilized samples compared to 10 mM DMSO stock solutions stored at 4 °C. Ilouga et al.5 studied a representative subset of the Evotec library, and predicted that in four years, the mean purity of the compound collection would decrease by about half in ambient liquid storage, while storage in frozen solutions would show more optimal results (<20%). In these studies, initial sample purity and storage in non-inert gas atmosphere were identified as factors in the decay of compounds, and supported that compound degradation is a concern in long term storage (>1 yr). 5
While the studies mentioned above have focused on analytical endpoints to measure changes due to compound storage, the manner in which an HTS assay campaign may be affected by these changes has not been addressed to date. Such an analysis would demonstrate how changes in compound quality over time are detected in a bioassay and provide additional guidelines for compound preparation during HTS or lead optimization studies. Therefore, we monitored the integrity of a compound library under ambient conditions using two robust bioassays over a period of 37 weeks. A typical HTS assay will show activity against only a small percent (typically < 1%) of the library. In our study, to ensure broad coverage of the chemical library, we used cytochrome P450 (CYP) isozymes. These enzymes have evolved to act as molecular incinerators in the liver and are known to interact with a wide range of substrates, making CYP assays ideal tools for quality assessment of diverse sets of compounds. In this study we employed CYP 1A2 and 2D6 isozymes as we have previously confirmed these assays perform well in 1,536-well microtiter plate formats, show low sensitivity to DMSO,11, 12 and are inhibited by a large number of compounds in typical compound collections (see PubChem AIDs:410 and 891 respectively). We examined compound storage in 1,536-well microtiter plates as this is central to our screening platform. Further, we used conditions that did not require investment in high-end storage units to make the study more comparable to what may be found in many academic screening laboratories that have proliferated in recent years.13, 14 The method of quantitative high throughput screening (qHTS)15, 16 was used to generate concentration-response curves (CRCs) for every library compound at each time point. Our endpoints monitored how potency, efficacy, and area under the curve (AUC) changed with compound storage time. In addition to an expected decrease in potency over time, we also found lower reproducibility of potency values and lower efficacy in older compound samples. We further examined the compound library using LC-MS in an attempt to address reasons why the potency of some compounds changed significantly over time. Based on this study we recommend ambient storage of 1,536-well compound plates at room temperature for < 4 months for screening collections. This study illustrates the magnitude of changes in apparent compound potency that can occur due to library storage.
Materials and Methods
Reagents
The luciferase-based P450-Glo™ Screening Systems were obtained from Promega (Madison, WI). The CYP 1A2 (cat# V9770) and CYP 2D6 (cat# V9890) screening systems were used in this study. DMSO and other solvents were analytical grade (Fisher ACS grade).
Preparation of compound libraries and control plates
The 1,120 compounds were from Prestwick Chemical Inc (Illkirch, France). Upon receipt, our compound management team subdivides the library into multiple, identical 384-well stock plates which are then heat sealed and stored at low temperature, −80°C. Inter-plate dilution series were prepared as described previously (see refs [15, 17]). Two stock plate sets of the Prestwick library from the same supplied batch were retrieved from low-temperature storage and thawed. To create a hydrated-DMSO copy, one stock plate set was diluted with water to a final composition of 67:33 (v/v) DMSO:water. Each of these 384-well compound library plate sets, DMSO-only and hydrated-DMSO, were reformatted onto 1,536-well plates, and serially diluted into separate working libraries and placed on Kalypsys rotating storage decks for subsequent assays. The twelve stock sample concentrations used in the working libraries were: 2,000 μM, 880 μM, 393 μM, 176 μM, 78.7 μM, 35.2 μM, 15.7 μM, 7.04 μM, 3.15 μM, 1.41 μM, 0.62 μM, 0.28 μM (final assay concentration range from 11.4 μM to 1.6 nM). After preparation of the working libraries, the remaining compound solutions in the 384-well plate sets were heat-sealed and placed back into the freezer at −80 °C below the glass transition temperature for the DMSO eutectic.18 These frozen 384-well copies are called “stored copies” throughout. These 384-well stored plate sets were thawed at the end of the experiment, characterized using CYP isozyme assays, and QC’d by LC-MS. To QC the working libraries, the highest concentration plate (2,000 μM) was re-formatted back into 384-well plates for compatibility with the LC-MS system. Additionally, an unused copy of the compound library was retrieved from low-temperature storage and characterized using the same procedure.
qHTS and storage conditions
Assays were repeated at elapsed times of 7, 42, 77, 105, 133 and 258 days; or 1, 6, 11, 15, 19, and 37 weeks following the initial measurements. Working compound library 1,536-well plates at the NIH Chemical Genomics Center (NCGC) are stored with Kalypsys lids, on revolving storage decks at room temperature, in ambient-atmosphere.17, 19 The “working copies” of the library using either DMSO or the hydrated- DMSO mixture were repeatedly accessed using a pin-tool20 at each time point of the assays.
Cytochrome P450 assays
Bioluminescent assays for CYP isozymes were based on conversion of a pro-luciferin substrate to D-luciferin, the substrate for firefly luciferase.21, 22 These assays were miniaturized to 1,536-well plates using a protocol where 2 μL of enzyme and the pro-luciferin substrate was added for final concentrations of either 10 nM or 5 nM enzyme (CYP 1A2 or 2D6, respectively). Either 100 μM or 30 μM of the pro-luciferin substrates Luc-ME (CYP 1A2) or Luc-ME EGE (CYP 2D6) were used in the assays. Using a pin-tool, 23 nL of compound was added to the assay wells.19, 20 The CYP enzyme reaction was then started by adding 2 μL of NADPH regeneration solution supplied by Promega (Madison, WI). Following 1 hour incubation at room temperature, 4 μL of the luciferin detection reagent was added. Twenty minutes later the luminescence signal was measured on a Perkin Elmer (Waltham, MA) ViewLux using a 60 second exposure time with 2x binning of the image pixels.
For the assays, controls were added from a separate 1,536-well compound plate as follows: Columns 1 and 2, 16-point titrations in duplicate of furafylline for CYP 1A2 or quinidine for CYP 2D6 (final starting assay concentration was 57 μM or 1.4 μM respectively in 1:2 dilutions); Column 3, the neutral control (DMSO); Column 4, was an IC100 concentration of the appropriate control inhibitor.
Calculation of MSR values
Concentration-response data from each screen were characterized similar to previous reports,15 using automated curve fitting software developed at the NCGC, available on the NCGC web site.23 Briefly, the software fits the data using a residual error minimization algorithm with automatic outlier determination, to a four-parameter Hill equation to obtain IC50 values. Apparent stimulators of P450 activity were minimal in both CYP 1A2 and 2D6 and showed weak activity that was treated here as inactive.
To ascertain the useable lifetime of the compound libraries, we calculated the MSR at each time point for each storage condition. In general, MSR≤3 is acceptable for a primary screening assay.24–26 Intra-run control-compound MSR values were calculated as described elsewhere,27 using the intra-run MSR formula.24, 25
Where σ is the standard deviation of log IC50 differences. Time-spaced MSR values,were calculated using the independent-run MSR equation.25
Mean ratios (MR) were also calculated at each time point as a measure of overall drift in apparent potency of the libraries:
Where d̄ is the average of the differences in log potency. If a library is apparently less potent overall than it was in the initial measurement, at that time point MR will be less than 1. Compounds with activity ratios above (LSA(+) = MR*MSR) or below (LSA(−) = MR/MSR) the limits of statistical agreement were identified as outliers. The week 0-week 1 MSR value was the baseline, and the MR from the time-spaced replicate of interest was used to set the LSA values.
As an additional test of library integrity, changes in compound assay performance were calculated by Riemann summation of the area under the curve (AUC) for each CRC.
Analytical Chemistry
Chromatography was performed on a Waters LC/MS system using MassLynx software. A BEH C18 column (1.7 micron, 2.1 × 50 mm) was heated to a temperature of 45 °C at a flow rate of 0.5 mL/min. A linear gradient from 2% to 100% acetonitrile over 1.3 minutes with a run time of 2.1 minutes was used. The mobile phase consisted of acetonitrile (0.025% trifluoroacetic acid) and HPLC Grade water (0.05% trifluoroacetic acid). Quantitation was determined by evaporative light scattering detector (ELSD) and ultraviolet absorbance (UV). Identification was determined using a Waters ZQ mass spectrometer (MS) with electrospray ionization.
Data was analyzed using Waters OpenLynx software. The primary detector for quantification was the ELSD. For compounds with a poor ELSD response, the AUC was determined by UV absorbance at 220 nm. Identification was determined by MS. Compounds which did not give sufficient MS signals to determine identity were reported as unknown. Compounds that gave no signal by any detection method were reported as not found.
Results and Discussion
Chemical library
To evaluate the use of a bioassay system to monitor compound sample integrity we used the Prestwick library that is available in adequate supply and represents a well-characterized set of compounds which is widely used in both pharmaceutical and academic screening laboratories.28 The Prestwick library contains small molecules of which 90% are marketed drugs and 10% are bioactive alkaloids that were selected to give a high degree of both structural and pharmacological diversity. We have analyzed this library for structural diversity which suggests that the Prestwick collection is very diverse, although more limited in scope because of its size compared to larger compound collections such as those found in PubChem.29
Activity of CYP isozyme assay
A large amount of activity was observed for each isozyme in the compound library regardless of storage conditions. Initially, the CYP 2D6 isozyme revealed inhibition by 32% of the library (357 compounds), while the CYP 1A2 isozyme was inhibited by 17% of the library (187 compounds). We found 52% of the CYP 1A2 isozyme active set was shared on average with the active set of the CYP 2D6 isozyme and 36% of the CYP 2D6 active set was shared with the CYP 1A2 active set at any time point of the experiment. The average potency of the compounds was found to be near 1 μM for both isozymes (average pIC50 = 5.8 ±0.6). Overall, both assays provided a large robust dataset to evaluate potency values across the time course of the experiment.
CYP assays show high performance
We evaluated the stability of the CYP isozyme assays themselves by calculating the MSR of control compounds at each time point. For the set of plates used to assay the library stored in DMSO, titrations of the CYP 1A2 control inhibitor furafylline yielded MSR values that varied from 1.7 to 2.9 over the 37 weeks of the experiment, with pIC50s that varied from 6.4 to 5.4. Comparable stability was observed in the plates used to assay the library stored in the hydrated-DMSO conditions, with control compound titrations yielding MSR values that varied between 1.2 and 2.8. The CYP 2D6 isozyme assay also showed good stability although several anomalous plates caused the control inhibitor titrations of quinidine during the sixth time point measurement to inflate the MSR value to 17.7. However, the CYP 2D6 control inhibitor MSR values at other points of the experiment in both hydrated-DMSO and DMSO working sets ranged between 1.3 and 1.9. The CYP 2D6 isozyme assay also had lower signal-to-background overall and showed a Z-factor of 0.62, compared to the CYP 1A2 assay which showed an Z-factor of 0.71. Overall, the average control baseline MSR value (excluding the one inflated point) for both assays was approximately 2.0.
As a further test of reproducibility of the assays, we compared CYP actives determined from an unused (e.g. newly received from compound management) preparation of the library in both CYP assays. The potencies of CYP 1A2 library actives determined from the freshly prepared library copy showed an MSR = 1.9. Similarly, the CYP 2D6 assay showed an MSR = 1.6 using the fresh library copy. Therefore, both CYP isozyme assays showed high performance throughout the time course of the experiment.
Time dependent performance of the CYP assays
We measured the MSR, MR and AUC for each time point for the libraries prepared in either DMSO or using a hydrated-DMSO mixture. The hydrated-DMSO mixture contained 67/33% v/v DMSO/water that yields a mole fraction of 0.63 in water, which was chosen due to its special properties of near maximal viscosity30–32 and proximity to the DMSO-hydrate/DMSO eutectic,18, 33 where compounds are expected to deviate most from ideal solubility.10, 34, 35
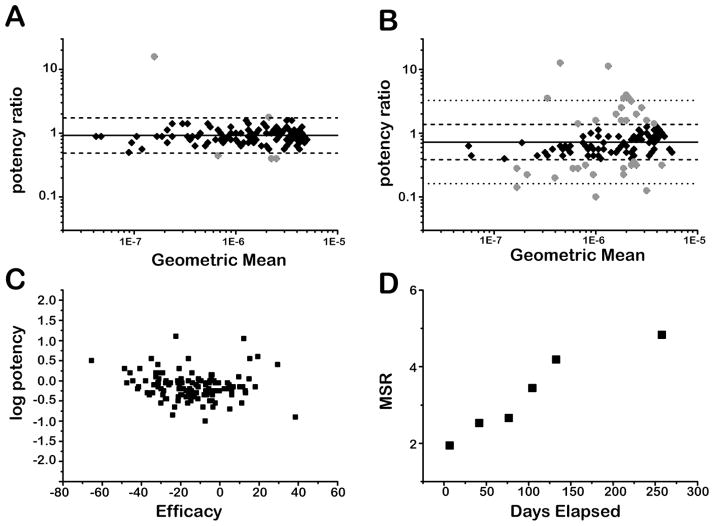
The overall trends in compound library performance as measured by the CYP 1A2 isozyme assayed against the DMSO working copy is shown in Figure 1. The reproducibility of IC50 values decreased as the experiment progressed, and consequently, the number of outliers increased. For example, in week 1, five outlying compounds were initially observed (Figure 1A) while at the conclusion of the experiment thirty-six outliers were observed (Figure 1B), out of 51 distinct outliers that appeared during the experiment. In general, compounds decreased in efficacy over time in concert with the decreases in potency (Figure 1C). Of the 126 compounds that had apparent CRCs in the initial as well as both replicates in the final time point assays, 103 showed a decrease in efficacy and 91 showed a decrease in potency, with 74 demonstrating a simultaneous decrease in potency and efficacy. Also indicative of the overall trend, 109 compounds showed a decrease in Hill AUC. The decrease in IC50 reproducibility is also reflected by the compound library MSR values, which show a progressive increase from 2.0 in week 1 to 4.5 in week 37 (Figure 1D).
Figure 1. CYP 1A2 assay over 37 weeks against the working-DMSO library copy.
A) IC50 reproducibility after 1 week of storage. Solid line: mean ratio (MR) = 0.92. Dashed lines: limits of statistical agreement (LSA(+)= 1.73, LSA(−) =0.49). Black diamonds: compounds with IC50s within LSA. Gray circles: outlier compounds with anomalous potency changes. B) IC50 reproducibility after 37 weeks of storage. Solid line: MR = 0.73. Dashed lines: LSA determined from week 1 MSR (LSA(+)=1.37, LSA(−) =0.39). Dotted lines: LSA using MSR from week 37 (LSA(+)= 3.27, LSA(−) = 0.16). Black diamonds: compounds with IC50s within LSA from week 1. Gray circles: outlier compounds with anomalous potency changes. C) Change in log potency vs. change in efficacy from week 1 to week 37. Negative changes in efficacy on this plot indicate a weaker overall response from a given compound. Negative changes in log potency indicate a higher IC50 value than the initial assay. D) MSR vs. time elapsed.
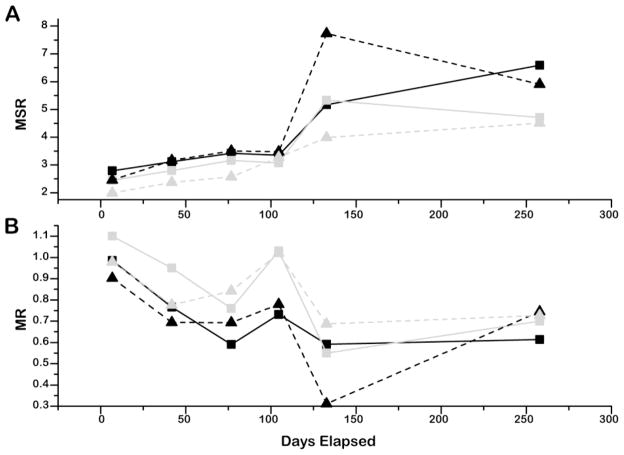
Examining both library conditions in the two CYP isozyme assays showed a similar trend (Figure 2). In all cases, the IC50 value reproducibility decreased over time, as indicated by increases in MSR values (Figure 2A). Additionally, in general for both assays, compound potency decreased over time, as tracked by decreases in the MRs (Figure 2B). The MRs represent the initial IC50 value divided by the IC50 value at the time point of interest for a compound, averaged over all compounds, such that MR<1 indicates less potency for the compound copy. After the initial assay, lower MR values were recorded, with an average of 0.84 over 37 weeks. Although the overall trend in MR values is downward, from measurement to measurement the values did increase at some time points.
Figure 2. Time-dependent performance of the two CYP assays against the DMSO and hydrated–DMSO working libraries.
A) Minimum Significant Ratios (MSR). B) Mean Ratios (MR). Black squares: CYP 2D6 assay with compounds assayed from the working hydrated-DMSO copy. Gray squares: CYP 2D6 assay with compounds assayed from the working DMSO copy. Black triangles: CYP 1A2 assayed from the working hydrated-DMSO copy. Gray triangles: CYP 1A2 assay with compounds assayed from the working DMSO copy. Lines are included as guides. The MR for week 15 was the only value to achieve the original MR (1.02), but the compound library increased MSR=3.4 suggesting the IC50 values were not as precise as in earlier measurements.
When we compared the potency differences between the two storage conditions, we observed that the changes occurred similarly in the two working copies. We compared the DMSO and hydrated-DMSO working copies to each other by taking the difference in log potencies between the two datasets at each time point for each CYP isozyme assay. For CYP 1A2, the DMSO to hydrated average MR was 0.92 +/− 0.20, and for CYP 2D6, the average MR was 0.93 +/− 0.12 suggesting similar changes in potency under either storage condition.
The hydrated-DMSO working library showed only slightly greater variance over time than the DMSO working copy. It is possible that the DMSO working copy achieved a water content of at least 10% within the first day of preparation, or equilibrated to hydrated conditions,36 as this can occur within a day in 1,536-well plates filled to a starting volume of 2 to 5 uL with 100% DMSO,37, 38 which would serve to minimize the differences between the two working copies. We found that in the CYP 1A2 assay, MSR values increased from 2.0 to 4.5 for the DMSO samples, while the hydrated-DMSO samples increased from 2.5 to 5.9. In the CYP 2D6 assay, MSR values increased from 2.5 to 4.7 for the DMSO working samples, with a maximum MSR of 5.3 (with 2.5% to 13.5% of active compounds identified as outliers), while the hydrated-DMSO working samples MSR values increased from 2.8 to 6.6. However, both storage conditions showed acceptable MSR values (library MSRs ≤ 3-fold) up to the 100 day time-point, after which IC50 variability became unacceptable as did potencies decreases relative to the starting point. As well, we note that in either condition a larger number of compounds became inactive after the first 100 days: 96±3% of the original actives were fit to high quality CRCs on average for the first 100 days while for the last two time points this average dropped to 79±7%.
Summary of Analytical QC results
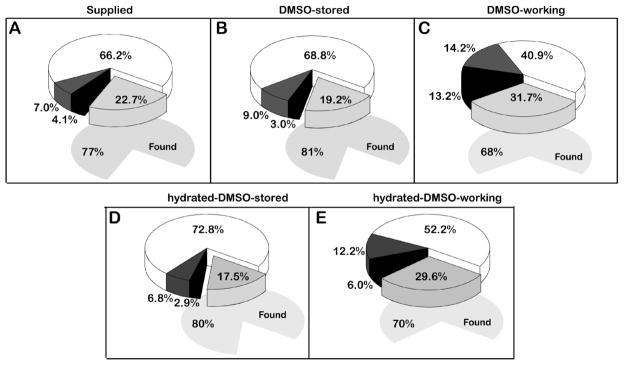
The QC result of the library as received from the supplier prepared in DMSO (Figure 3A, 77% found) was comparable to the DMSO stored copy (Figure 3B, 81% found) and the two stored copies showed a similar percentage of compounds found at the end of the experiment (Figure 3B,D). Differences between the two working conditions as observed by LC-MS were small. There was little difference in the numbers of compounds found in both the DMSO and hydrated-DMSO working copies: 763 and 786, respectively, at the conclusion of the experiment (approximately 70% found in either copy, Figure 3C,E). The number of compounds uncharacterized by LC-MS under hydrated conditions, n=212, and those with an apparent increase in purity, n=51, were comparable to the DMSO-working copy. However, the number of compounds showing >95% purity was higher in the hydrated-DMSO working (52%) copy than in the DMSO-working copy (41%). In contrast, the both working libraries conditions showed a reduction in compound QC, compared to the DMSO stored copies.
Figure 3. Analytical QC results for DMSO and hydrated-DMSO conditions.
A) Compound library as received from supplier, three days following plating. Light gray: samples not found (253); dark gray: purity 50–95% (78); black: purity < 50% (46); white: purity > 95% (740) B) Compound library plates stored sealed at −80°C for 37 weeks. Light gray: samples not found (214); dark gray: purity 50–95% (100); black: purity < 50% (34); white: purity > 95% (769) C) Working compound library plates stored in ambient saturated DMSO atmosphere for 37 weeks. Light gray: samples not found (354); dark gray: purity 50–95% (159); black: purity < 50% (147); white: purity > 95% (457). D) Hydrated-DMSO copy heat sealed and stored at −80°C for 37 weeks. Light gray: samples not found (189); dark gray: purity 50–95% (74); black: purity < 50% (31); white: purity > 95% (787). E) Hydrated-DMSO working compound library plates stored in ambient atmosphere for 37 weeks. Light gray: samples not found (331); dark gray: purity 50–95% (136); black: purity < 50% (67); white: purity > 95% (583). The total amount of samples found is represented by the underlying grey shaded area for each condition.
In a recent publication by Engeloch et al.37 duplicate copies of a 2mM, 1,404 member compound library were stored in a 90/10 DMSO/water mixture at 4 °C and observed sequentially over a 24-month period. This paper found that 85% of samples were found in excess of 85% pure -comparable to the values found for our stored copies described here. Similarly, we did not see an overall detrimental trend in the library QC data for the hydrated-DMSO samples compared to samples prepared with DMSO alone in either working or stored copies.
Correlation of LC-MS results with CYP assay results
We next examined if outlier compounds that showed anomalous potencies correlated with lower QC results. Of the 51 outliers observed from the CYP 1A2 assay in the DMSO-working copy, 31 (60%) were found by LC-MS as having a decrease in purity between samples from the stored copy and the working copy, with 5 compounds of the 51 not found in both plate copies. From a copy of 207 compounds showing decreases in potency, the number of compounds where a decrease in purity was measured by LC-MS totaled 84 (41%), while 100 (50%) were characterized as 100% pure both before and after the experiment. The remaining compounds were either not found by LC-MS in either plate copy (n=20) or showed an apparent increase in purity (n=3). For the CYP 2D6 assay against the DMSO copies, out of the 74 total outliers observed, 32 were found to have a decrease in purity (43%), 33 were found at full purity in both plate copies (45%), 5 showed an apparent increase in purity, and 4 were not found by QC in either copy. In the hydrated-DMSO copies, we noted that of the 44 outliers observed in the CYP 1A2 experiment, only 10 (23%) compounds showed a decrease in purity, with 28 (64%) at full purity in both stored and working plate sets. Three were not found by QC, and another 3 showed an apparent increase in purity. A similar distribution was found in the CYP 2D6 experiment, where out of 58 outliers, 14 (28%) compounds decreased in purity, and 39 (67%) were found at full purity in both stored and working plate sets. Two were not found, and 3 showed an apparent increase in purity. From this, it is evident that changes in compound purity measured with LC-MS do not necessarily predict variable CYP assay performance, perhaps due to degradation products acting as inhibitors of the CYP enzyme. However, this result also underscores the need to measure concentration in analytical library QC procedures as this would allow one to address losses in potencies due to precipitation of the compound.
Positional effects
The working library plates in this experiment used four-column flanking regions (the first four columns were reserved for controls) and four trailing rows (compound free/DMSO-only) along the long dimension of the plate. A plate-wise comparison of wells with outlier compounds at any point during the experiment did not show an obvious pattern, e.g., a tendency for compounds stored on plate edges to display high activity variability (data not shown). However, we note that edge-effects may have be more readily determined if a P450 isozyme such as CYP 3A4 was used as was recently demonstrated by Turner and co-workers39 in a study of nanospots formatted to 1,536 and 3,456-well plates.
Summary
We have shown how the reproducibility of potency values can change over time due to sample storage conditions. From this study we would recommend storage of 1,536-well compound library plates at room temperature for < 4 months. The Prestwick library used in this evaluation contains known drugs. Although there is certainly an increased effort to make compound libraries more “drug-like”, the optimal storage time may be different for other types of compound collections. The spot-checking of compound libraries using P450 assays as described here may serve as a useful tool for QC in addition to analytical chemistry profiling methods. Finally, our study found that differences, as measured with either traditional QC methods or two robust bioassays, between hydrated-DMSO and DMSO storage were small. Therefore, at least for collections of compounds with “drug-like” properties, the addition of water to the DMSO will likely have minimal detectable effects on bioassay results.
Acknowledgments
We thank Lino Ofiaza for archiving and retrieval of temperature and humidity records from the automated screening facility. This research was supported by the Molecular Libraries Initiative of the NIH Roadmap for Medical Research and the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health.
Abbreviations
- MSR
minimum significant ratio
- MR
mean potency ratio
- LSA
limits of statistical agreement
- CYP
cytochrome P450
- AUC
area under the curve
- CRC
concentration-response curve
- BEH
bridged ethyl hybrid
- DMSO
dimethyl sulfoxide
- LC
liquid chromatography
- MS
mass spectrometry
- HPLC
high performance liquid chromatography
- HTS
high-throughput screening
- qHTS
quantitative high-throughput screening
- QC
quality control
References
- 1.Bowes S, Sun DY, Kaffashan A, Zeng CH, Chuaqui C, Hronowski X, Buko A, Zhang X, Josia S. Quality Assessment and Analysis of Biogen Idec Compound Library. J Biomol Screen. 2006;11:828–835. doi: 10.1177/1087057106290993. [DOI] [PubMed] [Google Scholar]
- 2.Cheng XH, Hochlowski J, Tang H, Hepp D, Beckner C, Kantor S, Schmitt R. Studies on Repository Compound Stability in DMSO under Various Conditions. J Biomol Screen. 2003;8:292–304. doi: 10.1177/1087057103008003007. [DOI] [PubMed] [Google Scholar]
- 3.Darvas F, Karancsi T, Slegel P, Dorma G. Estimating stability for HTS library compounds. Genetic Engineering News. 2000;20:30–31. [Google Scholar]
- 4.Hochlowski J, Cheng X, Sauer D, Djuric S. Studies of the Relative Stability of TFA Adducts vs Non-TFA Analogues for Combinatorial Chemistry Library Members in DMSO in a Repository Compound Collection. J Comb Chem. 2003;5:345–349. doi: 10.1021/cc0300107. [DOI] [PubMed] [Google Scholar]
- 5.Ilouga PE, Winkler D, Kirchhoff C, Schierholz B, Wolcke J. Investigation of 3 industry-wide applied storage conditions for compound libraries. J Biomol Screen. 2007;12:21–32. doi: 10.1177/1087057106295507. [DOI] [PubMed] [Google Scholar]
- 6.Kozikowski BA, Burt TM, Tirey DA, Williams LE, Kuzmak BR, Stanton DT, Morand KL, Nelson SL. The Effect of Room-Temperature Storage on the Stability of Compounds in DMSO. J Biomol Screen. 2003;8:205–209. doi: 10.1177/1087057103252617. [DOI] [PubMed] [Google Scholar]
- 7.Kozikowski BA, Burt TM, Tirey DA, Williams LE, Kuzmak BR, Stanton DT, Morand KL, Nelson SL. The Effect of Freeze/Thaw Cycles on the Stability of Compounds in DMSO. J Biomol Screen. 2003;8:210–215. doi: 10.1177/1087057103252618. [DOI] [PubMed] [Google Scholar]
- 8.Chan JA, Hueso-Rodriguez JA. Compound Library Management. In: Janzen WP, editor. High Throughput Screening: Methods and Protocols. Vol. 190. Humana Press; 2002. pp. 117–127. [DOI] [PubMed] [Google Scholar]
- 9.Archer JR. History, Evolution, and Trends in Compound Management for High Throughput Screening. Assay Drug Develop Technol. 2004;2:675–681. doi: 10.1089/adt.2004.2.675. [DOI] [PubMed] [Google Scholar]
- 10.Oldenburg K, Pooler D, Scudder K, Lipinski C, Kelly M. High throughput sonication: Evaluation for compound solubilization. Comb Chem High Throughput Screen. 2005;8:499–512. doi: 10.2174/1386207054867364. [DOI] [PubMed] [Google Scholar]
- 11.Kariv I, Fereshteh MP, Oldenburg KR. Development of a miniaturized 384-well high throughput screen for the detection of substrates of cytochrome P450 2D6 and 3A4 metabolism. J Biomol Screen. 2001;6:91–99. doi: 10.1177/108705710100600205. [DOI] [PubMed] [Google Scholar]
- 12.Trubetskoy OV, Gibson JR, Marks BD. Highly miniaturized formats for in vitro drug metabolism assays using vivid fluorescent substrates and recombinant human cytochrome P450 enzymes. J Biomol Screen. 2005;10:56–66. doi: 10.1177/1087057104269731. [DOI] [PubMed] [Google Scholar]
- 13.Gordon EJ. Small-molecule screening: It takes a village. ACS Chemical Biology. 2007;2:9–16. doi: 10.1021/cb6004454. [DOI] [PubMed] [Google Scholar]
- 14.Lazo JS, Brady LS, Dingledine R. Building a Pharmacological Lexicon: Small Molecule Discovery in Academia. Mol Pharm. 2007;72:1–7. doi: 10.1124/mol.107.035113. [DOI] [PubMed] [Google Scholar]
- 15.Inglese J, Auld DS, Jadhav A, Johnson RL, Simeonov A, Yasgar A, Zheng W, Austin CP. Quantitative high-throughput screening: A titration-based approach that efficiently identifies biological activities in large chemical libraries. PNAS, USA. 2006;103:11473–11478. doi: 10.1073/pnas.0604348103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inglese J, Johnson RL, Simeonov A, Xia MH, Zheng W, Austin CP, Auld DS. High-throughput screening assays for the identification of chemical probes. Nat Chem Biol. 2007;3:466–479. doi: 10.1038/nchembio.2007.17. [DOI] [PubMed] [Google Scholar]
- 17.Yasgar A, Shinn P, Jadhav A, Auld D, Michael S, Zheng W, Austin CP, Inglese J, Simeonov A. Compound Management for Quantitative High-Throughput Screening. JALA. 2008;3:79–89. doi: 10.1016/j.jala.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasmussen DH. Phase Diagram for the System Water-Dimethylsulfoxide. Nature. 1968;220:1315–1317. doi: 10.1038/2201315a0. [DOI] [PubMed] [Google Scholar]
- 19.Michael S, Auld D, Klumpp C, Jadhav A, Zheng W, Thorne N, Austin CP, Inglese J, Simeonov A. A Robotic Platform for Quantitative High-Throughput Screening. Assay Drug Dev Technol. 2008;6:637–657. doi: 10.1089/adt.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cleveland PH, Koutz PJ. Nanoliter Dispensing for uHTS Using Pin Tools. Assay Drug Dev Technol. 2005;3:213–25. doi: 10.1089/adt.2005.3.213. [DOI] [PubMed] [Google Scholar]
- 21.Cali JJ, Ma D, Sobol M, Simpson DJ, Frackman S, Good TD, Daily WJ, Liu D. Luminogenic cytochrome P450 assays. Expert Opin Drug Metab Toxicol. 2006;2:629–45. doi: 10.1517/17425255.2.4.629. [DOI] [PubMed] [Google Scholar]
- 22.Cali JJ, Niles A, Valley MP, O’Brien MA, Riss TL, Shultz J. Bioluminescent assays for ADMET. Expert Opin Drug Metab Toxicol. 2008;4:103–20. doi: 10.1517/17425255.4.1.103. [DOI] [PubMed] [Google Scholar]
- 23.http://www.ncgc.nih.gov/pub/openhts/curvefit/.
- 24.Eastwood BJ, Chesterfield AK, Wolff MC, Felder CC. Methods for the Design and Analysis of Replicate-Experiment Studies to Establish Assay Reproducibility and the Equivalence of Two Potency Assays. In: Gad SC, editor. Drug Discovery Handbook. John Wiley & Sons, Inc; 2005. pp. 667–688. [Google Scholar]
- 25.Eastwood BJ, Farmen MW, Iversen PW, Craft TJ, Smallwood JK, Garbison KE, Delapp NW, Smith GF. The Minimum Significant Ratio: A Statistical Parameter to Characterize the Reproducibility of Potency Estimates from Concentration-Response Assays and Estimation by Replicate-Experiment Studies. J Biomolr Screen. 2006;11:253–261. doi: 10.1177/1087057105285611. [DOI] [PubMed] [Google Scholar]
- 26.AGM. Assay Guidance Manual Version 4.1. Eli Lilly and Company and NIH Chemical Genomics Center; 2005. [Google Scholar]
- 27.Shukla SJ, Nguyen D-T, MacArthur R, Simeonov A, Frazee WJ, Hallis TM, Marks BD, Singh U, Eliason HC, Printen J, Austin CP, Inglese J, Auld DS. Identification of Pregnane X Receptor Ligands Using Time-Resolved Fluorescence Resonance Energy Transfer and Quantitative High Throughput Screening. Assay Drug Dev Technol. 2009 doi: 10.1089/adt.2009.193. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.http://www.prestwickchemical.fr/index.php?pa=26.
- 29.http://pubchem.ncbi.nlm.nih.gov/
- 30.Cowie JMG, Toporowski PM. Association in the binary liquid system dimethyl sulphoxide-water. Canadian Journal of Chemistry-Revue Canadienne De Chimie. 1961;39:2240–2243. [Google Scholar]
- 31.Fort RJ, Moore WR. Viscosities of Binary Liquid Mixtures. Transactions of the Faraday Society. 1966;62:1112–1119. [Google Scholar]
- 32.Packer KJ, Tomlinson DJ. Nuclear Spin Relaxation and Self-Diffusion in the binary System, Dimethylsulphoxide (DMSO) + Water. Transactions of the Faraday Society. 1971;67:1302–1314. [Google Scholar]
- 33.Havemeyer RN. Freezing Point Curve of Dimethyl Sulfoxide - Water Solutions. Journal of Pharmaceutical Sciences. 1966;55:851–853. doi: 10.1002/jps.2600550822. [DOI] [PubMed] [Google Scholar]
- 34.Lipinski CA. Samples in DMSO: What an end user needs to know. Laboratory Robotics Interest Group; Somerset, NJ: 2006. [Google Scholar]
- 35.Fuchs R, McCrary GE, Bloomfield JJ. Mechanisms of Nucleophilic Displacement in Aqueous Dimethyl Sulfoxide Solutions. J American Chem Soc. 1961;83:4281–4284. [Google Scholar]
- 36.Ellson R, Stearns R, Mutz M, Brown C, Browning B, Harris D, Qureshi S, Shieh J, Wold D. In situ DMSO Hydration Measurements of HTS Compound Libraries. Combl Chemi High Throughput Screen. 2005;8:489–498. doi: 10.2174/1386207054867382. [DOI] [PubMed] [Google Scholar]
- 37.Engeloch C, Schopfer U, Muckenschnabel I, Le Goff F, Mees H, Boesch K, Popov M. Stability of Screening Compounds in Wet DMSO. J Biomol Screen. 2008;13:999–1006. doi: 10.1177/1087057108326536. [DOI] [PubMed] [Google Scholar]
- 38.Schopfer U, Hohn F, Hueber M, Girod M, Engeloch C, Popov M, Muckenschnabel I. Screening Library Evolution through Automation of Solution Preparation. J Biomol Screen. 2007;12:724–732. doi: 10.1177/1087057107301939. [DOI] [PubMed] [Google Scholar]
- 39.Turner BA, Evans BP, Pearson TT, Braden TK, Wise SC. Examination of Edge Effects with Different Storage Conditions of Preplated Dimethyl Sulfoxide Nanospots in ChemLib 1,536- and 3,456-Well Assay-Ready Plates. Assay Drug Dev Technols. 2008;6:811–818. doi: 10.1089/adt.2008.169. [DOI] [PubMed] [Google Scholar]