Abstract
The synthesis of pyridines from readily available α,β-unsaturated oximes and alkynes under mild conditions and low temperatures using Rh(III) catalysis has been developed. It was found that the use of sterically different ligands allows for complementary selectivities to be achieved.
Poly-substituted pyridines rank as one of the most common heterocycles encountered by medicinal chemists.1,2 The frequency with which this motif appears has necessitated the development of numerous approaches for generating pyridines of different substitution patterns.3 Transition metal catalysis is a powerful tool for this problem.4 Based on our experience with heteroatom containing metallacycles,5 we imagined we could use a nitrogen containing rhodacycle to access pyridines.
Our group,6 and others,7 have independently demonstrated that benzamides and acrylamides can be coupled with alkynes to access isoquinolones and pyridones using Rh(III)catalysis.8 This method affords access to a variety of nitrogen containing heterocycles including indoles,9 pyrroles,10 isoquinolines,11 and dihydro-isoquinolones.12 We envisioned that pyridines could be accessed from α,β-unsaturated oximes13 and alkynes, with the N–O bond of the oxime acting as an internal oxidant.
N–O bonds have been shown to function as internal oxidants in other C–H activation protocols.14,15,16,17 Guimond and Fagnou7a,b were the first to demonstrate that Rh (III) C–H activation functions with internal oxidants to prepare isoquinolones and dihydroisoquinolones. Inspired by this work, we believed that oximes might allow access to a similar 7-membered rhodacycle that can decompose to form pyridines with cleavage of the N–O bond. Notably, Cheng and workers have demonstrated a clever synthesis of pyridines using α,β-unsaturated oximes and alkynes under Rh (I) catalysis.18 Under these conditions unsymmetrical alkynes give low regioselectivity while requiring high temperatures (130 °C). We speculated that our approach would render more mild conditions and a broader substrate scope.19
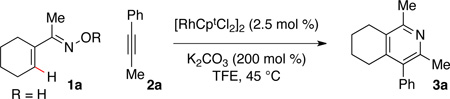
Table 1 describes modification to our standard reaction conditions. We found that oxime 1a and alkyne 2a in the presence of K2CO3 and [RhCptCl2]2 in 2,2,2-trifluoroethanol TFE) at 45 °C provide pyridine 3a in 87% yield with 4:1 regioselectivity. By comparison, the Rh(I) approach gives a similar yield, but 1.6:1 regioselectivity.18 The use of [RhCptCl2]2 is preferred, as [RhCp*Cl2]2 delivers similar yields but low regioselectivity (Table 1, entry 7).6b Importantly, the use of Cpt gives the alkyne regiosomer opposite of what is typically observed in Rh (III) C–H activation chemistry. Oxime methyl ethers afford no reaction (Table 1, entry 2). Furthermore, the acetyl oxime ester decomposes under the reaction to yield the free oxime within minutes.20 A base screen revealed CsOAc also functions well (Table 1, entry 3), but the reaction requires some base, presumably because the C–H activation proceeds through a concerted metallation-deprotonation (CMD) mechansim (Table 1, entry 4).21 More electron deficent ligands (Table 1, entry 8) are tolerated, but afford lower regioselectivity and yield. The enhanced selectivity provided by Cpt is likely due to steric interactions.
Table 1.
Reaction Optimizationa
 | |||
|---|---|---|---|
| Entry | Variations from “standard conditions” | Yield (%)b | Regioselectivity |
| 1 | none | 87 | 4:1 |
| 2 | R = Me | n.r.c | - |
| 3 | CsOAc instead of K2CO3 | 65 | 2.2:1 |
| 4 | No K2CO3 | n.r. | - |
| 5 | MeOH instead of TFE | 68 | 2.1:1 |
| 6 | (4:1) H2O/TFE instead of TFE | 70 | 4:1 |
| 7 | [RhCp*Cl2]2 instead of [RhCptCl2]2 | 83 | 1:2 |
| 8 | [RhCpCF3Cl2]2 instead of [RhCptCl2]2 | 20 | 1:1 |
Standard Reaction Conditions: oxime (0.2 mmol), alkyne (0.22 mmol), catalyst (0.005 mmol), base (0.4 mmol) in solvent (3 ml) stirred for 16 h.
Yields determined by GC/MS.
n.r. = no reaction.
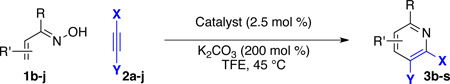
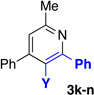
With optimized conditions in hand, we began a screen of the oxime components with 1-phenyl-1-propyne. The oxime was screened with [RhCptCl2]2 and [RhCp*Cl2]2 to determine the effect the ligand has on regioselectivity. Alkyl substituted oximes function well in this reaction with regioselectivities increasing when the Cpt ligand is employed (Table 2, entries 1–3).22 Aldoximes are tolerated under the reaction conditions, a constraint of the Cheng methodology (Table 2, entry 6).23 In contrast to alkyl-substituted oximes, β-aryl substituted oximes display higher selectivity when Cp* is used (Table 1, entries 4,5, and 7). Beyond giving high selectivity with Cp*, β-aryl groups also allow access to the opposite regioisomer in moderate regioselectivity. When electron-withdrawing groups are placed at the β-position of the substrate Cp* is a better ligand with good to excellent yield and regioselectivity (Table 2, entries 8 and 9).
Table 2.
Pyridine Scopea
 | ||||||
|---|---|---|---|---|---|---|
| entry | Cp* | Cpt | entry | Cp* | ||
| 1 | 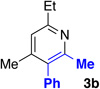 |
80% (1:1) |
83% (4:1) |
 |
||
| 2 | 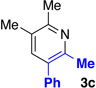 |
87% (2:1) |
85% (2.6:1) |
10 | Y = CH2OTBS |
80% (2.5:1) |
| 11 | Y = c-Pr |
81% (4.4:1) |
||||
| 12 | Y = CO2Et |
89% (5:1) |
||||
| 13 | Y = Ph |
89% - |
||||
| 3 | 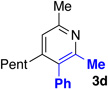 |
92% (1:1.2) |
93% (3.4:1) |
14 | 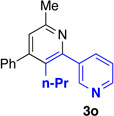 |
66% (2.5:1) |
| 4 | 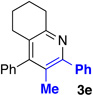 |
75% (4:1) |
70% (1:1) |
15 | 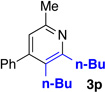 |
65% - |
| 5 | 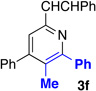 |
77% (3.5:1) |
76% (1:3.4) |
16 | 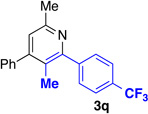 |
85% (3.5:1) |
| 6 | 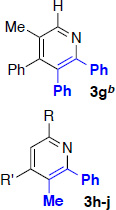 |
45% - |
- - |
17 | 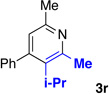 |
95% (10:1) |
| 7 | R = Me, R' = Ph |
91% (3.1:1) |
90% (1:3.3) |
18 | 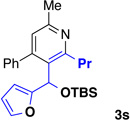 |
71% (9:1) |
| 8 |
72% (6.6:1) |
74% (1:1) |
||||
| 9 | R = Bu R' = CF3 |
72% (10:1) |
68% (1:1) |
|||
Standard Conditions: see Table 1. Cp* = [RhCp*Cl2]2, Cpt=[RhCptCl2]2
Reaction run at 80 °C with K2CO3 (50 mol %).
We explored a variety of alkyne components for the reaction with oxime 1j in the presence of [RhCp*Cl2]2. Protected propargyl alcohols function well under the reaction conditions, albeit with slightly lower regioselectivity (Table 2, entry 10). A variety of alkyl/aryl alkynes participate with good selectivity, including pyridine- and cyclopropyl-substituted alkynes. Phenyl propiolates provide pyridines in good yield and excellent regioselectivity (Table 2, entry 12). Dialkyl alkynes gave excellent selectivity. Pyridines 3r, 3s were isolated in high yield and excellent regioselectivity. In these cases the larger group is at the 2-position of the pyridine.
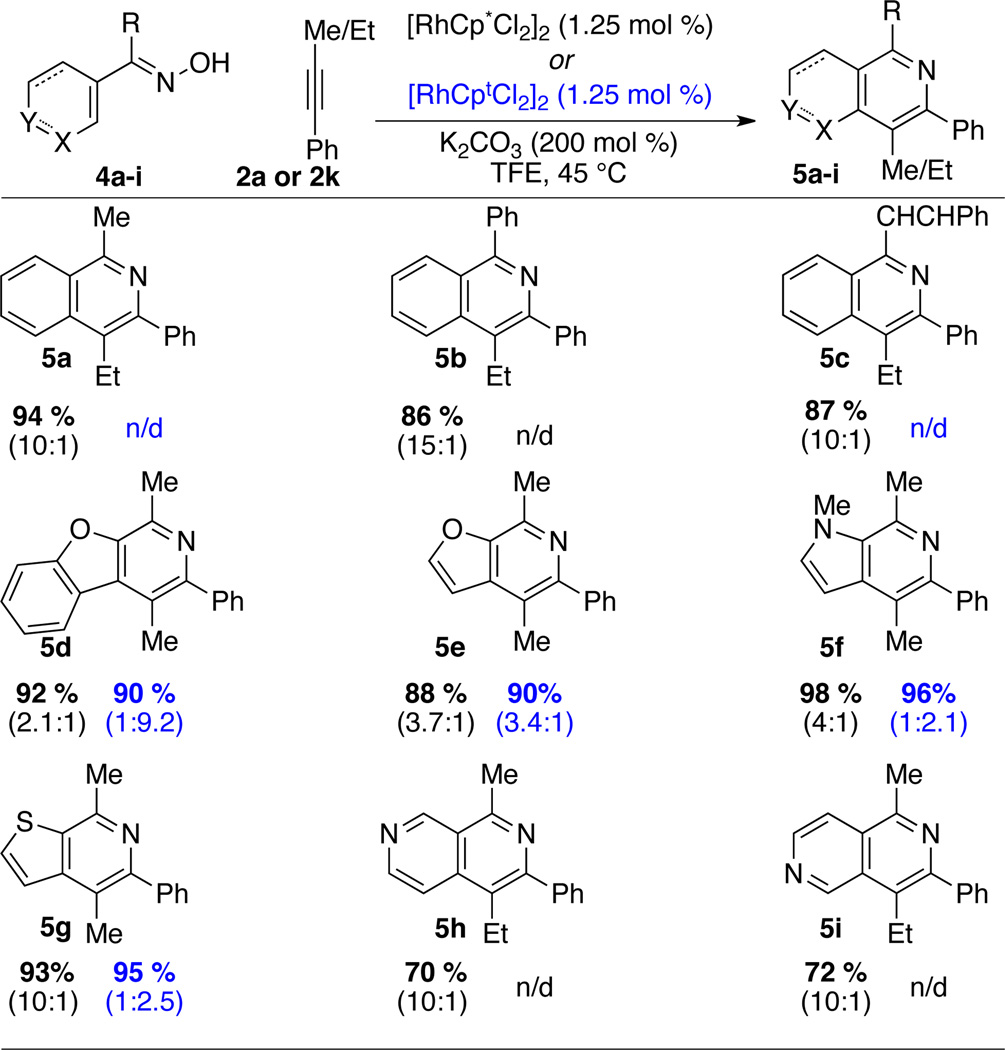
Previously, excellent work by Chiba demonstrated that O-Ac oximes can be used to access isoquinolones.11b Later, Li11a and Guimond/Fagnou11d demonstrated that free oximes can function as directing groups for this reaction. Using our conditions we were delighted to access isoquinolines. A variety of substitution is tolerated off the 1-position of the oxime, ranging from aryl, alkyl, and vinyl groups (Scheme 1, 5a–5c). We felt this method might also allow us to perform cyclizations with heterocyclic oximes. We were pleased to find that pyrroles, thiophenes, furans, and benzofurans are all tolerated in excellent yield (Scheme 1, 5d–5i). In most cases the regioselectivities with Cp* are quite pedestrian. Interestingly, changing to Cpt alters the intrinsic selectivities to favor the opposite isomer. In addition to these heterocycles, we found that pyridines are tolerated in this reaction. This is striking in light of the relatively few examples of Rh (III) C–H activation on heterocycles containing basic nitrogen.24 Furthermore, the C–H activation occurs predominately at the position adjacent to the nitrogen, apparently due to the increased kinetic acidity of that proton.
Scheme 1.
Isoquinoline Scope
Additionally we found a kinetic isotope effect of 18 in this transformation. While rare, a similar value was observed by Guimond/Fagnou in their internal oxidant rhodium (III) studies.7a
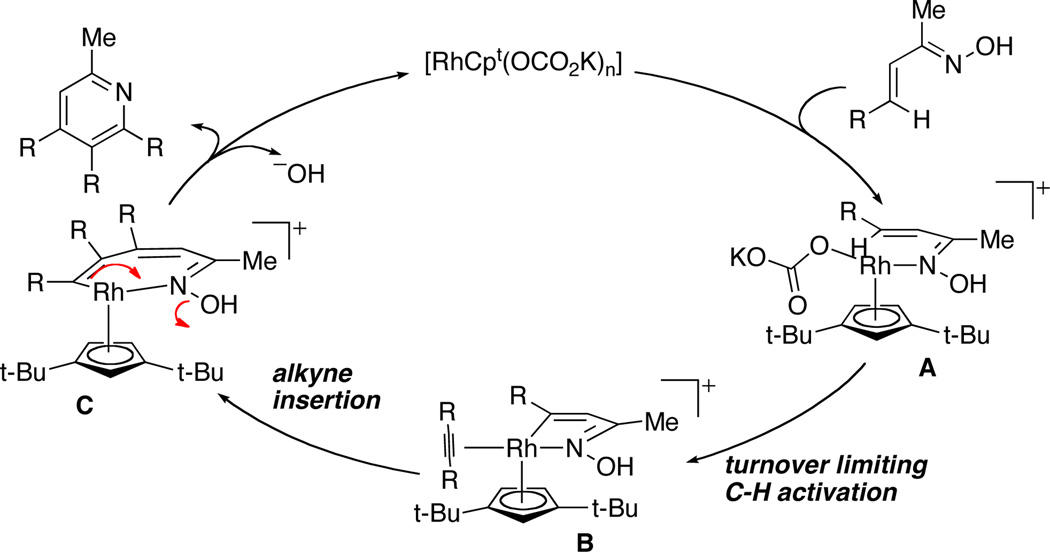
With C–H activation as the turnover-limiting event, we were interested in determining the mechanism of internal redox. In particular, we sought to probe the possibility of a 6π-electrocyclization event. Since α,β,γ,δ-unsaturated oximes undergo electro-cyclizations under mild conditions,25 we elected to generate the intermediate derived from an isoquinoline. Upon subjection of the 6π intermediate to our standard reaction conditions, we did not observe any pyridine product, suggesting that this reaction does not occur via a 6π-electrocyclization.
A possible product of this reaction is a polysubstituted pyridine N-oxide. This species could oxidize Rh(I) to Rh(III) under the reaction conditions and provide the desired product. When the reaction is conducted in the presence of 4-phenyl pyridine N-oxide, none of the reduced 4-phenyl pyridine is observed, suggesting that if the reductive elimination path is occurring, inner sphere oxidation is inherently rapid and the dominant pathway for reoxidizing Rh. Alternately, and perhaps more reasonably, N-O bond reduction occurs in concert with the C-N bond-forming event, as proposed by Guimond/Fagnou.7b
With these insights in hand, we propose the following mechanism. The monomeric rhodium catalyst coordinates to the basic nitrogen of the oxime A. A turnover limiting C–H activation event occurs, presumably facilitated by carbonate, to provide a 5-membered rhodacycle B. This metallacycle can insert an equivalent of alkyne to generate a 7-membered metallacycle, which undergoes a C–N bond formation with concomitant N-O bond cleavage D.
Supplementary Material
Scheme 2.
Acknowledgments
We thank NIGMS (GM80442) for support and Johnson Matthey for a loan of Rh salts. We thank Marie Trujillo (CSU) for early experiments.
Footnotes
Electronic Supplementary Information (ESI) available: Detailed experimental procedures and spectroscopic data. See DOI: 10.1039/b000000x/
Notes and references
- 1.(a) Mitscher LA. Chem. Rev. 2005;105:559. doi: 10.1021/cr030101q. [DOI] [PubMed] [Google Scholar]; (b) Torres M, Gil S, Parra M. Curr. Org. Chem. 2005;9:1757. [Google Scholar]; (c) Jones G. In: Comprehensive Heterocyclic Chemistry II. Katritzky AR, Rees CW, Scriven EFV, McKillop A, editors. Vol. 5. Oxford: Pergamon; 1996. p. 167. [Google Scholar]; (d) Henry GD. Tetrahedron. 2004;60:6043. [Google Scholar]; (e) Michael JP. Nat. Prod. Rep. 2005;104:3787. doi: 10.1039/b413750g. [DOI] [PubMed] [Google Scholar]
- 2.Roughley SD, Jordan AM. J. Med. Chem. 2011;54:3451. doi: 10.1021/jm200187y. [DOI] [PubMed] [Google Scholar]
- 3. Hill MD. Chem.-Eur. J. 2010;16:12052. doi: 10.1002/chem.201001100. Select examples: Movassaghi M, Hill MD, Ahmad OK. J. Am. Chem. Soc. 2007;129:10096. doi: 10.1021/ja073912a. Movassaghi M, Hill MD. J. Am. Chem. Soc. 2006;128:4592. doi: 10.1021/ja060626a.
- 4.(a) D’Souza BR, Lane TK, Louie J. Org. Lett. 2011;13:2936. doi: 10.1021/ol2009939. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Manning JR, Davies HML. J. Am. Chem. Soc. 2008;130:8602. doi: 10.1021/ja803139k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Barluenga J, Fernández-Rodríguez MA, García-García P, Aguilar E. J. Am. Chem. Soc. 2008;130:2764. doi: 10.1021/ja7112917. [DOI] [PubMed] [Google Scholar]; (d) Dash J, Lechel T, Reissig H-U. Org. Lett. 2007;9:5541. doi: 10.1021/ol702468s. [DOI] [PubMed] [Google Scholar]; (e) Trost BM, Gutierrez AC. Org. Lett. 2007;9:1473. doi: 10.1021/ol070163t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Wang Y-F, Chiba S. J. Am. Chem. Soc. 2009;131:12570. doi: 10.1021/ja905110c. [DOI] [PubMed] [Google Scholar]; (g) Oberg KM, Lee EE, Rovis T. Tetrahedron. 2009;65:5056. doi: 10.1016/j.tet.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Oberg KM, Rovis T. J. Am. Chem. Soc. 2011;133:4785. doi: 10.1021/ja200766k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Friedman RK, Oberg KM, Dalton DM, Rovis T. Pure Appl. Chem. 2010;82:1353. doi: 10.1351/PAC-CON-09-12-09. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Perreault S, Rovis T. Chem. Soc. Rev. 2009;38:3149. doi: 10.1039/b816702h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Hyster TK, Rovis T. J. Am. Chem. Soc. 2010;132:10565. doi: 10.1021/ja103776u. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hyster TK, Rovis T. Chem. Sci. 2011;2:1605. doi: 10.1039/C1SC00235J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Guimond N, Gorelsky SI, Fagnou K. J. Am. Chem. Soc. 2011;133:6449. doi: 10.1021/ja201143v. [DOI] [PubMed] [Google Scholar]; (b) Guimond N, Gouliaras C, Fagnou K. J. Am. Chem. Soc. 2010;132:6908. doi: 10.1021/ja102571b. [DOI] [PubMed] [Google Scholar]; (c) Mochida S, Umeda N, Hirano K, Satoh T, Miura M M. Chem. Lett. 2010;39:744. [Google Scholar]; (d) Su Y, Zhao M, Han K, Song G, Li X. Org. Lett. 2010;12:5462. doi: 10.1021/ol102306c. [DOI] [PubMed] [Google Scholar]; (e) Song G, Chen D, Pan C-L, Crabtree RH, Li X. J. Org. Chem. 2010;75:7487. doi: 10.1021/jo101596d. [DOI] [PubMed] [Google Scholar]
- 8.For a review, see; Satoh T, Miura M. Chem.-Eur. J. 2010;18:11212. doi: 10.1002/chem.201001363. Wencel-Delord J, Dröge T, Liu F, Glorius F. Chem. Soc. Rev. 2011;40:4740. doi: 10.1039/c1cs15083a.
- 9.(a) Stuart DR, Alsabeh P, Kuhn M, Fagnou K. J. Am. Chem. Soc. 2010;132:18326. doi: 10.1021/ja1082624. [DOI] [PubMed] [Google Scholar]; (b) Stuart DR, Bertrand-Laperle M, Burgess KMN, Fagnou K. J. Am. Chem. Soc. 2008;130:16474. doi: 10.1021/ja806955s. [DOI] [PubMed] [Google Scholar]; (c) Chen J, Pang Q, Sun Y, Li X. J. Org. Chem. 2011;76:3523. doi: 10.1021/jo1025546. [DOI] [PubMed] [Google Scholar]; (d) Chen J, Song G, Pan C-L, Li X. Org. Lett. 2010;12:5426. doi: 10.1021/ol1022596. [DOI] [PubMed] [Google Scholar]; (e) Huestis MP, Chan L, Stuart DR, Fagnou K. Angew. Chem. Int. Ed. 2011;50:1338. doi: 10.1002/anie.201006381. [DOI] [PubMed] [Google Scholar]
- 10. Rakshit S, Patureau PW, Glorius F. J. Am. Chem. Soc. 2010;132:9585. doi: 10.1021/ja104305s. (b) Ref. 9a
- 11.(a) Guimond N, Fagnou K. J. Am. Chem. Soc. 2009;131:12050. doi: 10.1021/ja904380q. [DOI] [PubMed] [Google Scholar]; (b) Too PC, Wang Y-F, Chiba S S. Org. Lett. 2010;12:5688. doi: 10.1021/ol102504b. [DOI] [PubMed] [Google Scholar]; (c) Too PC, Chua SH, Wong SH, Chiba S S. J. Org. Chem. 2011;76:6159. doi: 10.1021/jo200897q. [DOI] [PubMed] [Google Scholar]; (d) Zhang X, Chen D, Zhao M, Xhao J, Jia A, Li X. Adv. Synth. Catal. 2011;353:719. [Google Scholar]; (e) Wang Y-F, Toh KK, Lee J-Y, Chiba S. Angew. Chem. Int. Ed. 2011;50:5927. doi: 10.1002/anie.201101009. [DOI] [PubMed] [Google Scholar]; (f) Fukutani T, Umeda N, Hirano K, Satoh T, Miura M. Chem. Commun. 2009;74:7094. doi: 10.1039/b910198e. [DOI] [PubMed] [Google Scholar]; (g) Morimoto K, Hirano K, Satoh T, Miura M. Chem. Lett. 2011;40:600. [Google Scholar]
- 12. Rakshit S, Grohmann C, Besset T, Glorius F. J. Am. Chem. Soc. 2011;133:2350. doi: 10.1021/ja109676d. (b) ref 7a.
- 13.For select examples, see: Neufeldt SR, Sanford MS. Org. Lett. 2010;12:532. doi: 10.1021/ol902720d. Desai LV, Hull KL, Sanford MS. J. Am. Chem. Soc. 2004;126:9542. doi: 10.1021/ja046831c. Desai LV, Malik HA, Sanford MS. Org. Lett. 2006;8:1141. doi: 10.1021/ol0530272. Tsai AS, Brasse M, Bergman RG, Ellman JA. Org. Lett. 2011;13:540. doi: 10.1021/ol102890k. Sun C-L, Liu N, Li B-J, Yu D-G, Wang Y, Shi Z-J. Org. Lett. 2010;12:184. doi: 10.1021/ol902552v. For examples of olefination with Rh(III) catalysts, see: Mochida S, Hirano K, Satoh T, Miura M. J. Org. Chem. 2011;76:3024. doi: 10.1021/jo200509m. Besset T, Kuhl N, Patureau FW, Glorius F. Chem. Eur. J. 2011;17:7167. doi: 10.1002/chem.201101340.
- 14.For a review of, see: Patureau FW, Glorius F. Angew. Chem. Int. Ed. 2011;50:1977. doi: 10.1002/anie.201007241. For select examples see: Wu J, Cui X, Chen L, Jiang G, Wu Y. J. Am. Chem. Soc. 2009;131:13888. doi: 10.1021/ja902762a. Yoo EJ, Ma S, Mei T-S, Chan KSL, Yu J-Q. J. Am. Chem. Soc. 2011;133:7652. doi: 10.1021/ja202563w. Mei T-S, Wang X, Yu J-Q. J. Am. Chem. Soc. 2009;131:10806. doi: 10.1021/ja904709b.
- 15.(a) Tsutsui H, Narasaka K. Chem. Lett. 1999:45. [Google Scholar]; (b) Tsutsui H, Kitamura M, Narasaka K. Bull. Chem. Soc. Jpn. 2002;75:1451. [Google Scholar]; (c) Kitamura M, Narasaka K. Chem. Rec. 2002;2:268. doi: 10.1002/tcr.10030. [DOI] [PubMed] [Google Scholar]; (d) Tsutsui H, Narasaka K. Chem. Lett. 2001:526. [Google Scholar]
- 16.Tan Y, Hartwig JF. J. Am. Chem. Soc. 2010;132:3676. doi: 10.1021/ja100676r. [DOI] [PubMed] [Google Scholar]
- 17.Glorius has used internal oxidants in Rh (III) catalysts; see: (a) ref 10a. Willacher J, Rakshit S, Glorius F. Org. Biomol. Chem. 2011;9:4736. doi: 10.1039/c1ob05636k.
- 18.(a) Parthasarathy K, Jeganmohan M, Cheng C-H. Org. Lett. 2008;10:325. doi: 10.1021/ol7028367. [DOI] [PubMed] [Google Scholar]; (b) Parthasarathy K, Cheng C-H. J. Org. Chem. 2009;74:9359. doi: 10.1021/jo902084j. [DOI] [PubMed] [Google Scholar]
- 19.During the preparation of this manuscript, Chiba, Guimond/Fagnou, and Li each demonstrated that oximes may be used for the Rh(III)-catalyzed synthesis of isoquinolines; see: refs 7a, 10b-d.
- 20.Similar results were observed with aryl oximes; see: ref 7a.
- 21.Lapointe D, Fagnou K. Chem. Lett. 2010;39:1118. [Google Scholar]
- 22.More sterically encumbering groups at the β-position resulted in low yields.
- 23.Aldoxime isomerized to the primary amide.
- 24.Fukutani T, Hirano K, Satoh T, Miura M. J. Org. Chem. 2011;76:2867. doi: 10.1021/jo200339w. [DOI] [PubMed] [Google Scholar]
- 25.Trost BM, Gutierrez AC. Org. Lett. 2007;9:1473. doi: 10.1021/ol070163t. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.