Abstract
Monocyte chemoattractant protein 1 (MCP-1) plays a pivotal role in many inflammatory processes, including the progression of atherosclerosis and the response of the arterial wall to injury. We previously demonstrated that dexamethasone (Dex) inhibits MCP-1 mRNA accumulation in smooth muscle cells by decreasing its half-life. The effect of Dex was dependent upon the glucocorticoid receptor (GR) and independent of new transcription. Using RNA affinity and column chromatography, we have identified two proteins involved in regulating MCP-1 mRNA stability: Y-box binding protein 1 (YB-1), a multifunctional DNA/RNA-binding protein, and endoribonuclease UK114 (UK). By immunoprecipitation, YB and GR formed a complex present in equal amounts in extracts from untreated and Dex-treated cells. YB-1, UK, and GR small interfering RNA (siRNA) substantially inhibited the effect of Dex on MCP-1 mRNA accumulation. In addition, YB-1 antibody blocked the degradation of MCP-1 mRNA by cytoplasmic extracts from the Dex-treated cells. The degradative activity of extracts immunoprecipitated with antibodies to either YB-1 or GR was blocked with UK antibody. UK did not degrade MCP-1 mRNA; however, upon addition to nondegrading control extracts, it rapidly degraded MCP-1 mRNA. These studies define new roles for GR, YB-1, and UK in the formation of a molecular complex that degrades MCP-1 mRNA.
INTRODUCTION
Monocyte chemoattractant protein 1 (MCP-1) (also known as CCL2) is a CC chemokine that binds to the G protein-coupled 7 transmembrane spanning receptor CCR2. MCP-1 has been implicated in a variety of inflammatory processes, such as inflammatory bowel disease, rheumatoid arthritis, asthma, glomerulonephritides, and parasitic and viral infections (9, 10, 29, 42, 50, 59). MCP-1 is minimally expressed in normal arteries but is rapidly induced in smooth muscle cells (SMCs) by arterial injury (23) and expressed at high levels in intimal SMCs and macrophages in atherosclerotic plaques (60, 61). MCP-1 is also induced in cultured SMCs, fibroblasts, macrophages, and endothelial cells by a variety of agonists (18). Numerous studies, including those in genetically altered mice, have demonstrated the importance of MCP-1 and CCR2 in mediating macrophage accumulation in the development of atherosclerotic plaques (1, 4, 16, 17). The inhibition of macrophage accumulation in the vessel wall may have profound effects on the proliferative, inflammatory, and thrombotic components associated with arterial injury and atherosclerosis. Considering the potential role of MCP-1 in mediating vascular pathology, substantial effort has been expended to identify approaches to targeting MCP-1 (8, 54, 57).
Glucocorticoids (GCs) possess a wide variety of anti-inflammatory and antiproliferative properties. They are therefore used to suppress many types of allergic, inflammatory, and autoimmune disorders (19, 38, 48). GCs have been widely used to treat several cancers, such as leukemias, lymphomas, and multiple myelomas; to treat rheumatic disorders, such as rheumatoid arthritis and systemic lupus erythematosus; to treat acute allergic conditions, such as drug hypersensitivity reactions, allergic dermatitides, and asthma; in transplant recipients to prevent acute transplant rejection and graft-versus-host disease; and to treat inflammatory diseases of the skin, bowel, and nervous system. GCs are potent inhibitors of MCP-1 synthesis in a variety of cell types (21, 32, 36, 54), including SMCs (44–47). GCs are reported to suppress intimal hyperplasia (6, 55) and atherosclerosis (3, 43). GCs have been shown to decrease MCP-1 expression and macrophage accumulation in several animal models, including femoral arterial injury in cholesterol-fed rabbits (44–46), rat crescentic glomerulonephritis (41, 58), rat renal ischemia (46), and restraint-stressed mice (33).
We have previously reported that the GC dexamethasone (Dex) markedly reduces the accumulation of MCP-1 mRNA in SMCs and that the effect is almost exclusively due to changes in mRNA stability (a reduction in the half-life [t1/2] of MCP-1 mRNA from >3 h to <15 min) (43, 45, 46). We have also demonstrated that this effect is mediated by the glucocorticoid receptor (GR) and involves an apparently novel mechanism in which the GR binds directly to MCP-1 mRNA and facilitates its degradation (11). Employing an RNA affinity approach, we have now identified two proteins, Y-box binding protein 1 (YB-1) and RNase UK114 (UK), that mediate MCP-1 mRNA degradation. GR, YB-1 (a multifunctional DNA- and RNA-binding protein), and UK (an endoribonuclease) interact to form a molecular reactor that selectively targets and degrades MCP-1 mRNA.
MATERIALS AND METHODS
Reagents.
Recombinant human GR (rhGR; G1542) was from Sigma-Aldrich (St. Louis, MO). Recombinant YB-1 (H00004904-P01) and UK (H00010247-P01) were from Abnova (Littleton, CO). Human retinoic acid receptor (RAR; sc-4088), human mineralocorticoid receptor (MCR; sc-4419) and protein A/G Plus agarose beads (sc-2003) were from Santa Cruz Biotechnology (Santa Cruz, CA). Antibody (Ab) to GR (Ab3579) was obtained from Abcam (Cambridge, UK), and Ab to YB-1 (Y0396) was from Sigma (St. Louis, MO). Ab to UK was procured from five different vendors: Abcam (ab56669), Protein Tech (12930-1-AP), Sigma/Atlas (HPA022856 and HPA023489), Novus Biologicals (H00010247-M01), and Gene Tex (GTX 94993). A Bradford protein assay kit (500-0006, Bio-Rad, Hercules, CA) was used to determine protein concentration.
Cell culture and transfection.
Rat aortic SMCs were isolated from the thoracic aortas of male Sprague-Dawley rats by enzymatic dissociation and cultured in Dulbecco modified Eagle medium (DMEM; Gibco Laboratories, Gaithersburg, MD) and 10% heat-inactivated bovine serum as described previously (4). The response to Dex has been consistently seen with cells as early as passage 5 and as late as passage 23 (26); passages 12 to 18 were used for all experiments. YB-1, UK, and protein kinase Cδ (PKCδ) small interfering RNAs (siRNAs) were purchased from IDT (Coralville, IA). For YB-1, GCAGCAGACCGUAACAAUU corresponded to nucleotides (nt) 532 through 550 (GenBank accession no. NM031563). For PKCδ, GCUGAUGUGUGUGCAGUAU corresponded to nt 530 through 548 (GenBank accession no. BC076505). UK siRNA was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) as a pool of 3 target-specific 19- to 25-nt siRNAs designed to knock down gene expression (sc-146082; GenBank accession no. NM031714). GR siRNA was purchased from Dharmacon, Inc. (Lafayette, CO) as a SMARTpool ON-TARGETplus set of 4 siRNAs (MQ-089504-00-0005; GenBank accession no. NM012576). Scrambled siRNA was purchased from Santa Cruz Biotechnology, Inc., as control siRNA-A (sc-37007), consisting of a scrambled sequence that will not lead to the specific degradation of any known cellular mRNA. SMCs, at 60% confluence, were transfected with 20 nM double-stranded siRNA using Oligofectamine (Invitrogen, Carlsbad, CA) (28). After 72 h, the cells were collected and analyzed for mRNA and protein using real-time PCR (RT-PCR) and Western blots, respectively.
RNA preparation and RT-PCR.
Total RNA was isolated from SMCs using an RNeasy kit (Qiagen Inc., Valencia, CA). Three hundred nanograms of total RNA was used in each reaction, using the Masterscript RT-PCR system (5 PRIME Inc., Gaithersburg, MD). The parameters were as follows: 1 cycle at 54°C for 30 min and 94°C for 2 min; 18 cycles at 94°C for 22 s, 54°C for 22 s, and 73°C for 44 s; and 1 cycle at 73°C for 6 min. The primers spanned nt 145 through 595 for MCP-1, nt 801 through 1537 for GR, nt 83 through 673 for UK, and nt 420 through 988 for YB-1.
Monocyte chemotaxis assay.
Monocyte chemotaxis was measured using a 96-well microplate housing a polycarbonate filter with 5-μm pores (Neuro Probe, Inc., Gaithersburg, MD). This pore size has been shown to limit migration exclusively to monocytes (49). For each assay, 29 μl of vascular SMC-conditioned culture medium was loaded in triplicate wells. A framed filter was positioned on top of the wells on the microplate. A 50-μl aliquot of monocytes (4 × 106 cells/ml) was loaded on top of the filter. After a 90-min incubation in 5% CO2 at 37°C, the filter was removed. Preliminary experiments were done using N-formyl-methionyl-leucyl-phenylalanine (fMLP) (10 nmol/liter), a potent leukocyte chemotactic agent, as a positive control and 0.2% bovine serum albumin (BSA) as a negative control. Please note that we used a modification of the migration assay to count the cells. Instead of fixing and counting the cells on the bottom of the filter, we collected the actual number of cells that passed through the filter into the conditioned medium and then counted them using a hemocytometer. All experiments were performed in triplicate.
In vitro decay assays.
MCP-1 constructs were generated by PCR using the full-length rat MCP-1 cDNA (GenBank accession no. AF058786) as a template. Linearized cDNAs were transcribed in vitro with T3 RNA polymerase (Boehringer Mannheim, Indianapolis, IN) in the presence of [α-32P]UTP (800 Ci/mmol; New England Nuclear, Boston, MA) as previously described (26). c-fos (GenBank accession no. X06769) and Mena (mammalian homologue of Drosophila enabled; GenBank accession no. BC062927) mRNAs were also transcribed in vitro from their linearized full-length cDNAs. Probes were purified on 4% polyacrylamide gels. Cytosolic (S-100) extracts from untreated or agonist-treated SMCs were prepared as previously described (26) and stored at −80°C. In vitro RNA gel shifts and decay assays were performed and analyzed on polyacrylamide gels as previously described (26).
Immunoprecipitation.
Protein A/G beads (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) precoupled to primary Abs were used for immunoprecipitation. Thirty microliters of beads was first washed three times with cold S100 buffer (10 mM Tris [pH 7.4], 1.5 mM MgCl2, 10 mM KCl, 0.5 mM dithiothreitol) supplemented with a protease inhibitor cocktail tablet (Roche, Germany). After being washed, the beads were coated with the relevant Ab (2 μg) for 4 to 6 h at 4°C by gently mixing. After the beads were collected by centrifugation and washed three times with cold S100 buffer, whole-cell extracts (100 μg) were added and incubated overnight on a rotating platform at 4°C. The immunoprecipitates were collected by centrifugation at 600 × g for 5 min and washed three times with S100 buffer. For Western blotting, samples were run on a 4 to 20% SDS-PAGE gel and then immunoblotted with the appropriate Abs.
Purification of mRNA binding proteins.
SuperDex 75 (purchased from GE Healthcare, Piscataway, NJ) was loaded into a 1-ml tuberculin syringe to generate a separation column with a bed volume of 500 μl. Extracts (100 μl) from Dex-treated SMCs were loaded onto the column in S100 buffer. The first 400 μl was discarded as the void volume. Six fractions of 300 μl each were collected. Sequence analysis was performed by the Harvard Microchemistry and Proteomics Analysis Facility (Cambridge, MA) using microcapillary reverse-phase high-performance liquid chromatography-nanoelectrospray tandem mass spectrometry (μLC/MS/MS) on a Thermo Scientific LTQ-Orbitrap mass spectrometer.
RESULTS
Identification of YB1 and UK as proteins that interact with MCP-1 mRNA.
We previously reported that GCs reduce MCP-1 mRNA levels in cultured SMCs, chiefly by decreasing mRNA stability (reduction in t1/2 in the presence of serum from >3 h to ∼15 min) (46). In order to identify the responsible molecules, cytoplasmic extracts from Dex-treated SMCs were incubated for 5 min with radiolabeled MCP-1 mRNA and run on a 2% agarose gel (Fig. 1A). Four 3-mm sections, corresponding to three visible bands (labeled 1, 2, and 4), and a region midway between bands 2 and 4 (labeled 3) were cut, eluted overnight in S100 buffer, and analyzed for MCP-1 mRNA degradative activity. The eluate from one section (D1 in Fig. 1B) degraded MCP-1 mRNA. Eluates of sections derived from control extracts did not degrade MCP-1 mRNA under the conditions of the assay (data not shown). The degradative activity of D1 was selective for MCP-1 mRNA and was blocked by exogenous rhGR but not by other members of the steroid hormone superfamily (Fig. 1C). These properties are similar to those previously reported for the GC-sensitive MCP-1 mRNA degradative activity identified in SMCs (11).
Fig 1.
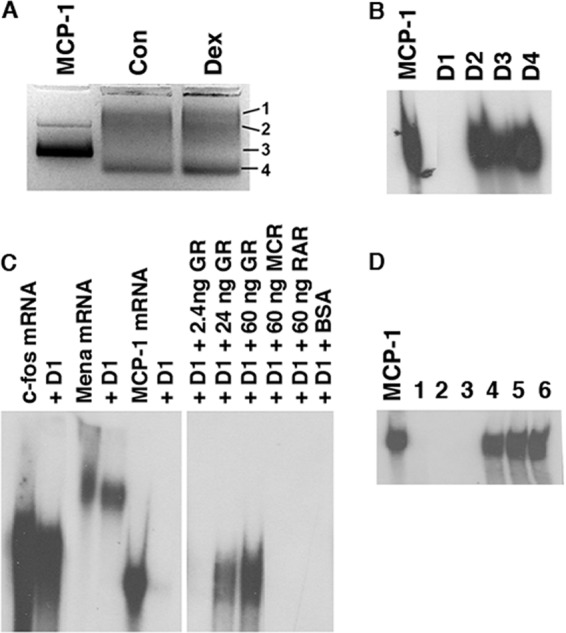
Purification of MCP-1 mRNA-binding proteins from Dex-treated SMCs. (A) Cytoplasmic extracts from untreated (Con) and Dex-treated (Dex) SMCs were incubated for 5 min with radiolabeled MCP-1 mRNA and run on 2% agarose gels. (B) Four shifted bands (D1 to D4 as shown in panel A) were eluted from the gels, incubated with radiolabeled MCP-1 mRNA for 30 min, and analyzed by 4% PAGE. (C) The eluate from D1 was incubated with radiolabeled c-fos, Mena, and MCP-1 mRNAs for 30 min and analyzed by 4% PAGE. In the right side of the panel (derived from the same gel), D1 was incubated with MCP-1 in the presence of either human recombinant GR, mineralocorticoid receptor (MCR), or retinoic acid receptor (RAR) at the amounts indicated. (D) D1 was loaded onto a 1-ml Superdex 75 column. Six 300-μl fractions were collected and tested for their ability to degrade MCP-1 mRNA as described for panel B.
Further purification of the activity from the D1 fraction was performed by gel filtration using a Superdex 75 column. Six 300-μl fractions were collected; the initial three degraded MCP-1 mRNA (Fig. 1D). Fractions 2 and 3 were combined and analyzed by mass spectroscopy. Fifty proteins were identified, including heat shock proteins, calbindin, fatty acid binding protein, ferritin heavy chain, and dermcidin. Of particular note were YB-1 and UK, both of which have been implicated in regulating mRNA expression and/or stability. YB-1 is a DNA/RNA-binding protein shown to regulate transcription and translation (25) and has also been reported to possess endoribonuclease activity (34). UK is an endoribonuclease that has been shown to inhibit translation by cleaving mRNA (33).
By RT-PCR, levels of YB-1 and UK mRNA were similar in control and Dex-treated SMCs (Fig. 2A). By Western blot analysis the YB1 protein appeared abundant, and the levels were also similar in control and Dex-treated SMCs (Fig. 2B). These data are consistent with our previous studies demonstrating that the effect of Dex on MCP-1 mRNA occurs in the presence of actinomycin D and cycloheximide (45). UK was undetectable on Western blots from either human or rat SMCs using five different Abs (Fig. 2C). These Abs detected rhUK (as in Fig. 2C), with amounts as small as 20 ng (data not shown), suggesting that the UK protein is present at low concentrations in SMCs.
Fig 2.
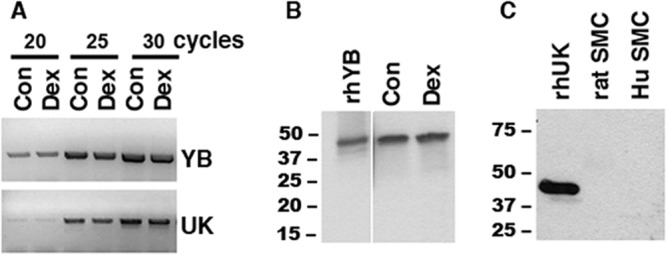
Analysis of YB-1 and UK mRNA and protein in SMCs. (A) RNA was harvested from untreated SMCs (Con) or SMCs treated with 1 μM Dex (Dex) for 3 h, and 300-ng aliquots were evaluated by RT-PCR for YB-1 and UK. (B and C) Total protein was harvested from control or Dex-treated SMCs (B) or untreated rat and human SMCs (C), and 50-μg aliquots were analyzed by Western blot analysis with the Abs to YB-1 (B) and UK (C). The results shown are representative of 3 experiments.
GR, YB-1, and UK mediate MCP-1 mRNA degradation.
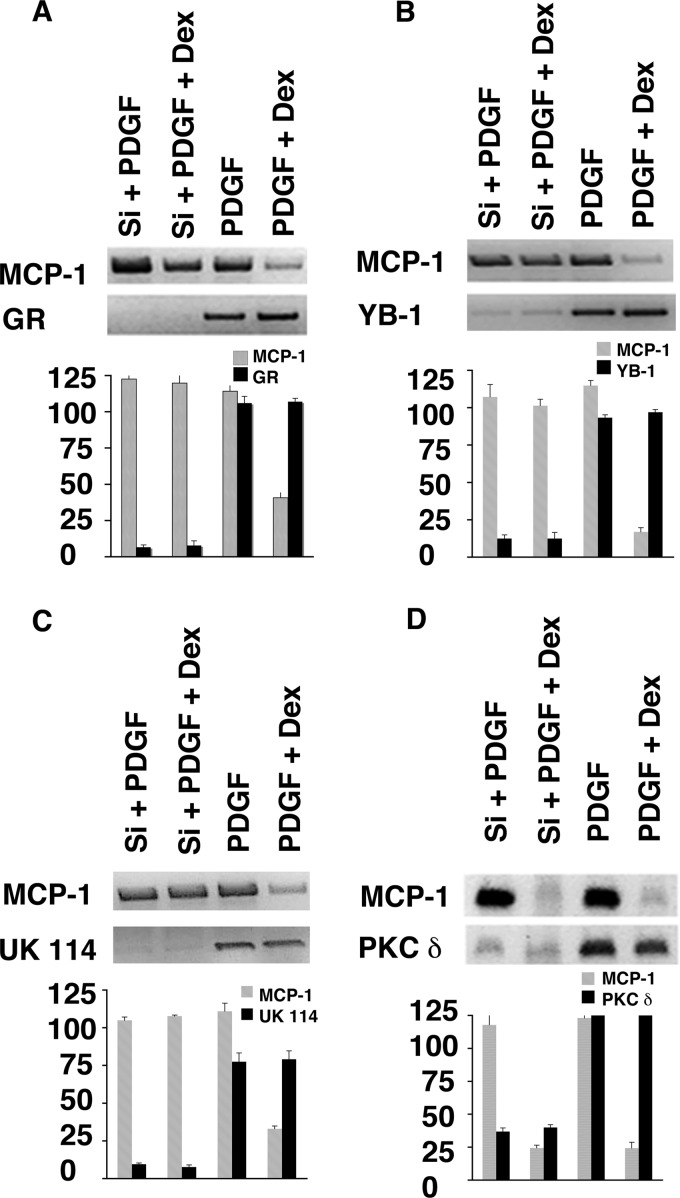
As a first approach to examining the roles of GR, YB-1, and UK in mediating MCP-1 mRNA degradation, SMCs were treated with siRNAs directed against each mRNA. As shown in Fig. 3, all three of the siRNAs substantially inhibited the effect of Dex on MCP-1 mRNA accumulation. siRNA to GR caused a 94.4% reduction in GR mRNA, as measured by densitometry. This completely blocked the effect of Dex on MCP-1 mRNA accumulation (Fig. 3A). siRNA to YB-1 caused a 90.1% reduction in YB-1 mRNA and an 85.8% inhibition of the Dex effect (Fig. 3B). siRNA to UK caused a 91.2% reduction in UK mRNA and a 94.9% inhibition of the Dex effect (Fig. 3C). As a control, siRNA to PKCδ caused a 95.1% reduction in PKCδ mRNA but did not block the Dex effect (Fig. 3D).
Fig 3.
SiRNA-mediated inhibition of MCP-1 mRNA expression. SMCs were transfected with siRNAs (Si) for GR (A), YB-1 (B), UK114 (C), or PKCδ (D) for 72 h and then treated for 3 h with 10 ng/ml platelet-derived growth factor (PDGF) (to induce MCP-1) alone or in the presence of 1 μM Dex (lanes 1 and 2, respectively). Duplicate cultures were treated with PDGF plus Dex in the absence of siRNA transfection (lanes 3 and 4, respectively). The MCP-1 level was evaluated by RT-PCR for MCP-1 (top lane on gel). Knockdown by GR (A), YB-1 (B), UK (C), and PKCδ (D) siRNA was also evaluated by RT-PCR (bottom lane on gel). The bar graph represents results of densitometry for all the SiRNAs. All four panels were run on the same gel, and lanes were cropped for easy representation. Each experiment was performed in triplicate.
To establish that the Dex-mediated degradation of MCP-1 mRNA is functionally relevant, we tested the ability of conditioned medium from SMCs to attract monocytes. As described above, siRNAs to YB-1, GR, or UK treatment effectively blocked Dex-mediated decay of MCP-1 mRNA (Fig. 4A). Dex treatment resulted in 84% inhibition of monocyte migration, which was completely reversed by all three of the siRNAs (Fig. 4B).
Fig 4.
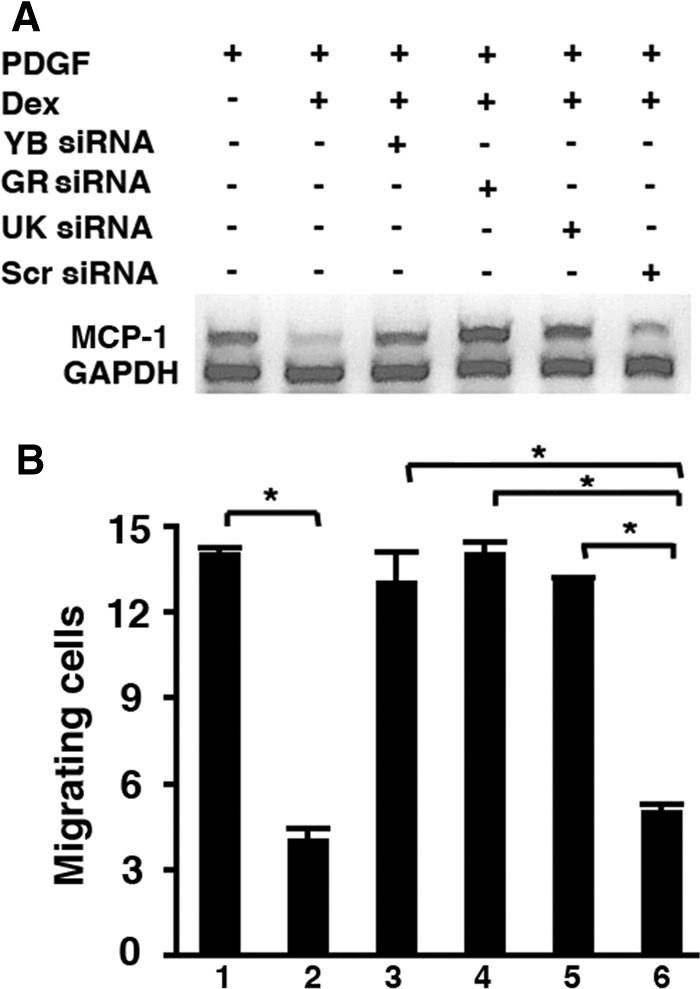
Inhibition of MCP-1 mRNA degradation and migration of monocytes by SiRNA treatment. SMCs were transfected with siRNAs for YB, GR, UK, or Scrambled (Scr) for 72 h and then treated for 16 h with 10 ng/ml platelet-derived growth factor (PDGF) (to induce MCP-1) plus 1 μM Dex. At the end of the incubation, conditioned medium was collected for migration assay (B), and RNA was harvested from the cells to perform RT-PCR for MCP-1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (A). Each experiment was performed in triplicate. *, P < 0.001.
To examine further the role of YB-1 in MCP-1 mRNA degradation, YB-1 Ab was added to extracts from Dex-treated SMCs (“Dex extracts”) prior to their use in mRNA degradation assays. The YB-1 Ab inhibited the ability of Dex extracts to degrade MCP-1 mRNA, whereas addition of the PKCδ Ab (P Ab) did not (Fig. 5A). The YB-1 Ab, by itself, did not affect the MCP-1 mRNA probe in the absence of Dex extracts (Fig. 5A, lane 1). In addition, immunodepletion of YB-1 from Dex extracts, using YB-1 Ab-coated protein A beads, abrogated their ability to degrade MCP-1 mRNA (Fig. 5B). In contrast, incubation with IgG-coated or bare protein A beads (Fig. 5B, no Ab lane) did not block the effect of Dex extracts.
Fig 5.
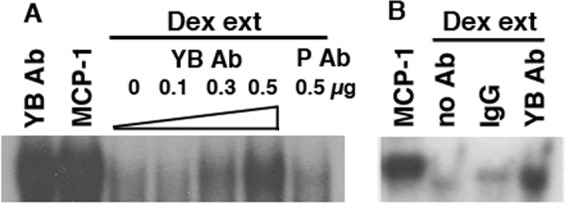
Inhibition of MCP-1 mRNA degradation by YB-1 Ab. (A) Dex extracts (ext) were incubated for 15 min with the YB-1 Ab (increasing amounts) and the PKCδ Ab (P Ab) and then assayed for MCP-1 mRNA degradative activity as in Fig. 1B. In lane 1, the YB-1 Ab was added to the MCP-1 probe in the absence of Dex extracts. (B) Dex extracts were immunodepleted overnight using the YB-1 Ab. Nonimmune IgG was used as a control. The no Ab lane contained extract and beads. Each experiment was performed in triplicate.
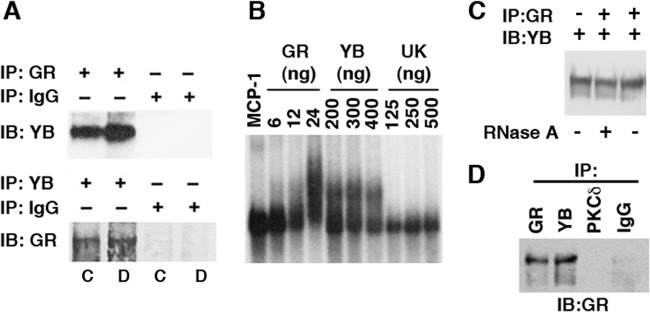
Bidirectional coimmunoprecipitation experiments using GR and YB-1 Abs suggested that GR and YB-1 interact in Dex extracts, as well as in extracts from untreated SMCs (“control extracts”) (Fig. 6A). It should be noted that in Fig. 6A, all lanes marked as control (C) represent equal aliquots of the same extract, as do all lanes marked as Dex (D). Gel shifts using in vitro-transcribed MCP-1 mRNA and rhGR, rhYB-1, or rhUK demonstrated that rhGR and rhYB-1 bound MCP-1 mRNA, whereas rhUK did not (Fig. 6B). Because it was possible that MCP-1 mRNA was acting as a bridge connecting GR and YB-1 and that the coimmunoprecipitation of GR and YB-1 was not due to direct interaction between the two proteins, coimmunoprecipitation experiments were performed in the presence or absence of 20 μg/ml RNase A. Treatment with RNase A had no effect on the ability of the GR Ab to coimmunoprecipitate YB-1 (Fig. 6C), suggesting that the interaction between the two proteins is independent of their binding to MCP-1 mRNA. It should be noted that the levels of YB-1 protein recovered after immunoprecipitating with the GR Ab were virtually the same as the level of YB-1 protein in an equal aliquot of the unprecipitated extract (compare all three lanes in Fig. 6C). In addition, the GR Ab was able to coimmunoprecipitate YB-1 from mixtures of purified rhGR and rhYB-1 proteins (Fig. 6D). This further demonstrates that the interaction between YB-1 and GR is not dependent upon cellular activation.
Fig 6.
Interactions among GR, YB-1, and MCP-1 mRNA. (A) Equal aliquots of the same extracts (Con [lanes C] or Dex [lanes D]) were immunoprecipitated (IP) with the Ab to GR, YB, or control IgG (as described in Materials and Methods) and then analyzed by immunoblotting (IB) on a 4 to 20% denaturing gel. Lanes 1 and 3 and lanes 2 and 4 were generated from the same extract. (B) In vitro-transcribed radiolabeled MCP-1 mRNA was incubated with rhGR, rhYB-1, and rhUK (amounts indicated) for 30 min and then run on nondenaturing gels. (C) Aliquots of the same Dex extract used for panel A were tested. Dex extracts were immunoprecipitated with the Ab to GR in the presence or absence of 20 μg/ml RNase A and then immunoblotted with the Ab to YB-1. The first lane represents the input (IB:YB-1). (D) Equal aliquots of rhGR and rhYB-1 were incubated together for 15 min followed by immunoprecipitation overnight with the indicated antibodies and then immunoblotted for GR Ab on a 4 to 20% denaturing gel. All experiments were performed in triplicate.
The above-described studies strongly support a role for YB-1 in mediating MCP-1 mRNA degradation. The levels of UK protein in our extracts were not sufficient to be detected by Western blotting; therefore, we sought alternative approaches to assess further the role of UK. To do so, we concentrated the MCP-1 mRNA degradative activity from Dex extracts by immunoprecipitation using the GR Ab affixed to protein A beads. As shown in Fig. 7A, the GR Ab immunoprecipitates degraded MCP-1 mRNA. However, this MCP-1 mRNA degradation was blocked when the GR Ab immunoprecipitates were preincubated with Abs to either YB-1 or UK. Similarly, the Ab to YB-1 also immunoprecipitated degradative activity, which was blocked by Abs to GR and UK (Fig. 7B). In addition, the Ab to UK also was able to immunoprecipitate degradative activity (Fig. 7B).
Fig 7.
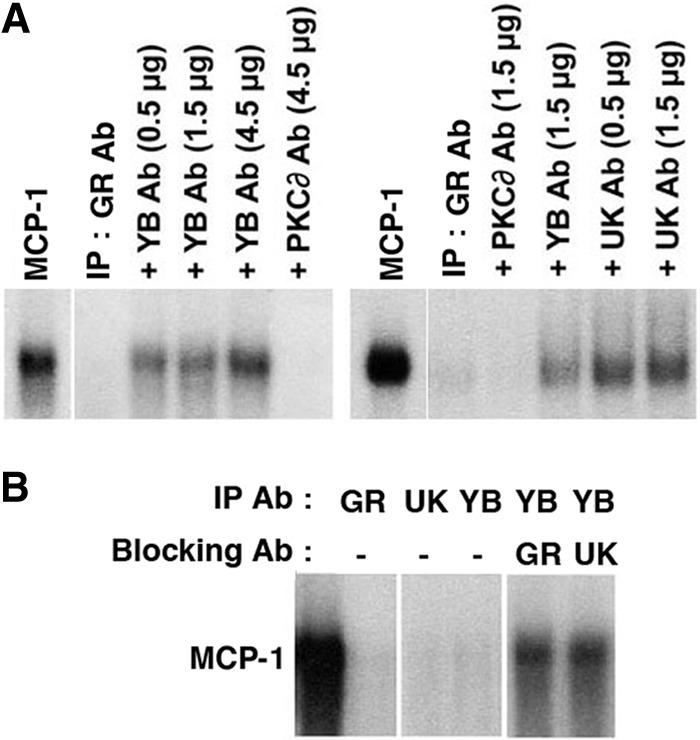
Immunoprecipitation of MCP-1 mRNA degradative activity. (A) Dex extracts were immunoprecipitated (IP) with the GR Ab, and the eluates were tested for their ability to degrade in vitro-transcribed MCP-1 mRNA alone (IP: GR Ab) or in the presence of the antibodies to YB-1, UK114, and PKCδ. Lanes labeled MCP-1 represent the probe alone and were run on the same gels as those to their immediate right. The two parts of panel A were derived from different gels. (B) Dex extracts were immunoprecipitated with the indicated antibodies, and the eluates were tested for their ability to degrade MCP-1 mRNA alone or in the presence of the antibodies to GR or UK. Panels were derived from the same gel. All experiments were performed in triplicate.
Our previous studies demonstrated that treatment of intact cells with GCs was necessary to stimulate MCP-1 mRNA degradative activity (45). To further address this, we attempted to reconstitute MCP-1 mRNA degradative activity using recombinant proteins. Of rhGR, rhYB-1, or rhUK, alone or in combination, none degraded MCP-1 mRNA in vitro (Fig. 8A and B). Although rhUK alone did not degrade in vitro-transcribed MCP-1 mRNA, it markedly enhanced degradation of the MCP-1 mRNA when added, at high concentrations, to control extracts (Fig. 8B). We previously demonstrated that rhGR, added in excess, could block the effect of Dex extracts on MCP-1 degradation; also, we hypothesized that the rhGR competes with the degradative complex for binding to the MCP-1 mRNA (11). Similarly, addition of rhYB-1 to the Dex extracts blocked MCP-1 mRNA degradation, whereas the addition of rhUK did not (Fig. 8C). Either rhGR or rhYB-1 blocked the ability of rhUK to enhance MCP-1 mRNA degradation in the presence of the control extracts (Fig. 8D), further supporting the concept that UK acts in concert with GR and YB-1 to reduce MCP-1 mRNA.
Fig 8.
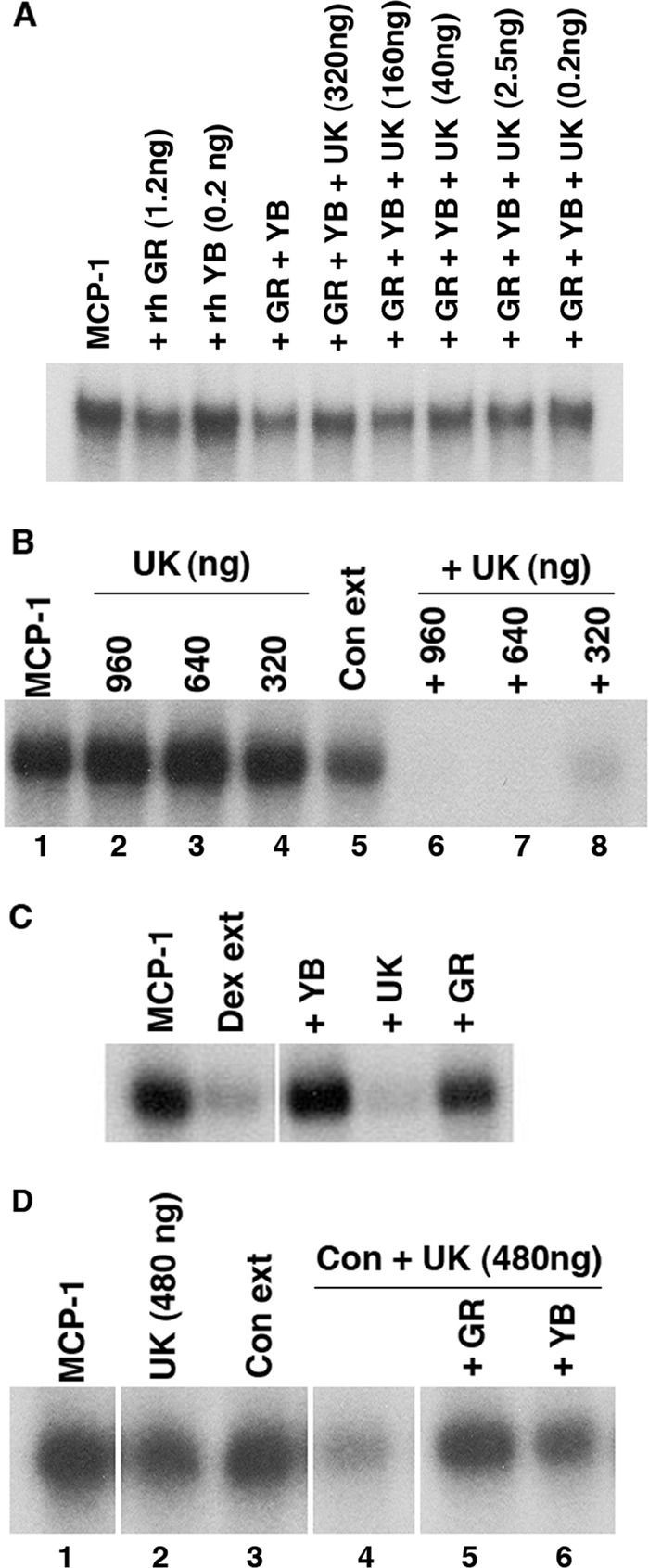
UK degrades MCP-1 mRNA in the presence of nondegrading SMC extracts. (A) In vitro-transcribed radiolabeled MCP-1 mRNA was incubated with rhGR or rhYB alone or in combination with rhUK at the concentrations indicated. Samples were then analyzed by 4% PAGE as in Fig. 1. (B) Radiolabeled MCP-1 mRNA (lane 1) was incubated with rhUK in the amounts indicated for 30 min (lanes 2 through 4), with the control extract (Con ext) alone (lane 5) or with the control extract and rhUK (lanes 6 through 8). (C) The Dex extracts were incubated with MCP-I mRNA alone or in the presence of recombinant proteins. (D) Radiolabeled MCP-1 mRNA (lane 1) was incubated for 30 min with rhUK alone (lane 2), control extract alone (lane 3), or control extract and rhUK (lanes 4 through 6) in the absence (lane 4) or presence of rhGR (lane 5) and rhYB-1 (lane 6). Panels were from the same gel. Each experiment was performed in triplicate.
DISCUSSION
MCP-1 plays a key role in mediating inflammation. In the vasculature, MCP-1 expression has been linked to the progression of atherosclerosis. We have previously shown that GCs decrease the expression of MCP-1 in vascular SMCs by altering mRNA stability (46). Intriguingly, the mechanism appeared to involve a nontranscriptional action of the GR. Abs to GR blocked the ability of Dex extracts to degrade MCP-1 mRNA (45). In addition, the rhGR bound specifically and selectively to MCP-1 mRNA (11). We hypothesized that GR was one component of a novel complex that degraded MCP-1 mRNA selectively. We now report that, using a combination of RNA affinity and gel filtration, we have identified two additional proteins (YB-1 and UK) that appear to act in concert with the GR to regulate MCP-1 mRNA decay.
YB-1 is a member of the nuclease-sensitive element-binding protein 1 (NSEP1)/YB-1 family of DNA-binding proteins named in recognition of its role as a transcription factor that recognizes the Y-box element in HLA class II gene promoters (12). YB-1 plays a significant role in the transcriptional regulation of genes encoding Fas, gelatinase A, collagen α1 (I), and multidrug resistance 1 (27, 30, 31, 39, 40). YB-1 regulates α-actin and CCL5 (RANTES) transcription in SMCs (24, 26, 62) and vascular endothelial growth factor expression in tumor cells (7). YB-1 protein binds to RNA and participates in many steps of mRNA biogenesis, including mRNA transcription, processing, and transport from the nucleus into the cytoplasm (52, 53, 56), where it can regulate mRNA localization, translation, and mRNA stability (13). YB-1 also functions as a structural protein involved in spatial organization of mRNA proteins (51) and binds in close proximity to the mRNA cap structure to displace eukaryotic translation initiation factor 4E (eIF4E) and eIF4G, thereby causing mRNA translational silencing and stabilization (14, 37). Inhibition of YB-1 with short hairpin RNA reduced intimal hyperplasia in response to arterial injury in apolipoprotein E−/− mice (26). Knockout of YB-1 induces a lethal phenotype in early embryos (15), suggesting a critical role for YB-1 during development.
In the present study, we found that treating SMCs with YB-1 siRNA substantially inhibited the effect of Dex on MCP-1 mRNA accumulation. Also, addition of the YB-1 Ab to Dex extracts or immunodepleting YB-1 from Dex extracts abrogated their ability to degrade MCP-1 mRNA. Therefore, YB-1 appears to be essential for MCP-1 mRNA degradation. Coimmunoprecipitation experiments suggest that the GR and YB-1 form a stable complex in control and Dex extracts. The binding of GR and YB-1 appears to be equimolar and does not appear to be a property of Dex activation, in that the amounts of YB-1:GR complexes are similar in control and Dex extracts. Purified rhGR and rhYB-1 proteins also form a stable association in vitro, further suggesting that their interactions are not dependent upon cellular activation. Although our data indicate that GR and YB-1 both play an important role in mediating MCP-1 mRNA degradation, neither molecule is “classically” a nuclease. There is a single report that YB-1 has endoribonuclease activity (against a synthetic 38-base oligomer) in vitro (34). Although SMC extracts could degrade the 38-mer, we were not able to block degradation with the YB-1 Ab under conditions in which it blocked MCP-1 mRNA degradation by the same extracts (data not shown). We were also unable to demonstrate endoribonuclease activity from commercially available rhYB-1. We therefore feel that it is unlikely that YB-1 is functioning as an endoribonuclease to regulate MCP-1 mRNA.
UK (also known as translational inhibitor protein p14.5 or heat-responsive protein 12) was purified from the perchloric acid-soluble fraction of goat liver (5). It shares a high degree of homology with other perchloric acid-soluble proteins (PSPs) extracted from the rat liver and kidney (2) and shows similarity to a newly hypothesized family of highly conserved proteins, YER057c/YJGFs (22). Studies suggest that UK has multiple functions. For example, UK was reported to inhibit protein synthesis in vitro in rabbit reticulocyte lysate (35). Overexpression of UK suppressed cell proliferation of normal rat kidney epithelial cells without any influence on cell viability (20). In addition, UK was reported to act as an endoribonuclease that inhibits translation by cleaving mRNA (35).
Like YB-1 and GR, UK mRNA levels were similar in untreated and Dex-treated SMCs. The UK protein was not detected by Western blotting, suggesting that the levels of UK protein in SMCs are very low, as might be expected for an intracellular nuclease. Our previous studies demonstrated that Dex-mediated degradation of MCP-1 mRNA in SMCs occurred in the presence of cycloheximide and actinomycin D (45), suggesting that the degradative activity did not involve transcription or translation. It is therefore not surprising that levels of GR, YB, and UK mRNA, and levels of GR and YB protein, were the same in control and Dex extracts. The Abs to UK were able to immunoprecipitate MCP-1 mRNA degradative activity from the Dex extracts. Like with YB-1 and GR, our siRNA data suggest that UK is involved in MCP-1 mRNA degradation. In addition, the Abs to UK blocked MCP-1 mRNA degradation by immunoprecipitates generated from Dex extracts using Abs to either GR or YB. rhUK did not degrade MCP-1 mRNA in vitro, either alone or in combination with rhGR or rhYB-1. However, the addition of very high levels of rhUK to control extracts (which includes GR:YB-1 complexes) induced the rapid degradation of MCP-1 mRNA. These data support our previous finding that cellular activation is critical for enhanced degradation of MCP-1 mRNA. We hypothesize that Dex treatment causes a modification in one or more of the three proteins that enhance MCP-1 mRNA degradation in the presence of a very small amount of UK (as found in cellular extracts). The addition of very large amounts of UK, such as those added to control extracts in the above-described experiments, may be sufficient to promote MCP-1 mRNA decay in the absence of prior activation by Dex.
Our data strongly suggest that UK is involved in MCP-1 mRNA degradation. However, rhUK alone did not degrade in vitro-transcribed MCP-1 mRNA. In addition, whereas both rhGR and rhYB-1 produced shifts with MCP-1 mRNA, suggesting direct binding, rhUK did not. These data suggest that the direct interaction of UK with MCP-1 mRNA may be weak or fleeting. It is possible that the GR:YB-1 complex acts as a scaffold that directs UK to MCP-I RNA for degradation. However, in vitro, rhUK failed to coimmunoprecipitate with either rhGR, rhYB-1, or both (data not shown). The apparent lack of interaction between UK and YB-1 or GR in vitro may indicate that there is another molecule that is required, that the interaction is very weak or short-lived, or that UK is acting coordinately with YB:GR but is not directly interacting with the two. It is also possible that there is a need for Dex-mediated cellular activation to affect a tripartite complex leading to MCP-1 mRNA degradation. Alternatively, the GR:YB-1 complex may bind to MCP-1 mRNA, causing a conformational change that facilitates a UK–MCP-1 mRNA interaction. In this scenario, cellular activation may be required for GR:YB-1 to produce the appropriate conformational change and/or for it to facilitate UK recruitment to MCP-1 mRNA.
Monocyte/macrophage accumulation is critical in inflammatory processes, including atherosclerosis. MCP-1 plays a major role in developing atherosclerotic lesions. Therefore, there has been immense interest in reducing MCP-1 accumulation. Our results have identified three proteins (GR, YB-1, and UK) that play a critical role in decreasing MCP-1 mRNA stability in SMCs in response to GCs. Further elucidation of the mechanism by which these proteins interact to regulate MCP-1 mRNA in the presence of Dex may allow the design of agents that mimic some of the anti-inflammatory properties of GCs without inducing their side effects.
ACKNOWLEDGMENTS
This work was supported by an American Heart Association Scientist Development grant (L.D.) and a grant from the National Institutes of Health (P01 HL77789) (M.B.T.).
Footnotes
Published ahead of print 16 July 2012
REFERENCES
- 1. Aiello RJ, et al. 1999. Monocyte chemoattractant protein-1 accelerates atherosclerosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 19: 1518–1525 [DOI] [PubMed] [Google Scholar]
- 2. Asagi K, et al. 1998. Purification, characterization and differentiation-dependent expression of a perchloric acid soluble protein from rat kidney. Nephron 79: 80–90 [DOI] [PubMed] [Google Scholar]
- 3. Asai K, et al. 1993. Dexamethasone-induced suppression of aortic atherosclerosis in cholesterol-fed rabbits. Possible mechanisms. Arterioscler. Thromb. 13: 892–899 [DOI] [PubMed] [Google Scholar]
- 4. Boring L, Gosling J, Cleary M, Charo IF. 1998. Decreased lesion formation in CCR2−/− mice reveals a role for chemokines in the initiation of atherosclerosis. Nature 394: 894–897 [DOI] [PubMed] [Google Scholar]
- 5. Ceciliani F, et al. 1996. The primary structure of UK114 tumor antigen. FEBS Lett. 393: 147–150 [DOI] [PubMed] [Google Scholar]
- 6. Chervu A, Moore WS, Quiñones-Baldrich WJ, Henderson T. 1989. Efficacy of corticosteroids in suppression of intimal hyperplasia. J. Vasc. Surg. 10: 129–134 [DOI] [PubMed] [Google Scholar]
- 7. Coles LS, et al. 2005. Phosphorylation of cold shock domain/Y-box proteins by ERK2 and GSK3beta and repression of the human VEGF promoter. FEBS Lett. 579: 5372–5378 [DOI] [PubMed] [Google Scholar]
- 8. Dawson J, Miltz W, Mir AK, Wiessner C. 2003. Targeting monocyte chemoattractant protein-1 signalling in disease. Expert Opin. Ther. Targets 7: 35–48 [DOI] [PubMed] [Google Scholar]
- 9. Dawson TC, Kuziel WA, Osahar TA, Maeda N. 1999. Absence of CC chemokine receptor-2 reduces atherosclerosis in apolipoprotein E-deficient mice. Atherosclerosis 143: 205–211 [DOI] [PubMed] [Google Scholar]
- 10. DeVries ME, Ran L, Kelvin DJ. 1999. On the edge: the physiological and pathophysiological role of chemokines during inflammatory and immunological responses. Semin. Immunol. 11: 95–104 [DOI] [PubMed] [Google Scholar]
- 11. Dhawan L, Liu B, Blaxall BC, Taubman MB. 2007. A novel role for the glucocorticoid receptor in the regulation of monocyte chemoattractant protein-1 mRNA stability. J. Biol. Chem. 282: 10146–10152 [DOI] [PubMed] [Google Scholar]
- 12. Didier DK, Schiffenbauer J, Woulfe SL, Zacheis M, Schwartz BD. 1988. Characterization of the cDNA encoding a protein binding to the major histocompatibility complex class II Y box. Proc. Natl. Acad. Sci. U. S. A. 85: 7322–7326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Evdokimova V, Ovchinnikov LP, Sorensen PH. 2006. Y-box binding protein 1: providing a new angle on translational regulation. Cell Cycle 5: 1143–1147 [DOI] [PubMed] [Google Scholar]
- 14. Evdokimova V, et al. 2001. The major mRNA-associated protein YB-1 is a potent 5′ cap-dependent mRNA stabilizer. EMBO J. 20: 5491–5502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fan L, Jones SN, Padden C, Shen Q, Newburger PE. 2006. Nuclease sensitive element binding protein 1 gene disruption results in early embryonic lethality. J. Cell. Biochem. 99: 140–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gosling J, et al. 1999. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J. Clin. Invest. 103: 773–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gu L, et al. 1998. Absence of monocyte chemoattractant protein-1 reduces atherosclerosis in low density lipoprotein receptor-deficient mice. Mol. Cell 2: 275–281 [DOI] [PubMed] [Google Scholar]
- 18. Gu L, Tseng SC, Rollins BJ. 1999. Monocyte chemoattractant protein-1. Chem. Immunol. 72: 7–29 [DOI] [PubMed] [Google Scholar]
- 19. Ito K, Chung KF, Adcock IM. 2006. Update on glucocorticoid action and resistance. J. Allergy Clin. Immunol. 117: 522–543 [DOI] [PubMed] [Google Scholar]
- 20. Kanouchi H, Tachibana H, Oka T, Yamada K. 2001. Recombinant expression of perchloric acid-soluble protein reduces cell proliferation. Cell. Mol. Life Sci. 58: 1340–1343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kawahara RS, Deng ZW, Deuel TF. 1991. Glucocorticoids inhibit the transcriptional induction of JE, a platelet-derived growth factor-inducible gene. J. Biol. Chem. 266: 13261–13266 [PubMed] [Google Scholar]
- 22. Kim JM, Yoshikawa H, Shirahige K. 2001. A member of the YER057c/yjgf/Uk114 family links isoleucine biosynthesis and intact mitochondria maintenance in Saccharomyces cerevisiae. Genes Cells 6: 507–517 [DOI] [PubMed] [Google Scholar]
- 23. Kim WJ, et al. 2003. MCP-1 deficiency is associated with reduced intimal hyperplasia after arterial injury. Biochem. Biophys. Res. Commun. 310: 936–942 [DOI] [PubMed] [Google Scholar]
- 24. Knapp AM, Ramsey JE, Wang SX, Strauch AR, Kelm RJ., Jr 2007. Structure-function analysis of mouse Pur beta II. Conformation altering mutations disrupt single-stranded DNA and protein interactions crucial to smooth muscle alpha-actin gene repression. J. Biol. Chem. 282: 35899–35909 [DOI] [PubMed] [Google Scholar]
- 25. Kohno K, Izumi H, Uchiumi T, Ashizuka M, Kuwano M. 2003. The pleiotropic functions of the Y-box-binding protein, YB-1. Bioessays 25: 691–698 [DOI] [PubMed] [Google Scholar]
- 26. Krohn R, et al. 2007. Y-box binding protein-1 controls CC chemokine ligand-5 (CCL5) expression in smooth muscle cells and contributes to neointima formation in atherosclerosis-prone mice. Circulation 116: 1812–1820 [DOI] [PubMed] [Google Scholar]
- 27. Lasham A, Lindridge E, Rudert F, Onrust R, Watson J. 2000. Regulation of the human fas promoter by YB-1, Puralpha and AP-1 transcription factors. Gene 252: 1–13 [DOI] [PubMed] [Google Scholar]
- 28. Liu B, Dhawan L, Blaxall BC, Taubman MB. 2010. Protein kinase Cä mediates MCP-1 mRNA stabilization in vascular smooth muscle cells. Mol. Cell. Biochem. 344: 73–79 [DOI] [PubMed] [Google Scholar]
- 29. MacDermott RP. 1999. Chemokines in the inflammatory bowel diseases. J. Clin. Immunol. 19: 266–272 [DOI] [PubMed] [Google Scholar]
- 30. Mertens PR, Harendza S, Pollock AS, Lovett DH. 1997. Glomerular mesangial cell-specific transactivation of matrix metalloproteinase 2 transcription is mediated by YB-1. J. Biol. Chem. 272: 22905–22912 [DOI] [PubMed] [Google Scholar]
- 31. Mertens PR, et al. 2002. Combinatorial interactions of p53, activating protein-2, and YB-1 with a single enhancer element regulate gelatinase A expression in neoplastic cells. J. Biol. Chem. 277: 24875–24882 [DOI] [PubMed] [Google Scholar]
- 32. Miyamasu M, et al. 1998. Glucocorticoids inhibit chemokine generation by human eosinophils. J. Allergy Clin. Immunol. 101: 75–83 [DOI] [PubMed] [Google Scholar]
- 33. Mizobe K, et al. 1997. Restraint stress-induced elevation of endogenous glucocorticoid suppresses migration of granulocytes and macrophages to an inflammatory locus. J. Neuroimmunol. 73: 81–89 [DOI] [PubMed] [Google Scholar]
- 34. Moraes KC, Quaresma AJ, Maehnss K, Kobarg J. 2003. Identification and characterization of proteins that selectively interact with isoforms of the mRNA binding protein AUF1 (hnRNP D). Biol. Chem. 384: 25–37 [DOI] [PubMed] [Google Scholar]
- 35. Morishita R, et al. 1999. Ribonuclease activity of rat liver perchloric acid-soluble protein, a potent inhibitor of protein synthesis. J. Biol. Chem. 274: 20688–20692. [DOI] [PubMed] [Google Scholar]
- 36. Natori Y, et al. 1997. Production of monocyte chemoattractant protein-1 by cultured glomerular epithelial cells: inhibition by dexamethasone. Exp. Nephrol. 5: 318–322 [PubMed] [Google Scholar]
- 37. Nekrasov MP, et al. 2003. The mRNA-binding protein YB-1 (p50) prevents association of the eukaryotic initiation factor eIF4G with mRNA and inhibits protein synthesis at the initiation stage. J. Biol. Chem. 278: 13936–13943 [DOI] [PubMed] [Google Scholar]
- 38. Newton R. 2000. Molecular mechanisms of glucocorticoid action: what is important? Thorax 55: 603–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Norman JT, et al. 2001. The Y-box binding protein YB-1 suppresses collagen alpha 1(I) gene transcription via an evolutionarily conserved regulatory element in the proximal promoter. J. Biol. Chem. 276: 29880–29890 [DOI] [PubMed] [Google Scholar]
- 40. Ohga T, et al. 1998. Direct involvement of the Y-box binding protein YB-1 in genotoxic stress-induced activation of the human multidrug resistance 1 gene. J. Biol. Chem. 273: 5997–6000 [DOI] [PubMed] [Google Scholar]
- 41. Ou ZL, Nakayama K, Natori Y, Doi N, Saito T. 2001. Effective methylprednisolone dose in experimental crescentic glomerulonephritis. Am. J. Kidney Dis. 37: 411–417 [DOI] [PubMed] [Google Scholar]
- 42. Panzer U, Stahl RA. 1999. Chemokines and renal inflammation. Nephrologie 20: 335–341 [PubMed] [Google Scholar]
- 43. Poon M, et al. 2001. Dexamethasone inhibits macrophage accumulation after balloon arterial injury in cholesterol fed rabbits. Atherosclerosis 155: 371–380 [DOI] [PubMed] [Google Scholar]
- 44. Poon M, Hsu WC, Bogadanov VY, Taubman MB. 1996. Secretion of monocyte chemotactic activity by cultured rat aortic smooth muscle cells in response to PDGF is due predominantly to the induction of JE/MCP-1. Am. J. Pathol. 149: 307–317 [PMC free article] [PubMed] [Google Scholar]
- 45. Poon M, Liu B, Taubman MB. 1999. Identification of a novel dexamethasone-sensitive RNA-destabilizing region on rat monocyte chemoattractant protein 1 mRNA. Mol. Cell. Biol. 19: 6471–6478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Poon M, et al. 1991. In vivo and in vitro inhibition of JE gene expression by glucocorticoids. J. Biol. Chem. 266: 22375–22379 [PubMed] [Google Scholar]
- 47. Pype JL, et al. 1999. Expression of monocyte chemotactic protein (MCP)-1, MCP-2, and MCP-3 by human airway smooth-muscle cells. Modulation by corticosteroids and T-helper 2 cytokines. Am. J. Respir. Cell Mol. Biol. 21: 528–536 [DOI] [PubMed] [Google Scholar]
- 48. Rhen T, Cidlowski JA. 2005. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N. Engl. J. Med. 353: 1711–1723 [DOI] [PubMed] [Google Scholar]
- 49. Rollins BJ, Walz A, Baggiolini M. 1991. Recombinant human MCP-1/JE induces chemotaxis, calcium flux, and the respiratory burst in human monocytes. Blood 78: 1112–1116 [PubMed] [Google Scholar]
- 50. Sato N, et al. 1999. Defects in the generation of IFN-gamma are overcome to control infection with Leishmania donovani in CC chemokine receptor (CCR) 5-, macrophage inflammatory protein-1 alpha-, or CCR2-deficient mice. J. Immunol. 163: 5519–5525 [PubMed] [Google Scholar]
- 51. Skabkin MA, et al. 2004. Structural organization of mRNA complexes with major core mRNP protein YB-1. Nucleic Acids Res. 32: 5621–5635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Skalweit A, et al. 2003. Posttranscriptional control of renin synthesis: identification of proteins interacting with renin mRNA 3′-untranslated region. Circ. Res. 92: 419–427 [DOI] [PubMed] [Google Scholar]
- 53. Sommerville J. 1999. Activities of cold-shock domain proteins in translation control. Bioessays 21: 319–325 [DOI] [PubMed] [Google Scholar]
- 54. Tesch GH. 2008. MCP-1/CCL2: a new diagnostic marker and therapeutic target for progressive renal injury in diabetic nephropathy. Am. J. Physiol. Renal Physiol. 294: F697–F701 [DOI] [PubMed] [Google Scholar]
- 55. Van Put DJ, et al. 1995. Dexamethasone influences intimal thickening and vascular reactivity in the rabbit collared carotid artery. Eur. J. Pharmacol. 294: 753–761 [DOI] [PubMed] [Google Scholar]
- 56. Wilkinson MF, Shyu AB. 2001. Multifunctional regulatory proteins that control gene expression in both the nucleus and the cytoplasm. Bioessays 23: 775–787 [DOI] [PubMed] [Google Scholar]
- 57. Xia Y, Frangogiannis NG. 2007. MCP-1/CCL2 as a therapeutic target in myocardial infarction and ischemic cardiomyopathy. Inflamm. Allergy Drug Targets 6: 101–107 [DOI] [PubMed] [Google Scholar]
- 58. Yamamoto-Shuda Y, Nakayama K, Saito T, Natori Y. 1999. Therapeutic effect of glucocorticoid on experimental crescentic glomerulonephritis. J. Lab. Clin. Med. 134: 410–418 [DOI] [PubMed] [Google Scholar]
- 59. Ying S, et al. 1999. Eosinophil chemotactic chemokines (eotaxin, eotaxin-2, RANTES, monocyte chemoattractant protein-3 (MCP-3), and MCP-4), and C-C chemokine receptor 3 expression in bronchial biopsies from atopic and nonatopic (Intrinsic) asthmatics. J. Immunol. 163: 6321–6329 [PubMed] [Google Scholar]
- 60. Yla-Herttuala S, et al. 1991. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc. Natl. Acad. Sci. U. S. A. 88: 5252–5256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yu X, et al. 1992. Elevated expression of monocyte chemoattractant protein 1 by vascular smooth muscle cells in hypercholesterolemic primates. Proc. Natl. Acad. Sci. U. S. A. 89: 6953–6957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang A, et al. 2005. YB-1 coordinates vascular smooth muscle alpha-actin gene activation by transforming growth factor beta1 and thrombin during differentiation of human pulmonary myofibroblasts. Mol. Biol. Cell 16: 4931–4940 [DOI] [PMC free article] [PubMed] [Google Scholar]