Abstract
Cell-cell fusion and cell invasion are essential for placental development. Human cytotrophoblasts in the chorionic villi may undergo cell-cell fusion to form syncytiotrophoblasts to facilitate nutrient-gas exchange or differentiate into extravillous trophoblasts (EVTs) to facilitate maternal-fetal circulation. The placental transcription factor glial cells missing 1 (GCM1) regulates syncytin-1 and -2 expression to mediate trophoblast fusion. Interestingly, GCM1 and syncytin-1 are also expressed in EVTs with unknown physiological functions. In this study, we performed chromatin immunoprecipitation-on-chip (ChIP-chip) analysis and identified the gene for high-temperature requirement protein A4 (HtrA4) as a GCM1 target gene, which encodes a serine protease facilitating cleavage of fibronectin and invasion of placental cells. Importantly, HtrA4 is immunolocalized in EVTs at the maternal-fetal interface, and its expression is decreased by hypoxia and in preeclampsia, a pregnancy complication associated with placental hypoxia and shallow trophoblast invasion. We further demonstrate that HtrA4 interacts with syncytin-1 and suppresses cell-cell fusion. Therefore, HtrA4 may be crucial for EVT differentiation by playing a dual role in prevention of cell-cell fusion of EVTs and promotion of their invasion into the uterus. Our study reveals a novel function of GCM1 and HtrA4 in regulation of trophoblast invasion and that abnormal HrtA4 expression may contribute to shallow trophoblast invasion in preeclampsia.
INTRODUCTION
Human placentation proceeds fast after embryo implantation, and different classes of specialized trophoblast cells have evolved to establish blood circulation for nutrient, gas, and waste exchange between mother and fetus. In brief, the mononuclear cytotrophoblasts in chorionic villi proliferate and differentiate through cell-cell fusion into a multinucleated syncytiotrophoblast layer, which is in direct contact with maternal blood to mediate the above-mentioned exchanges and produce hormones and growth factors for pregnancy maintenance. On the other hand, cytotrophoblasts in the chorionic villi that are anchored to uterine decidua proliferate into cell columns from which some cytotrophoblasts migrate and invade deeper layers of decidua. The migratory and invasive cytotrophoblasts, termed interstitial extravillous trophoblasts (EVTs), may further invade the uterine myometrium and replace the endothelial cells of spiral arteries. This phenomenon, called spiral artery remodeling, is essential for sufficient blood flow into intervillous spaces of the placenta, as remodeled arteries become dilated and nonvasoactive. Indeed, insufficient spiral artery remodeling due to shallow trophoblast invasion may result in placental hypoxia and pregnancy complications such as preeclampsia and intrauterine growth retardation with clinical features of gestational hypertension, proteinuria, and failure of optimal fetal growth (6).
Glial cells missing 1 (GCM1), also known as GCMa, is a transcription factor critical for placental development (2, 33). GCM1 regulates expression of syncytin-1 and -2 fusogenic proteins for syncytiotrophoblast differentiation and placental growth factor (PGF) for placental vasculogenesis (9, 19, 22, 37). The proteins syncytin-1 and -2 are encoded by envelope genes of the human endogenous retroviruses HERV-W and HERV-FRD, respectively (4, 5, 28). The syncytin polypeptide is posttranslationally cleaved into surface (SU) and transmembrane (TM) subunits, which mediate receptor recognition and membrane fusion, respectively. GCM1 activity is inhibited under hypoxia, where GSK-3β mediates Ser322 phosphorylation, leading to GCM1 ubiquitination and degradation (12). This may underscore decreased GCM1 and PGF expression in the hypoxic preeclamptic placentas. In contrast, cyclic AMP (cAMP) signaling stimulates GCM1 gene transcription (19) and enhances GCM1 stability by facilitating dual-specificity phosphatase 23-mediated Ser322 dephosphorylation and CREB-binding protein-mediated GCM1 acetylation, providing the underpinnings of the long-known stimulation of trophoblastic fusion by cAMP (8, 23).
In addition to their critical roles in syncytiotrophoblast differentiation, expression of GCM1 and syncytin-1 in EVTs has been reported (3, 25, 29). These observations raise questions about the functional role of GCM1 in EVT differentiation and why cell-cell fusion of EVTs is not observed at the maternal-fetal interface. In this study, we demonstrated that GCM1 upregulates the invasiveness of placental JAR and BeWo cells. By ChIP-chip analysis, we further identified a novel GCM1 target gene, the HtrA4 gene, whose product is a member of the high-temperature requirement protein A (HtrA) family of serine proteases capable of cleaving the extracellular matrix (ECM) protein fibronectin and mediating JAR and BeWo cell invasion. Immunohistochemistry revealed that HtrA4 and GCM1 are coexpressed in the interstitial EVTs at the maternal-fetal interface. Moreover, HtrA4 expression is decreased in BeWo cells under hypoxia and in preeclamptic placentas. The HtrA4 polypeptide is composed of an insulin growth factor-binding protein domain, a Kazal protease inhibitor domain, a trypsin protease domain, and a PDZ domain (13). Importantly, we found that HtrA4 binds to the SU subunit of syncytin-1 through its PDZ domain and that HtrA4 decreases the surface level of syncytin-1 and thereby suppresses syncytin-1-mediated cell-cell fusion. Therefore, GCM1 may regulate EVT differentiation by activating HtrA4 expression in order to stimulate EVT invasion and to counteract the fusogenic activity of syncytin-1. Our study reveals a novel function of GCM1 and HtrA4 in the regulation of trophoblast invasion and suggests that abnormal HtrA4 expression may contribute to the development of preeclampsia.
MATERIALS AND METHODS
Plasmid constructs.
A DNA fragment encoding wild-type HtrA4 with a C-terminal FLAG tag was cloned into pcDNA3 (Invitrogen, Carlsbad, CA) to generate the pHtrA4-FLAG expression plasmid. A serine-to-alanine mutation was introduced into the Ser326 residue at the active site of HtrA4 to generate the pHtrA4mt-FLAG expression plasmid for the protease-dead HtrA4 mutant. The pHtrA4mtδPDZ-FLAG expression plasmid was generated by deletion of the PDZ domain in the HtrA4mt cDNA. Human GCM1 cDNA fragment with an N-terminal hemagglutinin (HA) tag was subcloned into pcDNA3 to generate the pHA-GCM1 expression plasmid. For lentiviral expression, cDNA fragments for HA-GCM1, HtrA4-FLAG, and HtrA4mt-FLAG were subcloned into pCDH (SBI, Mountain View, CA) to generate pCDH-HA-GCM1, pCDH-HtrA4-FLAG, and pCDH-HtrA4mt-FLAG, respectively. For RNA interference, lentiviral pLKO.1-Puro short-hairpin RNA (shRNA) expression plasmids harboring a scrambled sequence (5′-CCTAAGGTTAAGTCGCCCTCG-3′) and sequences for GCM1 (5′-CCTCAGCAGAACTCACTAAAT-3′) and HtrA4 (5′-AAGCTACATACCCAGCCCTCC-3′) were provided by the National RNAi Core Facility of Taiwan. A human genomic DNA fragment containing the HtrA4 promoter region from nucleotide (nt) −971 to 29 (relative to the translational initiation site) was subcloned into pGL3E1B to generate the reporter construct pHtrA4-1kb. A proximal GCM1-binding site (GBS; nt −284 to −277) in the above-mentioned HtrA4 promoter region was mutated to generate the pHtrA4-1kb-GBSmt reporter construct. The pSyn1-HA expression plasmid, which encodes syncytin-1 with a C-terminal HA tag (syncytin-1–HA), has been described previously (11).
Cell culture, transfection, and lentivirus transduction.
The 293T, JAR, and BeWo cells were obtained from the American Type Culture Collection (Manassas, VA). For transient expression, cells were transfected with the indicated reporter and expression plasmids using Lipofectamine 2000 reagent (Invitrogen). For stable expression of exogenous HA-GCM1, HtrA4-FLAG, and HtrA4mt-FLAG, cells were infected with the recombinant lentivirus strains harboring the aforementioned pCDH constructs. In addition, stable GCM1 and HtrA4 knockdown cells were established using the recombinant lentivirus strains harboring the aforementioned pLKO.1-Puro shRNA expression plasmids. The infected cells were subjected to antibiotic selection using 1 μg/ml of puromycin, and the puromycin-resistant clones were pooled for studies. To study the effects of hypoxia on HtrA4 expression and promoter activity and cell invasion, cells were incubated under hypoxic (1% O2, 5% CO2, and 94% N2) or normoxic (21% O2, 5% CO2, and balance N2) conditions at 37°C for 72 h prior to further analysis.
Chromatin immunoprecipitation-on-chip (ChIP-chip) analysis.
BeWo cells stably expressing HA-GCM1 (BeWo31) (36) were treated with 50 μM forskolin for 24 h or left untreated before being subjected to ChIP assays using normal mouse serum or HA monoclonal antibodies (MAb) (Sigma, St. Louis, MO). The immunopurified genomic fragments were amplified by ligation-mediated PCR, and the resulting amplicons were fragmented and labeled with a terminal labeling kit (Affymetrix, Santa Clara, CA) according to the manufacturer's instructions. The chips of the human promoter 1.0R array (Affymetrix) were hybridized with the labeled amplicons, followed by data analysis using the Partek software package (St. Louis, MO). Furthermore, RefSeq mapping data were collected from the UCSC Genome Browser's RefFlat table (UCSC Genome Browser database, update 2007). The closest RefSeq 5′ end for each ChIP region was identified, regardless of whether the cluster was upstream or downstream of the 5′ end. The primer sequences for ChIP analysis of the interaction between GCM1 and HtrA4-GBS in BeWo cells are 5′-TGGAAACTGTTACGCTTCTCA-3′ and 5′-GTCTCTAGCCCTACCCG-3′. On the other hand, analysis of direct interaction between GCM1 and HtrA4-GBS was performed by electrophoretic mobility shift assay (EMSA) as previously described (22). In brief, recombinant GCM1-FLAG (8) was incubated with a radiolabeled oligonucleotide probe harboring the wild-type (5′-GTGAGCTTTCTTATCAGTCTGCCCTCATTGTCGGTTTT-3′) or mutant (5′-GTGAGCTTTCTTATCAGTCTGAACTACTTGTCGGTTTT-3′) HtrA4-GBS in the presence of GCM1 or unrelated syncytin-2 Ab (11). The reaction mixtures were analyzed by electrophoresis on 5% nondenaturing polyacrylamide gels.
Cell invasion assay.
Cell invasion analysis of GCM1- or HtrA4-expressing JAR cells and GCM1 or HtrA4 knockdown BeWo cells was performed using Matrigel invasion chambers (BD Biosciences, Bedford, MA) according to the manufacturer's instructions. In brief, cells were plated in the chambers and incubated for 48 h. Migrating cells in the lower surface of the filters were fixed with paraformaldehyde, visualized by crystal violet staining, and counted. Four microscopic fields per sample were randomly selected for quantification in each of three independent experiments. Images were prepared for presentation using Adobe Photoshop v7.0.
Immunohistochemistry, flow cytometry analysis, and single-cell RT-PCR.
Normal and preeclamptic placental tissue biopsy specimens were fixed in neutral buffered formalin, embedded in paraffin, and sectioned. Tissue sections were deparaffinized and subjected to immunostaining by incubation with normal serum, cytokeratin 7 (CK7; Millipore, Billerica, MA), HtrA4, and GCM1 Abs, respectively. The sections were then incubated sequentially with biotinylated secondary antibody and horseradish peroxidase (HRP)-conjugated streptavidin. Antigenic detection was performed using chromogenic substrate 3,3′-diaminobenzidine tetrahydrochloride (DAB), and the sections were further counterstained with hematoxylin. The HtrA4 antibody was prepared from guinea pigs immunized with His-tagged recombinant protein containing the region from amino acids 170 to 476 of the HtrA4 polypeptide.
To purify EVTs, villous tissues in the basal plate of term placenta were collected, trypsinized, and subjected to Percoll gradient centrifugation to enrich trophoblast cells (11). The trophoblast cells were then incubated with HLA-G Ab (Abcam, Cambridge, MA) and Alexa Fluor 488-conjugated secondary Ab (Invitrogen) and subsequently analyzed in a FACSAria II flow cytometer (BD Biosciences) to obtain the HLA-G-positive EVTs. Increasing number of EVTs (5, 50, and 500 cells) were lysed using a RealTime ready cell lysis kit (Roche Applied Science, Indianapolis, IN) and reverse transcribed, followed by analysis of GCM1 and HtrA4 transcripts in a LightCycler 480 real-time PCR instrument (Roche Applied Science). The sequences of the primer sets for PCR analysis in this study are as follows: 5′-GGACTACATCCAGACCGAC-3′ and 5′-TGGGACTCCGTGAGGAAC-3′ for HtrA1, 5′-TCTGGAGGTCCCCTGGTTA-3′ and 5′-AGCATCATCACCCCAATGT-3′ for HtrA2, 5′-GAGGGCTGGTCACATGAAGA-3′ and 5′-GCTCCGCTAATTTCCAGT-3′ for HtrA3, 5′-GTCAGCACCAAACAGCG-3′ and 5′-GGAGATTCCATCAGTCACCC-3′ for HtrA4, 5′-CTGACAAGGCTTTTTTCTTCACA-3′ and 5′-CCAGACGGGACAGGTTT-3′ for GCM1, and 5′-AACTCCATCATGAAGTGTGACG and 5′-GATCCACATCTGCTGGAAGG-3′ for β-actin.
Preparation of GST fusion proteins and HtrA4 recombinant proteins.
The glutathione S-transferase (GST) fusion proteins GST-SU and GST-TM, which contain the SU and the TM subunits of syncytin-1, respectively, were prepared in Escherichia coli using the pGEX6P-1 vector (GE Healthcare, Piscataway, NJ). Recombinant HtrA4-FLAG and HtrA4mt-FLAG proteins were prepared in a vaccinia virus expression system. In brief, 293T cells were infected with vTF7-3, a vaccinia virus expressing T7 RNA polymerase, followed by transfection of pHtrA4-FLAG, pHtrA4mt-FLAG, and pHtrA4mtδPDZ-FLAG, respectively. Recombinant HtrA4-FLAG, HtrA4mt-FLAG, and HtrA4mtδPDZ-FLAG proteins were purified from the culture media of transfected 293T cells using agarose beads conjugated with a FLAG MAb (Sigma). Cleavage of ECM protein by HtrA4 was performed by incubation of fibronectin (Calbiochem, La Jolla, CA) and HtrA4-FLAG or HtrA4mt-FLAG at 37°C for 16 h, followed by immunoblotting with fibronectin Ab (Sigma). Pulldown experiments were performed by incubation of GST-SU- or GST-TM-preloaded glutathione agarose beads with HtrA4mt-FLAG to identify the interaction domain in syncytin-1 for HtrA4. Likewise, GST-SU-preloaded glutathione agarose beads were incubated with HtrA4mt-FLAG or HtrA4mtδPDZ-FLAG to identify the interaction domain in HtrA4 for syncytin-1. HtrA4mt-FLAG was used in the pulldown experiments instead of wild-type HtrA4-FLAG to prevent cleavage of the GST fusion proteins.
Cell-cell fusion analysis and analysis of surface syncytin-1.
To test the effect of HtrA4 on syncytin-1-mediated cell-cell fusion, 293T cells were first infected with the vTF7-3 vaccinia virus. The infected 293T cells were then split into different populations for transfection of the green fluorescent protein expression plasmid pEGFP-N1 (Clontech, Mountain View, CA) plus pcDNA3, pHtrA4-FLAG, or pHtrA4mt-FLAG. An additional population of infected 293T cells was transfected with pSyn1-HA. The 293T cells expressing syncytin-1–HA were cocultured with the 293T cells coexpressing enhanced green fluorescent protein (EGFP) plus the wild-type or mutant HtrA4. After 16 h, cell-cell fusion was examined under an immunofluorescence microscope (Olympus, Tokyo, Japan) equipped with a cooled charge-coupled device camera (DP50). To analyze the syncytin-1–HA on cell surfaces, a separate set of the aforementioned cocultured cells was subjected to surface biotinylation using sulfosuccinimidyl 2-(biotinamido)-ethyl-1,3′-dithiopropionate (sulfo-NHS-SS-biotin; Thermo Scientific, Rockford, IL). Streptavidin-conjugated agarose beads were added to pull down the biotinylated proteins, followed by immunoblotting with HA MAb. Likewise, BeWo cells expressing scrambled or HtrA4 shRNA were subjected to a biotinylation reaction, followed by immunoprecipitation with an antibody against the TM subunit of syncytin-1 (7) and immunoblotting with HRP-conjugated streptavidin.
RESULTS
GCM1 regulates placental cell invasion.
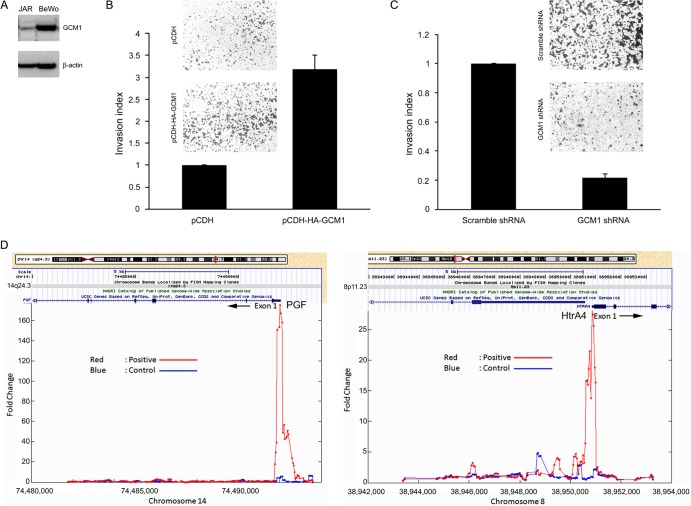
The fact that GCM1 is expressed in EVTs raises the possibility that GCM1 may play an important role in regulation of trophoblast invasion. To test this hypothesis, we examined whether GCM1 regulates the invasion activity of placental JAR and BeWo cells. As the endogenous GCM1 protein level is much lower in JAR cells than in BeWo cells (Fig. 1A), exogenous HA-tagged GCM1 (HA-GCM1) was introduced into JAR cells using a lentiviral expression system to establish JAR cells stably expressing HA-GCM1. For invasion analysis, cells harboring the pCDH empty vector (referred to here as mock cells) and HA-GCM1-expressing JAR cells were plated in Matrigel-coated Transwells. As shown in Fig. 1B, invasion of the HA-GCM1-expressing JAR cells was significantly enhanced compared to that of mock cells. As a complementary approach, we tested the effect of GCM1 knockdown on the invasion activity of BeWo cells. Indeed, compared with BeWo cells stably expressing scrambled shRNA, the invasion activity was significantly decreased in BeWo cells stably expressing GCM1 shRNA (Fig. 1C). These results suggested that GCM1 may participate in placental cell invasion.
Fig 1.
GCM1 regulates placental cell invasion. (A) Expression of GCM1 in JAR and BeWo cells. JAR and BeWo cells were subjected to immunoblotting with GCM1 and β-actin Abs. (B) Stimulation of JAR cell invasion by GCM1. Mock (pCDH) and HA-GCM1-expressing (pCDH-HA-GCM1) JAR cells were plated in Matrigel-coated chambers for invasion analysis. Representative pictures of cells that migrated to and invaded the lower surface of filters are provided. Means and standard deviations (SD) obtained from three independent experiments are presented. (C) GCM1 knockdown suppresses BeWo cell invasion. BeWo cells stably expressing scrambled or GCM1 shRNA were subjected to invasion analysis as described for panel A. (D) Identification of GCM1 target genes. BeWo31 cells were treated with 50 μM forskolin for 24 h, followed by ChIP-chip analysis as described in Materials and Methods. HtrA4 was identified as a candidate GCM1 target gene with strong hybridization signals (red versus blue) in the region upstream of exon1 of the HtrA4 gene (right). PGF, encoded by a known GCM1 target gene, was also identified in the analysis (left).
The HtrA4 gene is a GCM1 target gene.
We reasoned that GCM1 may transactivate novel target genes encoding the critical downstream effectors in the regulation of placental cell invasion. We performed ChIP-chip experiments using BeWo31 cells, which stably express HA-GCM1, to identify the candidate GCM1 target genes involved in placental cell invasion. As expected, placental growth factor (PGF) was identified as one of the GCM1 target genes in our ChIP-chip experiments (Fig. 1D, left). We concentrated on a novel GCM1 target gene, the HtrA4 gene (Fig. 1D, right), which encodes a serine protease primarily expressed in placenta, for further investigation of its role in regulation of placental cell invasion.
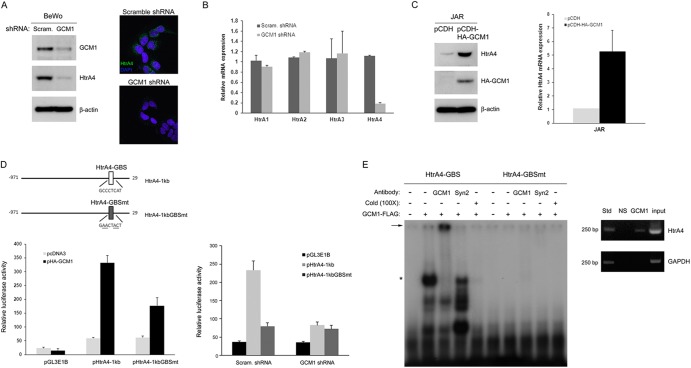
To confirm that the HtrA4 gene is a bona fide target gene of GCM1, we tested whether GCM1 regulates HtrA4 expression. An HtrA4 antibody was generated that does not cross-react with the other HtrA family members (data not shown). We measured the HtrA4 protein levels in the GCM1 knockdown and scrambled control BeWo cells. As shown in Fig. 2A (left), the HtrA4 protein level measured by immunoblotting was significantly decreased by GCM1 knockdown. Correspondingly, lower HtrA4 signals were detected in the cytoplasm of GCM1 knockdown BeWo cells by immunofluorescence microscopy (Fig. 2A, right). Moreover, the transcript level of HtrA4, but not HtrA1, -2, or -3, was also significantly decreased by GCM1 knockdown (Fig. 2B). Immunoblotting and quantitative PCR analyses for HtrA4 expression were also performed in the HA-GCM1-expressing and mock JAR cells. Indeed, GCM1 overexpression positively stimulated HtrA4 expression at both the protein and transcript levels in JAR cells (Fig. 2C).
Fig 2.
Regulation of HtrA4 expression by GCM1. (A) GCM1 knockdown decreases HtrA4 expression. BeWo cells stably expressing scrambled or GCM1 shRNA were subjected to immunoblotting with GCM1, HtrA4, and β-actin Abs, respectively. In a separate experiment, cells were immunostained with HtrA4 Ab (green) and nuclei were stained by DAPI (blue), followed by confocal microscopy analysis. Note that HtrA4 signals in the cytoplasm were decreased in the GCM1 knockdown BeWo cells. (B) Expression of HtrA4, but not other HtrA family members, is decreased by GCM1 knockdown. BeWo cells stably expressing scrambled or GCM1 shRNA were harvested for quantitative PCR analysis of the transcript levels of HtrA family members. Means and SD obtained from three independent experiments are presented. (C) Overexpression of GCM1 stimulates HtrA4 expression. Mock and HA-GCM1-expressing JAR cells were harvested for immunoblotting and quantitative PCR analysis of HtrA4 protein and transcript levels, respectively. (D) Regulation of HtrA4 promoter activity by GCM1. Schematic representation of HtrA4 promoter region with the wild-type and mutant GCM1-binding site (GBS) is provided (top). 293T cells were transfected with pHtrA4-1kb or pHtrA4-1kbGBSmt with or without pHA-GCM1 expression plasmid (left). BeWo cells expressing scrambled or GCM1 shRNA were transfected with pHtrA4-1kb or pHtrA4-1kbGBSmt (right). At 48 h posttransfection, cells were harvested for luciferase assays. Means and SD obtained from three independent experiments are presented. (E) Interaction of GCM1 and the GBS in HtrA4 promoter. Recombinant GCM1-FLAG was incubated with radiolabeled HtrA4-GBS or HtrA4-GBSmt probes in the presence of GCM1 or syncytin-2 (Syn2) Ab in EMSA. The asterisk and arrow indicate the GCM1-FLAG-DNA complex and its supershifted complex, respectively. Association of GCM1 and HtrA4-GBS in BeWo cells was analyzed by ChIP using normal rabbit serum (NS) or GCM1 Ab for immunoprecipitation and designated primer pairs for PCR.
We next studied whether GCM1 directly regulates the promoter activity of HtrA4 by transient-expression experiments. The pHtrA4-1kb reporter plasmid, which harbors a 1-kb HtrA4 promoter region proximal to the translational initiation site, was cotransfected with pHA-GCM1 into 293T cells. The luciferase reporter activity directed by pHtrA4-1kb was positively stimulated by HA-GCM1 (Fig. 2D, left). In line with our ChIP-chip study, a potential GCM1-binding site (HtrA4-GBS) was identified within the 1-kb HtrA4 promoter region (Fig. 2D, top). When the HtrA4-GBS was mutated in pHtrA4-1kbGBSmt, the luciferase reporter activity stimulated by GCM1 was compromised (Fig. 2D, left), indicating that the identified HtrA4-GBS is a functional GCM1-responsive element in the HtrA4 promoter. This was further supported by the fact that the luciferase activity directed by pHtrA4-1kb, but not pHtrA4-1kbGBSmt, was significantly decreased in the GCM1 knockdown BeWo cells (Fig. 2D, right). Of note, the possibility of additional binding sites for GCM1 and/or GCM1-interacting factors within the proximal 1 kb region of HtrA4 promoter cannot be ruled out, as GCM1 still partially stimulates the reporter activity directed by pHtrA4-1kbGBSmt (Fig. 2D, left). We next performed EMSA to characterize the interaction between GCM1 and HtrA4-GBS. To this end, recombinant GCM1-FLAG was incubated with a radiolabeled oligonucleotide probe harboring the HtrA4-GBS or HtrA4-GBSmt sequence. As shown in Fig. 2E (left), GCM1-FLAG specifically bound to HtrA4-GBS but not HtrA4-GBSmt, and the complex of GCM1 and HtrA4-GBS was supershifted by the GCM1 antibody, supporting a direct interaction between GCM1 and HtrA4-GBS. Correspondingly, in vivo interaction between GCM1 and HtrA4-GBS was confirmed by ChIP analysis in BeWo cells (Fig. 2E, right). Taken together, these results suggested that HtrA4 is a bona fide GCM1 target gene.
Expression of HtrA4 in human placenta.
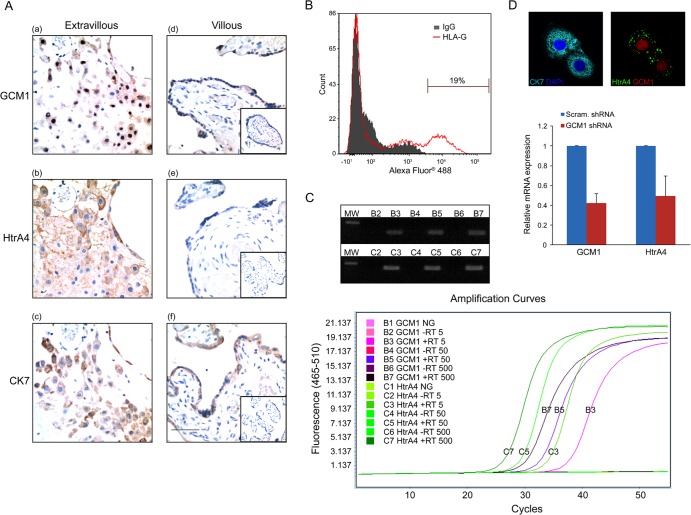
We next examined the expression of GCM1 and HtrA4 in human placenta by immunohistochemistry. Term placental tissue sections were immunostained using antibodies to GCM1, HtrA4, and CK7 (a trophoblast marker). While GCM1 was detected in the nuclei of both the villous syncytiotrophoblast layer and cells in the basal plate (Fig. 3A, panels a and d), HtrA4 was primarily detected in the cytoplasm of cells and the extracellular space between cells in the basal plate (Fig. 3A, compare panels b and e). Of note, both GCM1- and HtrA4-positive cell populations corresponded to the population of CK7-positive EVTs in the consecutive sections of basal plate (Fig. 3A, panels a to c).
Fig 3.
Coexpression of GCM1 and HtrA4 in the interstitial EVTs of human placenta. (A) Immunostaining of GCM1 and HtrA4 in term placenta. Term placental tissues sections were subjected to immunohistochemistry using GCM1 (a and d), HtrA4 (b and e), and CK7 (c and f) Abs and further counterstained with hematoxylin. Note that expression of GCM1 corresponded with that of HtrA4 in the CK7-positive EVTs in the consecutive sections of basal plate (a to c). The insets in panels d to f show sections immunostained with normal rabbit, guinea pig, and mouse serum, respectively. Bar, 100 μm. (B) Purification of EVTs. Primary trophoblast cells prepared from term placenta were subjected to flow cytometry analysis using HLA-G Ab as described in Materials and Methods. (C) Expression of HtrA4 and GCM1 in EVTs. HLA-G-positive EVTs were subjected to single cell RT-PCR as described in Materials and Methods. Amplification curves of real-time PCRs of increasing input cell numbers are presented. Of note, neither GCM1 nor HtrA4 transcript was detected in the samples without reverse transcriptase (RT) or in the mock reaction mixtures without input sample (NG). The PCR products were analyzed by agarose gel electrophoresis (top). MW, molecular weight marker. (D) Colocalization of GCM1 and HtrA4 in EVTs. HLA-G-positive EVTs were immunostained with GCM1, HtrA4, and CK7 Abs, followed by confocal microscopy analysis. In a separate experiment, cells were transduced with lentivirus harboring scrambled or GCM1 shRNA and subsequently harvested for quantitative PCR analysis of the HtrA4 and GCM1 transcripts. Means and SD from three independent experiments are presented.
To examine whether both GCM1 and HtrA4 are coexpressed in the interstitial EVTs of basal plate, we purified EVTs from primary trophoblast cells by flow cytometry analysis using an Ab against human leukocyte antigen G (HLA-G), which is an EVT surface marker (27) (Fig. 3B). Expression of GCM1 and HtrA4 transcripts was analyzed in increasing numbers of purified EVTs (5, 50, and 500 cells) by real-time PCR analysis with SYBR green and a LightCycler instrument. As shown in Fig. 3C (bottom), the amplification curves of GCM1 and HtrA4 transcripts were detected only in the RNA samples converted to cDNA by reverse transcriptase (RT). Importantly, the amplification cycle numbers of initial detection of both GCM1 and HtrA4 transcripts were inversely proportional to the input cell numbers. The PCR-amplified GCM1 and HtrA4 fragments were also analyzed by gel electrophoresis and were detected only in the reverse-transcribed samples (Fig. 3C, top). We further performed immunofluorescence microscopy of the purified EVTs with CK7, GCM1, and HtrA4 Abs. As shown in Fig. 3D (top), confocal analysis revealed that GCM1 and HtrA4 are colocalized in CK7-positive EVTs. Moreover, the HtrA4 transcript level was decreased in the purified EVTs when GCM1 was knocked down (Fig. 3D, bottom). Taken together, these results suggested that GCM1 regulates HtrA4 expression in the EVTs of basal plate of human placenta.
Regulation of placental cell invasion by HtrA4.
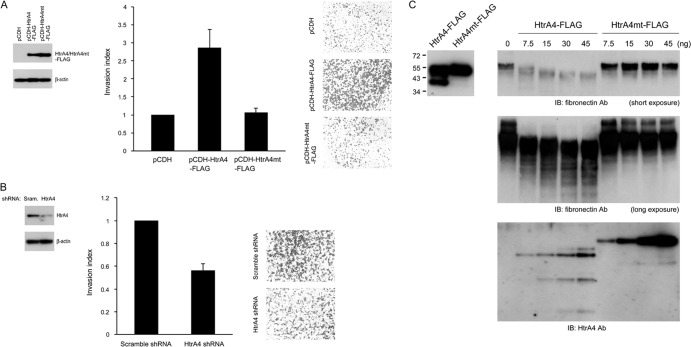
We next tested whether HtrA4 regulates placental cell invasion. Because the endogenous level of HtrA4 protein is low in JAR cells and high in BeWo cells (Fig. 2A and B), JAR and BeWo cells stably expressing HtrA4-FLAG and HtrA4 shRNA, respectively, were established for invasion assays. Compared with the mock JAR cells, the invasion activity was stimulated in JAR cells expressing the wild-type HtrA4-FLAG but not the protease-dead mutant HtrA4mt-FLAG (Fig. 4A). Correspondingly, knockdown of HtrA4 in BeWo cells suppressed the invasion activity of BeWo cells (Fig. 4B). We reasoned that HtrA4 may cleave extracellular matrix proteins to promote cell invasion. Therefore, we purified wild-type HtrA4-FLAG and HtrA4mt-FLAG proteins from the culture media of 293T cells transfected with pHtrA4-FLAG and pHtrA4mt-FLAG, respectively. The purified HtrA4-FLAG and HtrA4mt-FLAG proteins were incubated with recombinant fibronectin, followed by immunoblotting with antifibronectin Ab. As shown in Fig. 4C, cleavage of fibronectin by HtrA4-FLAG, but not HtrA4mt-FLAG, was observed in a dose-dependent manner. Therefore, HtrA4 may facilitate cell invasion through digestion of the ECM protein fibronectin and affect the interaction between fibronectin and its integrin receptor, which is critical for placental cell invasion (14).
Fig 4.
HtrA4 regulates placental cell invasion. (A) Overexpression of HtrA4 stimulates placental cell invasion. Mock and HtrA4-HA-expressing JAR cells were plated in Matrigel-coated chambers for invasion analysis. Expression of HtrA4-HA was analyzed by immunoblotting with HA MAb (left). (B) HtrA4 knockdown suppresses placental cell invasion. BeWo cells stably expressing scrambled or HtrA4 shRNA were subjected to invasion analysis as described for panel A. Knockdown of HtrA4 expression in BeWo cells was analyzed by immunoblotting with HtrA4 Ab (left). (C) Proteolytic cleavage of fibronectin by HtrA4. Purified HtrA4-FLAG and HtrA4mt-FLAG proteins from culture medium of 293T cells transfected with pHtrA4-FLAG and pHtrA4mt-FLAG, respectively, were analyzed by immunoblotting with FLAG MAb (left). Fibronectin was incubated with increasing amounts of HtrA4-FLAG or HtrA4mt-FLAG at 37°C for 16 h. The reaction mixture was then subjected to immunoblotting with HtrA4 or fibronectin Ab. Of note, partial cleavage of HtrA4-FLAG, but not HtrA4mt-FLAG, in the reaction was detected (bottom).
Decreased expression of HtrA4 by hypoxia and in preeclamptic placentas.
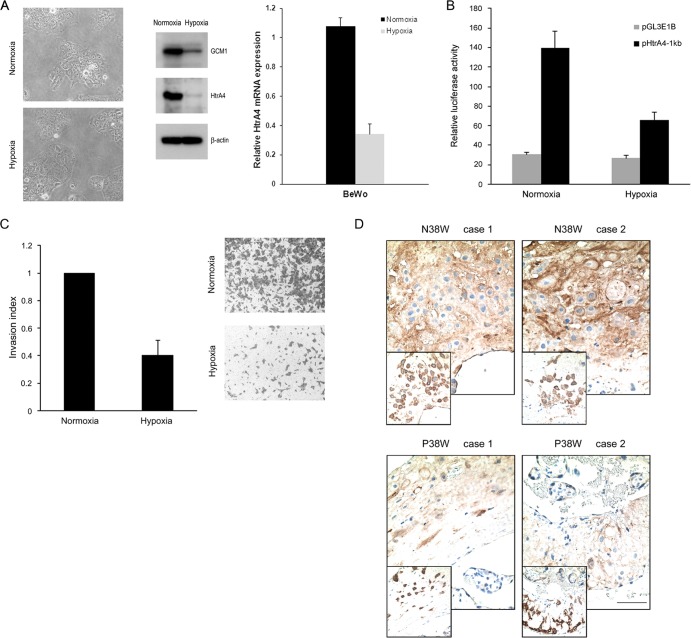
Our previous studies indicated that GCM1 activity is downregulated by hypoxia and in preeclampsia (10, 12). Accordingly, we measured the levels of HtrA4 protein and transcript in BeWo cells exposed to normoxic and hypoxic conditions, respectively. The morphology of BeWo cells under normoxic and hypoxic conditions was not significantly different (Fig. 5A). In line with our previous study (12), the GCM1 protein level was decreased in the hypoxic BeWo cells. Importantly, both the HtrA4 protein and transcript levels were also decreased in the hypoxic BeWo cells (Fig. 5A). Moreover, the promoter activity of HtrA4 was suppressed by hypoxia as the luciferase activity directed by pHtrA4-1kb was significantly decreased in BeWo cells transfected with pHtrA4-1kb and incubated under hypoxic conditions (Fig. 5B). Accordingly, the invasion activity of BeWo cells was significantly impaired under hypoxia (Fig. 5C). Because preeclampsia is associated with placental hypoxia, we demonstrated by immunohistochemistry that HtrA4 expression was significantly lower in preeclamptic placentas than in the gestation age-matched normal placentas (Fig. 5D). These results suggested that trophoblast invasion impaired by hypoxia or in preeclampsia may be attributed to the suppression of HtrA4 expression caused by decreased GCM1 activity.
Fig 5.
HtrA4 expression under hypoxia and in preeclampsia. (A) Downregulation of HtrA4 expression by hypoxia. BeWo cells were incubated under normoxic or hypoxic conditions. After 72 h of incubation, cells were analyzed by immunoblotting with GCM1, HtrA4, and β-actin Abs, respectively. Phase-contrast images for the morphology of normoxic and hypoxic cells were provided (left). In a separate experiment, cells were subjected to quantitative PCR analysis of the HtrA4 transcript level. (B) Suppression of HtrA4 promoter activity by hypoxia. BeWo cells were transfected with pHtrA4-1kb and incubated under normoxic and hypoxic conditions and subsequently harvested for luciferase assays. (C) Suppression of placental cell invasion by hypoxia. BeWo cells plated in Matrigel-coated chambers were incubated under normoxic or hypoxic condition for invasion analysis. Means and SD from three independent experiments are presented in panels A to C. (D) Decreased HtrA4 expression in preeclamptic placentas. Sections of two normal (N) and two preeclamptic (P) placentas at 38 weeks of gestation were immunostained with HtrA4 Ab. Note that the insets show consecutive sections immunostained with CK7 Ab. Bar, 100 μm.
HtrA4 suppresses cell-cell fusion mediated by syncytin-1.
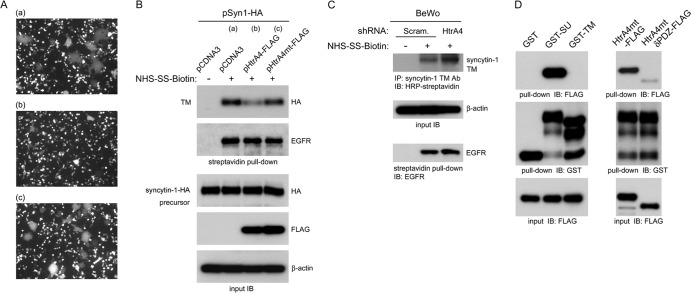
Although GCM1 regulates syncytin-1 expression to mediate cell-cell fusion for syncytiotrophoblast formation, cell-cell fusion of EVTs does not occur given that syncytin-1 is expressed in these cells. To reconcile this discrepancy, we investigated whether HtrA4 affects the cell-cell fusion mediated by syncytin-1. To this end, a coculture system was established to monitor cell-cell fusion by plating in the same cell culture dish a population of 293T cells expressing syncytin-1–HA and a second population of 293T cells coexpressing EGFP plus empty vector or EGFP plus HtrA4-FLAG. As expected, syncytia were detected in the coculture of syncytin-1–HA-expressing 293T cells with 293T cells coexpressing EGFP plus empty vector (Fig. 6A, panel a). Interestingly, syncytium formation was significantly decreased when syncytin-1–HA-expressing 293T cells were cocultured with 293T cells coexpressing EGFP plus HtrA4-FLAG (Fig. 6A, panel b), suggesting that HtrA4 suppresses syncytin-1–HA-mediated cell-cell fusion. Moreover, this suppressive effect of HtrA4 depended on its protease activity as expression of HtrA4mt-FLAG did not significantly affect cell-cell fusion mediated by syncytin-1–HA (Fig. 6A, panel c). We further studied how HtrA4 suppresses syncytin-1 activity by analysis of the protein level of syncytin-1 on cell surfaces. The 293T cells of the above-mentioned coculture studies were subjected to biotinylation, followed by pulldown assay with streptavidin-conjugated matrix and HA MAb. As shown in Fig. 6B, the protein level of the TM subunit of syncytin-1–HA decreased in the presence of HtrA4-FLAG but not HtrA4mt-FLAG. The observed negative effect of HtrA4 was specific to surface-bound syncytin-1–HA, as the level of surface-bound epidermal growth factor receptor (EGFR) was not affected (Fig. 6B). We also examined the level of surface-bound syncytin-1 in BeWo cells expressing scrambled or HtrA4 shRNA. To this end, an antibody recognizing the TM subunit of syncytin-1 was used to precipitate the biotinylated TM subunit of syncytin-1 on cell surfaces. Indeed, knockdown of HtrA4 significantly increased the protein level of syncytin-1 TM subunit on the surface of BeWo cells (Fig. 6C). Therefore, HtrA4 may cleave syncytin-1 to impair its fusogenic activity.
Fig 6.
Regulation of syncytin-1-mediated cell-cell fusion by HtrA4. (A) HtrA4 suppresses cell-cell fusion mediated by syncytin-1. 293T cells coexpressing empty vector and EGFP (a), HtrA4-FLAG and EGFP (b), or HtrA4mt-FLAG and EGFP (c) were cocultured with 293T cells expressing syncytin-1–HA for 24 h. Cell-cell fusion was examined by fluorescence microscopy. (B) HtrA4 decreases the protein level of surface syncytin-1. A separate set of the cocultured 293T cells described for panel A were subjected to biotinylation, followed by streptavidin pulldown and immunoblotting with HA or EGFR MAb. As a loading control, whole-cell lysates were subjected to immunoblotting with HA, FLAG, and β-actin MAbs, respectively. (C) HtrA4 regulates syncytin-1 expression in placental cells. BeWo cells stably expressing scrambled or HtrA4 shRNA were subjected to biotinylation, followed by immunoprecipitation with a syncytin-1 TM Ab and then immunoblotting with HRP-conjugated streptavidin. Note that the surface EGFR protein level was not affected by HtrA4. (D) Characterization of interaction between HtrA4 and syncytin-1. Purified HtrA4mt-FLAG was incubated with agarose matrix preloaded with GST, GST-SU, or GST-TM, followed by immunoblotting with FLAG MAb. On the other hand, HtrA4mt-FLAG or HtrA4mtδPDZ-FLAG was subjected to pulldown analysis with GST-SU demonstrating that the PDZ domain of HtrA4 is critical for recognition of the SU domain of syncytin-1.
We further tested the role of SU and TM subunits of syncytin-1 in interaction with HtrA4 by pulldown analysis. We used HtrA4mt-FLAG as prey to prevent proteolysis of the bait proteins, GST-SU and GST-TM, which are GST fusion proteins harboring the SU and TM subunits, respectively. As shown in Fig. 6D (left), HtrA4mt-FLAG was pulled down by GST-SU but not GST-TM. We also investigated the role of PDZ domain in HtrA4 for interaction with the SU subunit of syncytin-1. HtrA4mt-FLAG or its PDZ deletion variant HtrA4mtδPDZ-FLAG was incubated with GST-SU in pulldown analysis. Interestingly, deletion of the PDZ domain significantly decreased the interaction between HtrA4 and the SU subunit of syncytin-1 (Fig. 6D, right). Taken together, these results suggested that HtrA4 recognizes the SU subunit of syncytin-1 through its PDZ domain.
DISCUSSION
Successful pregnancy requires proper migration and invasion of trophoblasts into the maternal uterine tissue, which is a complicated process involving multiple intrinsic and extrinsic factors to regulate the interaction between EVTs and the decidual stromal cells. Both matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs), which inhibit the function of MMPs, are expressed in trophoblasts and decidual cells to regulate trophoblast migration and invasion (20). In addition, the STAT3 transcription factor is activated in the leukemia inhibitory factor (LIF) signaling pathway to promote trophoblast cell migration (16). Nevertheless, activation of STAT3 and overexpression of MMPs was also commonly found in invasive tumors of different tissue origins (17, 35), suggesting that additional regulatory mechanisms specific to placenta may be involved in control of trophoblast migration and invasion. In the present study, we demonstrated that the placental transcription factor GCM1 is able to regulate placental cell invasion through transactivation of HtrA4 serine protease. We further found that HtrA4 may cleave the ECM fibronectin to facilitate placental cell invasion. Collectively, these findings revealed a novel regulatory mechanism of EVT invasion by GCM1 and HtrA4.
Members of HtrA family are involved in different biological functions. HtrA2 is a mitochondrial serine protease that promotes apoptosis via interaction with and proteolysis of the IAP proteins, which contributes to caspase activation (13). Expression of both HtrA1 and -3 is detected at high levels in decidual cells and villous trophoblasts but is barely detectable in interstitial EVTs (21, 31). In contrast, here we demonstrated that HtrA4 is highly expressed in the interstitial EVTs, which can be attributed to a functional GCM1-responsive element in the proximal region of HtrA4 promoter. Interestingly, HtrA1 and -3 have been reported to inhibit trophoblast migration and invasion, and the protease activity of HtrA1 has been shown to be dispensable in the process (1, 34). The underlying mechanisms of inhibition of trophoblast migration and invasion by HtrA1 and -3 remain elusive. In the present study, we provide evidence that HtrA4 is a downstream effector of GCM1 stimulating placental cell invasion, most likely through proteolysis of ECM proteins. Clinically, elevated expression of both HtrA1 and -3 is detected in preeclampsia (1, 21). Moreover, HtrA3 expression is enhanced under hypoxic conditions (21). Here we show that HtrA4 expression is suppressed in BeWo cells by hypoxia, and so too is the invasiveness of BeWo cells. Correspondingly, we also show that HtrA4 expression decreases in preeclampsia. As placental hypoxia is associated with preeclampsia and enhances GCM1 degradation, we speculate that HtrA4 expression is compromised by decreased GCM1 activity, which may contribute to the incomplete trophoblast invasion commonly found in preeclamptic placentas. Just how HtrA1 and -3 expression is elevated in preeclampsia is currently unknown. Further investigations are required to clarify whether there are functional interactions between HtrA4, -1, and -3 in the regulation of trophoblast invasion and the development of preeclampsia. Of note, Drewlo et al. (15) recently showed that TIMP4 expression is increased in GCM1 knockdown BeWo cells and preeclampsia. However, we performed immunoblotting analysis to compare the protein levels of TIMP4 in BeWo cells expressing scrambled shRNA and GCM1 shRNA. Neither set of cells exhibited any significant change of TIMP4 expression (data not shown). This discouraged us from pursuing the issue of whether TIMP4 is a direct target gene of GCM1 and is involved in GCM1-regulated trophoblast invasion.
Immunohistochemistry indicated that HtrA4 is primarily expressed in interstitial EVTs but not villous trophoblasts. Because GCM1 is expressed in both cell types, we speculate that a cell/promoter context-dependent mechanism may be involved to favor HtrA4 expression in EVTs over villous trophoblasts. Moreover, expression of GCM1 target genes in the two cell types may be differentiated due to the different environmental cues in the villus and uterine decidua. Interestingly, we observed that forskolin, a cAMP stimulant, exerts a higher stimulatory effect on syncytin-1 expression than HtrA4, which correlates with the stimulatory effect of forskolin on cell-cell fusion (data not shown). Investigation of the differential expression of HtrA4 in EVTs is under way in our laboratory. Previous studies have demonstrated that syncytin-1, but not syncytin-2, is expressed in EVTs (24, 25, 29). That both HtrA4 and syncytin-1 are expressed in EVTs seems to be paradoxical in the regulation of EVT differentiation. In the present study, we demonstrated that syncytin-1 is a substrate of HtrA4. In this scenario, the PDZ domain of HtrA4 is crucial for recognition of the SU subunit of syncytin-1. As a result, HtrA4 decreases the level of surface syncytin-1 to suppress cell-cell fusion mediated by syncytin-1. These observations support an additional function of HtrA4 in the suppression of EVT fusion and may reconcile the above-described paradox (Fig. 7).
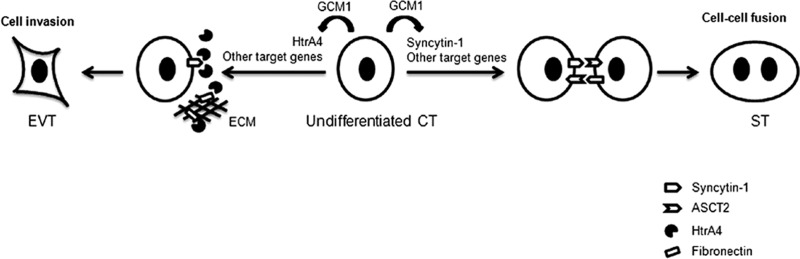
Fig 7.
Model of regulation of EVT differentiation by GCM1. The mononuclear cytotrophoblasts (CT) in chorionic villi may proliferate and differentiate into a multinucleated syncytiotrophoblast (ST) layer or invasive extravillous trophoblasts (EVT). GCM1 can regulate ST differentiation through transactivation of syncytin-1, which interacts with its cognate receptor, the sodium-dependent neutral amino acid transporter type 2 (ASCT2), to facilitate cell-cell fusion. In addition, GCM1 can participate in EVT differentiation through transactivation of HtrA4, which may cleave fibronectin in the extracellular matrix (ECM) and facilitate cell migration and invasion. Importantly, HtrA4 may also cleave syncytin-1 to prevent cell-cell fusion of EVTs.
PDZ domains in numerous proteins are responsible for specific protein-protein interactions via interaction with the C-terminal peptide sequence or structurally related internal peptide sequence of target proteins (18). Interestingly, studies have shown that the binding of peptide to the PDZ domain in HtrA1, -2, and -3 activates their protease activities (26, 30, 32). Accordingly, based on our finding that the PDZ domain of HtrA4 interacts with the SU subunit of syncytin-1, we believe that the protease activity of HtrA4 may be activated by this interaction to facilitate the proteolysis of surface-bound syncytin-1. Future structure-functional analysis is required to test this speculation and to identify and characterize the critical peptide motif in the SU subunit that is recognized by the PDZ domain of HtrA4.
Autocrine and paracrine factors such as cytokines and hormones have been reported to positively or negatively regulate the migration and invasion of EVTs. For instance, epidermal growth factor and LIF are able to stimulate, whereas transforming growth factor β and gamma interferon inhibit, trophoblast migration and invasion (20). The present study identified a novel function of GCM1 and HtrA4 in regulation of placental cell invasion. Future studies are warranted to investigate whether these autocrine and paracrine factors act on the axis of GCM1 and HtrA4 to accomplish their regulation duties on placental cell migration and invasion.
ACKNOWLEDGMENTS
This work was supported by grants (to H.C.) from the National Science Council (grant 100-2311-B-001-010-MY3), the National Health Research Institutes (grant NHRI-EX100-10049SI), and Academia Sinica, Taiwan.
Footnotes
Published ahead of print 9 July 2012
REFERENCES
- 1. Ajayi F, et al. 2008. Elevated expression of serine protease HtrA1 in preeclampsia and its role in trophoblast cell migration and invasion. Am. J. Obstet. Gynecol. 199:557.e1–557.e10 [DOI] [PubMed] [Google Scholar]
- 2. Anson-Cartwright L, et al. 2000. The glial cells missing-1 protein is essential for branching morphogenesis in the chorioallantoic placenta. Nat. Genet. 25:311–314 [DOI] [PubMed] [Google Scholar]
- 3. Baczyk D, et al. 2004. Complex patterns of GCM1 mRNA and protein in villous and extravillous trophoblast cells of the human placenta. Placenta 25:553–559 [DOI] [PubMed] [Google Scholar]
- 4. Blaise S, de Parseval N, Benit L, Heidmann T. 2003. Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc. Natl. Acad. Sci. U. S. A. 100:13013–13018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Blond JL, et al. 2000. An envelope glycoprotein of the human endogenous retrovirus HERV-W is expressed in the human placenta and fuses cells expressing the type D mammalian retrovirus receptor. J. Virol. 74:3321–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cartwright JE, Fraser R, Leslie K, Wallace AE, James JL. 2010. Remodelling at the maternal-fetal interface: relevance to human pregnancy disorders. Reproduction 140:803–813 [DOI] [PubMed] [Google Scholar]
- 7. Chang C, Chen PT, Chang GD, Huang CJ, Chen H. 2004. Functional characterization of the placental fusogenic membrane protein syncytin. Biol. Reprod. 71:1956–1962 [DOI] [PubMed] [Google Scholar]
- 8. Chang CW, Chuang HC, Yu C, Yao TP, Chen H. 2005. Stimulation of GCMa transcriptional activity by cyclic AMP/protein kinase A signaling is attributed to CBP-mediated acetylation of GCMa. Mol. Cell. Biol. 25:8401–8414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chang M, et al. 2008. Glial cell missing 1 regulates placental growth factor (PGF) gene transcription in human trophoblast. Biol. Reprod. 78:841–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen CP, Chen CY, Yang YC, Su TH, Chen H. 2004. Decreased placental GCM1 (glial cells missing) gene expression in pre-eclampsia. Placenta 25:413–421 [DOI] [PubMed] [Google Scholar]
- 11. Chen CP, et al. 2008. Functional characterization of the human placental fusogenic membrane protein syncytin 2. Biol. Reprod. 79:815–823 [DOI] [PubMed] [Google Scholar]
- 12. Chiang MH, et al. 2009. Mechanism of hypoxia-induced GCM1 degradation: implications for the pathogenesis of preeclampsia. J. Biol. Chem. 284:17411–17419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Clausen T, Southan C, Ehrmann M. 2002. The HtrA family of proteases: implications for protein composition and cell fate. Mol. Cell 10:443–455 [DOI] [PubMed] [Google Scholar]
- 14. Damsky CH, et al. 1994. Integrin switching regulates normal trophoblast invasion. Development 120:3657–3666 [DOI] [PubMed] [Google Scholar]
- 15. Drewlo S, Czikk M, Baczyk D, Lye S, Kingdom J. 2011. Glial cell missing-1 mediates over-expression of tissue inhibitor of metalloproteinase-4 in severe pre-eclamptic placental villi. Hum. Reprod. 26:1025–1034 [DOI] [PubMed] [Google Scholar]
- 16. Fitzgerald JS, et al. 2005. Leukemia inhibitory factor triggers activation of signal transducer and activator of transcription 3, proliferation, invasiveness, and altered protease expression in choriocarcinoma cells. Int. J. Biochem. Cell Biol. 37:2284–2296 [DOI] [PubMed] [Google Scholar]
- 17. Holtan SG, Creedon DJ, Haluska P, Markovic SN. 2009. Cancer and pregnancy: parallels in growth, invasion, and immune modulation and implications for cancer therapeutic agents. Mayo Clin. Proc. 84:985–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hung AY, Sheng M. 2002. PDZ domains: structural modules for protein complex assembly. J. Biol. Chem. 277:5699–5702 [DOI] [PubMed] [Google Scholar]
- 19. Knerr I, et al. 2005. Stimulation of GCMa and syncytin via cAMP mediated PKA signaling in human trophoblastic cells under normoxic and hypoxic conditions. FEBS Lett. 579:3991–3998 [DOI] [PubMed] [Google Scholar]
- 20. Knofler M, Pollheimer J. 2012. IFPA Award in Placentology lecture: molecular regulation of human trophoblast invasion. Placenta 33(Suppl):S55–S62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li Y, et al. 2011. Placental HtrA3 is regulated by oxygen tension and serum levels are altered during early pregnancy in women destined to develop preeclampsia. J. Clin. Endocrinol. Metab. 96:403–411 [DOI] [PubMed] [Google Scholar]
- 22. Liang CY, et al. 2010. GCM1 regulation of the expression of syncytin 2 and its cognate receptor MFSD2A in human placenta. Biol. Reprod. 83:387–395 [DOI] [PubMed] [Google Scholar]
- 23. Lin FY, et al. 2011. Dual-specificity phosphatase 23 mediates GCM1 dephosphorylation and activation. Nucleic Acids Res. 39:848–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malassine A, et al. 2007. Expression of the fusogenic HERV-FRD Env glycoprotein (syncytin 2) in human placenta is restricted to villous cytotrophoblastic cells. Placenta 28:185–191 [DOI] [PubMed] [Google Scholar]
- 25. Malassine A, et al. 2005. Expression of HERV-W Env glycoprotein (syncytin) in the extravillous trophoblast of first trimester human placenta. Placenta 26:556–562 [DOI] [PubMed] [Google Scholar]
- 26. Martins LM, et al. 2003. Binding specificity and regulation of the serine protease and PDZ domains of HtrA2/Omi. J. Biol. Chem. 278:49417–49427 [DOI] [PubMed] [Google Scholar]
- 27. McMaster MT, et al. 1995. Human placental HLA-G expression is restricted to differentiated cytotrophoblasts. J. Immunol. 154:3771–3778 [PubMed] [Google Scholar]
- 28. Mi S, et al. 2000. Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 403:785–789 [DOI] [PubMed] [Google Scholar]
- 29. Muir A, Lever AM, Moffett A. 2006. Human endogenous retrovirus-W envelope (syncytin) is expressed in both villous and extravillous trophoblast populations. J. Gen. Virol. 87:2067–2071 [DOI] [PubMed] [Google Scholar]
- 30. Murwantoko, et al. 2004. Binding of proteins to the PDZ domain regulates proteolytic activity of HtrA1 serine protease. Biochem. J. 381:895–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nie G, et al. 2006. Distinct expression and localization of serine protease HtrA1 in human endometrium and first-trimester placenta. Dev. Dyn. 235:3448–3455 [DOI] [PubMed] [Google Scholar]
- 32. Runyon ST, et al. 2007. Structural and functional analysis of the PDZ domains of human HtrA1 and HtrA3. Protein Sci. 16:2454–2471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schreiber J, et al. 2000. Placental failure in mice lacking the mammalian homolog of Glial Cells Missing, GCMa. Mol. Cell. Biol. 20:2466–2474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Singh H, Makino SI, Endo Y, Nie G. 2010. Inhibition of HTRA3 stimulates trophoblast invasion during human placental development. Placenta 31:1085–1092 [DOI] [PubMed] [Google Scholar]
- 35. Xie TX, et al. 2004. Stat3 activation regulates the expression of matrix metalloproteinase-2 and tumor invasion and metastasis. Oncogene 23:3550–3560 [DOI] [PubMed] [Google Scholar]
- 36. Yang CS, et al. 2005. FBW2 targets GCMa to the ubiquitin-proteasome degradation system. J. Biol. Chem. 280:10083–10090 [DOI] [PubMed] [Google Scholar]
- 37. Yu C, et al. 2002. GCMa regulates the syncytin-mediated trophoblastic fusion. J. Biol. Chem. 277:50062–50068 [DOI] [PubMed] [Google Scholar]