Abstract
Integrin-mediated focal adhesions connect the extracellular matrix and cytoskeleton to regulate cell responses, such as migration. Protein tyrosine phosphatase α (PTPα) regulates integrin signaling, focal adhesion formation, and migration, but its roles in these events are incompletely understood. The integrin-proximal action of PTPα activates Src family kinases, and subsequent phosphorylation of PTPα at Tyr789 acts in an unknown manner to promote migration. PTPα-null cells were used in reconstitution assays to distinguish PTPα-Tyr789-dependent signaling events. This showed that PTPα-Tyr789 regulates the localization of PTPα and the scaffolding protein Cas to adhesion sites where Cas interacts with and is phosphorylated by Src to initiate Cas signaling. Linking these events, we identify BCAR3 as a molecular connector of PTPα and Cas, with phospho-Tyr789 PTPα serving as the first defined cellular ligand for the BCAR3 SH2 domain that recruits BCAR3-Cas to adhesions. Our findings reveal a novel role of PTPα in integrin-induced adhesion assembly that enables Src-mediated activation of the pivotal function of Cas in migration.
INTRODUCTION
Integrin engagement by extracellular matrix (ECM) components triggers a complex network of signaling events that regulates the assembly and function of multicomponent protein complexes (16). These complexes are set in the physical context of cytoplasmic adhesion sites located at ECM-integrin contact points. Adhesion complexes signal to the cytoskeleton to promote its reorganization and also integrate signals that determine their own dynamic assembly and disassembly that are required for cell movement (29).
Protein tyrosine phosphorylation is a major mechanism that regulates integrin signaling and cell movement, with at least nine protein tyrosine kinases and the same number of protein tyrosine phosphatases (PTPs) identified as regulators of integrin-linked adhesion site function (47). Among the latter is PTPα (PTPRA), a widely expressed receptor-like PTP belonging to the classical tyrosine-specific family of PTPs (41). PTPα-null mouse embryonic fibroblasts (MEFs) exhibit delayed integrin-mediated spreading and migration (40, 44, 48). This is associated with reduced focal adhesion formation and actin stress fiber assembly and a decreased responsiveness to force-dependent strengthening of integrin-cytoskeletal linkages. Impaired focal adhesion turnover in PTPα-deficient cells accompanies and/or underlies these defects, as the presence of enlarged, more stable focal adhesions at the cell periphery suggests that adhesion site remodelling and the redistribution of focal adhesion proteins to nascent adhesion structures are defective (19).
At the molecular level, PTPα catalyzes the dephosphorylation and activation of Src family tyrosine kinases (SFKs) (31, 40). Integrin engagement induces the interaction of PTPα with integrins and PTPα-mediated activation of Src and Fyn to promote the interaction of these SFKs with FAK (40, 44, 48). Src phosphorylates sites in the kinase domain of FAK to induce full FAK activation and also target other sites in FAK and in key FAK-associated scaffolding proteins to create phosphotyrosyl sites that recruit additional molecules to the complex (27, 49). These events are impaired in PTPα-null MEFs, including downstream signaling events such as tyrosine phosphorylation of the scaffolding/adaptor proteins p130Cas (Cas) and paxillin, activation of the RhoGTPase Rac1, and activation of the Erk mitogen-activated protein kinases (8, 19, 40). In MEFs and hippocampal neurons, force transduction in the integrin-mediated matrix rigidity response requires PTPα-dependent recruitment of Fyn and tyrosine phosphorylation of Cas at the leading edge to regulate early spreading of fibroblasts or neurite extension (22, 23).
In addition to the early catalytic role of PTPα as an integrin proximal activator of Src and Fyn, PTPα plays an unknown second role in integrin signaling that is dependent upon its phosphorylation at a tyrosine residue, Tyr789, in its C-terminal tail. Stimulation of integrin signaling upregulates PTPα-Tyr789 phosphorylation in an SFK- and FAK-dependent manner, suggesting that it occurs downstream of integrin-induced and PTPα-mediated SFK-FAK activation (8). Consistent with this, the phosphorylation of PTPα at Tyr789 is not required for PTPα-dependent activation of Src and Fyn or for tyrosine phosphorylation of FAK in response to integrin stimulation. However, PTPα-Tyr789 phosphorylation is required for some subsequent integrin signaling events, as mutant PTPα-Y789F, which is catalytically active, is unable to support effective integrin-induced cytoskeletal rearrangement, focal adhesion formation, and, ultimately, cell migration (8). Although the precise nature of the phosphorylation-dependent role of PTPα is unclear, it likely involves a focal adhesion-specific function of PTPα since Tyr789 is required for the presence of PTPα at these structures (24). PTPα-phosphoTyr789 is also a binding site that can be recognized by the SH2 domains of Grb2 and Src (11, 39, 50), although the significance of these protein interactions to PTPα function in integrin signaling is not known.
To elucidate the function of phospho-PTPα, we identified integrin signaling events that were defective in PTPα−/− MEFs and assessed whether these could be rescued by adenovirus-mediated expression of wild-type (WT) PTPα or an unphosphorylatable mutant (Y789F) form of PTPα. We find that integrin-induced tyrosine phosphorylation of the scaffolding protein Cas is dependent upon PTPα Tyr789 phosphorylation. Our study describes a noncatalytic mechanism of action of PTPα mediated through phospho-Tyr789 that positions Cas for phosphorylation and activation of its signaling function to promote cell migration.
MATERIALS AND METHODS
Cell lines and cell culture/stimulation.
Wild-type (PTPα+/+) and PTPα−/− mouse embryonic fibroblasts (MEFs) have been described previously (48). All cells were grown in uncoated culture-grade dishes (adherent cells) in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum and penicillin-streptomycin. Prior to timed stimulation of integrin signaling, the cells were starved in medium containing 0.5% fetal bovine serum for 18 h. They were then detached from the dish using 0.05% trypsin-EDTA (Invitrogen). The trypsinization was stopped with 0.5 mg/ml soybean trypsin inhibitor (Invitrogen) in DMEM, and the cells were washed twice with serum-free medium. After centrifugation, the cells were resuspended in serum-free medium containing 0.1% bovine serum albumin and maintained at 37°C for 1 h in suspension. Suspended cells were plated onto fibronectin-coated plates (105 cells/ml) and incubated at 37°C for 15 min before harvesting. The fibronectin-coated dishes were prepared ahead of time by incubating dishes containing 10 μg/ml fibronectin (Chemicon International, Inc.) in phosphate-buffered saline (PBS) overnight at 4°C. Before use, the dishes were washed twice with PBS and then incubated with serum-free DMEM at 37°C for 1 h.
Antibodies and immunological detection reagents.
PTPα and PTPα-phospho-Tyr789 antibodies have been described previously (8). Antibodies to Cas, Crk, FAK, Rac1, and Cdc42 were from BD Transduction Laboratories. Antibody to Src (v-Src) was from Calbiochem. Antibody to BCAR3 was from Bethyl Laboratories, Inc. Antibodies to PAK, RhoGDI, and GST were from Santa Cruz Biotechnology, Inc. Anti-VSVG and -actin antibodies were from Cell Signaling. Antiphosphotyrosine antibody (4G10) was from Upstate Biotechnology. Phosphorylation site-specific antibodies to Cas Tyr410 and PAK Ser144 were from Cell Signaling and those to Src Tyr416 and Tyr527 were from Biosource.
BCAR3 expression constructs.
The plasmid pCMV6-AC-BCAR3 containing full-length human BCAR3 cDNA (OriGene Technologies, Inc., Rockville, MD) was used as a template in a PCR (primers F1, 5′-ACTGAAGCTTATGGCTGCAGGAAAATT-3′, and R1, 5′-AAAAAAAGCGGCCGCTCAAAGCTCTGCCTG-3′) to generate full-length wild-type BCAR3 cDNA with a 5′ HindIII site and a 3′ NotI site. This PCR product was cloned into pXJ40-FLAG vector that had been digested with HindIII and NotI to generate the expression plasmid pXJ40-FLAG-BCAR3 (WT). Site-directed mutagenesis using overlap extension PCR of the pCMV6-AC-BCAR3 template was performed to generate full-length BCAR3 cDNA with mutations in the codon for Arg177 (changing the expressed amino acid to Lys) in the SH2 domain. Separate PCRs were performed to produce two pieces of the complete BCAR3 cDNA that overlapped in sequence around the mutated region, using the primer pairs F1 (described above) with R2 (5′-GGACAGAGAGTCTTTAACTAGGAAGTC-3′, mutated bases underlined) and F2 (5′-GACTTCCTAGTTAAAGACTCTCTGTCC-3′, mutated bases underlined) with R1 (described above). These PCR products were gel purified and mixed to serve as templates in another PCR using primers F1 and R1 to generate full-length mutant BCAR3 cDNA. This was cloned into HindIII- and NotI-digested pXJ40-FLAG to produce the expression plasmid pXJ40-FLAG-BCAR3 (R177K). The same procedure was used to generate the plasmid pXJ40-FLAG-BCAR3 (R478A) encoding full-length BCAR3 cDNA with mutations in the codon for Arg478 (changing the expressed amino acid to Ala) in the GEF domain. The primer pairs used for first-round PCRs were F1 and R3 (5′-GGCCATGAATGCCGCTGTTGCCAA-3′, mutated bases underlined) and F3 (5′-TTGGCAACAGCGGCATTCATGGCC-3′, mutated bases underlined) and R1, followed by second-round PCR on the mixed templates using primer pairs F1 and R1. The pXJ40-mCherry-BCAR3 plasmids expressing WT BCAR3 or BCAR3 with a mutant SH2 domain (R177K) were generated by EcoRI/HindIII excision of the FLAG tag from the appropriate BCAR3-expressing plasmids and its replacement with the PCR-generated mCherry sequence that was prepared from another plasmid template by using appropriate primers with added EcoRI and HindIII sequences. All wild-type and mutant BCAR3 cDNA insert sequences and their in-frame cloning with the FLAG or mCherry tags were verified by sequencing.
PTPα and BCAR3 expression and BCAR3 depletion.
The generation and use of adenovirus to express WT PTPα and Y789F PTPα in PTPα−/− cells were previously described (8). The pXJ41-neo-VSVG-PTPα plasmid (WT) (2), pXJ41-neo-VSVG-PTPα plasmid (Y789F) (8), and BCAR3-expressing plasmids were transfected into cells using Lipofectamine LTX reagent (Invitrogen). Infected and transfected cells were cultured for 24 h prior to further experimentation. Small interfering RNA (siRNA) targeting murine BCAR3 (ON-TARGETplus SMARTpool BCAR3) and control nontargeting siRNA (ON-TARGETplus nontargeting pool) were from Dharmacon. The cells were transfected with 100 nM siRNA using Lipofectamine RNAiMAX reagent from Invitrogen.
Cell lysis, immunoprecipitation, and immunoblot analysis.
Cells were harvested in lysis buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 2 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride [PMSF], 10 μg/ml leupeptin, and 10 μg/ml aprotinin) and processed for immunoprecipitation and immunoblotting experiments as described previously (48).
Membrane translocation assay.
The preparation of cell membrane and cytosolic fractions was carried out as described previously (10). The PTPα+/+ and PTPα−/− MEFs were placed on fibronectin-coated dishes for 15 min as described above, and then the dishes were chilled on ice. The cells were washed twice with ice-cold PBS and lysed with hypotonic lysis buffer (10 mM Tris-HCl [pH 7.5], 1.5 mM MgCl2, 5 mM NaCl, 1 mM NaF, 0.2 mM Na3VO4). Cells were then scraped from the dishes, and after 20 s of sonication the lysate was centrifuged at 16,000 × g at 4°C for 15 min. The supernatant was transferred to a fresh tube, and this fraction is referred to as the cytosolic fraction (C); the insoluble pellets were dissolved in 2× SDS sample buffer (membrane fraction [M]). Fractionation was verified by probing for IGF1Rβ (membrane protein) and RhoGDIα (cytsolic protein).
Focal adhesion enrichment assay.
The preparation of focal adhesion-enriched (insoluble) and detergent-soluble (soluble) cell fractions was carried out as described previously (20, 34). The cells were placed on fibronectin-coated dishes for 15 min as described above, and then the dishes were chilled on ice. The cells were washed twice with ice-cold PBS, lysed with cytoskeleton stabilizing buffer (CSB) [0.3 M sucrose, 0.5% Triton X-100, 10 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES; pH 6.8), 100 mM KCl, 1 mM CaCl2, 2.5 mM MgCl2, 1 mM Na3VO4, and protease inhibitors (1 mM PMSF, 10 μg/ml leupeptin, 10 μg/ml aprotinin)] for 5 min, and collected into tubes. This fraction is referred to as the detergent-soluble or soluble fraction. The dishes were rinsed briefly (<5 s) twice with CSB, and RIPA buffer (50 mM HEPES [pH 7.4], 1% sodium deoxycholate, 1% Triton X-100, 0.1% SDS, 150 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, and protease inhibitors as described above) was added to the dishes, and they were kept for 5 min on ice. The dishes were scraped, and the resulting solution was centrifuged at 16,000 × g at 4°C for 15 min to clear cell debris. The supernatant is referred to as the insoluble or focal adhesion-enriched fraction. Following Western blotting of the fractions and densitometric quantification, the percentage of insoluble protein representing focal adhesion-localized protein was calculated as (insoluble amount × 100)/(soluble amount + insoluble amount).
Immunofluorescence and TIRF microscopy.
Cells were transiently cotransfected with pEGFP-Cas and mCherry-vinculin (where EGFP is enhanced green fluorescent protein), or in other experiments with mCherry-BCAR3, using Lipofectamine LTX (Invitrogen). The cells were placed on fibronectin-coated glass coverslips for 15 min, washed twice with cold PBS, and fixed for 30 min in 4% paraformaldehyde in PBS. Fixed cells were washed twice with PBS and imaged using the Olympus IX81 CellT̂IRF (total internal reflection fluorescent) system equipped with a CoolSnap HQ2 charge-coupled-device (CCD) camera. The corresponding fluorescent-labeled proteins were visualized with a 60× TIRF objective lens (numerical aperture [NA] of 1.49) using the 488-nm and 561-nm lasers set to achieve a calibrated penetration depth of 75 nm and 80 nm, respectively. Images were analyzed using the software ImageJ, and colocalization of Cas and vinculin was measured using Pearson's correlation.
Pulldown assays.
Rac1 and Cdc42 activities were measured by GST–PAK-binding domain (PBD) pulldown assays performed with 100 μg cell lysate using a Rac1 activation kit from Stressgen. To assess BCAR3-SH2 domain interactions, cell lysates were mixed with recombinant GST-BCAR3-SH2 (Signosis, Inc.) and GST-Bind resin (Novagen) for 18 h at 4°C. The resin was collected, washed three times with cell lysis buffer, resolved by SDS-PAGE, and analyzed by immunoblotting.
Far-Western blotting.
Anti-VSVG-PTPα (WT and Y789F) immunoprecipitates were resolved by SDS-PAGE and transferred to a polyvinylidene difluoride (PVDF) membrane. The membrane was probed with the GST-BCAR3-SH2 domain fusion protein at 4°C. After overnight incubation, the membrane was washed three times with PBS containing 0.1% Tween 20 (PBST), followed by the addition of horseradish peroxidase (HRP)-conjugated anti-GST antibody at room temperature for 2 h. After the membrane was washed three times in PBST, signal was detected using ECL Plus (Amersham).
Statistical analyses.
Densitometric quantification of immunoblots from at least three independent experiments was statistically analyzed using Student's t test.
RESULTS
PTPα-Tyr789 is necessary for efficient integrin-induced Cas tyrosine phosphorylation and Cas-Crk association.
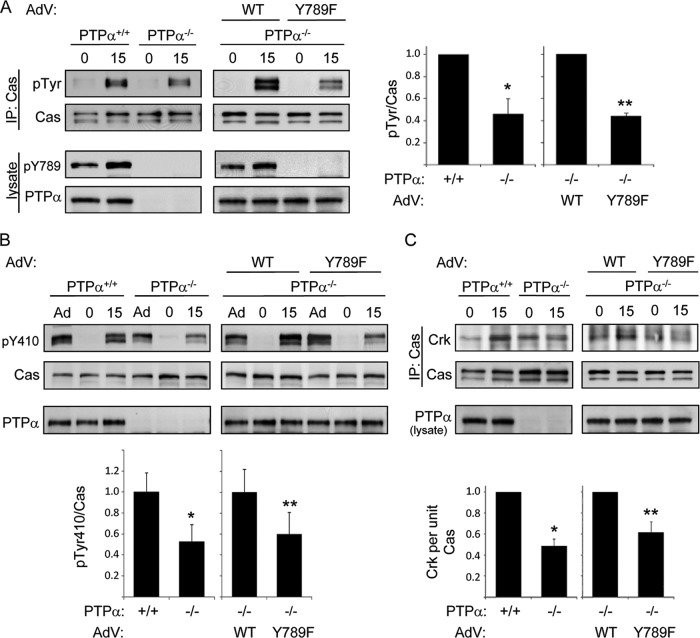
Integrin-induced cell migration is defective in the absence of PTPα Tyr789 phosphorylation (8). The catalytic activity of PTPα is required for the upstream integrin signaling events of Src and Fyn activation, FAK tyrosine phosphorylation, and paxillin tyrosine phosphorylation (8, 48). However, these events are independent of PTPα Tyr789 phosphorylation (8), suggesting that phospho-Tyr789 PTPα mediates a unique signaling action that promotes integrin-induced migration. To elucidate the role of phospho-Tyr789 PTPα, we determined whether other integrin signaling events that are defective in PTPα−/− MEFs could be rescued by adenovirus-mediated expression of wild-type (WT) PTPα or an unphosphorylatable mutant (Y789F) form of PTPα. The integrin-induced tyrosine phosphorylation of the scaffolding protein Cas, an important regulator of cell migration (7, 21), is impaired in MEFs lacking PTPα due to the reduced activity of the PTPα-regulated Src family kinases (Src and Fyn) that phosphorylate Cas (22, 40). Expressing WT PTPα in PTPα−/− MEFs restored the defective Cas tyrosine phosphorylation when suspended cells were placed on dishes coated with the integrin ligand fibronectin (FN) (Fig. 1A). We have previously shown (8), and reconfirmed (data not shown), that mutant Y789F PTPα catalyzes integrin-induced Src and Fyn activation as effectively as WT PTPα. However, the reduced tyrosine phosphorylation of Cas in PTPα−/− MEFs was not rescued by expression of Y789F PTPα (Fig. 1A), indicating a distinct Tyr789-dependent action of PTPα.
Fig 1.
PTPα Tyr789 is required for integrin-induced Cas phosphorylation and recruitment of Crk. (A) Wild-type (PTPα+/+ or +/+) and PTPα−/− (−/−) MEFs or PTPα−/− MEFs infected with adenovirus (AdV) expressing WT or Y789F PTPα were placed in suspension for 1 h (0) and then plated on FN-coated dishes for 15 min (15). Cas immunoprecipitates were probed for phosphotyrosine (pTyr) and Cas. Lysates were probed for phospho-Tyr789 PTPα and PTPα. Results from 3 experiments were quantified by densitometry and the tyrosine phosphorylation per unit of Cas is shown on the graphs to the right as means ± standard deviations (SD). Asterisks indicate significant differences (*, P = 3 × 10−7; **, P = 2.2 ×10−4). (B) As described in the legend to panel A, except cell lysates (where Ad represents adherent cells grown on plastic dishes) were probed for phospho-Tyr410 Cas, Cas, and PTPα. The graph shows phospho-Tyr410 Cas per unit of Cas as means ± SD (n = 3 experiments). Asterisks indicate significant differences (*, P = 0.008; **, P = 0.037). (C) As described in the legend to panel A, except that Cas immunoprecipitates were probed for Crk and Cas and lysates were probed for PTPα. The graph shows associated Crk per unit of Cas as means ± SD (n = 4 experiments with PTPα+/+ and PTPα−/− cells; n = 3 experiments with the cells reconstituted by adenovirus infection). Asterisks indicate significant differences (*, P = 6.9 × 10−6; **, P = 4.4 ×10−4).
Integrin-stimulated tyrosine phosphorylation of Cas at multiple sites, particularly within a central region known as the substrate domain (SD), enables Cas association with the SH2 domain-containing adaptor protein Crk to promote downstream signaling and cell migration (3, 9, 21). We found that the phosphorylation of Cas at a specific tyrosine residue within the SD, Tyr410, was reduced in PTPα−/− cells plated on FN, and this was also defective in PTPα−/− cells expressing Y789F PTPα compared to those expressing WT PTPα (Fig. 1B). In accord with the impaired tyrosine phosphorylation of Cas in PTPα−/− and Y789F PTPα-expressing MEFs, the association of Cas and Crk was reduced in these cells (Fig. 1C). Together, these results indicate that PTPα-Tyr789 regulates the integrin-mediated functional modification of Cas.
PTPα-Tyr789 regulates Cas downstream signaling events.
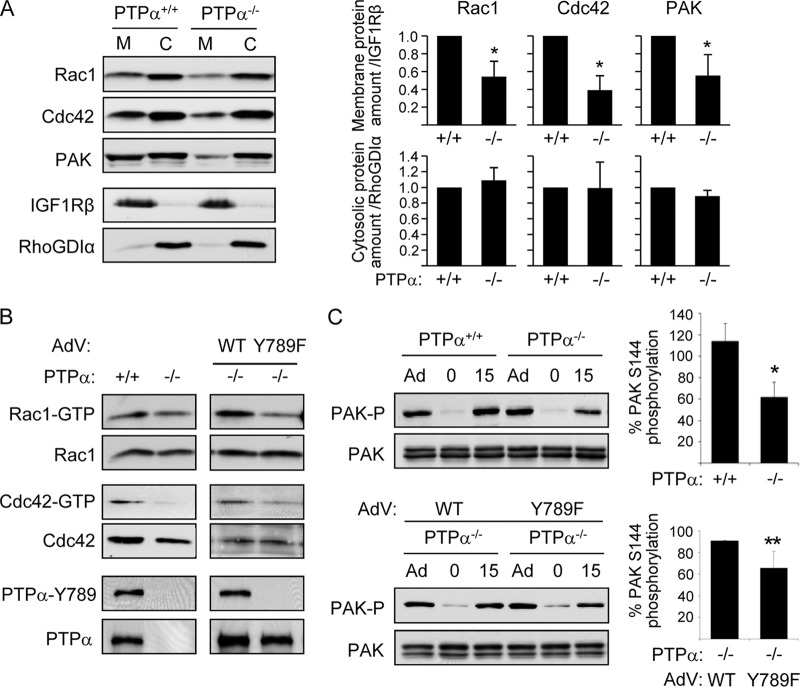
Cas-associated Crk couples with GEFs such as DOCK180 to activate Rac1/Cdc42 and promote cell migration (3, 9, 21). To verify that PTPα-Tyr789-dependent Cas activation defects impacted downstream Cas signaling, we examined the activation of the GTPases Rac1 and Cdc42, which are integrin-responsive regulators of the cytoskeleton and of the Rac/Cdc42 effector p21-activated kinase (PAK). Catalytically active PTPα is required for integrin-induced Rac1 activation and Rac1 relocalization to focal adhesions (19). Consistent with this, we found that after stimulating integrin signaling by plating cells on fibronectin for 15 min, there was less Rac1 and less Cdc42 in the membrane fraction of integrin-stimulated PTPα−/− MEFs compared to levels in wild-type MEFs (Fig. 2A). The PTPα−/− cells also contained less membrane-associated PAK (Fig. 2A). No corresponding significant differences in the cytosolic localization of these proteins were detected (Fig. 2A), as might be expected since the relocalizable populations are much smaller than those that remained in the cytosol. Integrin-stimulated PTPα−/− cells also contained smaller amounts of active GTP-bound Rac and Cdc42 (Fig. 2B, left panels) and exhibited reduced PAK activation as determined by the reduced phosphorylation of PAK at Ser144 (Fig. 2C). We determined the abilities of WT and Y789F PTPα to rescue these latter defects. Expression of WT PTPα in PTPα-null cells was significantly more effective than mutant Y789F PTPα in restoring integrin-induced activation of Rac1, Cdc42, and PAK (Fig. 2B and C). These findings suggest that Tyr789 of PTPα promotes integrin-induced cell migration (8) by facilitating Cas tyrosine phosphorylation and Crk-coupled Rac1/Cdc42-PAK signaling.
Fig 2.
Lack of PTPα Tyr789 impairs integrin-induced signaling to Rac, Cdc42, and PAK. (A) Wild-type (PTPα+/+) and PTPα−/− MEFs were placed on FN-coated dishes for 15 min. Cell membrane and cytosolic fractions were prepared and probed for the indicated proteins. The distributions of the membrane-associated protein IGF-1Rβ and the cytosolic protein RhoGDI were probed to validate the fractionation as well as the loading amounts (IGF1Rβ in PTPα+/+ membrane = 1.00 versus 1.08 ± 0.08 in PTPα−/− membrane, and RhoGDIα in PTPα+/+ cytosol = 1.00 versus 0.98 ± 0.05 in PTPα−/− cytosol; n = 3 experiments). Results from 3 experiments were quantified by densitometry. The relative amounts of Rac, Cdc42, and PAK per unit IGF1Rβ in the membrane fractions, and per unit RhoGDIα in the cytosolic fractions, are shown in the graphs as means ± SD. Asterisks indicate significant differences (from left to right: P = 0.010, 0.003, 0.031). (B) Wild-type (+/+) and PTPα−/− (−/−) MEFs or PTPα−/− MEFs infected with adenovirus (AdV) expressing WT or Y789F PTPα were placed on FN-coated dishes for 15 min. Cell lysates were used in GST-PBD pulldown assays, and the pulldowns were probed for Rac1 or Cdc42 to detect active GTP-bound proteins. Lysates were probed for Rac1, Cdc42, phospho-Tyr789 PTPα (pY789), and PTPα. (C) The indicated MEFs were grown as adherent (Ad) cultures on plastic dishes and then placed in suspension for 1 h (0) before plating on FN-coated dishes for 15 min (15). Lysates were probed for phospho-Ser144 PAK (PAK-P) and PAK. Results from 3 experiments were quantified by densitometry, and the PAK Ser144 phosphorylation per unit of PAK is shown on the graphs to the right as means ± SD. Asterisks indicate significant differences (*, P = 4.1 × 10−3; **, P = 0.047).
Cas and Src association is impaired in cells expressing mutant Y789F PTPα.
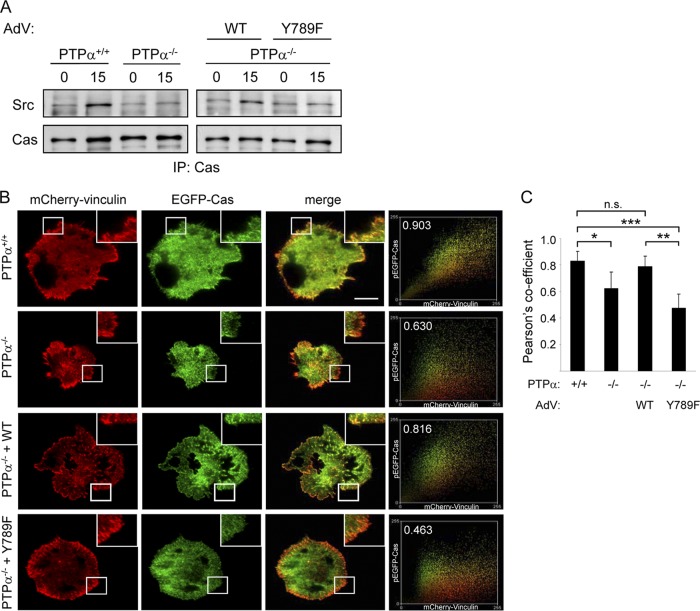
Cas substrate domain phosphorylation is carried out by Src that can directly and, through FAK, indirectly associate with Cas (33). Since we have shown that Y789F PTPα is at least as efficient as WT PTPα in activating Src in integrin-stimulated MEFs (8), the defective Cas phosphorylation in Y789F PTPα-expressing cells is not due to defective kinase activity of Src. We therefore investigated whether the reduced Cas tyrosine phosphorylation in Y789F PTPα-expressing cells was due to the impaired association of Cas and Src. Cas immunoprecipitates from wild-type and PTPα−/− MEFs and from PTPα−/− MEFs reexpressing WT and Y789F PTPα were probed for associated Src. Integrin stimulation induced the association of Src with Cas in wild-type MEFs but not in PTPα−/− MEFs, and the defective Src-Cas association was restored by expression of WT but not mutant Y789F (Fig. 3A). We conclude that Tyr789 of PTPα is required for the Cas-Src association that enables Src to phosphorylate Cas and promote downstream signaling.
Fig 3.
Cas-Src association and Cas-vinculin colocalization in focal adhesions requires PTPα-Tyr789. (A) Wild-type (PTPα+/+ or +/+) and PTPα−/− (−/−) MEFs or PTPα−/− MEFs infected with adenovirus (AdV) expressing WT or Y789F PTPα were placed in suspension for 1 h (0) and then on FN-coated dishes for 15 min (15). Cas immunoprecipitates were probed for Src and Cas. (B) The cell types in panel A were cotransfected with mCherry-vinculin and EGFP-Cas. After 24 h, the cells were suspended for 1 h and then placed on FN-coated dishes for 15 min. Vinculin and Cas signals were imaged using TIRF microscopy. Scale bar (in top merge panel) = 30 μm. The extent of colocalization between the EGFP and mCherry signals from one area of the cell (inset) was scatter plotted (far right panels) and calculated using Pearson's correlation coefficient (fraction number within plot). (C) Pearson's correlation coefficient was calculated from two areas per cell (representative images shown in panel B) and from 10 cells of each type and is shown as the means ± SD for 20 areas analyzed. Asterisks indicate significant differences (*, P = 2 × 10−4; **, P = 4 × 10−7; ***, P = 5 × 10−8), and n.s. indicates no significant difference (P = 0.210).
PTPα-Tyr789 regulates Cas localization to focal adhesions.
Integrin engagement induces Cas to move to nascent focal adhesions, where it is phosphorylated (1, 15, 30, 45). The defective Cas-Src association in mutant Y789F PTPα-expressing cells suggested that in the absence of PTPα-Tyr789 phosphorylation, Cas and Src might not colocalize properly in newly forming focal adhesions. We investigated whether Cas recruitment to focal adhesions was dependent on PTPα-Tyr789 using fluorescent protein-tagged microscopy. Wild-type and PTPα−/− MEFs, and PTPα−/− MEFs expressing WT or Y789F PTPα, were cotransfected with plasmids expressing mCherry-vinculin and EGFP-Cas. Vinculin is an early resident in nascent focal adhesions (46), and its colocalization with Cas indicates the presence of Cas in focal adhesions. This was visualized in FN-stimulated cells using total internal reflection fluorescence (TIRF) microscopy that selectively images signals at the cell-substratum interface. Cas and vinculin colocalization was impaired in cells lacking PTPα, as indicated by the lower Pearson's coefficient calculated for this population of cells (Fig. 3B and C). Cas colocalization with vinculin was restored by expression of WT PTPα in these cells but not by expression of Y789F PTPα (Fig. 3B and C), suggesting that Cas movement to focal adhesions is dependent on PTPα with an intact Tyr789.
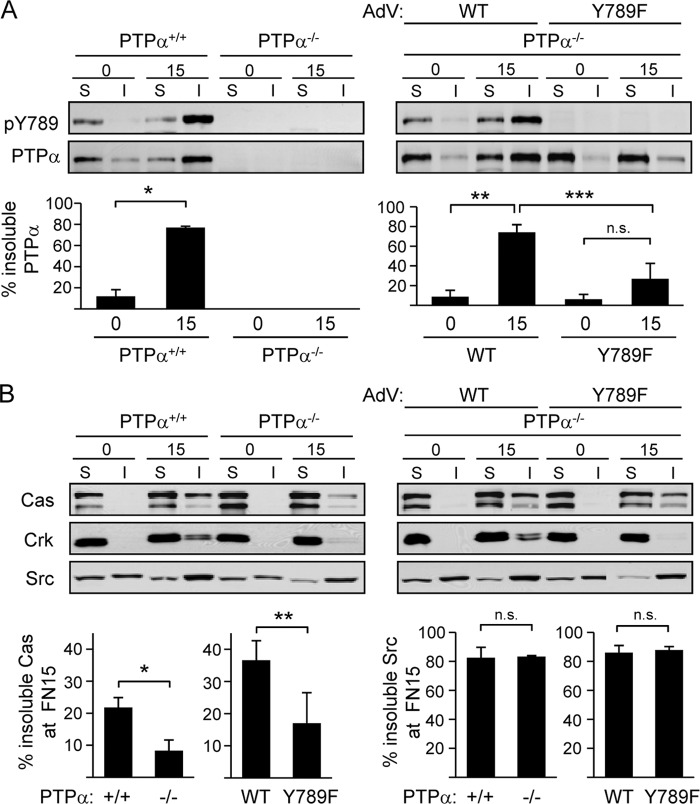
Immunofluorescent visualization of PTPα indicates that PTPα-Tyr789 directs the localization of PTPα to adhesion sites or focal adhesions (24), raising the possibility that Cas or Src recruitment to focal adhesions is regulated by phospho-PTPα-dependent recruitment to these structures. We determined the integrin-induced redistribution of endogenous PTPα and expressed WT and Y789F PTPα by fractionating suspended or integrin-stimulated cells into soluble and focal adhesion-enriched fractions (20, 34). In wild-type MEFs, most endogenous PTPα was located in the soluble fraction of suspended cells that lack integrin-mediated substrate attachment and signaling. Plating these cells on FN induced PTPα Tyr789 phosphorylation and the relocalization of a majority of PTPα to the insoluble focal adhesion-enriched fraction (Fig. 4A, left). In PTPα−/− cells expressing introduced WT PTPα, a similar integrin-stimulated phosphorylation and relocalization of WT PTPα was detected. However, the integrin-induced relocalization of expressed mutant Y789F PTPα to the focal adhesion-enriched fraction was severely impaired (Fig. 4A, right).
Fig 4.
PTPα-Tyr789 directs PTPα, Cas, and Crk, but not Src, to integrin-mediated focal adhesion-enriched fractions. Wild-type (PTPα+/+ or +/+) and PTPα−/− (−/−) MEFs or PTPα−/− MEFs infected with adenovirus (AdV) expressing WT or Y789F PTPα were placed in suspension for 1 h (0) and then plated on FN-coated dishes for 15 min (15). Cell lysates were fractionated into soluble (S) and insoluble (I) focal adhesion-enriched fractions. (A) Cell fractions were probed for phospho-Tyr789 PTPα (pY789) and PTPα. Results from 3 experiments were quantified by densitometry, and the amount of total PTPα in the soluble and insoluble fractions was taken as 100%. Graphs show the percentage of PTPα in insoluble fractions as means ± SD. Asterisks indicate significant differences (*, P = 0.0001; **, P = 0.0004; ***, P = 0.0094), and n.s. indicates no significant difference (P = 0.0934). (B) As described in the legend to panel A, except that fractions were probed for Cas, Crk, and Src. Results from 3 experiments were quantified by densitometry, and the amount of total Cas or Src in the soluble and insoluble fractions was taken as 100%. Graphs show the percentage of Cas (left graphs) and Src (right graphs) in insoluble fractions of FN-plated cells as means ± SD. Asterisks indicate significant differences (*, P = 0.0070; **, P = 0.0397), and n.s. indicates no significant differences (left, P = 0.8535; right, P = 0.6001).
Examination of the localization of Cas, Crk, and Src in the above-described cell types revealed that in wild-type MEFs, populations of all these proteins moved to focal adhesion-enriched fractions upon integrin stimulation. Strikingly, the relocalization of Cas and Crk, but not Src, was PTPα dependent, as this was significantly reduced in PTPα−/− MEFs (Fig. 4B). Expression of WT PTPα in the PTPα-null cells restored Cas and Crk relocalization to the focal adhesion-enriched fraction, whereas expressed Y789F PTPα was much less effective (Fig. 4B). These results indicate that integrin-stimulated PTPα phosphorylation at Tyr789 promotes the recruitment of PTPα and Cas to focal adhesions, while the movement of Src to these structures is PTPα independent.
Integrin-induced focal adhesion localization of the Cas-binding protein BCAR3 is regulated by PTPα-Tyr789.
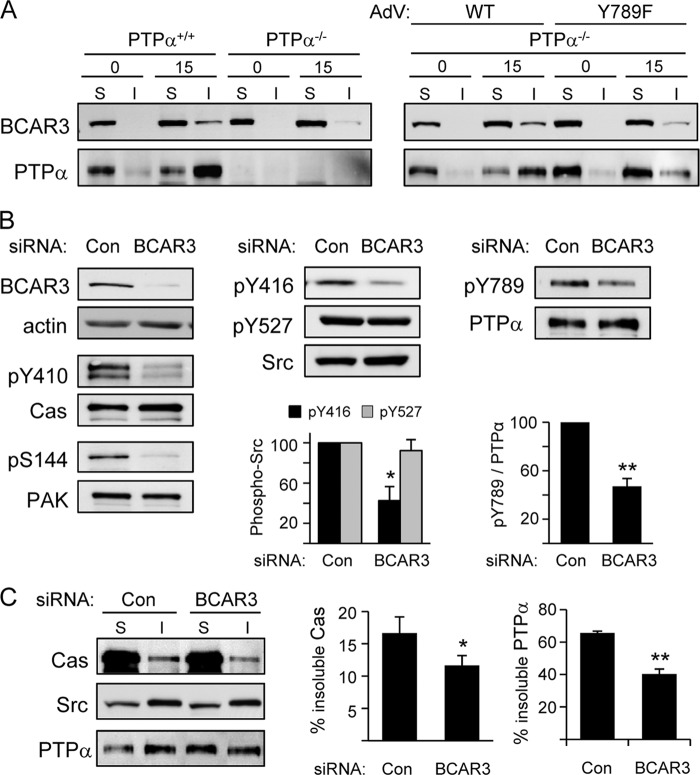
These findings suggest that the phosphorylation of PTPα at Tyr789 not only directs PTPα to forming focal adhesions but also plays an important role in bringing Cas to focal adhesions, where it interacts with Src and undergoes Src-mediated phosphorylation and functional activation. A remarkably similar phenotype to Y789F PTPα-expressing cells is reported for cells depleted in a protein called BCAR3 (also known as AND-34 in mice) (5), including impaired Src-Cas association, tyrosine phosphorylation of Cas, relocalization of Cas to the cell membrane, and cell migration (37, 38). Thus, BCAR3 and phospho-Tyr789 PTPα may act within the same signaling pathway. Interestingly, BCAR3 is a Cas-binding protein that interacts with the C-terminal region of Cas through a GEF-like domain (17) and possesses an SH2 domain in its N-terminal region (5, 42) that interacts with an unknown cellular ligand. To test the notion that BCAR3 might be an intermediate in PTPα-Tyr789-dependent recruitment of Cas to focal adhesions, we investigated if BCAR3 indeed underwent integrin-stimulated localization to focal adhesion-enriched fractions and if this required PTPα and an intact Tyr789 site within PTPα. As shown in Fig. 5A, a portion of BCAR3 moved into focal adhesion fractions upon integrin engagement of wild-type MEFs, but much less BCAR3 relocated to these fractions in cells lacking PTPα. Moreover, expression of WT PTPα in PTPα−/− cells effectively restored BCAR3 movement into the focal adhesion-enriched fractions, whereas expression of Y789F PTPα induced 3-fold less BCAR3 relocalization.
Fig 5.
Functional interactions of PTPα and BCAR3. (A) Wild-type (PTPα+/+) and PTPα−/− (−/−) MEFs or PTPα−/− MEFs infected with adenovirus (AdV) expressing WT or Y789F PTPα were placed in suspension for 1 h (0) and then plated on FN-coated dishes for 15 min (15). Cell lysates were fractionated into soluble (S) and insoluble (I) focal adhesion-enriched fractions. (A) Cell fractions were probed for BCAR3 and PTPα. The results are representative of 2 experiments. (B) Wild-type MEFs were transfected with control nontargeting or BCAR3-targeted siRNA. The cells were suspended for 1 h and then plated on FN for 15 min. Cell lysates were probed for BCAR3, actin, phospho-Tyr410 Cas, Cas, phospho-Ser144 PAK, and PAK (left panels); for phospho-Tyr416 and phospho-Tyr527 Src and Src (middle panels); and for phospho-Tyr789 PTPα and PTPα (right panels). The graphs show the means ± SD. Src phosphorylation at Tyr416 and Tyr527 per unit of Src (middle) and the phospho-Tyr789 per unit PTPα as determined by densitometric quantification of 3 experiments. Asterisks indicate significant differences with the control (*, P = 0.001; **, P = 7.3 × 10−5). (C) As described in the legend to panel B, but soluble (S) and insoluble (I) focal adhesion-enriched fractions were prepared and probed for Cas, Src, and PTPα. The results from 3 experiments were quantified by densitometry, and the amount of total Cas and total PTPα in the soluble and insoluble fractions was taken as 100%. The graphs show the percentage of Cas and PTPα in the insoluble cell fractions as means ± SD. The asterisks denote a significant difference (*, P = 0.042; **, P = 1.8 × 10−4).
Mutant Y789F PTPα-dependent defects are phenocopied by BCAR3 knockdown.
To confirm the role of BCAR3 in regulating Cas tyrosine phosphorylation and localization, we depleted ∼90% of BCAR3 from MEFs using siRNA. As reported (38), the depletion of BCAR3 inhibited the integrin-induced tyrosine phosphorylation of Cas. It also inhibited PAK activation (Fig. 5B), in accord with the latter being a downstream target of BCAR3-Cas signaling (6). BCAR3 is reported to regulate Src activity (32, 38), and integrin-induced Src phosphorylation at its activating Tyr416 site was indeed abrogated in BCAR3-deficient cells (Fig. 5B). Notably, integrin-induced phosphorylation of PTPα Tyr789 was also reduced in these cells (Fig. 5B).
Integrin-induced Cas relocalization to focal adhesion-enriched fractions was significantly reduced by about one-third in the BCAR3-deficient cells (Fig. 5C). While Src relocalization was unaffected, PTPα relocalization to the focal adhesion-enriched fractions was impaired by BCAR3 siRNA treatment (Fig. 5C). This is likely due to the reduced phosphorylation of PTPα Tyr789, which in turn may underlie the reduced Cas relocalization in the BCAR3-depleted cells.
All together, these results reveal that BCAR3-mediated activation of Src, possibly acting in concert with PTPα-mediated activation of Src (40, 48), promotes PTPα-Tyr789 phosphorylation to induce the relocalization of PTPα to focal adhesions. Thus, not only is PTPα-phospho-Tyr789 important for recruitment of BCAR3 and Cas to focal adhesions as shown in Fig. 5A, but BCAR3 also acts upstream of PTPα, regulating PTPα and Cas relocalization to focal adhesions by stimulating Src and Src-dependent PTPα-Tyr789 phosphorylation.
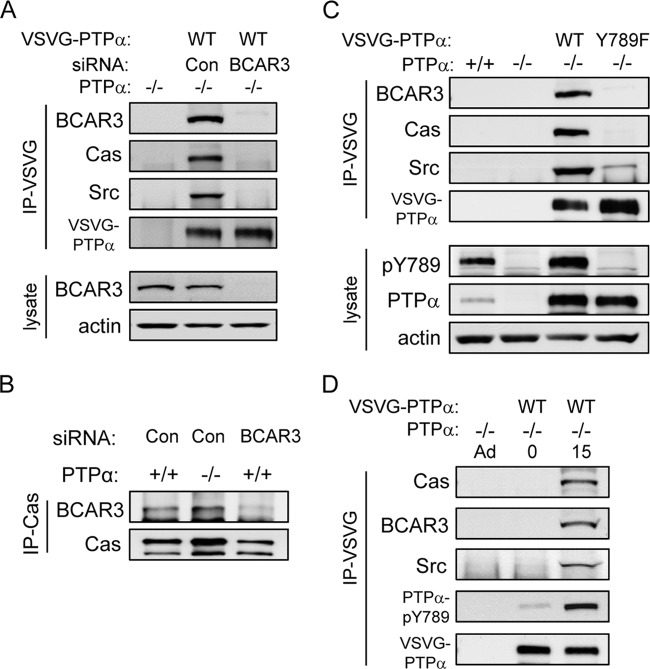
PTPα-Tyr789 mediates an integrin- and BCAR3-dependent interaction of PTPα with Cas and Src.
We addressed the question of whether PTPα physically interacted with BCAR3 and, via BCAR3, with Cas. To this end, we expressed VSVG-tagged WT PTPα in PTPα−/− cells that were depleted of BCAR3 or treated with control nontargeting siRNA. Probing VSVG-PTPα immunoprecipitates showed that BCAR3 indeed associated with PTPα, and that Cas and Src were also present in the immunoprecipitates from the control cells expressing BCAR3 (Fig. 6A). However, depleting cells of BCAR3 virtually abolished the association of Cas and Src with PTPα (Fig. 6A). This could be due to a role of BCAR3 in forming a molecular bridge between PTPα and Cas. It might also be due to reduced PTPα tyrosine phosphorylation in the absence of BCAR3 (Fig. 5B), although it seemed unlikely that this ∼50% reduction in phospho-PTPα would virtually abolish the interaction of PTPα with Cas. PTPα was not required for the interaction of BCAR3 and Cas, since Cas immunoprecipitates from wild-type and PTPα-deficient MEFs contained equivalent amounts of associated BCAR3 (Fig. 6B).
Fig 6.
PTPα Tyr789 and BCAR3 are required for integrin-induced formation of a PTPα-BCAR3-Cas-Src complex. (A) PTPα−/− MEFs were transfected with control nontargeting or BCAR3-targeted siRNA and with or without VSVG-tagged WT PTPα. Anti-VSVG immunoprecipitates from adherent cell lysates were probed for BCAR3, Cas, Src, and VSVG (VSVG-PTPα). Lysates were probed for BCAR3 and actin. (B) Cas immunoprecipitates were prepared from wild-type (+/+) or PTPα−/− (−/−) MEFS and, as a control, from wild-type MEFs from which BCAR3 was depleted using siRNA. Immunoprecipitates were probed for BCAR3 and Cas. (C) Lysates from adherent cultures of wild-type MEFs (+/+) and PTPα−/− MEFs (−/−) and from PTPα−/− MEFs transfected with VSVG-tagged WT PTPα or Y789F PTPα were used to prepare VSVG immunoprecipitates. These were probed as indicated, and lysates were probed for phospho-Tyr789 PTPα, PTPα, and actin. (D) PTPα−/− MEFs were untransfected or transfected with VSVG-WT PTPα. The transfected cells from cultures in uncoated dishes (Ad) were placed in suspension for 1 h (0) and then plated on FN-coated dishes for 15 min (15). Anti-VSVG immunoprecipitates from cell lysates were probed as indicated.
To distinguish between the actions of BCAR3 to (i) activate Src and promote PTPα Tyr789 phosphorylation that enabled PTPα-Cas association or to (ii) potentially form a physical link between phospho-PTPα and Cas, we determined whether Tyr789 of PTPα mediated its association with BCAR3, Cas, and Src. We prepared VSVG immunoprecipitates from VSVG-tagged WT and Y789F PTPα-expressing cells from which BCAR3 was not depleted (so PTPα Tyr789 was phosphorylated). As shown in Fig. 6C, BCAR3 was detectable in association with phospho-WT PTPα but was not detected in association with Y789F PTPα. Cas and Src were also found in association with WT PTPα, but no Cas and a greatly reduced amount of Src interacted with Y789F PTPα. Together, these results indicated that phospho-Tyr789 is necessary for PTPα interaction with BCAR3, Cas, and Src. In keeping with this interaction being an integrin-regulated event that requires PTPα Tyr789 phosphorylation, we found that in suspended cells where integrin signaling is not operational, PTPα was not associated with BCAR3, Cas, or Src. Stimulating integrin signaling by placing these cells on FN induced the phosphorylation of PTPα-Tyr789 and the association of BCAR3, Cas, and Src with PTPα (Fig. 6D).
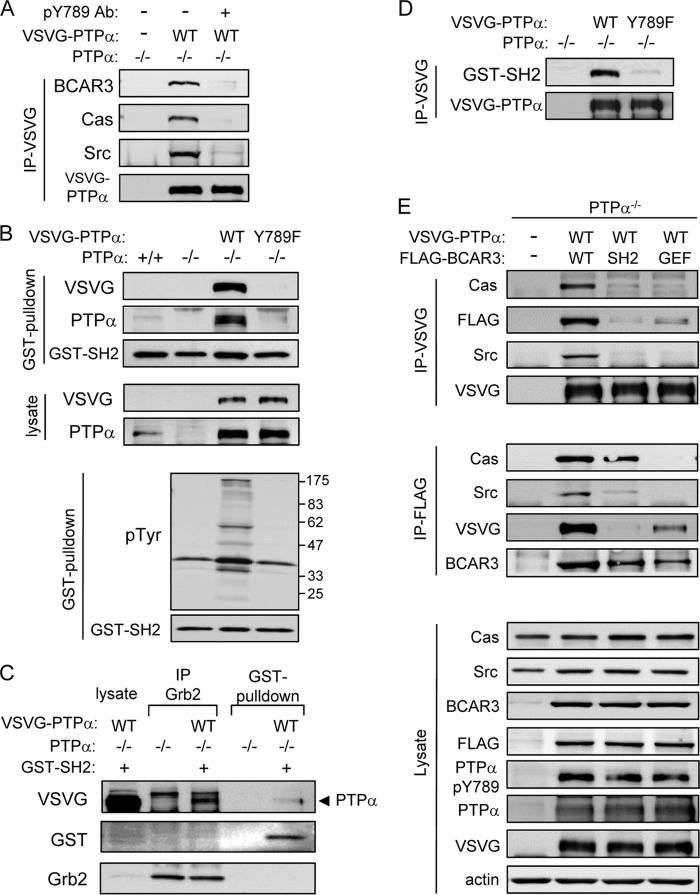
The BCAR3 SH2 domain directly binds PTPα-Tyr789.
To confirm the role of PTPα phospho-Tyr789 in the PTPα-BCAR3 interaction, we first examined whether the PTPα phospho-Tyr789-specific antibody could compete with BCAR3 for PTPα binding. Lysates of cells expressing VSVG-tagged WT PTPα were incubated with or without the anti-phospho-Tyr789 antibody, and PTPα was immunoprecipitated using bead-conjugated anti-VSVG antibody. Probing the immunoprecipitates showed that the interaction of BCAR3, as well as that of Cas and Src, with PTPα was virtually eliminated in the presence of the phospho-Tyr789 antibody (Fig. 7A), strongly suggesting that phospho-Tyr789 was the site for BCAR3 binding.
Fig 7.
PTPα phospho-Tyr789 directly interacts with the BCAR3-SH2 domain. (A) PTPα−/− MEFs were untransfected or transfected with VSVG-tagged WT PTPα. Lysates were mixed with anti-phospho-Tyr789 PTPα antibody as indicated, and anti-VSVG immunoprecipitates were prepared and probed as shown. (B) Lysates from the indicted cells were used for GST-BCAR3-SH2 pulldowns. Input lysates and pulldowns and starting lysates were probed for VSVG (PTPα) and PTPα or GST (BCAR3-SH2). Other GST-SH2 pulldowns (bottom two panels) were probed for phosphotyrosine and GST (BCAR3-SH2). (C) PTPα−/− MEFs were untransfected or transfected with VSVG-tagged WT PTPα. Lysates of VSVG-WT PTPα-expressing cells were mixed with recombinant GST-SH2 (BCAR3) fusion protein as indicated, and then these and the untransfected cell lysates were used to prepare anti-Grb2 immunoprecipitates or GST-SH2 pulldowns. Samples were probed for VSVG (PTPα), GST (BCAR3-SH2), and Grb2. (D) VSVG immunoprecipitates from adherent PTPα−/− MEFs that were untransfected or transfected with VSVG-tagged WT and 789F PTPα were probed with GST-SH2 (BCAR3) fusion protein. Bound GST-SH2 domain was detected using anti GST antibody. (E) PTPα−/− MEFs were untransfected or cotransfected with VSVG-WT PTPα and FLAG-tagged WT BCAR3 or mutant forms of BCAR3 (SH2, R177K mutation in SH2 domain; GEF, R748A mutation in GEF domain). Anti-VSVG (top panels) and anti-FLAG (middle panels) immunoprecipitates and cell lysates (bottom panels) were probed for the indicated proteins or epitope tags.
The role of the BCAR3 SH2 domain in mediating this binding was investigated using GST pulldown assays where GST-SH2 (BCAR3) fusion protein was mixed with lysates of wild-type and PTPα−/− MEFs or of PTPα−/− MEFs expressing VSVG-WT PTPα or VSVG-Y789F PTPα. Glutathione-mediated pulldowns of the GST-SH2 domain contained associated WT PTPα but not Y789F PTPα (Fig. 7B). A smaller amount of endogenous PTPα was also detected in pulldowns from wild-type MEFs (Fig. 7B, left). These results indicate that the BCAR3-SH2 domain interacts with PTPα phospho-Tyr789. Other GST-SH2 pulldowns were probed with antiphosphotyrosine (Fig. 7B, bottom two panels), but besides a band of ∼38 to 40 kDa that appeared in all samples, additional tyrosine phosphorylated proteins were detected only in the pulldown from VSVG-WT PTPα-expressing cells. This suggests that they are phosphoproteins that associate with phospho-Tyr789 PTPα rather than directly interacting with the SH2 domain of BCAR3 and that no other readily detectable phosphotyrosyl proteins interacted with the GST-SH2 domain of BCAR3.
Since the adaptor protein Grb2 also binds through its SH2 domain to PTPα phospho-Tyr789 (11, 39), it would be predicted that BCAR3 and Grb2 binding to phospho-Tyr789 would be mutually exclusive. We therefore tested whether complexes of PTPα and Grb2 or BCAR3-SH2 could be distinguished. The GST-SH2 protein was added to lysates of cells expressing VSVG-WT PTPα, and portions of the mixture were immunoprecipitated with anti-Grb2 antibody or used for GST pulldown. The Grb2 immunoprecipitates contained PTPα and Grb2 but not GST-SH2 (BCAR3), whereas the GST pulldowns contained PTPα and GST-SH2 (BCAR3) but not Grb2 (Fig. 7C), providing further evidence that the BCAR3 SH2 domain recognizes and binds to PTPα phosphoTyr789.
To confirm that BCAR3 SH2 directly bound to phospho-Tyr789 PTPα, we conducted a Far-Western analysis, where VSVG immunoprecipitates from lysates of VSVG-WT or -Y789F PTPα-expressing cells were resolved by SDS-PAGE and transferred to a membrane and then incubated with GST-SH2 (BCAR3) fusion protein. Subsequent probing with anti-GST antibody detected GST-SH2 in the WT PTPα sample at a position that coincided with that of WT PTPα, whereas virtually no binding of BCAR3 SH2 to Y789F PTPα was observed (Fig. 7D). These results demonstrate that the BCAR3 SH2 domain is sufficient to directly interact with PTPα Tyr789.
The SH2 and GEF domains of BCAR3 are, respectively, required for the physical linkage of PTPα with Cas and Src.
We tested whether mutant forms of BCAR3 with a point mutation in the SH2 domain (R177K, a position in human BCAR3 analogous to a conserved Arg residue in other SH2 domains that is required for target binding) (14) or in the GEF domain that mediates association with Cas (R748A in human BCAR3) (43) could bind to phospho-Tyr789 PTPα and support the interaction of PTPα with Cas and Src. PTPα−/− cells were untransfected or cotransfected with VSVG-WT PTPα and FLAG-tagged WT or mutant (SH2, R177K; GEF, R748A) BCAR3, and anti-VSVG and anti-FLAG immunoprecipitates were analyzed for associated proteins (Fig. 7E). Both anti-VSVG (Fig. 7E, top panels) and anti-FLAG (Fig. 7E, middle panels) immunoprecipitates from cells coexpressing WT forms of VSVG-PTPα and FLAG-BCAR3 contained the members of the protein complex we had previously detected: PTPα, BCAR3, Cas, and Src. The anti-VSVG immunoprecipitates from cells coexpressing tagged WT PTPα and the SH2 mutant of BCAR3 contained almost no BCAR3, Cas, or Src, as would be expected if BCAR3-SH2 mediated the association of PTPα with Cas and Src. In accord, virtually no PTPα was detectable in the anti-FLAG immunoprecipitates from these cells despite the presence of phospho-Tyr789 PTPα in the lysates (Fig. 7E, bottom panels). However, Cas was present, consistent with its interaction with BCAR3 being independent of the BCAR3 SH2 domain, as was a reduced amount of Src. Reciprocal anti-VSVG or anti-FLAG immunoprecipitates from cells coexpressing tagged WT PTPα and the GEF mutant of BCAR3, respectively, contained associated BCAR3 and PTPα, but the association appeared reduced compared to that detected in anti-VSVG immunoprecipitates from cells expressing WT BCAR3. Virtually no Cas or Src was coimmunoprecipitated. The GEF mutation in BCAR3 interferes with BCAR3-Cas association (43), suggesting that Cas binding to BCAR3 may modulate BCAR3 localization or conformation for optimal BCAR3-SH2-mediated interaction with PTPα. Together, the above results demonstrate that an intact SH2 domain within the BCAR3 protein is required for binding to phospho-Tyr789 PTPα, confirm that an intact GEF domain within BCAR3 is required for its binding to Cas, and show that these interactions mediate formation of the PTPα-BCAR3-Cas/Src complex.
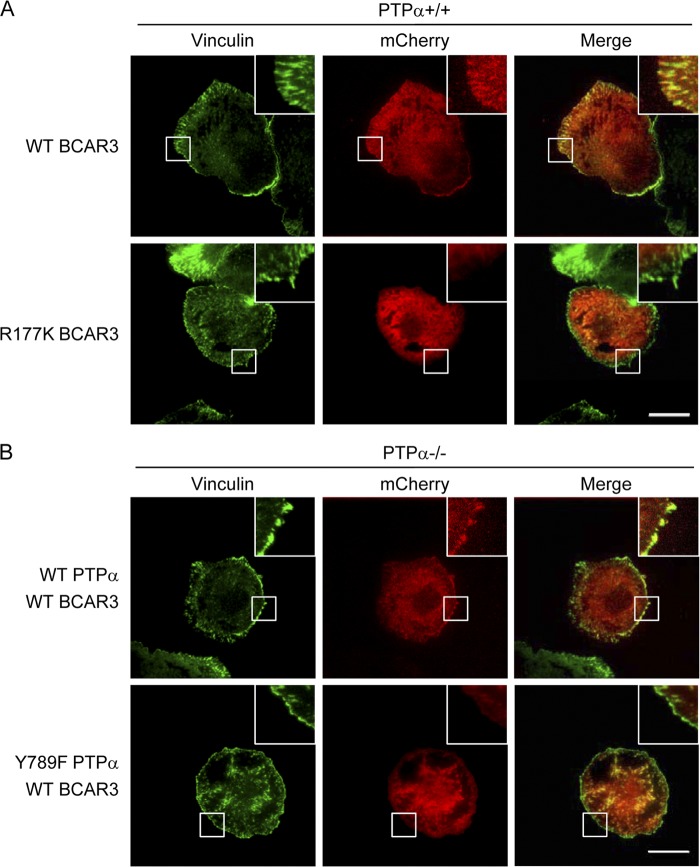
An intact BCAR3-SH2 domain and Tyr789 of PTPα are required for integrin-induced recruitment of BCAR3 to focal adhesions.
To test the role of the SH2 domain of BCAR3 in integrin-induced localization of BCAR3 to focal adhesions, we generated mCherry-tagged WT or mutant R177K BCAR3 and expressed these in wild-type MEFs. The cells were plated on FN for 15 min, fixed and immunostained for vinculin, and imaged for vinculin staining and mCherry using TIRF microscopy. The WT BCAR3 colocalized with vinculin in focal adhesion structures (Fig. 8A, top panels), but BCAR3 with a mutant SH2 domain did not colocalize with vinculin in focal adhesions (Fig. 8A, bottom panels). To determine if Tyr789 of PTPα was required for this recruitment of WT BCAR3 to focal adhesions, mCherry-tagged WT BCAR3 was expressed in PTPα−/− cells that had been infected with adenovirus expressing either WT PTPα or Y789F PTPα. After plating the cells on FN for 15 min, mCherry-BCAR3 colocalized with vinculin in the WT PTPα-expressing cells (Fig. 8B, top panels) but not in the Y789F PTPα-expressing cells (Fig. 8B, bottom panels). Together, these results demonstrate that both Tyr789 of PTPα and the SH2 domain of BCAR3 mediate BCAR3 recruitment to focal adhesions upon integrin stimulation, supporting the functionality of the demonstrated interaction of PTPα-phospho-Tyr789 and BCAR3-SH2.
Fig 8.
BCAR3 focal adhesion localization is dependent on BCAR3-SH2 domain and PTPα-Tyr789. (A) Wild-type (+/+) MEFs were transfected with mCherry-WT BCAR3 or mCherry-R177K BCAR3. Cells were starved, placed in suspension for 1 h, and then placed on FN-coated dishes for 15 min. Cells were stained with vinculin, and vinculin and mCherry (BCAR3) signals were imaged using TIRF microscopy. (B) PTPα−/− MEFS infected with adenovirus expressing WT PTPα or Y789F PTPα were transfected with mCherry-WT BCAR3 and treated as described in the legend to panel A. Scale bar = 30 μm.
DISCUSSION
Our study describes a novel noncatalytic mechanism of action of PTPα in integrin-mediated cell migration that is regulated by phosphorylation of PTPα at a site in its C-terminal tail region, Tyr789. We show through cell fractionation that Tyr789 phosphorylation regulates integrin-induced focal adhesion localization of PTPα. It also functions as a molecular recognition site that, via binding BCAR3, recruits Cas to PTPα in focal adhesions, where Cas is activated.
Integrin engagement induces the phosphorylation of PTPα at Tyr789, and this is required for efficient cell migration (8). Through reconstituting WT PTPα or mutant Y789F PTPα expression in PTPα−/− MEFs and evaluating their abilities to rescue progressively more upstream integrin signaling defects in the parental PTPα-null cells, we pinpointed the tyrosine phosphorylation of Cas as the most integrin-proximal event that is dependent on an intact Tyr789 residue in PTPα. Impaired Cas phosphorylation is due to the compromised interaction of Src and Cas in Y789F PTPα-expressing cells. Biochemical localization studies demonstrated that WT but not Y789F PTPα moved into focal adhesion-enriched fractions upon integrin engagement, consistent with the Tyr789-dependent immunofluorescent detection of PTPα in focal adhesions reported by Lammers et al. (24). Furthermore, the reduced presence of Y789F PTPα in focal adhesions was associated with the impaired localization of Cas, but not Src, to focal adhesions. Together, these results indicate that PTPα phospho-Tyr789 functions to provide a signal and/or recruitment mechanism not only for PTPα but also for Cas to assemble in newly forming adhesions where the latter is in proximity to and can be phosphorylated by Src.
Since Cas does not possess SH2 or PTB domains that could directly interact with phospho-Tyr789, we postulated the involvement of an intermediary protein. BCAR3 (also known as Nsp2, Shep2, or AND-34) (5, 42) is a member of an SH2 domain-containing family of Cas-binding proteins that includes Nsp1 and Shep1 (12, 26, 35), and numerous features of BCAR3 suggested it as an attractive candidate. Structurally, in addition to its SH2 domain of unknown specificity, BCAR3 contains a GEF domain that binds to Cas. Functionally, BCAR3 depletion phenocopies many of the defects of Y789F PTPα-expressing cells. Importantly, BCAR3 can regulate cell spreading and movement. Its overexpression promotes breast cancer cell or fibroblast spreading on fibronectin, migration, and invasion, while depletion of BCAR3 inhibits these cell properties (32, 37, 38). These actions of BCAR3 are dependent upon its ability to bind to Cas, since BCAR3-enhanced migration is abolished or reduced when the BCAR3 GEF domain responsible for Cas binding is deleted or mutated (32, 43). In support of BCAR3 mediating the interaction of PTPα and Cas, we detect a complex of PTPα-BCAR3-Cas-Src and show that the presence of Cas and Src in this complex is dependent on BCAR3, since Cas and Src do not interact with PTPα in BCAR3-depleted MEFs. As PTPα Tyr789 is not phosphorylated due to impaired Src activation in the BCAR3-depleted cells, this impaired interaction could be due to the absence of the phospho-Tyr789 binding site on PTPα. However, the interaction of PTPα with BCAR3, Cas, and Src is also abolished in cells expressing BCAR3 with a mutant SH2 domain, even though Tyr789 of PTPα is phosphorylated. In addition, the interaction of BCAR3 with Cas/Src is eliminated by a mutation in the BCAR3 GEF domain, while this mutant BCAR3 can still interact with phospho-Tyr789 PTPα. All together, this evidence confirms BCAR3 as an activator of Src (38) and reveals BCAR3 as a molecular linker of PTPα and Cas.
Our study identifies phospho-Tyr789 PTPα as the first cellular ligand for the SH2 domain of BCAR3. Not only does formation of the complex require PTPα Tyr789 and an intact SH2 domain of BCAR3, but the recombinant BCAR3 SH2 domain interacts directly with WT PTPα and not with Y789F PTPα. The ability of the BCAR3 SH2 domain to bind to PTPα phospho-Tyr789 was unexpected, as this SH2 domain is not closely related to either of two other SH2 domains, those of Grb2 and Src (25), that can bind phospho-Tyr789. However, virtually nothing is known of BCAR3-SH2 specificity, and besides PTPα, no other target phosphotyrosyl proteins have been found.
Two domains of Cas mediate its targeting to adhesion complexes, and both domains are required for optimal focal adhesion recruitment and tyrosine phosphorylation of Cas (13, 18, 28). The N-terminal SH3 domain of Cas associates with FAK to recruit Cas to focal adhesions. The C-terminal region of Cas also mediates its focal adhesion localization, although the molecular basis for this has been elusive. Functionally, we have demonstrated that the interaction of Tyr789 of PTPα with the SH2 domain of BCAR3 is required for PTPα-BCAR3-Cas-Src interaction and promotes the integrin-induced movement of BCAR3 and Cas to focal adhesion-enriched fractions. Our results support a model (Fig. 9) where integrin activation induces Src-mediated phosphorylation of PTPα at Tyr789, resulting in formation and localization of a PTPα-BCAR3-Cas complex at nascent adhesion sites. Since BCAR3 interacts with the C-terminal region of Cas, specifically with the helix-containing Cas family C-terminal homology domain (CCH) region of Cas required for its optimal focal adhesion localization (13, 17), we propose that this mechanism underlies the C-terminal-dependent focal adhesion localization of Cas. Consistent with this, BCAR3 has been suggested to regulate migration by promoting Cas localization to the membrane or leading edge (37). BCAR3 and Cas colocalize at the cell periphery and in migration-associated structures such as ruffles and lamellipodia. Conversely, Cas is lost from membrane ruffles in BCAR3-depleted and migration-impaired cells. In our model, the assembly of the PTPα phospho-Tyr789-BCAR3-Cas complex in the adhesions places Cas in proximity to Src and provides a mechanistic explanation for the reported role of BCAR3 in regulating Cas-Src interaction that promotes Src-mediated phosphorylation of Cas (38). In respect to the latter, the PTPα-BCAR3 C-terminal Cas anchor could cooperate with FAK-mediated N-terminal anchoring of Cas to provide opposing traction points that allow the mechanical force-dependent conformational extension of Cas that facilitates its SD phosphorylation by Src (36). Notably, PTPα regulates Src-mediated FAK activation (48), suggesting that PTPα functions as a master coordinator of both arms of the spatial two-pronged anchoring of Cas in focal adhesions.
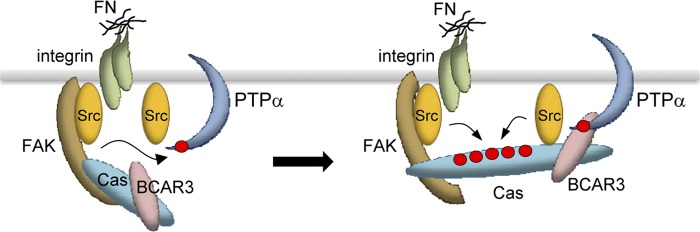
Fig 9.
Schematic diagram of the mechanism by which phospho-Tyr789 PTPα coordinates Cas positioning and phosphorylation in focal adhesions. Left, fibronectin (FN) engagement of integrins induces PTPα phosphorylation at Tyr789 by activated Src in the Src-FAK complex. Right, phospho-Tyr789 PTPα is localized to forming focal adhesions. BCAR3 forms a molecular bridge between phospho-PTPα and Cas (with the SH2 domain of BCAR3 binding to phosphoTyr789 on PTPα and the GEF domain of BCAR3 associating with the C-terminal region of Cas), thus positioning Cas in focal adhesions. The PTPα-BCAR3-dependent C-terminal anchoring of Cas promotes Cas interaction with Src and Src-mediated phosphorylation and activation of Cas. This may be facilitated by FAK-mediated anchoring of the N-terminal of Cas in focal adhesions so that dual opposing Cas anchors enable stretching and increased SD region accessibility to phosphorylation. Cas phosphorylation enables optimal signaling to Crk, Rac1, Cdc42, and PAK and cell migration (not shown).
In summary, we have described a novel molecular complex of PTPα-BCAR3-Cas-Src. This complex forms in response to PTPα Tyr789 phosphorylation and mediates Cas localization to focal adhesions and Cas downstream signaling to promote cell migration. Given that Cas is also a critical regulator of cell movement in pathological contexts such as cancer metastasis (4), the interdependent PTPα-BCAR3-Cas module may also operate to enable the invasion and spreading of malignant cells and could thus present multiple aspects for potential therapeutic perturbation.
ACKNOWLEDGMENTS
We thank Greg Longmore for the EGFP-Cas-expressing plasmid.
This work was supported by grants from the Canadian Institutes of Health Research (MOP-49410) (C.J.P.) and from the Leukemia and Lymphoma Society of Canada and the Canada Foundation for Innovation (C.J.L.). S.Y.S.C. was supported by a Child & Family Research Institute Graduate Award, C.J.L. was supported by the Michael Cuccione Foundation, and C.J.P. holds an Investigatorship from the Child & Family Research Institute.
Footnotes
Published ahead of print 16 July 2012
REFERENCES
- 1. Ballestrem C, et al. 2006. Molecular mapping of tyrosine-phosphorylated proteins in focal adhesions using fluorescence resonance energy transfer. J. Cell Sci. 119:866–875 [DOI] [PubMed] [Google Scholar]
- 2. Bhandari V, Lim KL, Pallen CJ. 1998. Physical and functional interactions between receptor-like protein-tyrosine phosphatase alpha and p59fyn. J. Biol. Chem. 273:8691–8698 [DOI] [PubMed] [Google Scholar]
- 3. Bouton AH, Riggins RB, Bruce-Staskal PJ. 2001. Functions of the adapter protein Cas: signal convergence and the determination of cellular responses. Oncogene 20:6448–6458 [DOI] [PubMed] [Google Scholar]
- 4. Cabodi S, del Pilar Camacho-Leal M, Di Stefano P, Defilippi P. 2010. Integrin signalling adaptors: not only figurants in the cancer story. Nat. Rev. Cancer 10:858–870 [DOI] [PubMed] [Google Scholar]
- 5. Cai D, Clayton LK, Smolyar A, Lerner A. 1999. AND-34, a novel p130Cas-binding thymic stromal cell protein regulated by adhesion and inflammatory cytokines. J. Immunol. 163:2104–2112 [PubMed] [Google Scholar]
- 6. Cai D, et al. 2003. AND-34/BCAR3, a GDP exchange factor whose overexpression confers antiestrogen resistance, activates Rac, PAK1, and the cyclin D1 promoter. Cancer Res. 63:6802–6808 [PubMed] [Google Scholar]
- 7. Cary LA, Han DC, Polte TR, Hanks SK, Guan JL. 1998. Identification of p130Cas as a mediator of focal adhesion kinase-promoted cell migration. J. Cell Biol. 140:211–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen M, Chen SC, Pallen CJ. 2006. Integrin-induced tyrosine phosphorylation of protein-tyrosine phosphatase-alpha is required for cytoskeletal reorganization and cell migration. J. Biol. Chem. 281:11972–11980 [DOI] [PubMed] [Google Scholar]
- 9. Defilippi P, Di Stefano P, Cabodi S. 2006. p130Cas: a versatile scaffold in signaling networks. Trends Cell Biol. 16:257–263 [DOI] [PubMed] [Google Scholar]
- 10. del Pozo MA, Price LS, Alderson NB, Ren XD, Schwartz MA. 2000. Adhesion to the extracellular matrix regulates the coupling of the small GTPase Rac to its effector PAK. EMBO J. 19:2008–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. den Hertog J, Tracy S, Hunter T. 1994. Phosphorylation of receptor protein-tyrosine phosphatase alpha on Tyr789, a binding site for the SH3-SH2-SH3 adaptor protein GRB-2 in vivo. EMBO J. 13:3020–3032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dodelet VC, Pazzagli C, Zisch AH, Hauser CA, Pasquale EB. 1999. A novel signaling intermediate, SHEP1, directly couples Eph receptors to R-Ras and Rap1A. J. Biol. Chem. 274:31941–31946 [DOI] [PubMed] [Google Scholar]
- 13. Donato DM, Ryzhova LM, Meenderink LM, Kaverina I, Hanks SK. 2010. Dynamics and mechanism of p130Cas localization to focal adhesions. J. Biol. Chem. 285:20769–20779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Felekkis KN, et al. 2005. AND-34 activates phosphatidylinositol 3-kinase and induces anti-estrogen resistance in a SH2 and GDP exchange factor-like domain-dependent manner. Mol. Cancer Res. 3:32–41 [PubMed] [Google Scholar]
- 15. Fonseca PM, et al. 2004. Regulation and localization of CAS substrate domain tyrosine phosphorylation. Cell Signal. 16:621–629 [DOI] [PubMed] [Google Scholar]
- 16. Geiger B, Yamada KM. 2011. Molecular architecture and function of matrix adhesions. Cold Spring Harb. Perspect. Biol. 3:a005033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gotoh T, Cai D, Tian X, Feig LA, Lerner A. 2000. p130Cas regulates the activity of AND-34, a novel Ral, Rap1, and R-Ras guanine nucleotide exchange factor. J. Biol. Chem. 275:30118–30123 [DOI] [PubMed] [Google Scholar]
- 18. Harte MT, Macklem M, Weidow CL, Parsons JT, Bouton AH. 2000. Identification of two focal adhesion targeting sequences in the adapter molecule p130(Cas). Biochim. Biophys. Acta 1499:34–48 [DOI] [PubMed] [Google Scholar]
- 19. Herrera Abreu MT, et al. 2008. Tyrosine phosphatase PTPalpha regulates focal adhesion remodeling through Rac1 activation. Am. J. Physiol. Cell Physiol. 294:C931–C944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaplan KB, et al. 1994. Association of the amino-terminal half of c-Src with focal adhesions alters their properties and is regulated by phosphorylation of tyrosine 527. EMBO J. 13:4745–4756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klemke RL, et al. 1998. CAS/Crk coupling serves as a “molecular switch” for induction of cell migration. J. Cell Biol. 140:961–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kostic A, Sap J, Sheetz MP. 2007. RPTPalpha is required for rigidity-dependent inhibition of extension and differentiation of hippocampal neurons. J. Cell Sci. 120:3895–3904 [DOI] [PubMed] [Google Scholar]
- 23. Kostic A, Sheetz MP. 2006. Fibronectin rigidity response through Fyn and p130Cas recruitment to the leading edge. Mol. Biol. Cell 17:2684–2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lammers R, Lerch MM, Ullrich A. 2000. The carboxyl-terminal tyrosine residue of protein-tyrosine phosphatase alpha mediates association with focal adhesion plaques. J. Biol. Chem. 275:3391–3396 [DOI] [PubMed] [Google Scholar]
- 25. Liu BA, et al. 2006. The human and mouse complement of SH2 domain proteins—establishing the boundaries of phosphotyrosine signaling. Mol. Cell 22:851–868 [DOI] [PubMed] [Google Scholar]
- 26. Lu Y, Brush J, Stewart TA. 1999. NSP1 defines a novel family of adaptor proteins linking integrin and tyrosine kinase receptors to the c-Jun N-terminal kinase/stress-activated protein kinase signaling pathway. J. Biol. Chem. 274:10047–10052 [DOI] [PubMed] [Google Scholar]
- 27. Mitra SK, Schlaepfer DD. 2006. Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr. Opin. Cell Biol. 18:516–523 [DOI] [PubMed] [Google Scholar]
- 28. Nakamoto T, et al. 1997. Requirements for localization of p130cas to focal adhesions. Mol. Cell. Biol. 17:3884–3897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parsons JT, Horwitz AR, Schwartz MA. 2010. Cell adhesion: integrating cytoskeletal dynamics and cellular tension. Nat. Rev. Mol. Cell Biol. 11:633–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Petch LA, Bockholt SM, Bouton A, Parsons JT, Burridge K. 1995. Adhesion-induced tyrosine phosphorylation of the p130 src substrate. J. Cell Sci. 108(Pt 4):1371–1379 [DOI] [PubMed] [Google Scholar]
- 31. Ponniah S, Wang DZ, Lim KL, Pallen CJ. 1999. Targeted disruption of the tyrosine phosphatase PTPalpha leads to constitutive downregulation of the kinases Src and Fyn. Curr. Biol. 9:535–538 [DOI] [PubMed] [Google Scholar]
- 32. Riggins RB, Quilliam LA, Bouton AH. 2003. Synergistic promotion of c-Src activation and cell migration by Cas and AND-34/BCAR3. J. Biol. Chem. 278:28264–28273 [DOI] [PubMed] [Google Scholar]
- 33. Ruest PJ, Shin NY, Polte TR, Zhang X, Hanks SK. 2001. Mechanisms of CAS substrate domain tyrosine phosphorylation by FAK and Src. Mol. Cell. Biol. 21:7641–7652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sakai R, et al. 1994. A novel signaling molecule, p130, forms stable complexes in vivo with v-Crk and v-Src in a tyrosine phosphorylation-dependent manner. EMBO J. 13:3748–3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sakakibara A, Hattori S. 2000. Chat, a Cas/HEF1-associated adaptor protein that integrates multiple signaling pathways. J. Biol. Chem. 275:6404–6410 [DOI] [PubMed] [Google Scholar]
- 36. Sawada Y, et al. 2006. Force sensing by mechanical extension of the Src family kinase substrate p130Cas. Cell 127:1015–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schrecengost RS, Riggins RB, Thomas KS, Guerrero MS, Bouton AH. 2007. Breast cancer antiestrogen resistance-3 expression regulates breast cancer cell migration through promotion of p130Cas membrane localization and membrane ruffling. Cancer Res. 67:6174–6182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schuh NR, Guerrero MS, Schrecengost RS, Bouton AH. 2010. BCAR3 regulates Src/p130 Cas association, Src kinase activity, and breast cancer adhesion signaling. J. Biol. Chem. 285:2309–2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Su J, Batzer A, Sap J. 1994. Receptor tyrosine phosphatase R-PTP-alpha is tyrosine-phosphorylated and associated with the adaptor protein Grb2. J. Biol. Chem. 269:18731–18734 [PubMed] [Google Scholar]
- 40. Su J, Muranjan M, Sap J. 1999. Receptor protein tyrosine phosphatase alpha activates Src-family kinases and controls integrin-mediated responses in fibroblasts. Curr. Biol. 9:505–511 [DOI] [PubMed] [Google Scholar]
- 41. Tonks NK. 2006. Protein tyrosine phosphatases: from genes, to function, to disease. Nat. Rev. Mol. Cell Biol. 7:833–846 [DOI] [PubMed] [Google Scholar]
- 42. van Agthoven T, et al. 1998. Identification of BCAR3 by a random search for genes involved in antiestrogen resistance of human breast cancer cells. EMBO J. 17:2799–2808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Vanden Borre P, Near RI, Makkinje A, Mostoslavsky G, Lerner A. 2011. BCAR3/AND-34 can signal independent of complex formation with CAS family members or the presence of p130Cas. Cell Signal 23:1030–1040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. von Wichert G, et al. 2003. RPTP-alpha acts as a transducer of mechanical force on alphav/beta3-integrin-cytoskeleton linkages. J. Cell Biol. 161:143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vuori K, Ruoslahti E. 1995. Tyrosine phosphorylation of p130Cas and cortactin accompanies integrin-mediated cell adhesion to extracellular matrix. J. Biol. Chem. 270:22259–22262 [DOI] [PubMed] [Google Scholar]
- 46. Zaidel-Bar R, Ballestrem C, Kam Z, Geiger B. 2003. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J. Cell Sci. 116:4605–4613 [DOI] [PubMed] [Google Scholar]
- 47. Zaidel-Bar R, Geiger B. 2010. The switchable integrin adhesome. J. Cell Sci. 123:1385–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zeng L, et al. 2003. PTP alpha regulates integrin-stimulated FAK autophosphorylation and cytoskeletal rearrangement in cell spreading and migration. J. Cell Biol. 160:137–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhao X, Guan JL. 2011. Focal adhesion kinase and its signaling pathways in cell migration and angiogenesis. Adv. Drug Deliv. Rev. 63:610–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zheng XM, Resnick RJ, Shalloway D. 2000. A phosphotyrosine displacement mechanism for activation of Src by PTPalpha. EMBO J. 19:964–978 [DOI] [PMC free article] [PubMed] [Google Scholar]