Abstract
The present study investigated the capacity of formulated Berberis vulgaris extract/β-cyclodextrin to protect liver against CCl4-induced hepatotoxicity in mice. Formulated and non-formulated extracts were given orally (50 mg/kg/day) to mice for 7 days and were then intra-peritoneally injected with 1.0 mL/kg CCl4 on the 8th day. After 24 h of CCl4 administration, an increase in the levels of apartate-amino-transferase (AST), alanine-amino-transferase (ALT) and malondialdehyde (MDA) was found and a significant decrease in superoxide-dismutase (SOD), catalase (CAT), glutathione (GSH) and glutathione-peroxidase (GPx) levels could be detected. This was accompanied by extended centrilobular necrosis, steatosis, fibrosis and an altered ultrastructure of hepatocytes. Pre-treatment with formulated or non-formulated extract suppressed the increase in ALT, AST and MDA levels and restored the level of antioxidant enzymes at normal values. Histopathological and electron-microscopic examination showed milder liver damage in both pre-treated groups and the protective effect was more pronounced after the formulated extract was administered. Internucleosomal DNA fragmentation induced by CCl4 was reduced in the group which received non-formulated extract and absent in the group which received formulated extract. Taken together, our results suggest that Berberis vulgaris/β-cyclodextrin treatment prevents hepatic injury induced by CCl4 and can be considered for further nutraceutical studies.
Keywords: Berberis vulgaris extract, β-cyclodextrin, hepatoprotection, oxidative stress
1. Introduction
The liver is prone to xenobiotic-induced injury because of its central role in xenobiotic metabolism and its portal location within circulation [1].
Several xenobiotics, such as: arsenic, cadmium, chloroform, carbon tetrachloride, pesticides, dioxins, nitrosamines [2] become hepatotoxic through metabolisation and bioactivation processes. The first phase reactions (oxidation, reduction or hydrolysis, etc.), followed by conjugation ones, play an important role in converting xenobiotics into a form suitable for elimination in order to prevent xenobiotic-induced liver injury [3,4]. But these metabolic processes require multiple biochemical transformations, and in some cases, the intermediates mediate toxic responses. Therefore, the liver is potentially susceptible to injury during the act of performing its function [5].
Carbon tetrachloride (CCl4) is the best characterized model of xenobiotic-induced hepatotoxicity and commonly used for the screening of hepatoprotective effects of drugs and natural products. Liver damage induced by carbon tetrachloride (CCl4) is based on metabolisation to trichloromethyl radicals (CCl3· and/or CCl3OO·) under cytocrome P450 action [6,4].
Many herbs are used by traditional medicine to prevent and treat liver diseases. Several hundred plants have been examined for use in a wide variety of liver disorders, but just a part have been well investigated, such as: Silybum marianum (milk thistle), Picrorhiza kurroa (kutkin), Curcuma longa (turmeric), Camellia sinensis (green tea), Chelidonium majus (greater celandine), Glycyrrhiza glabra (licorice), Allium sativa (garlic) [7–9].
Berberry (Berberis vulgaris L., family Berberidaceae) is a herb growing in Europe and Asia, which has been less investigated from a therapeutic point of view. The root, bark, leaf and fruit have been used in traditional medicine to treat diarrhea, colitis, gastroenteritis, and hepatic disorders. Several alkaloid constituents with an isoquinolinic nucleus, such as berberine, berbamine and palmatine were isolated [10]. Other compounds like terpenoids lupeol, oleanolic acid, stigmasterol and stigmasterol glucoside [11] as well as polyphenols [12] were also identified. Nevertheless, berberine is the most important alkaloid that is generally claimed to be responsible for their beneficial effects [12]. There are multiple pharmacological effects of berberine, such as antimicrobial [13,14], anti-tumor [15–17], and anti-inflammatory effects [18–20]. It also has effects on the gastro-intestinal [11,21–23], cardiovascular [24–26] and nervous [27] systems.
Several studies revealed that berberine exerts preventive and curative effects on liver against experimental injuries. Feng et al [28] reported a significant decrease of hepatic marker enzymes in CCl4 treated rats after oral administration of berberine at 80, 120, 160 mg/kg daily doses compared to control animals, whereas a lower dose (4 mg/kg) was not effective [29]. On the other hand, intraperitoneal administration of berberine in rats in a dose of 0.5–5 mg/kg counteracted the damaging effect of tert-butyl hydroperoxide by reducing the generated oxidative stress [30]. A few studies concerning the oral treatment of rats with extract of Berberis vulgaris root have been reported [31]. The dose that effectively protected the liver was 900 mg/kg, 30 times higher than average dose (THD) used in some traditional systems of medicine.
There is no report in the literature on the protective effect of formulated Berberis vulgaris L. extract/β-cyclodextrin against CCl4-induced liver injury. Therefore, the present study was carried out to evaluate the preventive effect of Berberis vulgaris L. extract against CCl4-induced acute hepatotoxicity in mouse liver as well as the effects of β-cyclodextrin complexation, in hepatoprotective therapeutical formulations.
2. Results and Discussion
2.1. The Complex of Berberis vulgaris Extract and β Cyclodextrin
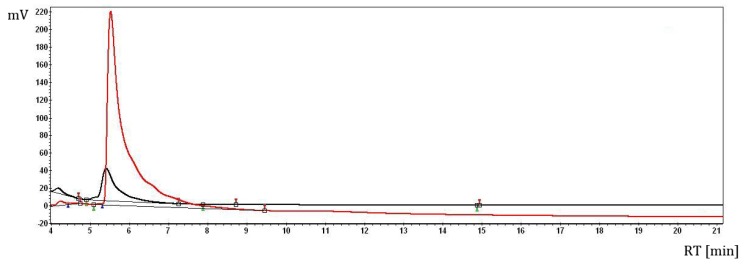
The presence of the berberine (the main compound from Berberis vulgaris L. bark) in the B. vulgaris L./β-cyclodextrin complex was identified by means of HPLC analysis, according to the previous study (Figure 1) [32].
Figure 1.
HPLC analysis of the re-extracted (ethanolic solution) Berberis vulgaris L. sample from the β-cyclodextrin complex (lower chromatogram—black) in comparison with the berberine standard solution (upper chromatogram—red).
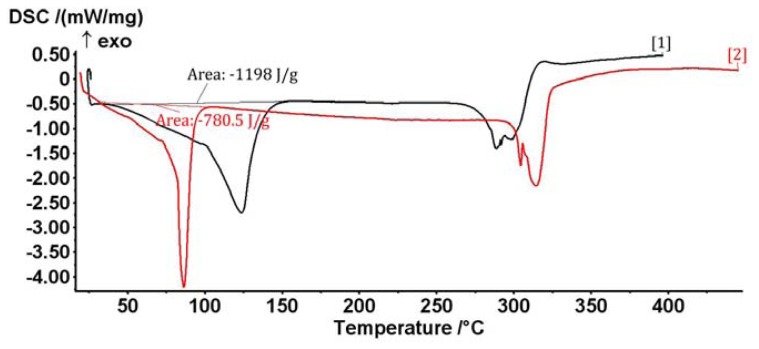
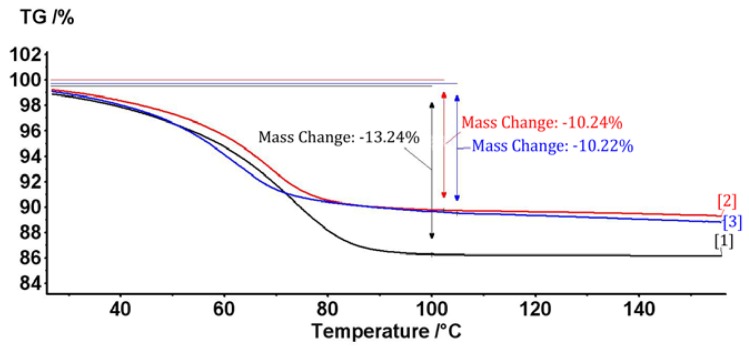
The B. vulgaris L. extract/β-cyclodextrin complex formation is proved by thermal and calorimetric analyses. Thus, the overall profile of the DSC (differential scanning calorimetry) and TG (thermogravimetry) analyses of the complex are very different in comparison with the β-cyclodextrin case; the formation of the complex is especially revealed by the shifting of the water dissociation calorimetric process to lower temperatures in the DSC analysis. This is explained by the replacing of these water molecules from β-cyclodextrin with the bioactive B. vulgaris compounds (such as berberine, which was previously identified by HPLC analysis) (Figure 2). Furthermore, the water concentration in the β-cyclodextrin complex is approximately 3% lower than in the case of initial β-cyclodextrin, which are revealed by both DSC and TG analyses (1200 J/g for B.v./βCD complex and 780 J/g for βCD in the DSC analysis; 10.2% mass loss for B.v./βCD complex and 13.2% for βCD in the TG analysis; Figures 2 and 3). These means that some water molecules (especially from the β-cyclodextrin cavity) are replaced by Berberis vulgaris L. extract compounds (especially by the most concentrated berberine, revealed by HPLC analysis) in the nanoencapsulation process, which leads to the formation of Berberis vulgaris extract/β-cyclodextrin complex.
Figure 2.
Differential scanning calorimetry (DSC) analysis of the Berberis vulgaris L. extract/β-cyclodextrin complex (red curve) and the corresponding β-cyclodextrin sample (black curve).
Figure 3.
Thermogravimetry (TG) analysis of the Berberis vulgaris L. extract/β-cyclodextrin complexes (upper curves) and the corresponding β-cyclodextrin sample (lower curve).
2.2. Effects of Berberis vulgaris Extract/β Cyclodextrin Pre-Treatment on Serum ALT, AST, γ-GT, Total and Direct Bilirubin
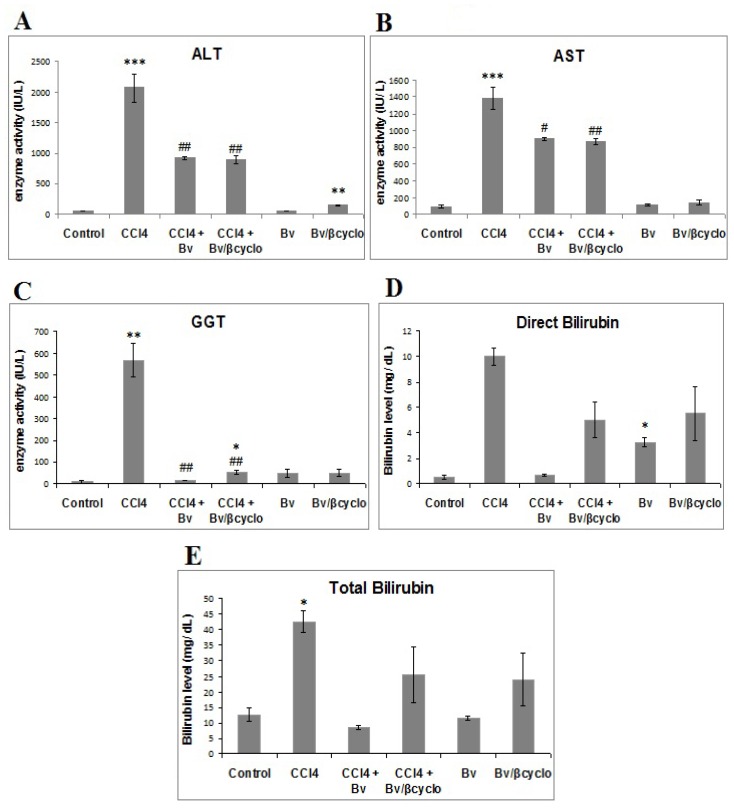
Effects of non-formulated and formulated Berberis vulgaris extract on serum ALT, AST, γ-GT activities, as well as total and direct bilirubin, in mice from various treatment groups are shown in Figure 4. The dose of 50 mg/kg formulated and non-formulated extract of Berberis vulgaris was chosen according to previous experiments [31], taking into account that the maximum concentration of berberine was found in bark (the plant used for our extract preparation) followed by root, leaves and fruit [32].
Figure 4.
The effects of a 50 mg/kg dose of formulated and non-formulated Berberis vulgaris extract against liver injuries induced by CCl4 for 24 h on serum (A) alanine-amino-transferase (ALT); (B) apartate-amino-transferase (AST); (C) γ-glutamyl transferase (γ-GT); (D) direct bilirubin; (E) total bilirubin. Values are expressed as mean ± SD (n = 8). * p < 0.05 significantly different from the control group; # p < 0.05 significantly different from the CCl4-treated group.
After 24 h of CCl4 treatment, the ALT (Figure 4A), AST (Figure 4B) and γ-GT (Figure 4C) serum activities significantly increased (p < 0.001) by 40.46, 14.86 respectively 59.82 folds, whereas the total bilirubin and direct bilirubin levels significantly raised by 3.4 and 20 times compared to control group.
Pre-treatment with 50 mg/kg of Berberis vulgaris extract/β-cyclodextrin decreased significantly the CCl4-induced elevation of serum aminotransferases by 50% for ALT (Figure 4A). The non-formulated Berberis vulgaris extract showed no increase of ALT activity whereas the formulated one significantly raised it compared to control group. In the case of AST, both formulated and non-formulated Berberis vulgaris extracts reduced the activity elevation induced by CCl4 treatment by about 35%, whereas in the groups where only one type of extract was administered, AST levels were the same as in the control group (Figure 4B).
In addition, the up-regulation of γ-GT activity determined by CCl4 exposure was diminished by about 10 times by pre-treatment with formulated and non-formulated extracts (Figure 4C).
In the same time, a significant decrease of serum total and direct bilirubin by about 50% was observed in the pre-treated mice with formulated and non-formulated Berberis vulgaris extracts.
It is well known that chemical agents produce liver damage, causing high increases in bilirubin and enzymes activity presented above, which are released into serum [4,33,34]. The elevated activities of ALT, AST and γ-GT as well as total and direct bilirubin are indicative for cellular leakage and loss of the functional integrity of the liver cell membrane [35,36], and mitochondrial disruption respectively [37].
In our study, the pre-treatment with both formulated and non-formulated extracts, suppressed the increment of these parameters induced by CCl4 administration, results similar with those obtained by 160 mg/kg berberine pre-treatment [28]. It seems that the Berberis vulgaris extract entrapped in β cyclodextrin was more efficient at a lower dose than the alkaloid itself.
2.3. Effects of Berberis vulgaris Extract/β Cyclodextrin Pre-Treatment on Antioxidant Status and Lipid Peroxidation
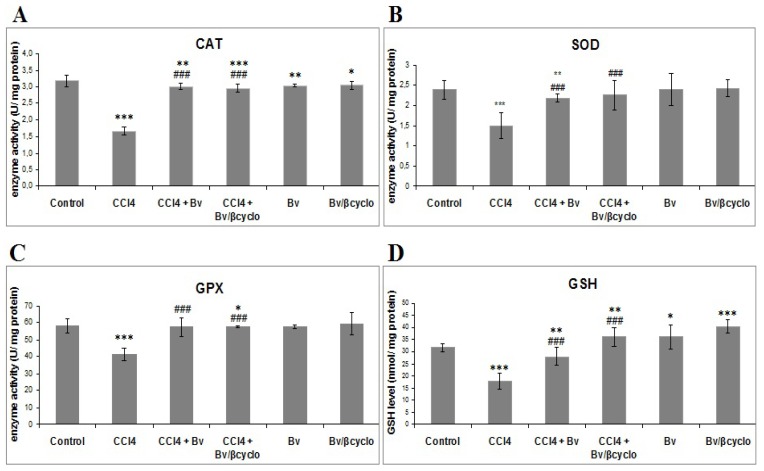
As shown in Figure 5, activities of CAT, SOD and GPx were significantly decreased in CCl4 treated mice compared to control ones. Formulated and non-formulated Berberis vulgaris extract pre-treatment significantly prevented inhibition of these enzymes activities caused by CCl4, and SOD activity of non-formulated Berberis vulgaris extract pre-treatment was slightly decreased compared to formulated extract and CCl4 treated group. In addition, CCl4 toxicity was confirmed by depletion of cellular GSH content in liver cells. Pre-treatment with non-formulated and Berberis vulgaris/β cyclodextrin extract determined a significant increase of GSH concentration compared to CCl4 groups. It should be mentioned that CAT, SOD and GPX activities in mice liver treated only with formulated or non-formulated Berberis vulgaris extract were approximately equal to those from control group, and GSH content was higher in these two groups compared to control. The level of this tripeptide, important in redox homeostasis and free radical scavenging detoxification of electrophils, increased probably by stimulation of γ-glutamyl cycle and/or enzymatic regeneration of GSH from oxidized glutathione.
Figure 5.
Protective effect of a 50 mg/kg dose of non-formulated Berberis vulgaris extract and Berberis vulgaris extract/βcyclodextrin on CCl4-induced oxidative stress in mice liver. Oxidative stress was assessed by measuring the (A) catalase (CAT); (B) superoxide dismutase (SOD) and (C) glutathione peroxidase (GPX) activity; (D) glutathione (GSH) level. * p < 0.05 significantly different from the control group; # p < 0.05 significantly different from the CCl4-treated group.
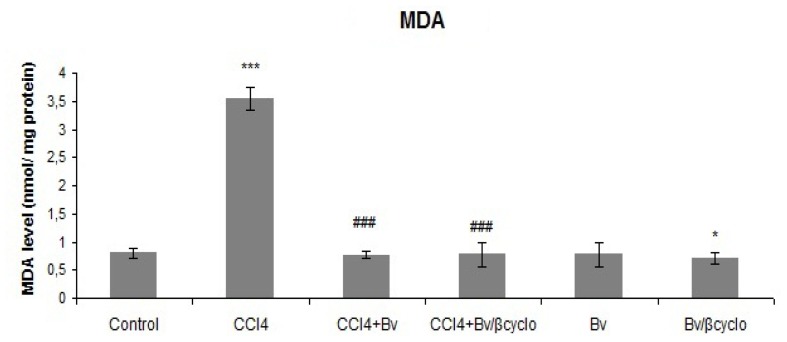
Lipid peroxidation was determined by measuring the concentration of malondialdehyde. As Figure 6 illustrates, after 24 h of CCl4 treatment, MDA content significantly increased, but its high level was markedly reduced through treatment with formulated and non-formulated Berberis vulgaris extracts to levels almost similar to control group. In the case of treatment with Berberis vulgaris extracts alone, MDA levels close to normal group were observed.
Figure 6.
Protective effect of a 50 mg/kg dose of non-formulated Berberis vulgaris extract and Berberis vulgaris extract/βcyclodextrin CCl4-induced lipid peroxidation in mice liver. * p < 0.05 significantly different from the control group; # p < 0.05 significantly different from the CCl4-treated group.
Previous studies showed that CCl4 caused hepatocellular damages through an increase in the formation of DNA strand breaks, 8-oxodeoxiguanozine and MDA-DNA adducts under conditions of increased lipid peroxidation, and high depletion in glutathione content [38].
It is well known that antioxidant enzymes and GSH deficiency enhances the possibility for lipid peroxidation, and may cause irreversible damage to the liver tissue [39]. The protective effects of Berberis vulgaris extracts on the liver of CCl4 treated mice highlighted their elevated antioxidant activity, polyphenolic content, and rich nutritional composition, which sustain redox properties of extracts [40–42]. The antioxidant effects of Berberis vulgaris extract shown here were in agreement with a previous report [31].
We have shown that GPx and GSH levels of non-formulated extract pre-treatment group were statistically similar with those measured in the liver of Berberis vulgaris extract/β-cyclodextrin pre-treated mice. The pre-treatment with both Berberis vulgaris extracts attenuated CCl4 toxicity in a very efficient way, improving the antioxidant status of liver tissue.
It seems that they did not stimulate the antioxidant enzymes because CAT, SOD and GPx activities were not increased in mice treated only with formulated or non-formulated Berberis vulgaris extract.
2.4. Histopathology
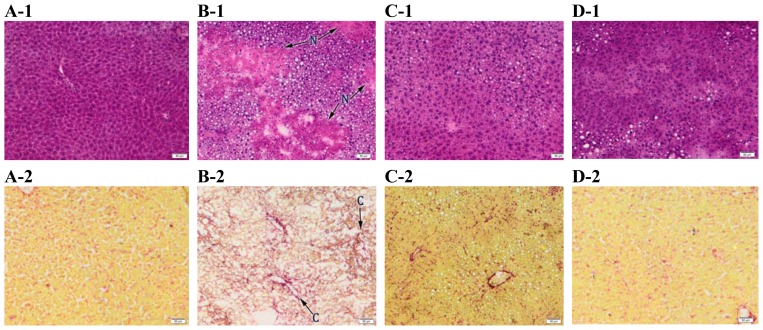
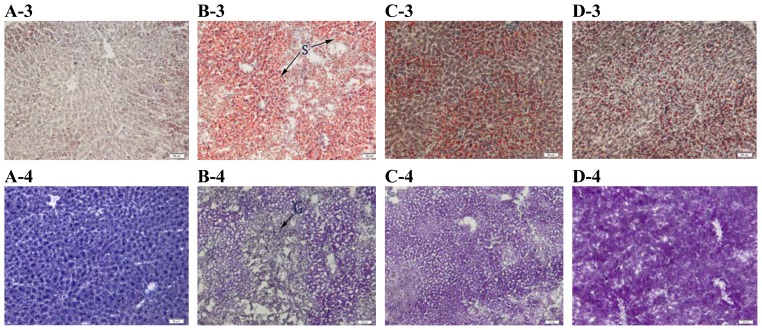
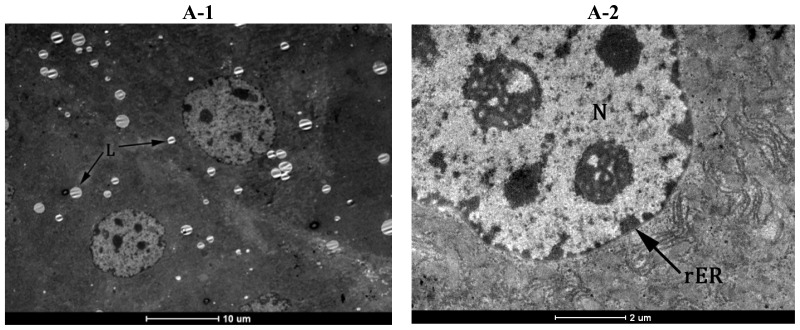
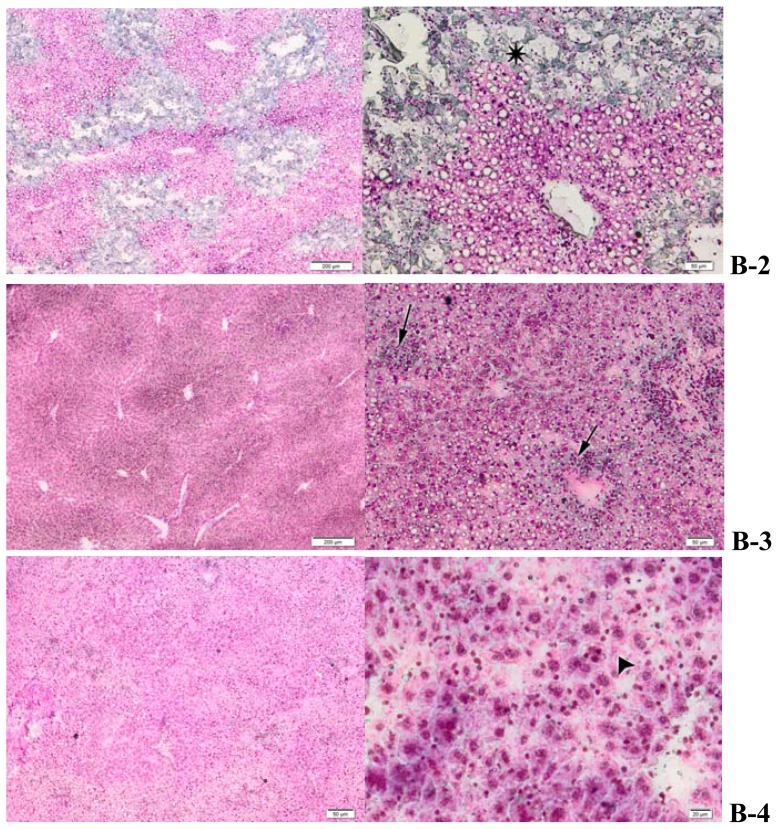
Light microscopic evaluation of liver tissues showed normal liver architecture (Figure 7A-1) without steatosis (Figure 7A-3) and fibrosis aspects (Figure 7A-2) in control group. Normal glycogen deposits are present into hepatocytes cytoplasm (Figure 7A-4).
Figure 7.
Protective effect of a 50 mg/kg dose of Berberis vulgaris extract and Berberis vulgaris extract/βcyclodextrin on structure of hepatocytes against liver injuries induced by CCl4. (A) Control group; (B) CCl4 group; (C) Berberis vulgaris extract + CCl4 group; (D) Berberis vulgaris extract/β cyclodextrin + CCl4 group. 1. H & E staining (nuclei-blue; cytoplasm-red), 2. Fouchet Van Gieson staining (nuclei-red; cytoplasm-yellow; collagen- red), 3. Oil Red staining (nuclei-blue; lipid drops–red), 4. P.A.S Periodic Acid Schiff staining (glycogen deposits—pink violet). N: centrilobular necrosis; C: collagen proliferation; S: steatosis; G: glycogen deposits depletion. Scale: 50 μm.
After 24 h of CCl4 exposure, necrobiotic changes of hepatocytes including vacuolar degeneration, nuclear pyknosis and necrosis (Figure 7B-1) were observed, which were in agreement with previous reports [36,43]. The necrosis was more pronounced in the centrilobular area with the formation of bridges from one central vein to another (Figure 7B-1), followed by infiltration of inflammatory cells, sinusoid congestion, macro and microvesicular steatosis of hepatocytes (Figure 7B-3). Inflammatory cell infiltration and slight fibrosis around central vein and sinusoids were detected (Figure 7B-2). Glycogen deposits were severely diminished compared to the control group (Figure 7B-4), probably due to the high-energy demand of the hepatocytes for the possible repair processes in CCl4-given animals [44].
The seven-day preventive treatment with 50 mg/kg of formulated and non-formulated Berberis vulgaris extract, showed mild to moderate accumulation of lipid drops and reduction of inflammatory infiltrates compared to the CCl4 group (Figure 7C-3, D-3). In both Berberis pre-treated groups, an absence of hepatocellular necrosis areas (Figure 7C-1, D-1) was observed. Vacuolated hepatocytes were seen in some centrilobular areas but to a lesser extent compared to CCl4 group (Figure 7C-1, D-1). Similarly, Feng et al. [28] reported that fatty content change, necrosis and lymphocyte infiltration were improved in the histological sections from 80, 12 and 160 mg/kg berberine pre-treated and post-treated mice in a dose-dependent manner.
Fibrosis around central vein and sinusoids was reduced in both pre-treated groups (Figure 7C-2, D-2). Similar results were observed with berberine that prevented liver fibrosis due to the decreased number of hepatic stellate cells (HSCs) [45] or by regulating the lipid peroxidation and antioxidant system [46].
Also, glycogen deposits were highly restored in the Berberis vugaris extract/β-cyclodextrin group (Figure 7D-4) than in non-formulated Berberis vulgaris extract pre-treatment (Figure 7C-4). Our results are in accordance with previous data which have shown that hepatic glycogen levels increased after 75 mg/kg of berberine were administered to rats [47].
2.5. Electron Microscopy
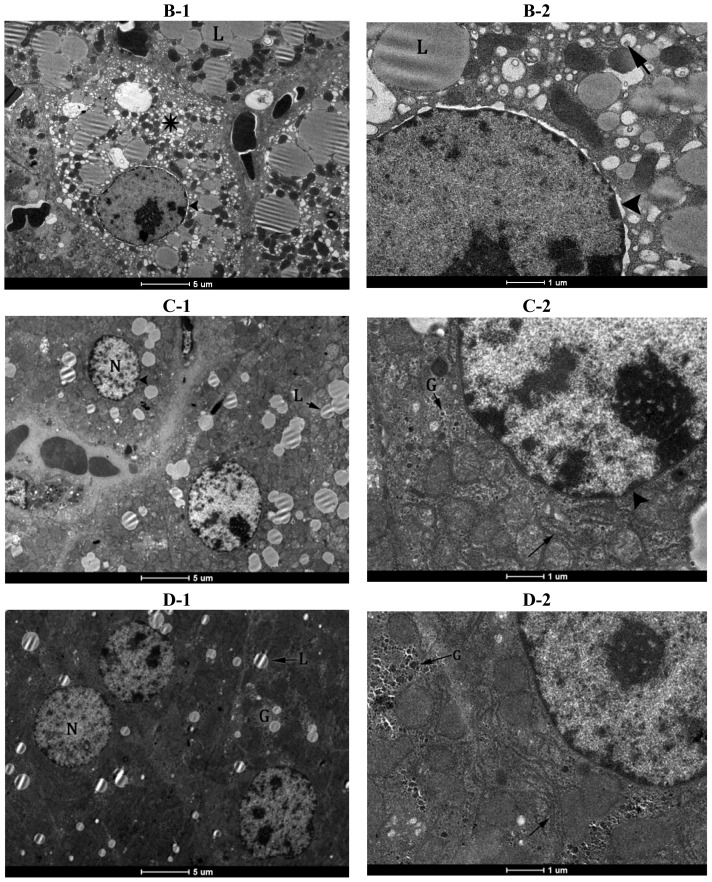
The ultrastructure of hepatocytes was normal in the control group with regular aspect of nuclear shape and rER’s profiles (Figure 8A-2), few lipid globules, and normal glycogen deposits into cytoplasm (Figure 8A-1). In the CCl4 treated group most of the hepatocytes showed large lipid globules and glycogen loss, according to optic microscopy analysis. Some hepatocytes showed an oedematous cytoplasm matrix with two populations of lipid globules -large and small, degenerated organelles and proliferation of smooth reticulum vesicles (Figure 8B-1). There were irregular lamellar organization and large dilatations with focal breaks in rERs of hepatocytes of CCl4 treated group in many areas, associated with dilated perinuclear space and enlarged pores (Figure 8B-2). The changes of hepatocytes ultrastructure [48] were probably due to the injuries in membrane structure caused by lipid peroxidation.
Figure 8.
Protective effect of a 50 mg/kg dose of Berberis vulgaris extract and Berberis vulgaris extract/βcyclodextrin on ultrastructure of hepatocytes against liver injuries induced by CCl4. (A) Control group; normal aspect of nucleus (N), glycogen deposits (G) and few lipid drops (L); (B) CCl4 group; oedematous cytoplasm matrix with sER proliferation (asterix); dilated rER profiles (arrow) and enlarged nuclear space (arrowhead); increased number and size of lipid drops (L); (C) Berberis extract + CCl4 group; normal aspect of nuclear shape (arrowhead) and rER (arrow);Mild reduction of number and size of lipid drops (L) and moderate recovery of glycogen deposits (G); (D) Berberis vulgaris extract/βcyclodextrin + CCl4 group; Glycogen deposits recovery (G) and normal aspect of rER (arrow).
Pre-treatment with 50 mg/kg of formulated and non-formulated Berberis vulgaris extract significantly reduced the volume and number of lipid globules in the hepatocytes (Figure 8C-1, D-1), more obvious than in non-formulated Berberis vulgaris extract (Figure 8C-1). The organelle and cytoplasm structures were widely protected against the effects of CCl4. Large dilatations with focal breaks in rERs of hepatocytes, associated with dilated perinuclear space and enlarged pores were not seen in either pre-treated group (Figure 8C-2, D-2). Although glycogen deposits are increased both in formulated and non-formulated Berberis vulgaris groups compared to CCl4 (Figure 8C-2, D-2).
2.6. Effects of Berberis vulgaris Extract/β-Cyclodextrin Pre-Treatment on DNA Damage
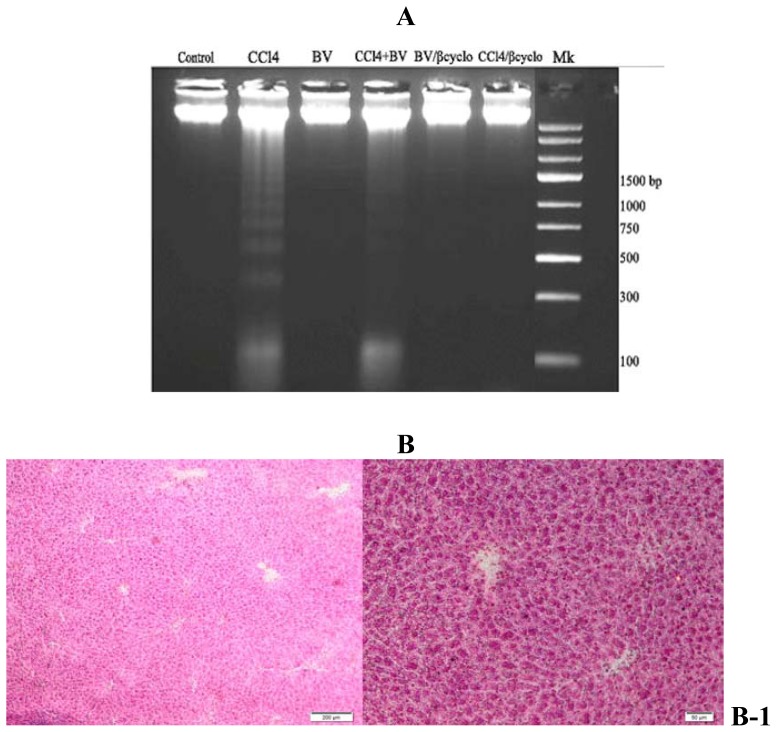
DNA internucleosomal fragmentation was seen in liver samples of CCl4 treated group and a reduced level of it appeared in the mice group pre-treated with non-formulated Berberis vulgaris extract. At the same time, the pre-treatment with Berberis vulgaris extract/β-cyclodextrin completely suppressed this process (Figure 9A). Also, no DNA internucleosomal fragmentation was induced by either formulated and non-formulated Berberis vulgaris extracts in the absence of CCl4 treatment.
Figure 9.
Effects of Berberis vulgaris extract/β-cyclodextrin on CCl4-induced DNA damage. (A) Lane 1-DNA isolated from normal liver; Lane 2: DNA isolated from CCl4 intoxicated liver; Lane 3: DNA isolated from liver treated with Berberis vulgaris; Lane 4: DNA isolated from liver pre-treated with Berberis vulgaris extract followed by i.p. CCl4 injection; Lane 5: DNA isolated from liver treated with Berberis vulgaris extract/β cyclodextrin; Lane 6: DNA isolated from liver pre-treated with Berberis vulgaris extract/β cyclodextrin followed by i.p. CCl4 injection; Lane 7: Marker (1-kb DNA ladder); (B) Methyl-green pyronin staining of liver. B-1: Control group; B-2: CCl4 group; extended ssDNA areas (asterix); B-3: Berberis vulgaris extract + CCl4; semnificative reduction of ssDNA areas (arrow); B-4: Berberis vulgaris extract/β cyclodextrin + CCl4; ssDNA areas loss and normal RNA distribution (arrowhead) (DNA—pale green; RNA—pink).
The methyl-green pyronin (MGP) histochemical investigation of liver tissue was in agreement with DNA fragmentation assay. Large pale green areas of damaged DNA were seen in the CCl4 group especially in the centrilobular area with the formation of bridges from one central vein to another (Figure 9B-2) which overlap with areas of the liver affected by necrobiosis observed in H & E micrographs. These areas are more reduced in the group pre-treated with non-formulated Berberis vulgaris extract (Figure 9B-3) and are absent in the group pre-treated with Berberis vulgaris extract/β-cyclodextrin (Figure 9B-4), as in the case of normal mice, where intense pink areas of RNA are also present (Figure 9B-1).
DNA strand breaks are a cause of direct modification of DNA by chemical agents or their metabolites, which occurs when reactive oxygen species (ROS) interact with DNA [49,50]. In our study, the type of cell death induced by CCl4 was investigated by DNA fragmentation assay and methyl-green pyronin (MGP) histochemical staining, which highlights the presence of RNA and ssDNA into liver tissue [51–53]. Internucleosomal DNA fragmentation was observed in liver samples of 1.0 mL/kg CCl4 intoxicated group, which is the indication that cells were undergoing apoptotic or necrotic death as in previous studies [54–56]. The absence of DNA ladder and of MGP staining in the liver of mice pretreated with Berberis vulgaris extract/β-cyclodextrin and intoxicated with CCl4 indicated the hepatoprotective role of the formulated extract. The non-formulated extract counteracted the damaging effect of CCl4 but to a lesser extent, possibly due to the increased bioavilability of β cyclodextrin formulation as already it was proved in the case of liver injuries [57–59].
3. Experimental Section
3.1. Berberis vulgaris Extract/β-Cyclodextrin Formulation
Samples of Berberis vulgaris L. were collected from the Botanical Garden of Vasile Goldis Western University of Arad during October 2008 and certified at the herbarium in the Department of Botany, Faculty of Natural Sciences, where a voucher specimen already exist. The bark of Berberis vulgaris L. was separated and frozen until extraction. Bioactive compounds from Berberis vulgaris samples were separated by solid-liquid extraction in 96% ethanol, according to the method described by Hadaruga et al [32]. The final extract was subjected to HPLC analysis [32] followed by β-cyclodextrin nanoencapsulation [32,60]. The evaluation of Berberis vulgaris/β-cylodextrin complex formation was proved by differential scanning calorimetry (DSC) and thermogravimetry (TG) according to the previous study [32]. The dried form of the formulated and non-formulated extract, dissolved in distilled water was administered orally (50 mg/kg b.w.) to mice so as to check hepatoprotective activities.
3.2. Animals and Experimental Procedure
Adult male Swiss mice weighing 25 ± 3 g were obtained from Animal House of the Vasile Goldis Western University of Arad. The animals were left for 2 days for acclimatization to animal room conditions maintained on standard pellet diet and water ad libitum at a temperature of 20–25 °C under a 12 h light/dark cycle throughout the experiment. The food was withdrawn on the day before the sacrifice. All animals received human care and study protocols complied with the guidelines of the Animal House. Throughout the experiments, animals were processed according to the international ethical guidelines for the care of laboratory animals.
Forty-eight animals were divided into 6 groups, as follows: group 1 (control group), group 2 (CCl4 treated group), group 3 (pre-treated with 50 mg/kg b.w. of Berberis vulgaris extract and subsequently intraperitoneally injected with CCl4), group 4 (pre-treated with 50 mg/kg b.w. of Berberis vulgaris extract/β-cyclodextrin and subsequently i.p. injected with CCl4), group 5 (treated with 50 mg/kg b.w. of Berberis vulgaris extract), group 6 (treated with 50 mg/kg b.w. of Berberis vulgaris extract/β-cyclodextrin). Non-formulated Berberis vulgaris extract and Berberis vulgaris extract/β-cyclodextrin complex were orally administrated to mice from groups 3 and 5 and to groups 4 and 6 for 7 days. Mice from groups 2, 3, and 4 were intraperitoneally (i.p.) injected with CCl4 at a dose of 1.0 mL/kg body weight in 50% olive oil (1:1) on the 8th day. Control and CCl4 treated groups received the equivalent volume of distilled water orally for 7 days.
24 h after CCl4 i.p. injection the mice were sacrificed by cervical dislocation. Serum and tissue samples were used for histopathology, electron microscopy and biochemical analyses.
3.3. Animals and Experimental Procedure
Homogenates (prepared as 1 g of tissue per 10 volumes of buffer) of mice livers were prepared in ice-cold buffer (0.1 M TRIS-HCl, 5 mM EDTA buffer, pH 7.4) and homogenized for 2 min at 16 Hz using a ball mill (type MM 301, Retsch GmbH & Co, Haan, Germany). The homogenate was centrifuged at 10,000 rpm for 30 min in a Hettich centrifuge at 4 °C to remove the cell debris A few crystals of the protease inhibitor phenylmethylsulfonyl fluoride were added in the homogenates. The supernatant was decanted and used for biochemical assays.
Blood was collected from retro-orbital venous plexus. The tubes with whole blood were incubated for 60 min at room temperature to allow clotting. They were then centrifuged for 15 min at 2000 rpm. The supernatant (serum) was aliquoted and used immediately for biochemical analyses.
3.4. Histopathology
Frozen liver sections were cut at 8 μm at SLEE MNT cryotome, fixed in 10% buffered formaldehide and stained with hematoxylin-eosin, oil red, P.A.S. periodic acid Schiff, Fouchet van Gieson and methyl-green pyronin, according to the methods of Bio-Optica staining kits. Mounted slides were examined under a light microscope (Olympus BX43 microscope) and photographed using a digital camera Olympus XC30.
3.5. Electron Microscopy
For ultrastructural investigations, liver samples were prefixed in 2.7% glutaraldehyde solution in 0.1 M phosphate buffer for 1.5 h, at 4 °C. Then they were washed in 0.15 M phosphate buffer (pH 7.2). Postfixation was performed in 2% osmic acid solution in 0.15 M phosphate buffer for 1 h at 4 °C. Dehydration was performed in acetone, and inclusion in the epoxy embedding resin Epon 812. The blocks have been cut at an ultramicrotome type LKB, at 70 nm thickness. The sections were doubly contrasted with solutions of uranyl acetate and lead citrate and were analyzed with TEM Tecnai 12 Biotwin electron microscope.
3.6. Biochemical Assays
3.6.1. Activities of Serum Hepatic Markers
The serum activities of aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transferase (γ-GT), direct and total bilirubin were evaluated by the spectrophotometric method using commercially available kits (Roche reagents, Meylan, France) according to the manufacturer’s instructions.
3.6.2. Assesment of Antioxidant Status
Hepatic catalase (CAT) activity was determined by the Aebi method [61] which records the decomposition of H2O2 by decrease in the absorbance at 240 nm. One unit of CAT activity is equal to the decomposition of one μmole H2O2/min/mL. Liver superoxide dismutase (SOD) activity was measured by the method described by Beauchamp and Fridovich [62]. One unit of SOD activity is defined as the amount of enzyme that inhibits the oxidation of NADH by 50% at 37 °C. The measurement of GPx activity was performed according to the method of Beutler et al. [63] which is based on monitoring of the oxidation of NADPH at 340 nm (V-530 JASCO spectrophotometer). All enzymatic activities, calculated as specific activities (units/mg of protein) were expressed as % from controls.
GSH content was measured with a commercial kit (Sigma-Aldrich, Taufkirchen, Germany) according to the manufacturer’s instructions. The absorbance was measured at 405 nm using a microtiter plate reader (GENIOS Tecan) and the concentration was expressed in nmoles GSH/mg protein.
3.6.3. Assay of Lipid Peroxidation
The hepatic malondialdehyde (MDA) content, a measure of lipid peroxidation, was assayed using a fluorimetric method described by Del Rio et al. [64]. Briefly, the sample (200 μL) was incubated with 0.1 M HCl (700 μL) for 20 min at room temperature. A volume of 900 μL of 0.025 M thiobarbituric acid was added, and the mixture was maintained at 37 °C for 65 min. Then the samples were subjected to fluorescence analysis (λex = 520 nm; λem = 549 nm) (Spectrofluorometer FP-6300 JASCO). Relative fluorescence units (RFU) were converted to nmoles malondialdehyde (MDA) using 1,1,3,3-tetramethoxypropane as standard.
3.6.4. Protein Concentration Measurement
The protein content was determined using Lowry’s method with bovine serum albumine as standard [65].
3.7. DNA Fragmentation Assays
Following the method of Zhou et al. [66], 0.15 g of frozen liver was homogenized in 1.5 mL lysis buffer [10 mM Tris-HCl pH 7.5; 100 mM EDTA; 0.5% SDS; 0.1 mg/L proteinase K]. RNA-free DNA from each sample was extracted twice with phenol, once with phenol-chloroform-isoamyl alcohol (PCI 25:24:1) and once with chloroform. After centrifugation, DNA was precipitated with cold ethanol and sodium acetate. The pellet was dissolved in TE buffer (10 mM Tris-HCl; 1.0 mM EDTA) and incubated with RNase (0.1 mg/L) at 37 °C for 45 min. Subsequently the suspension was re-extracted with PCI and chloroform and precipitated as described above. A quantity of 3 μg of DNA was loaded on 1.5% agarose gel and run at 100 V. The gel was stained with ethidium bromide and photographed under UV light using UVP BioDoc-IT Imaging System.
3.8. Statistical Analysis
All results were expresed as mean ± SEM. The data were analyzed for statistical significance using Student’s t test. A value of p < 0.05 was considered significant: *; distinct significant at p < 0.01: **; very significant at p < 0.001: ***.
4. Conclusions
In conclusion, this study showed that Berberis vulgaris extract/β-cyclodextrin presented better hepatoprotective effects than free extract on oral administration probably due to its increased bioavailability. It seems that formulated extract could be used as a low cost phytotherapeutical supplement suitable for acute or chronic conditions of liver diseases or as a supportive treatment in addition to conventional therapies of serious hepatic diseases. Taken together, our results suggest that Berberis vulgaris extract/β-cyclodextrin should be further investigated regarding clinical applications.
Acknowledgment
This work was supported by Ministry of Education and Research from Romania, PNII 62072/2008.
References
- 1.Jones A.L. Anatomy of the Normal Liver. In: Zakin D., Boyer T.D., editors. Hepatology: A Textbook of Liver Disease. 3rd ed. WB Saunders; Philadelphia, PA, USA: 1996. pp. 3–32. [Google Scholar]
- 2.Zimmerman H.J. Hepatotoxicity: The Adverse Effects of Drugs and other Chemicals on the Liver. Appleton-Century Crofts Publisher; New York, NY, USA: 1978. [Google Scholar]
- 3.Kaminski M., Wiaderkiewicz R. The role of the liver in xenobiotic biotransformation. Part I. The role of the liver and its cells and their interactions. Probl. Forensic Sci. 2007;72:357–378. [Google Scholar]
- 4.Weber L.W., Boll M., Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: Carbon tetrachloride as a toxicological model. Crit. Rev. Toxicol. 2003;33:105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 5.Merrick B.A. Toxicoproteomics in liver injury and inflammation. Ann. N. Y. Acad. Sci. 2006;1076:707–717. doi: 10.1196/annals.1371.017. [DOI] [PubMed] [Google Scholar]
- 6.Manibusan M.K., Odin M., Eastmond D.A. Postulated carbon tetrachloride mode of action: A review. J. Environ. Sci. Health C. 2007;25:185–209. doi: 10.1080/10590500701569398. [DOI] [PubMed] [Google Scholar]
- 7.Luper S. A review of plants used in the treatment of liver disease: Part 1. Altern. Med. Rev. 1998;3:410–421. [PubMed] [Google Scholar]
- 8.Luper S. A review of plants used in the treatment of liver disease: Part 2. Altern. Med. Rev. 1999;4:178–188. [PubMed] [Google Scholar]
- 9.Murriel P., Rivera-Espinoza Y. Beneficial drugs for liver diseases. J. Appl. Toxicol. 2008;28:93–103. doi: 10.1002/jat.1310. [DOI] [PubMed] [Google Scholar]
- 10.Ivanovska N., Philipov S. Study on the anti-inflamatory action of Berberis vulgaris root extract, alkaloid fractions and pure alkaloids. Int. J. Immunopharmacol. 1996;18:553–561. doi: 10.1016/s0192-0561(96)00047-1. [DOI] [PubMed] [Google Scholar]
- 11.Saied S., Begum S. Phytochemical studies of Berberis vulgaris. Chem. Nat. Compd. 2004;40:137–140. [Google Scholar]
- 12.Imanshahidi M., Hosseinzadeh H. Pharmacological and therapeutical effects of Berberis vulgaris and its active constituent, berberine. Phytother. Res. 2008;22:999–1012. doi: 10.1002/ptr.2399. [DOI] [PubMed] [Google Scholar]
- 13.Freile M.L., Giannini F., Pucci G. Antimicrobial activity of aqueolus extracts and of berberine isolates from Berberis heterophylla. Fitoterapia. 2003;74:702–705. doi: 10.1016/s0367-326x(03)00156-4. [DOI] [PubMed] [Google Scholar]
- 14.Mahady G.B., Pendland S.L., Stoia A., Chaadwick L.R. In vitro susceptibility of Helicobacter pylori to isoquinoline alkaloids from Sanguinaria canadensis and Hydrastis canadensis. Phytother. Res. 2003;17:217–221. doi: 10.1002/ptr.1108. [DOI] [PubMed] [Google Scholar]
- 15.Iizuka N., Miyamoto K., Okita K., Tangoku A., Hayashi H., Yosino S. Inhibitory effect of Coptidis rhizoma and berberine on the proliferation of human esophageal cancer cell lines. Cancer Lett. 2000;148:19–25. doi: 10.1016/s0304-3835(99)00264-5. [DOI] [PubMed] [Google Scholar]
- 16.Thirupurasundari C.J., Padmini R., Devaraj S.N. Effect of berberine on the antioxidant status, ultrastructural modifications and protein bound carbohydrates in azoxymethane-induced colon cancer in rats. Chem. Biol. Interact. 2009;177:190–195. doi: 10.1016/j.cbi.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 17.Wang N., Feng Y., Zhu M., Tsang C.M., Man K., Tong Y., Tsao S.W. Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: The cellular mechanism. J. Cell. Biochem. 2010;111:1426–1436. doi: 10.1002/jcb.22869. [DOI] [PubMed] [Google Scholar]
- 18.Kuo C.L., Chi C.W., Liu T.Y. The anti-inflamatory potential of berberine in vitro and in vivo. Cancer Lett. 2004;203:127–137. doi: 10.1016/j.canlet.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Kupeli E., Kosar M., Yesilada E., Husnu K., Baser C. A comparative study on the anti-inflamatory, antinociceptive and antipyretic effects of isoquinoline alkaloids from the root of Turkish Berberis species. Life Sci. 2002;72:645–657. doi: 10.1016/s0024-3205(02)02200-2. [DOI] [PubMed] [Google Scholar]
- 20.Singh A., Duggal S., Kaur N., Singh J. Berberine: Alkaloid with wide spectrum of pharmacological activities. J. Nat. Prod. 2010;3:64–75. [Google Scholar]
- 21.Hobara N., Watanabe A. Berberine-Induced bile bilirubin secretion in the rat. Curr. Ther. Res. Clin. Exp. 1984;35:663–667. [Google Scholar]
- 22.Rabbani G.H., Butler T., Knight J., Sanyal S.C., Alam K. Randomized controlled trial of berberine sulfate therapy for diarrhea due to enterotoxigenic Escherichia coli and Vibrio cholera. J. Infect. Dis. 1978;155:979–984. doi: 10.1093/infdis/155.5.979. [DOI] [PubMed] [Google Scholar]
- 23.Zhou H., Mineshita S. The effect of berberine chloride on experimental cholitis in rats in vivo and in vitro. J. Pharmachol. Exp. Ther. 2000;294:822–829. [PubMed] [Google Scholar]
- 24.Fatehi M., Saleh T.M., Fatehi-Hassanabad Z., Farrokhfal K., Jafarzadeh M., Davodi S. A pharmacological study on Berberis vulgaris fruit extract. J. Ethnopharmacol. 2005;102:46–52. doi: 10.1016/j.jep.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 25.Wong K.K. Mechanism of the aortic relaxation induced by low concentrations of berberine. Planta Med. 1998;64:756–757. doi: 10.1055/s-2006-957575. [DOI] [PubMed] [Google Scholar]
- 26.Zeng X.H., Zeng X.J., Li Y.Y. Efficacy and safety of berberine for congestive heart failure secondary to ischemic or idiopathic dilated cardiomyophathy. Am. J. Cardiol. 2003;92:173–176. doi: 10.1016/s0002-9149(03)00533-2. [DOI] [PubMed] [Google Scholar]
- 27.Lee B., Yang C.H., Hahm D.H., Choe E.S., Lee H.J., Pyun K.H., Shim I. Inhibitory Effects of Coptidis rhizoma and Berberine on Cocaine-induced Sensitization. Evid. Complement. Altern. Med. 2009;6:85–90. doi: 10.1093/ecam/nem070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feng Y., Siu K.Y., Ye X., Wang N., Yuen M.F., Leung C.H., Tong Y., Kobayashi S. Hepatoprotective effects of berberine on carbon tetrachloride-induced acute hepatotoxicity in rats. Chin. Med. 2010;5:1–6. doi: 10.1186/1749-8546-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janbaz K.H., Gilan A.H. Studies on preventive and curative effects of berberine on chemical-induced hepatotoxicity in rodents. Fitoterapia. 2000;71:25–33. doi: 10.1016/s0367-326x(99)00098-2. [DOI] [PubMed] [Google Scholar]
- 30.Hwang J.M., Wang C.J., Chou F.P. Inhibitory effect of berberine on tert-butyl hydroperoxide-induced oxidative damage in rat liver. Arch. Toxicol. 2002;76:664–670. doi: 10.1007/s00204-002-0351-9. [DOI] [PubMed] [Google Scholar]
- 31.Fallah H., Zarrei M., Ziai M., Mehrazma M., Alavian S.M., Kianbakht S., Mehdizadeh M. The effects of Taraxacum officinale L. and Berberis vulgaris L. root extracts on carbon tetrachloride induced liver toxicity in rats. J. Med. Plants. 2010;9:45–52. [Google Scholar]
- 32.Hadaruga D.I., Hadaruga N.G., Bandur G.N., Rivis A., Costescu C., Ordodi V.L., Ardelean A. Berberis vulgaris extract/β cyclodextrin nanoparticles synthesis and characterization. Rev. Chim. (Bucharest) 2010;61:669–675. [Google Scholar]
- 33.Chaung S.S., Lin C.C., Lin J., Yu K.H., Hsu Y.F., Yen M.H. The hepatoprotective effects of Limonium sinense against carbon tetrachloride and beta-D-galactosamine intoxication in rats. Phytother. Res. 2003;17:784–791. doi: 10.1002/ptr.1236. [DOI] [PubMed] [Google Scholar]
- 34.Clawson G.A. Mechanism of carbon tetrachloride hepatotoxicity. Pathol. Immunopathol. Res. 1989;8:104–112. doi: 10.1159/000157141. [DOI] [PubMed] [Google Scholar]
- 35.Bhadauria M. Propolis prevents hepatorenal injury induced by chronic exposure to carbon tetrachloride. Evid. Complement. Altern. Med. 2012 doi: 10.1155/2012/235358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaker E. Sylimarin, the antioxidant component and Silybum marianum extracts prevent liver damage. Food Chem. Toxicol. 2010;48:803–806. doi: 10.1016/j.fct.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Xu L., Gao J., Wang Y., Yu W., Zhao X., Yang X., Zhong Z., Qian Z.M. Myrica rubra extracts protect the liver from CCl4-induced damage. Evid. Complement. Altern. Med. 2011 doi: 10.1093/ecam/nep196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beddowes E.J., Faux S.P., Chipman J.K. Chloroform, carbon tetrachloride and glutathione depletion induce secondary genotoxicity in liver cells via oxidative stress. Toxicology. 2003;187:101–115. doi: 10.1016/s0300-483x(03)00058-1. [DOI] [PubMed] [Google Scholar]
- 39.Kohen R., Nyska A. Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2010;30:620–650. doi: 10.1080/01926230290166724. [DOI] [PubMed] [Google Scholar]
- 40.Motalleb G., Hanachi P., Kua S.H., Fauziah O., Asmah R. Evaluation of phenolic content and total antioxidant activity in Berberis vulgaris fruit extract. J. Biol. Sci. 2005;5:648–653. [Google Scholar]
- 41.Parichehr H. Using HPLC to determination the composition and antioxidant activity of Berberis vulgaris. Eur. J. Sci. Res. 2009;29:47–54. [Google Scholar]
- 42.Zovko M.K., Kremer D., Karlović K., Kosalec I. Evaluation of antioxidant activities and phenolic content of Berberis vulgaris L. and Berberis croatica Horvat. Food Chem. Toxicol. 2010;48:2176–2180. doi: 10.1016/j.fct.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 43.Ozturk F., Gul G., Ates B., Ozturk I.C., Cetin A., Vardi N., Otlu A., Yilmaz I. Protective effect of apricot (Prunus armeniaca L.) on hepatic steatosis and damage induced by carbon tetrachloride in Wistar rats. Br. J. Nutr. 2009;102:1767–1775. doi: 10.1017/S0007114509991322. [DOI] [PubMed] [Google Scholar]
- 44.Junnila M., Rahko T., Sukura A. Reduction of carbon tetrachloride-induced hepatotoxic effects by oral administration of betaine in male Han-Wistar rats: A morphometric histological study. Vet. Pathol. 2000;37:231–238. doi: 10.1354/vp.37-3-231. [DOI] [PubMed] [Google Scholar]
- 45.Sun X., Zhang X., Hu H., Lu Y., Chen J., Yasuda K., Wang H. Berberine inhibits hepatic stellate cell proliferation and prevents experimental liver fibrosis. Biol. Pharm. Bull. 2009;32:1533–1537. doi: 10.1248/bpb.32.1533. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Q., Xiao X., Feng K., Wang T., Li W., Yuan T., Sun X., Sun Q., Xiang H., Wang H. Berberine moderates glucose and lipid metabolism through multipathway mechanism. Evid. Complement. Altern. Med. 2011 doi: 10.1155/2011/924851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou J.Y., Zhou S.W., Zhang K.B., Tang J.L., Guang L.X., Ying Y., Xu Y., Zhang L., Li D.D. Chronic effects of berberine on blood, liver glucolipid metabolism and liver PPARs expression in diabetic hyperlipidemic rats. Biol. Pharm. Bull. 2008;31:1169–1176. doi: 10.1248/bpb.31.1169. [DOI] [PubMed] [Google Scholar]
- 48.Tasci I., Mas N., Mas M.R., Tuncer M., Comert B. Ultrastructural changes in hepatocytes after taurine treatment in CCl4 induced liver injury. World Gastroenterol. 2008;14:4897–4902. doi: 10.3748/wjg.14.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Folkmann J.K., Risom L., Jacobsen N.R., Loft H.W.S., Moller P. Oxidatively damaged DNA in rats exposed by oral gavage to C60 fullerenes and single-walled carbon nanotubes. Environ. Health Perspect. 2009;117:703–708. doi: 10.1289/ehp.11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moller P., Wallin H. Adduct formation, mutagenesis and nucleotide excision repair of DNA damage produced by reactive oxygen species and lipid peroxidation product. Mutat. Res. 1998;410:271–290. doi: 10.1016/s1383-5742(97)00041-0. [DOI] [PubMed] [Google Scholar]
- 51.Iseki S., Mori T. Methyl green pyronin stain distinguishes prolifferating from differentiated nonproliferating cell nuclei after acid denaturation of DNA. J. Histochem. 1986;34:683–687. doi: 10.1177/34.5.3701031. [DOI] [PubMed] [Google Scholar]
- 52.Sen J.Y., Huang Q.Y., Gao H.Y., Liu Y.L., Cheng C.F. Modification and application of methyl green-pyronin stain after acid denaturation of DNA. Prog. Anat. Sci. 1999;5:272–273. [Google Scholar]
- 53.Wang H.M., Zheng N.G., Wu J.L., Gong C.C., Wang Y.L. Dual effects of 8-Br-cAMP on differentiation and apoptosis of human esophageal cancer. World J. Gastroenterol. 2005;7:6538–6542. doi: 10.3748/wjg.v11.i41.6538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Manna P., Bhattacharyya S., Das J., Ghosh J., Parames C., Sil C. Phytomedicinal role of Pithecellobium dulce against CCl4-mediated hepatic oxidative impairments and necrotic cell death. Evid. Complement. Altern. Med. 2011 doi: 10.1093/ecam/neq065. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Sakr S.A., El-Abd S.F., Osman M., Kandil A.M., Helmy M.S. Ameliorative effect of aqueous leave extract of Ocimum basilicum on CCl4-induced hepatotoxicity and apoptosis in albino rats. J. Am. Sci. 2011;7:116–127. [Google Scholar]
- 56.Sengupta M., Sharma G.D., Chakraborty B. Effect of aqueous extract of Tinospora cordifolia on functions of peritoneal macrophages isolated from CCl4 intoxicated male albino mice. BMC Complement. Altern. Med. 2011;11:1–9. doi: 10.1186/1472-6882-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Linn T.T., Wang B.M., Li X.Y., Pan Y.W., Liu J.Q., Shen J.C., Luo G.M. An insight into the protection of rat liver against ischemia/reperfusion injury by 2-selenium-bridged beta-cyclodextrin. Hepatol. Res. 2009;39:1125–1136. doi: 10.1111/j.1872-034X.2009.00545.x. [DOI] [PubMed] [Google Scholar]
- 58.Liu Y., Sakagami H., Hasimoto K., Kikuchi H., Amano O., Ishara M., Kanda Y., Kunii S., Kochi M., Zhang W., Yu G. Tumor-specific cytotoxicity and type of cell death induced by beta-cyclodextrin benzaldehyde inclusion compound. Anticancer Res. 2008;28:229–236. [PubMed] [Google Scholar]
- 59.Yadav V.R., Prasad S., Kannappan R., Ravindran J., Chatuvedi M.M., Vaahtera L., Parkkinen J., Aggarwal B.B. Cyclodextrin-complexed cucurmin exhibits anti-inflamatory and antiproliferative activities superior to those of cucurmin through higher cellular uptake. Biochem. Pharmacol. 2010;80:1021–1032. doi: 10.1016/j.bcp.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Hadaruga D.I., Hadaruga N.G., Hermenean A., Rivis A., Paslaru V., Codina G. Biomaterials: Thermal stability of the oleic acid/α and β cyclodextrin complexes. Rev. Chim. (Bucharest) 2008;59:994–998. [Google Scholar]
- 61.Aebi H. In: Catalase in Methods of Enzymatic Analysis. 2nd ed. Bergmayer H.U., editor. Academic Press; New York, NY, USA: 1974. pp. 673–677. [Google Scholar]
- 62.Beauchamp C.O., Fridovich I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971;44:276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 63.Beutler E. Red Cell Metabolism: A Manual of Biochemical Methods. 3rd ed. Grune & Stratton Inc; New York, NY, USA: 1974. [Google Scholar]
- 64.Del Rio D., Pellegrini N., Colombi B., Bianchi M., Serafini M., Torta F., Tegoni F., Musci M., Brighenti F. Rapid fluorimetric method to detect total plasma malondialdehyde with mild derivatization conditions. Clin. Chem. 2003;49:690–692. doi: 10.1373/49.4.690. [DOI] [PubMed] [Google Scholar]
- 65.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 66.Zhou B.R., Gumenscheimer M., Freudenberg M., Galanos C. A sriking correaltion between lethal activity and apoptotic DNA fragmentation of liver in response of D-galactosamine-sintetized mice to a non-lethal amount of lipopolysaccharide. Acta Pharmacol. Sin. 2003;24:193–198. [PubMed] [Google Scholar]