Abstract
When analyzing the secretome of the plant pathogen Pseudomonas syringae pv. tomato DC3000, we identified hemolysin-coregulated protein (Hcp) as one of the secreted proteins. Hcp is assumed to be an extracellular component of the type VI secretion system (T6SS). Two copies of hcp genes are present in the P. syringae pv. tomato DC3000 genome, hcp1 (PSPTO_2539) and hcp2 (PSPTO_5435). We studied the expression patterns of the hcp genes and tested the fitness of hcp knockout mutants in host plant colonization and in intermicrobial competition. We found that the hcp2 gene is expressed most actively at the stationary growth phase and that the Hcp2 protein is secreted via the T6SS and appears in the culture medium as covalently linked dimers. Expression of hcp2 is not induced in planta and does not contribute to virulence in or colonization of tomato or Arabidopsis plants. Instead, hcp2 is required for survival in competition with enterobacteria and yeasts, and its function is associated with the suppression of the growth of these competitors. This is the first report on bacterial T6SS-associated genes functioning in competition with yeast. Our results suggest that the T6SS of P. syringae may play an important role in bacterial fitness, allowing this plant pathogen to survive under conditions where it has to compete with other microorganisms for resources.
INTRODUCTION
Gram-negative bacteria possess a number of secretion systems to transport proteins through the inner and outer membranes to the surrounding milieu to carry out a wide range of functions, such as biogenesis of organelles (i.e., flagella), nutrient acquisition, pathogenesis, and efflux of toxins or drugs (36, 51). The type VI secretion system (T6SS) was only recently discovered and found to be conserved in many Gram-negative bacterial genera (6, 31, 38). The T6SS secretory machinery includes an ATPase (ClpB/ClpV), a serine/threonine protein kinase (PpkA), a phosphatase (PppA), a regulatory forkhead-associated domain protein (Fha), tubulus-forming structural proteins VipA and VipB, and IcmF/IcmH (DotU)-like proteins that are homologous to the T4SS membrane components (3, 4, 8, 44). The proteins secreted by this route are still largely uncharacterized. The T6SS has been demonstrated to have a role in the ecological success or virulence of a bacterial pathogen during its interaction with animal host cells (21, 38, 46). It has also been shown to contribute to virulence or fitness in plant hosts (27, 54). The T6SS is also important for the survival of attacks by predatory amoeba (29, 38). In some cases, T6SS was found to function as the secretion route for toxins that inhibit the growth of other bacteria (16, 26, 32, 42). The common T6SS-secreted proteins encoded by all T6SS-harboring bacteria are Hcp (hemolysin coregulated) and VgrG (valine-glycine rich) protein variants. Hcp and VgrG proteins are assumed to be extracellular components of the secretion machinery (37). The C-terminal part of some VgrG proteins has been associated with virulence-related functions such as actin cross-linking and ADP-ribosylation in eukaryotic host cells (37, 50). The N-terminal part of VgrG proteins shows structural similarity to phage tail tip proteins gp27 and gp5, and Hcp resembles the phage tail tube proteins, which suggests that the T6SS might have evolved from bacteriophages (20, 34, 37, 52). The finding of phage tail homology led to the hypothesis that the T6SS may also function like the T4 bacteriophage tail and puncture the target cell membrane to deliver effector proteins (37). Hcp proteins have been observed to form hexameric donut-like structures and VgrG proteins were found to form trimers (31, 37). The Hcp hexamers could theoretically form larger tubular structures which could serve as a conduit for secretion, and tube-like Hcp multimers have been observed in in vitro studies (20). However, no T6SS-associated stable cell surface structure resembling the pili of T3SS or T4SS (12, 41) or a phage tail has been observed thus far. Instead, an intracellular tubular structure consisting of VipA/B proteins and functioning like a contractile phage tail was recently found (3).
Pseudomonas syringae is a plant-pathogenic bacterium found in a wide variety of agricultural and nonagricultural environments such as clouds, rivers, and snowpack (30). More than 50 pathogenic varieties have been described that can infect numerous plant species, causing diseases such as leaf spots, blight, and speck and cankers and galls in shoots. Although it is typically regarded as a leaf- and shoot-infecting pathogen, it can also infect the roots of plants (2). Because of the economic importance of the pathogen in causing disease in so many plant species, it has emerged as the model bacterial pathogen for studying plant pathogenicity. Studies of P. syringae have revealed that the T3SS and its effector proteins are the major virulence determinants of this phytopathogen, allowing the bacteria to suppress plant defenses and multiply inside the host tissues (13). However, the plant signal for induction of the virulence factors is still unknown. To further understand how Pseudomonas reacts to the conditions inside the host plants and therefore define factors important for colonization and virulence, a proteomic approach was conducted using an hrp-inducing medium supplemented with tomato cell extracts (15; this study). In this modified minimal medium, Hcp2 was identified as one of the most abundant secreted proteins and its secretion was shown to be dependent on T6SS. The three P. syringae strains whose full genomic sequences are available were all found to carry putative T6SS gene clusters, and the two chromosomal T6SS gene clusters in P. syringae pv. tomato DC3000 were named HSI-I and HSI-II, after Hcp secretion island (44). Here we show that only one of the two Hcp proteins is secreted in P. syringae pv. tomato DC3000. By using deletion mutants, we also found that neither of the hcp genes is involved in virulence in host plants. Instead, a novel activity was revealed in this phytopathogenic bacterium suggesting that Hcp2 plays a role in intermicrobial competition.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture media.
The bacterial strains and plasmids used in this study are listed in Table 1. Strains of P. syringae were grown in King's medium B (KB) (19) with appropriate antibiotics at 22 to 28°C or in Hrp-inducing minimal medium (HIM) (17) at 18°C unless otherwise specified. Escherichia coli strains and the other enterobacterial species were cultured in Luria-Bertani (LB) medium. The concentrations of the antibiotics used for selection were as follows: kanamycin, 50 μg/ml; rifampin, 100 μg/ml; tetracycline, 25 μg/ml.
Table 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| Escherichia coli DH5α | F− ϕ80lacZΔM15 Δ(lacZYA-argF)U169 recA1 endA1 hsdR17 (rK− mK+) phoA supE44 λ− thi-1 gyrA96 relA1 | Invitrogen |
| Escherichia coli BL21(DE3) | Lambda DE3 lysogen | Invitrogen |
| Escherichia coli S17-1 | recA pro hsdR RP4-2-Tc::Mu-Km::Tn7 | 49 |
| Pectobacterium carotovorum subsp. carotovorum SCC1 | Field isolate from Finland; old name, Erwinia carotovora subsp. carotovora | 43 |
| Pectobacterium wasabiae SCC3193 | Field isolate from Finland; old name, Erwinia carotovora SCC3193 | 35 |
| Pseudomonas syringae pv. tomato DC3000 strains | ||
| Wild type | Field isolate (rifampin sensitive) | D. Cupples |
| Rifr mutant | Rifampin-resistant derivative of field isolate | D. Cupples |
| Δhcp1 mutant | In-frame deletion of hcp1 (Pto_2539); Rifr | This study |
| Δhcp2 mutant | In-frame deletion of hcp2 (Pto_5435); Rifr | This study |
| Δhcp1 Δhcp2 mutant | In-frame deletion of hcp1 and hcp2; Rifr | This study |
| ΔicmF1 mutant | In-frame deletion of icmF1 (Pto_2554); Rifr | This study |
| ΔicmF2 mutant | In-frame deletion of icmF2 (Pto_5418); Rifr | This study |
| ΔicmF1 ΔicmF2 mutant | In-frame deletion of icmF1 and icmF2; Rifr | This study |
| Plasmids | ||
| pK18mobsac | RP4mob; Kmr | 45 |
| pPP | Promoter probe plasmid with beetle luciferase gene; Tetr | 14 |
| phcp2-LUC | hcp2 promoter in pPP | This study |
Analysis of secreted proteins under Hrp-inducing conditions.
The P. syringae pv. tomato DC3000 wild-type and hrcC mutant strains were first cultured in KB starting at an optical density at 600 nm (OD600) of 0.1 to 0.3 (3.5 h at 28°C) and then in HIM starting at an OD600 of 0.3. After 19 h of incubation at 18°C, the cultures were centrifuged at 8,000 × g for 15 min at 4°C. The culture supernatants were filtered through a 0.45-μm filter to abolish all of the bacterial cells, and phenylmethylsulfonyl fluoride was added to a 0.5 mM concentration. Proteins from the cell-free supernatants of 100-ml liquid cultures were concentrated by using Amicon ultrafiltration devices and extracted with phenol as previously described (33). Protein samples were focused overnight on isoelectric focusing strips in a pH range 3 to 10 and then run on 12% polyacrylamide gels, which were subsequently silver stained. Samples of induced proteins were picked for matrix-assisted laser desorption ionization–time of flight (TOF) mass spectrometry (MS) and quadrupole TOF MS. Protein identification and data analysis were performed in a manner similar to that previously described (28).
Purification of Hcp1 and Hcp2 proteins for antiserum preparation.
The genes that encode Hcp1 and Hcp2 were cloned by PCR from P. syringae pv. tomato DC3000 genomic DNA. The primers used for amplification of the coding region of hcp1 as an NdeI-HindIII fragment were 5′-GGCCCATATGCCTACACCCGCATTTC-3′ (forward) and 5′-GTCAAGCTTCAGTCCAGACCTAAGTCG-3′ (reverse), and hcp2 was amplified as an NdeI-BamHI fragment with primers 5′-CAGGCATATGGCTACGCCAGCGTA-3′ (forward) and 5′-CGAGGATCCAGACTTAGCCAGCGAC-3′ (reverse). PCR products were ligated into the expression vector pJC40 (10) and transformed first into E. coli DH5α and then to E. coli BL21(DE3). Hcp overproduction in E. coli BL21(DE3) was induced by 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG). After probe sonication of the cells nine times with 5-s pulses and 3-s breaks, proteins were precipitated and dissolved in 6 M guanidine hydrochloride solution. Hcp proteins with an N-terminal His10 tag were purified by Ni-nitrilotriacetic acid affinity chromatography under denaturing conditions according to the Qia-Expressionist protocol (Qiagen, Valencia, CA). Rabbit antisera against the Hcp1 and Hcp2 proteins were prepared by LabAs Ltd. in Tartu, Estonia.
Construction of hcp1, hcp2, icmF1, and icmF2 single and double deletion mutants of P. syringae pv. tomato DC3000.
To make in-frame deletions of hcp1 and hcp2 in P. syringae pv. tomato DC3000, pΔhcp1 and pΔhcp2 were constructed as previously described (53), with some modifications. First, a 1.6-kb fragment containing the upstream region of hcp1 was amplified by PCR using primers Pto_2539mt-1 and Pto_2539mt-2 (Table 2). A 2-kb DNA fragment containing the downstream region of hcp1 was amplified using primers Pto_2539mt-3 and Pto_2539mt-4. Then, a crossover PCR was performed to amplify a 3.6-kb fragment containing both the upstream and downstream fragments of hcp1 using the PCR products from the first two steps and primers Pto_2539mt-1 and Pto_2539mt-4. This fragment was digested with EcoRI and HindIII and cloned into the EcoRI/HindIII sites of pK18mobsac (45) to generate pΔhcp1, which was then transformed into E. coli S17-1 λ pir (49). The kanamycin-resistant P. syringae pv. tomato DC3000 strain with pΔhcp1 inserted into the upstream or downstream region by homologous recombination was obtained via conjugation and subsequently plated onto KB plates containing 20% sucrose to counterselect the integration. The Δhcp1 mutant was first screened by PCR with primers Pto_2539mt-1 and Pto_2539mt-4 and further confirmed by Southern blot analysis (data not shown). Similarly, pΔhcp2 was constructed by using the corresponding primers Pto_5435mt-1, Pto_5435mt-2, Pto_5435mt-3, and Pto_5435mt-4. pΔhcp2 was transferred from E. coli S17-1 λ pir into the P. syringae pv. tomato DC3000 wild-type and Δhcp1 mutant strains via conjugation, and the deletion of hcp2 was screened for by PCR with primers Pto_5435mt-1 and Pto_5435mt-4, followed by confirmation by Southern blot analysis. This produced the Δhcp2 and Δhcp1 Δhcp2 mutants.
Table 2.
Primers used in PCRs to create plasmid constructs for generation of in-frame chromosomal deletions in P. syringae pv. tomato DC3000
| Primer | Sequence (5′→3′) | Feature of underlined sequence |
|---|---|---|
| Pto_2539mt-1 | CCGAATTCGCGGAAAAACGCCTTGGAAAGG | EcoRI site |
| Pto_2539mt-2 | GCGAGATTATTTCGGACTGCATTGGTTTCTC CTGGAAGGTTA | Region complementary to primer Pto_2539mt-3 |
| Pto_2539mt-3 | ATGCAGTCCGAAATAATCTCGC | |
| Pto_2539mt-4 | CCAAGCTTTTATCCA TTGCGCGACCTCT | HindIII site |
| Pto_5435mt-1 | CCGGATCCTTCCAGCTCGACCAAGAGATC | BamHI site |
| Pto_5435mt-2 | TGATTGGCCGGCGCGAACATGGAGGTGCTCCTTGCTGGAT | Region complementary to primer Pto_5435mt-3 |
| Pto_5435mt-3 | ATGTTCGCGCCGGCCAATCA | |
| Pto_5435mt-4 | CCTCTAGATCAGATCGCACACGCACCGT | XbaI site |
| Pto_2554mt-1 | CCGGATCCATGAATTCGCACAAGGTTATCTG | BamHI site |
| Pto_2554mt-2 | CTAAAGATCCCGTGACATCAGACTTTTCTCTTGTCAGGATCGAG | Region complementary to Pto_2554mt-3 |
| Pto_2554mt-3 | TGATGTCACGGGATCTTTAGAACCG | |
| Pto_2554mt-4 | CCTCTAGATCAACCCGCGGTACTGGTCG | XbaI site |
| Pto_5418mt-1 | CCTCTAGAACACCCATGAACCGCTCGCC | XbaI site |
| Pto_5418mt-2 | CAAGGGCTGGCTACCAGCATTTACGGCTGTGACTGCGGCTGGAC | Region complementary to Pto_5418mt-3 |
| Pto_5418mt-3 | ATGCTGGTAGCCAGCCCTTGGCGC | |
| Pto_5418mt-4 | CCAAGCTTGCTGCGCCGACATCTGCTGA | HindIII site |
In the P. syringae pv. tomato DC3000 genome, PSPTO_2554 and PSPTO_5418 were identified as two genes that encode proteins homologous to the T6SS membrane protein IcmF (44), which is required for the secretion of proteins that belong to the Hcp family (24, 58). The genes were named icmF1 and icmF2, respectively. In-frame deletions of icmF1 and icmF2 in P. syringae pv. tomato DC3000 were obtained by using an approach similar to that used to construct the Δhcp1 and Δhcp2 mutants. The primers and restriction sites used to generate pΔicmF1 and pΔicmF2 for deletion of the chromosomal icmF1 and icmF2 genes are listed in Table 2. After the ΔicmF1 mutant was acquired, it was used to construct the ΔicmF1 ΔicmF2 double mutant. All of the mutations were confirmed by PCR and Southern blot analysis.
Luciferase fusion construct.
A region 558 bp upstream from the first ATG codon of the hcp2 gene was amplified as a KpnI-BamHI fragment by using primers 5′-GTGGTACCTGACTATCTAAGCGAATG-3′ (forward) and 5′-GGCGGATCCATGGAGGTGCTCCTTG-3′ (reverse) and cloned into the promoter probe plasmid pPP (14) 5′ of the luciferase gene LUCGR coding region. The resulting construct was named phcp2-LUC.
In vivo luciferase activity assay.
To study luciferase expression in vivo from the phcp2-LUC fusion construct carried on a plasmid, susceptible tomato (cv. Agriset) leaves were infiltrated with a P. syringae pv. tomato/phcp2-LUC cell suspension at an OD600 of 1.0 (109 CFU/ml) in 10 mM potassium phosphate buffer (PPB), pH 7.2. For sample preparation, the leaves with infiltrated areas were detached and surface sterilized with 15% H2O2 in phosphate-buffered saline (PBS) for 10 min and then rinsed with sterile PBS. Two leaf discs 10 mm in diameter were removed from the infiltrated leaf areas with a cork borer and homogenized in 1.5 ml ice-cold 10 mM PPB, pH 7.2, with an Ultra-Turrax T25 grinder (IKA, Stauffen, Germany). Luciferase activity expressed by the P. syringae LUC transformant strains was determined by a method similar to one previously described (15). Briefly, a 100-μl bacterial sample, in this case, the undiluted leaf disc homogenate, was quickly mixed with 100 μl of cell lysis mix (Promega, Madison, WI) and 100 μl of luciferin substrate solution (luciferin from Sigma-Aldrich, St. Louis, MO) in a luminometer cuvette. Photon counts were recorded immediately after the insertion of the cuvette into a Biocounter M1500 luminometer (Lumac, St. Paul, MN). To determine the number of viable bacteria, the homogenized leaf samples were diluted 10-fold in a series in 100 mM PPB, pH 7.2, with 0.1% soybean peptone and plated on KB agar supplemented with rifampin at 75 μg/ml. Colonies were counted after 3 days of incubation at 28°C.
Virulence assay on tomato plants.
Tomato (Solanum lycopersicum) cultivar Moneymaker was grown in a growth chamber set at 24°C/22°C (day/night) with a 16-h photoperiod and 70% relative humidity. Four-week-old tomato plants were dip inoculated with different P. syringae pv. tomato DC3000 strains at a level of 2 × 107 CFU/ml in a 10 mM MgCl2 solution containing 0.02% Silwet-77. Determination of bacterial populations in tomato leaves was done as described previously (22).
Arabidopsis colonization assay.
Arabidopsis thaliana ecotype Colombia plants were spray inoculated separately with the P. syringae pv. tomato DC3000 parent strain and the Δhcp1 Δhcp2 double mutant at an OD600 of 0.1 (108 cells/ml) in 10 mM MgCl2; six plants per strain were sprayed until runoff. After 7 days, the leaf rosettes were cut and weighed and each rosette was homogenized in 3 ml of ice-cold PPB with soybean peptone without surface sterilization. Dilutions for plating were made in the same buffer. Samples were spread on KB agar with rifampin, and colonies were counted after 3 days of incubation at 22°C. To test the competitive fitness of the hcp double mutant with the parent strain, eight Arabidopsis plants were spray inoculated with a 1:1 mixture of the P. syringae pv. tomato DC3000 rifampin-sensitive strain and the Δhcp1 Δhcp2 double mutant strain, both transformed with the pPP plasmid carrying tetracycline resistance genes, at an OD600 of 0.1 (total cell density, 108/ml) in 10 mM MgCl2. After 7 days, inoculated plant rosettes were homogenized without surface sterilization. Bacteria from the plant samples were plated on KB agar with tetracycline (12.5 μg/ml) and with or without rifampin. Colonies were counted after 4 days of incubation at 22°C.
Bacterial competition assay.
The P. syringae pv. tomato DC3000 rifampin-resistant parent strain and its mutant derivatives were precultured on KB agar. Enterobacterial strains were precultured on LB agar and then suspended in KB broth to an OD of 0.1, and 100 μl of the suspension was spread onto each KB agar plate to obtain an even cell layer. Droplets of a similarly prepared P. syringae cell suspension were added on top with the aid of paper discs to prevent the droplets from spreading too wide. After overnight incubation at 25°C, both the enterobacterial growth inhibition and the fluorescence under UV light of P. syringae were detected visually. To obtain quantitative results, the double-inoculated area of the nutrient agar was cut out with a sterilized 10-mm cork borer and the bacteria were resuspended in 5 ml KB broth, diluted, and spread onto fresh KB agar plates for CFU counting. The enterobacteria were cultured at 37°C overnight, and P. syringae strains were cultured with rifampin (75 μg/ml) at 25°C for 3 to 4 days.
Yeast competition assay.
The P. syringae pv. tomato DC3000 rifampin-resistant parent strain and its mutant derivatives were precultured on KB agar, and the Cryptococcus carnescens yeast was cultured in liquid PYG medium (per liter, 15 g proteose peptone, 2.5 g yeast extract, 18 g glucose, 280 mg NaHPO4, 272 mg KH2PO4, 240 mg NaCl, 8 mg MgSO4, and 12 mg CaCl2). P. syringae pv. tomato cells were suspended in 10 mM MgCl2 and diluted to an OD of 0.01, corresponding to 107 cells/ml. Ten microliters of a P. syringae pv. tomato cell suspension and 10 μl of yeast cell culture were applied together to PYG agar in 24-well cell culture plates. After 7 days of incubation at 23°C, all of the cells in a well were suspended in 5 ml of salt solution (per liter, 280 mg NaHPO4, 272 mg KH2PO4, 240 mg NaCl, 8 mg MgSO4, 12 mg CaCl2) and the yeast cells were counted with a Bürker cell counting chamber. For each sample, six 0.04-mm squares with a 0.1-mm depth were counted.
RESULTS
Hcp2 is expressed and secreted into the culture medium.
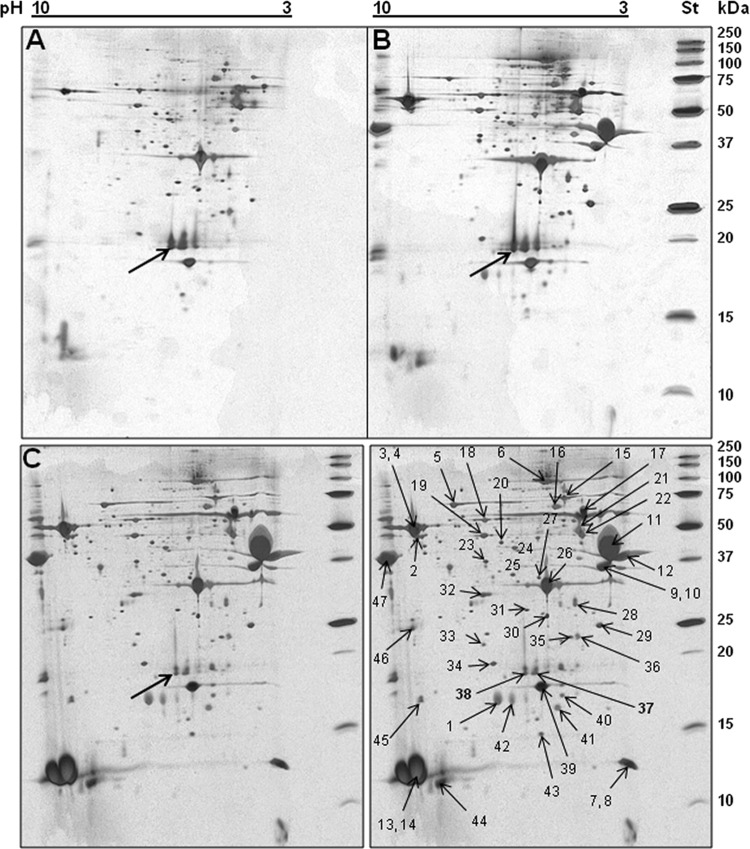
To achieve our goal of identifying bacterial factors that contribute to plant colonization and virulence, we specifically aimed to find plant-inducible proteins secreted by P. syringae pv. tomato DC3000 in HIM—a minimal medium that mimics the conditions in the plant apoplast—and in the same medium supplemented with tomato cell exudates. Among the proteins secreted into the minimal medium, with and without tomato exudates, several known T3SS-dependent proteins were identified by MS, i.e., the pilin HrpA; accessory proteins HrpZ, HrpK, HopAK1, HopP1, and HrpW; effector proteins AvrPto and HopAM1-1; the flagellin FliC; and the flagellar hook protein FliD. The only protein whose production was clearly upregulated by the tomato cell exudates was the T3SS pilin HrpA. In addition to these previously characterized T3SS-secreted proteins, the T6SS-related hemolysin-coregulated protein Hcp2 was identified in two silver-stained spots on the gel (Fig. 1 and Table 3). Other T6SS-associated proteins were not found in the culture medium. A comparison of the two-dimensional gel spots and MS identification of the proteins from the P. syringae pv. tomato DC3000 wild-type and hrcC mutant strains showed that Hcp2 secretion was not dependent on T3SS and not enhanced or repressed by tomato exudates. Because of the discovery of a T6SS-related protein being expressed in HIM, we extended our focus to characterize Hcp and to determine if it also plays a role in virulence or plant colonization.
Fig 1.
Proteins detected in the culture supernatant of P. syringae pv. tomato DC3000 grown in HIM. (A) The P. syringae pv. tomato DC3000 hrcC mutant is unable to secrete the T3SS-dependent proteins. (B) The P. syringae pv. tomato DC3000 parent strain cultured in HIM. (C) The P. syringae pv. tomato DC3000 parent strain cultured in HIM supplemented with tomato cell culture exudates. At the right edge of the gels, in a separate lane, are the standard proteins. In the right half of panel C, the spots analyzed by MS are indicated by arrows and the numbering corresponds to that in Table 2. The single arrows in the other panels point to the spots at which Hcp2 was identified.
Table 3.
Proteins identified by MS in supernatant of P. syringae pv. tomato DC3000 culture in HIM
| Sample(s) | ID code | Protein | Characterization | % Sequence coverageb | Observed mass (kDa) | Calculated pI | Predicted localization |
|---|---|---|---|---|---|---|---|
| 1 | PSPTO_4001 | AvrPto | T3SS-secreted effector | 63 | 18.309 | 6.02 | Secreted |
| 2, 3, 4 | PSPTO_1951 | FliD | Flagellar hook protein | 48, 26 | 49.290 | 8.99 | Membrane |
| 5 | PSPTO_4101 | HopAK1 | T3SS helper, putative pectate lyase | 37 | 59.048 | 6.85 | Secreted |
| 6 | PSPTO_1405 | HrpK1 | T3SS putative translocase | 11 | 81.044 | 5.27 | Secreted |
| 7, 8 | PSPTO_1589 | AAO55109 | Putative lipoprotein | 30 | 33.749 | 7.66 | Membranea |
| 9, 10 | PSPTO_2678 | HopP1 | T3SS helper, putative lytic transglycosylase | 39, 25 | 32.349 | 4.33 | Secreted |
| 11, 12 | PSPTO_1382 | HrpZ1 | T3SS-secreted harpin | 28, 22 | 36.463 | 4.01 | Secreted |
| 13, 14 | PSPTO_1381 | HrpA1 | T3SS pilin | 70 | 11.580 | 8.96 | Secreted |
| 15 | PSPTO_2841 | Transposase | ISPsy14 transposase | 19 | 58.143 | 9.46 | Intracellular |
| 16 | Not identified | ||||||
| 17 | PSPTO_4504 | DnaJ | Chaperone (Hsp40) | 15 | 41.009 | 6.34 | Cytoplasma |
| 18 | PSPTO_2678 | HopP1 | T3SS helper | 37 | 32.349 | 4.33 | Secreted |
| 19 | PSPTO_1534 | RpsB | Ribosomal protein S2 | 22 | 27.441 | 8.56 | Intracellular |
| 20 | Not identified | ||||||
| 21 | PSPTOA0032 | Lsc-3 | Levansucrase | 29 | 47.970 | 4.74 | Secreted |
| 21 | PSPTO_1373 | HrpW1 | T3SS-secreted harpin | 29 | 42.885 | 4.63 | Secreted |
| 22, 23 | Not identified | ||||||
| 24 | PSPTO_3615 | Recombinase | Site-specific recombinase | 14 | 60.488 | 7.29 | Intracellular |
| 25 | Not identified | ||||||
| 26 | PSPTO_1949 | FliC | Flagellin | 52 | 29.158 | 5.25 | Secreted |
| 27-31 | Not identified | ||||||
| 32 | PSPTO_1022 | HopAM1-1 | T3SS-secreted effector | 61 | 31.340 | 6.26 | Secreted |
| 33 | PSPTO_2713 | CheR | Putative chemotaxis methyltransferase | 18 | 33.582 | 6.26 | Intracellular |
| 34 | PSPTO_5098 | Hypothetical | Putative acyltransferase | 21 | 36.400 | 9.38 | Membranea |
| 35 | PSPTO_0945 | TerE | Tellurium resistance protein | 59 | 20.267 | 4.84 | Membranea |
| 36 | PSPTO_0944 | TerD | Tellurium resistance protein | 38 | 20.495 | 4.69 | Cytoplasma |
| 37 | PSPTO_4001 | Hcp2 | Secreted protein Hcp | 38 | 19.132 | 5.53 | Secreted |
| PSPTO_5435 | AvrPto | T3SS-secreted effector | 34 | 18.375 | 5.92 | Secreted | |
| 38 | PSPTO_4001 | Hcp2 | Secreted protein Hcp | 38 | 19.132 | 5.53 | Secreted |
| PSPTO_5435 | AvrPto | T3SS-secreted effector | 34 | 18.375 | 5.92 | Secreted | |
| 39 | PSPTO_4001 | AvrPto | T3SS-secreted effector | 45 | 18.375 | 5.92 | Secreted |
| 40 | PSPTO_2713 | CheR | Putative methyltransferase (fragment?) | 21 | 33.382 | 6.46 | Intracellular |
| 41 | PSPTO_1950 | FlaG | Flagellar protein | 33 | 14244 | 5.19 | Secreted |
| 42 | PSPTO_4001 | AvrPto | T3SS-secreted effector | 63 | 18375 | 5.92 | Secreted |
| 43 | Not identified | ||||||
| 44 | PSPTO_1925 | FlgM | Negative regulator of flagellin synthesis | 63 | 10.979 | 8.04 | Cytoplasma |
| 45, 46 | Not identified | ||||||
| 47 | PSPTO_1382 | HrpZ1 | T3SS-secreted harpin | 19 | 36.463 | 4.01 | Secreted |
Prediction by PSORT algorithm.
Two values indicate sequence coverage for the second spot from which the same protein was identified, when different from the first spot sample.
P. syringae pv. tomato DC3000 Hcp2 is expressed as covalently linked dimers.
Because of the detection of several presumably intracellular proteins in the culture supernatant, which suggests the occurrence of cell lysis during sample preparation, we needed to confirm whether Hcp2 is actively secreted into the extracellular medium. The P. syringae pv. tomato DC3000 genome harbors two putative hcp genes (1, 44), but only Hcp2 was detected in the culture medium. To find out if only one or both of the hcp genes were actively expressed, antisera against the Hcp1 and Hcp2 proteins were produced. The proteins were overproduced in E. coli, purified, and used to immunize rabbits. Both the Hcp1 and Hcp2 antisera recognized a 19-kDa protein from the culture supernatants of P. syringae pv. tomato DC3000, and each antiserum prepared against one of the two proteins also recognized the other affinity-purified protein by cross-reaction (data not shown). The two Hcp proteins of P. syringae pv. tomato DC3000 show 53% amino acid sequence identity, so it is not surprising that the antiserum prepared against one Hcp cross-reacted with the other. Besides that, the sizes of the two proteins differ by only one amino acid and they cannot be distinguished by standard gel electrophoresis. Hence, single deletion (Δhcp1 or Δhcp2) and double deletion (Δhcp1 Δhcp2) chromosomal mutants were created to study the expression of Hcp1 and Hcp2 separately. Protein immunoblot assays revealed that the Δhcp2 mutant did not produce any protein recognizable by either of the two antisera and thus resembled the Δhcp1 Δhcp2 double mutant (Fig. 2, left panels). Since the amount of recognized protein produced and secreted by the Δhcp1 mutant seemed equal to that of the parent strain, we concluded that the Hcp1 protein was not produced by P. syringae pv. tomato DC3000 under these experimental conditions whereas Hcp2 was abundantly produced and secreted.
Fig 2.
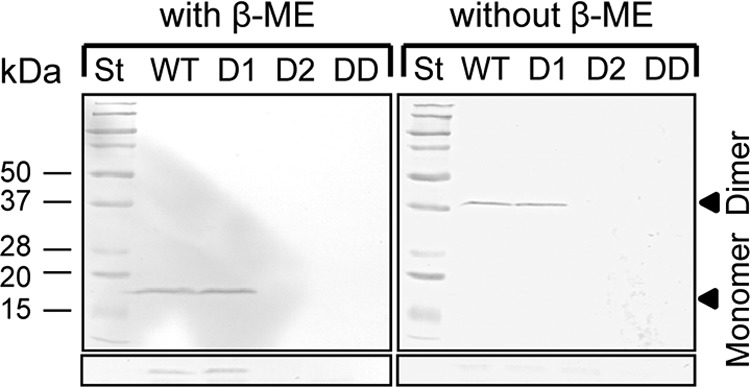
The Hcp2 protein is secreted by P. syringae pv. tomato DC3000. Protein samples were prepared from supernatants and cell pellets of fresh overnight cultures in KB. β-ME was added to the samples on the left and omitted from the samples on the right. The proteins were detected by using anti-Hcp1 antiserum and an anti-rabbit monoclonal antibody conjugated with alkaline phosphatase, which degrades the Nitro Blue Tetrazolium–5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (BCIP) substrate, producing the color violet. The lower panels show immunodetection of Hcp from the cell fractions of the same samples with and without β-ME. Only the monomeric form could be detected in the cell fractions treated with β-ME. St, standard proteins; WT, P. syringae pv. tomato DC3000 parent strain; D1, Δhcp1 deletion mutant; D2, Δhcp2 deletion mutant; DD, Δhcp1 Δhcp2 double deletion mutant.
Further analysis of the Hcp2 protein was carried out to determine whether it is monomeric or oligomeric. In a Tris-glycine-SDS-polyacrylamide gel system with β-mercaptoethanol (β-ME) in the sample buffer, Hcp2 always appeared as a 19-kDa monomer (Fig. 2, left panel). When the reducing agent was omitted from the sample buffer, the antiserum no longer detected a 19-kDa band but instead recognized a 38-kDa band, corresponding to a Hcp2 dimer, from the culture supernatant (Fig. 2, right panel). The dimer was not detectable in the cell fractions, suggesting that the reducing conditions in the cytoplasm do not allow Hcp2 dimerization. In nondenaturing gels using perfluorooctanoic acid instead of SDS, Hcp2 also appeared as a dimer and no higher-order oligomers were detectable (data not shown).
Secretion of Hcp2 is dependent on the T6SS membrane component IcmF2.
To confirm that the observed release of Hcp2 into the culture medium is dependent on T6SS, we created new mutants of P. syringae pv. tomato DC3000 in which the genes homologous to the T6SS membrane protein component IcmF are deleted. We found that P. syringae pv. tomato ΔicmF2 mutant was unable to secrete Hcp2 protein into the culture medium (KB) (Fig. 3). In contrast, the ΔicmF1 mutant was still fully capable of secreting Hcp2. These results suggest that the secretion of Hcp2 is dependent on the function of the T6SS built up of the components encoded by HSI-II.
Fig 3.

Secretion of Hcp2 is dependent on IcmF2. The Hcp2 protein was immunologically detected in the cell fraction of wild-type P. syringae pv. tomato DC3000 and all three of the ΔicmF mutants grown in KB overnight. In the culture medium, Hcp2 was detected only in the wild-type strain and the ΔicmF1 mutant, suggesting that the secretion of Hcp2 requires IcmF2 function. RpoA is a nonsecreted reference protein.
The Hcp2 expression level is dependent on the bacterial growth phase.
P. syringae pv. tomato DC3000 secretome analysis showed that Hcp2 was expressed in minimal medium. We sought to confirm this by examining the expression of the hcp2 gene and also to determine whether this gene is constitutively expressed or regulated by the culture conditions or bacterial growth phase. We constructed a plasmid with the hcp2 promoter linked to a luciferase reporter gene, called phcp2-LUC, and transformed it into P. syringae pv. tomato DC3000. The luciferase activity under the control of the hcp2 promoter was determined in both HIM and a rich medium, KB. Luminescence counts and OD600 were measured at 24, 48, and 72 h. The relative expression level in KB was lower than that in the minimal medium containing fructose or mannitol, but it increased with culture age and finally reached a higher level when the cultures reached the late stationary phase (Fig. 4A). hcp2 promoter activity in minimal medium supplemented with glucose was expressed at the same levels as with mannitol (data not shown). The hcp2 promoter was also active in P. syringae pv. tomato DC3000 growing on solid KB, and the luminescence per unit of OD600 was slightly higher in plate cultures than in liquid cultures (Fig. 4B). In contrast to hcp2, reporter gene constructs with the hcp1 promoter failed to detect any activity in vitro (data not shown).
Fig 4.
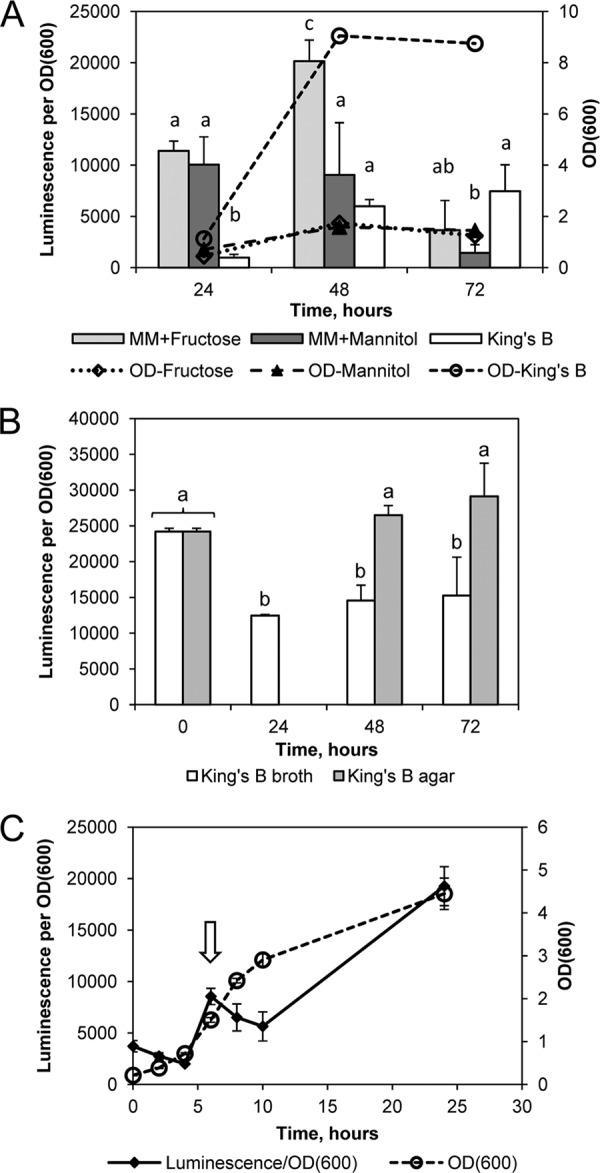
The level of hcp2 expression shows variation depending on the culture conditions and growth phase. (A) After 24 h, luciferase expression under the control of the hcp2 promoter was significantly higher in the minimal medium (MM) than in KB (P < 0.0001, Student's t test). After 48 h, luminescence counts per unit of OD600 were significantly higher in MM plus fructose than in the two other media (P < 0.0001 in comparison with KB, P < 0.01 in comparison with MM plus mannose), and after 72 h, both growth and luminescence were declining in the minimal medium while they were still maintained in KB. Three parallel cultures were started in each medium, KB and MM with 10 mM fructose or 10 mM mannitol at an OD600 of 0.3, and incubated with shaking for 3 days at 22°C. Error bars indicate SDs, and statistically significant differences are indicated by different letters. (B) The activity of the hcp2 promoter was higher on solid medium than in liquid medium. The P. syringae pv. tomato/phcp2-LUC strain and the promoterless control P. syringae pv. tomato/pPP strain were precultured on KB agar and inoculated at an OD600 of 0.1 into fresh KB liquid medium or spread as a lawn on KB agar. The relative luminescence of the control strain stayed at a constant low level below 100. In agar cultures, luciferase activity could not be reliably determined before 48 h because the cell numbers were too low. Two separate cultures of each kind were sampled, and error bars indicate SDs. Results that are different at a confidence level of P < 0.05 (Student's t test) are marked with different letters. (C) The activity of the hcp2 promoter changed with the growth phase. Five parallel liquid cultures of the P. syringae pv. tomato/phcp2-LUC strain were started at an OD600 of 0.2 in KB and grown overnight at 22°C. n = 5; error bars indicate SDs. The arrow points to the peak of hcp2 promoter activity detected in the mid-logarithmic growth phase.
Because putative RhlR binding sites were identified in both the hcp1 and hcp2 promoter sequences (see Fig. S1 and Table S1 in the supplemental material), suggesting that hcp gene expression could be regulated by quorum-sensing signals, we studied more closely whether hcp2 expression is growth phase dependent. The activity of the hcp2 promoter, observed as a function of luciferase activity per unit of cell density at several time points in liquid culture in KB, was at the lowest level during the early logarithmic phase and increased during the late logarithmic phase (Fig. 4C). Surprisingly, hcp2 promoter activity did not increase at a constant rate and oscillated between expression and repression during population growth. From the lowest level detected during the early logarithmic phase, the activity increased >3-fold by the mid-logarithmic phase and then declined before increasing again in the late logarithmic phase. Because cultures started at different cell densities had the same relative expression level at the same time points (data not shown), a higher cell density per se does not seem to enhance hcp2 promoter activity. Also, culture supernatant taken from a dense P. syringae pv. tomato DC3000 culture and mixed into fresh culture medium before starting a new culture did not enhance hcp2 expression, suggesting that the hcp2 expression level is not regulated by a diffusible quorum-sensing signal. However, dilution of a culture with fresh medium always caused a drop in the relative expression level.
Altogether, the results indicate that hcp2 expression was highest after 24 h of growth, when the bacterial population entered the stationary phase, and higher in minimal medium or on a solid surface than in rich medium or in a liquid culture with shaking. This may indicate that hcp2 expression is linked to the depletion of nutrients.
hcp2 expression is not upregulated in planta and does not significantly contribute to colonization of or virulence in the plant host.
The discovery that Hcp2 was expressed in a minimal medium that mimics the plant apoplast raised the possibility that hcp2 expression is upregulated in the plant. To analyze the expression of the hcp2 gene in P. syringae pv. tomato DC3000 during host plant infection, we injected the phcp2-LUC strain into the leaf tissue of susceptible tomato plants (cv. Agriset). Leaf disc samples were taken from the infiltrated areas, luciferase activity was measured, and bacteria were plated on KB agar to determine viable cell numbers. We found that the activity of the hcp2 promoter in bacteria inoculated into tomato leaves was lower than the expression level in culture medium and declined over time (Fig. 5). Thus, differently from the T3SS components, hcp2 expression was not promptly induced in the plant apoplast. Because of the localized necrosis of leaf tissue caused by the high bacterial inoculum concentration, it was not possible to obtain samples at later time points to see if hcp2 expression would increase after the initial decline.
Fig 5.
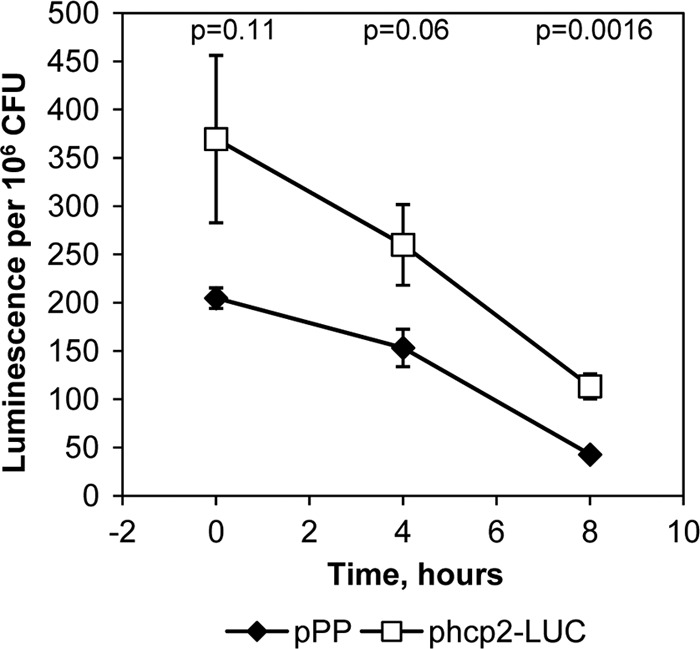
hcp2 promoter activity is very low in bacteria inoculated into tomato leaves. pPP, luciferase gene with no promoter; phcp2-LUC, promoter fusion construct. Luminescence counts per 106 viable cells, determined as CFU, are shown. n = 4; error bars indicate SDs, and P values indicate the statistical significance of the difference between the hcp2 promoter and the promoterless construct (Student's t test).
To determine whether hcp1 or hcp2 of P. syringae pv. tomato DC3000 plays a role in plant colonization or virulence, bacterial populations and disease development in the tomato cultivar Moneymaker were monitored over 7 days after dip inoculation with the P. syringae pv. tomato DC3000 wild-type or Δhcp1, Δhcp2, or Δhcp1 Δhcp2 mutant strain at 2 × 107 CFU/ml. Deletion of hcp1 or hcp2 did not significantly affect bacterial growth in tomato plants, indicating that these two genes play no or minor roles in P. syringae pv. tomato DC3000 virulence (see Fig. S2 in the supplemental material). The experiment was repeated three times with similar results, and data analysis was performed using Duncan's multiple-range test.
A similar result was obtained with another host of P. syringae pv. tomato DC3000, A. thaliana, when the population sizes of the parent strain and the Δhcp1 Δhcp2 mutant were determined 7 days after spray inoculation of A. thaliana plants with pure and mixed cultures. After separate spray inoculations with pure cultures, the average population size of the P. syringae pv. tomato DC3000 parent strain in Arabidopsis leaves was 1.0 × 108 (standard deviation [SD], 7.19 × 107) CFU/g of fresh weight and that of the Δhcp1 Δhcp2 mutant was 7.25 × 107 (SD, 5.13 × 107) CFU/g of fresh weight (see Fig. S2 in the supplemental material). A comparison of means by Student's t test indicated that the difference in the mean population sizes was not statistically significant. Variations in fitness can be revealed by the more sensitive competitive index (CI) assay that uses mixed inoculations (25). The CI of the Δhcp1 Δhcp2 double mutant was 0.893 (SD, 0.482), which is not significantly different from 1 according to a one-sample t test (P = 0.27 with 95% confidence). Thus, the Δhcp1 Δhcp2 mutant did not significantly differ from the wild type in plant colonization efficiency under these conditions. These data therefore suggest that hcp1 and hcp2 of P. syringae pv. tomato DC3000 might have a function other than to promote host plant colonization.
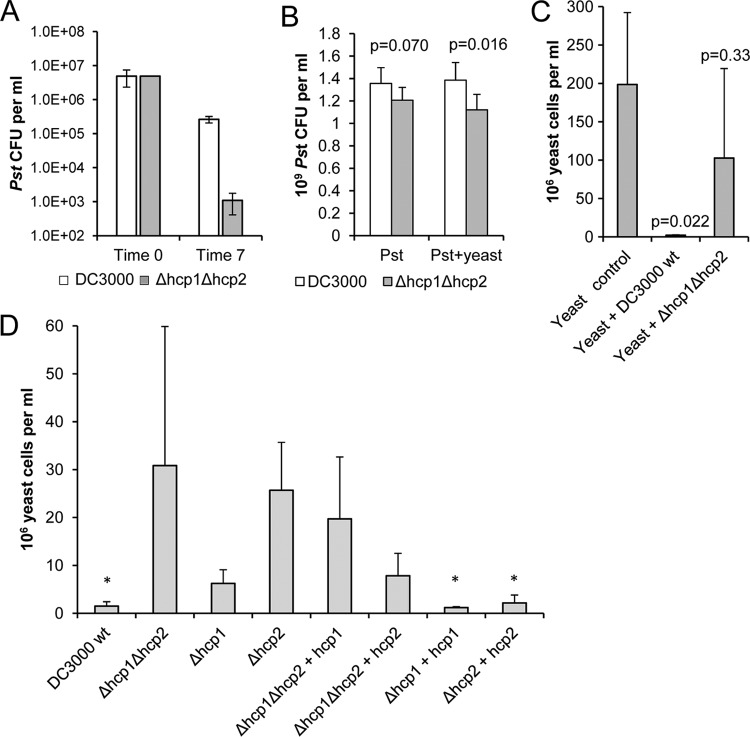
Hcp2 of P. syringae is required for survival in competition with enterobacteria.
Since the T6SS is commonly present in Gram-negative bacteria and was recently discovered to have a role in suppressing the growth of competitive bacteria (16, 32, 47), we hypothesized that T6SS in phytopathogenic bacteria may be used to enhance competitive fitness instead of virulence or pathogenicity. When P. syringae pv. tomato DC3000 was cocultured with a range of different bacteria on solid medium, we found that it was able to inhibit the growth of several species of enterobacteria, including E. coli, Proteus vulgaris, and the plant pathogens Pectobacterium carotovorum subsp. carotovorum (SCC1) and Pectobacterium wasabiae (SCC3193). Deletion of hcp2 abolished the ability of P. syringae pv. tomato to inhibit enterobacterial growth and also decreased its growth in the mixed culture (Fig. 6; see Fig. S3 in the supplemental material). Complementation of the Δhcp2 mutation fully restored the ability to inhibit enterobacterial growth and allowed the complemented strain to grow well in the mixed culture and be more competitive. The inverse correlation between cell counts of E. coli and P. syringae pv. tomato DC3000 strains carrying the hcp2 gene is significant (P < 0.01) according to a Pearson two-tailed correlation test and a paired-sample test. In pure culture without competition, the Δhcp2 mutant grew as well as the P. syringae pv. tomato DC3000 parent strain (data not shown), indicating that the mutation does not noticeably impair any basic cellular functions of P. syringae pv. tomato DC3000. Suppression of enterobacterial growth could be visually detected as a zone with a thinner translucent cell layer at the P. syringae pv. tomato application site, and since a viable P. syringae pv. tomato population emits strong greenish fluorescence under UV light due to siderophore production, it was easy to confirm which bacterial species was dominating in a mixed culture (Fig. 7A). Later, after 2 days in coculture, E. coli was able to grow despite the inhibitory activity of P. syringae pv. tomato. This delayed growth of E. coli could not displace P. syringae pv. tomato at the site of inoculation, but the two bacterial species reached approximately equal cell densities. While the parent and Δhcp1 mutant strains survived the competition, the Δhcp2 mutant population declined. The complemented Δhcp2 strain was able to recover the ability to compete with E. coli (Fig. 7B). In contrast to the Gram-negative species, the Δhcp2 mutant was not impaired in the ability to compete with the Gram-positive Arthrobacter and Bacillus species on solid KB (data not shown).
Fig 6.
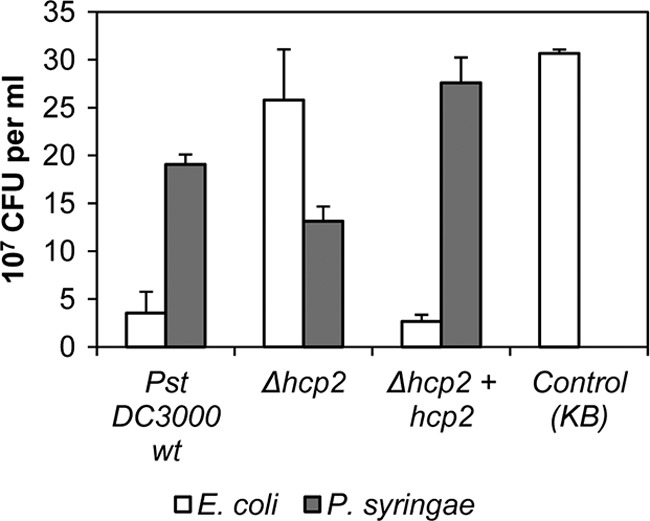
The ability of P. syringae pv. tomato DC3000 to inhibit E. coli growth in a mixed culture is dependent on hcp2. Reintroduction of the hcp2 gene into the Δhcp2 mutant resulted in functional complementation of the mutation. The P. syringae strains and E. coli BL21 were cocultured on KB agar plates for 16 h at 25°C before samples were taken. The control sample is E. coli cultured alone. For detection of CFU, the cocultures were suspended in liquid medium and replated. E. coli colonies were counted after 20 h of incubation at 37°C, and P. syringae colonies were counted after 4 days of incubation at 21°C with rifampin. The results shown are averages of samples from three separate cocultures, and error bars indicate SDs. The negative correlation between P. syringae pv. tomato growth and that of E. coli in a mixed culture is significant at a level of P = 0.01 (Pearson two-tailed correlation test). wt, wild type.
Fig 7.
Hcp2 is required for the survival of P. syringae pv. tomato DC3000 in a coculture with E. coli, whereas Hcp1 is not required. P. syringae pv. tomato strains were added as droplets on top of an E. coli cell layer in the following order: 1, DC3000 parent strain; 2, Δhcp1 mutant; 3, Δhcp2 mutant; 4, Δhcp1 Δhcp2 double mutant; 5, Δhcp1 + hcp1 complemented mutant; 6, Δhcp2 + hcp2 complemented mutant; 7, Δhcp1 Δhcp2 + hcp1 complemented double mutant; 8, Δhcp1 Δhcp2 + hcp2 complemented double mutant; 9, control (KB only). The bacteria were cocultured on KB agar plates at 25°C for 48 h before photographs were taken, and samples were subsequently prepared for the determination of viable counts. (A) In daylight, the inhibition of E. coli BL21 growth by P. syringae can be detected as a thinner cell layer zone (VIS). Under UV light, P. syringae cells are fluorescent whereas E. coli cells are not. (B) For CFU detection, cocultures were suspended in liquid medium and replated. E. coli colonies were counted after 20 h of incubation at 37°C, and P. syringae colonies were counted after 3 days of incubation at 25°C with rifampin. The results shown are averages of samples from two separate cocultures, and error bars indicate SDs. wt, wild type.
When the P. syringae pv. tomato DC3000 parent and the Δhcp2 mutant strains were cocultured with E. coli in liquid KB with shaking at 200 rpm, no differences in the viable cell numbers of the two strains could be detected (data not shown). Also, cell-free culture filtrates from a P. syringae pv. tomato DC3000 overnight liquid culture in KB did not inhibit E. coli growth when added to an E. coli cell layer on solid medium. To test whether the hcp2-associated function requires direct contact between the cells of the two competing species, a cell-impermeable filter (pore size, 0.2 μm) was placed between the two bacterial cell layers on solid KB. Both the P. syringae pv. tomato DC3000 parent strain and the hcp2 mutant grew equally well on the filter laid on top of the E. coli cell layer. When wild-type P. syringae pv. tomato DC3000 or the hcp2 mutant was first spread on the agar, E. coli grew equally well on the filter regardless of which P. syringae pv. tomato strain was growing beneath it. Without the filter, if P. syringae pv. tomato was spread first on KB agar and E. coli added on top, the Δhcp2 mutant was cleared away within the E. coli application area whereas parent strain DC3000 was not cleared away (see Fig. S4 in the supplemental material). Thus, the difference in competitive fitness between the parent strain and the Δhcp2 mutant was observed only when P. syringae pv. tomato cells were in contact with E. coli on solid medium.
P. syringae hcp genes are required for competition against eukaryotic microbes.
In other studies, active T6SSs have been found to influence the growth of eukaryotic organisms such as amoebae (38). We therefore tested the P. syringae pv. tomato DC3000 parent strain and the Δhcp1 Δhcp2 double deletion mutant in cocultures with a range of eukaryotic microorganisms. We found that deletion of the hcp genes from P. syringae pv. tomato DC3000 resulted in increased susceptibility to grazing by the amoeba Acanthamoeba polyphaga (Fig. 8A). In contrast, no difference between wild-type P. syringae pv. tomato DC3000 and the hcp double mutant was seen when they were tested for nematode grazing (data not shown). Of the yeast species tested, Saccharomyces cerevisiae (baker's yeast) was unable to compete against either the wild-type or the Δhcp1 Δhcp2 double mutant P. syringae pv. tomato strain in a mixed culture. However, competition against Cryptococcus carnescens and Rhodotorula glutinis revealed a clear difference between the two P. syringae pv. tomato strains. Competitive fitness assays against the Cryptococcus yeast were done with both a rich medium (PYG agar) and a minimal medium (HIM). On PYG agar, the P. syringae pv. tomato strains grew quickly and then finally lost their viability during the 7-day incubation while the yeast still slowly multiplied in the cultures. In contrast, on HIM agar, the P. syringae pv. tomato strains remained viable for several weeks, which allowed us to determine the effect of competition with the yeast on the population size of P. syringae pv. tomato in a mixed culture. The Δhcp1 Δhcp2 double mutant strain displayed reduced fitness in a mixed culture with the yeast (Fig. 8B). We found that wild-type P. syringae pv. tomato DC3000 was able to inhibit yeast growth, whereas the Δhcp1 Δhcp2 mutant strain was dramatically impaired in this activity (Fig. 8C). Thus, the hcp genes seem to be required for yeast growth suppression.
Fig 8.
Survival of P. syringae pv. tomato DC3000 (Pst) in mixed cultures with amoebae and yeast cells is dependent on Hcp function. (A) The hcp genes are required for full survival of predation by amoebae. Bacterial populations of the P. syringae pv. tomato DC3000 wild-type and hcp double mutant strains were enumerated at time point 0 and after 7 days of coincubation with 3 × 105 A. polyphaga cells on PYG agar. The values shown are means of three replicates, and error bars indicate the standard errors of the means. At time point 7, the difference between the wild-type (wt) and mutant P. syringae pv. tomato strains is significant at a level of P = 0.0053 (5 df) by analysis of variance. (B) The P. syringae pv. tomato Δhcp1 Δhcp2 double mutant has reduced fitness in a mixed culture with yeast cells. P. syringae pv. tomato strains were inoculated onto HIM agar with the Cryptococcus yeast (105 P. syringae pv. tomato cells and 7.4 × 104 yeast cells per spot), and after 7 days of incubation, the cells were collected and plated onto KB agar. n = 6; error bars indicate SDs. P values (Student's t test) indicate the significance of differences between the P. syringae pv. tomato parent strain and the hcp mutant. (C) hcp genes are required for yeast growth inhibition. Yeast cells were counted after 7 days of culture on PYG agar. Each culture was started with 7.4 × 104 yeast cells, and in the mixed cultures, 105 P. syringae pv. tomato cells were added. n = 3; error bars show SDs. P values (t test) indicate the significance of differences between the mixed culture and the control yeast culture. (D) Hcp2 is a major factor in yeast growth inhibition. Yeast cells were counted after 7 days of incubation on PYG agar together with one of the P. syringae pv. tomato strains. Each of the mixed cultures was started with 105 P. syringae pv. tomato cells and 5.8 × 104 yeast cells. n = 3; error bars show SDs. The asterisks indicate cultures in which yeast growth was inhibited by P. syringae pv. tomato. According to one-way analysis of variance, the differences between the P. syringae pv. tomato strains are significant at a level of P = 0.04 (F = 23.9). wt, wild type.
To further characterize the impact of the P. syringae pv. tomato DC3000 T6SS on eukaryotes, we focused on the yeast competition phenotype. P. syringae pv. tomato DC3000 strains with individual deletions of hcp1 and hcp2 were tested for fitness for competition against the Cryptococcus yeast. Deletion of hcp2 resulted in a significant (P < 0.0001) decrease in P. syringae pv. tomato fitness, which was observed as increased yeast growth. Surprisingly, deletion of hcp1 also resulted in a small but statistically significant (P = 0.0008) increase in yeast growth. The reduced inhibitory activity of the mutants was restored to the wild-type level by genetic complementation of the hcp gene mutations (Fig. 8D). When both of the hcp genes were present, as in the parent strain and in the Δhcp1 + hcp1 and Δhcp2 + hcp2 complementation strains, the yeast growth was effectively suppressed. According to a Pearson two-tailed correlation test, the correlation between yeast growth inhibition and the presence of hcp genes is significant at P < 0.01 (see Fig. S5 in the supplemental material).
DISCUSSION
We used a proteomic approach to analyze the secretome of P. syringae pv. tomato DC3000 for the identification of potential novel plant colonization and virulence factors. In addition to known T3SS effectors, Hcp2, probably a component of the T6SS, was found abundantly in hrp-inducing medium. Unlike T3SS effectors whose expression is tightly controlled under the regulation of an unknown plant signal(s) and the alternate sigma factor hrpL (55), the expression of hcp2 could be detected in P. syringae pv. tomato DC3000 grown in both rich medium and minimal medium. Immunoblotting results suggest that, unlike Hcp2, the Hcp1 protein is not produced in any of the culture media used in this study or it is produced at a very low level that is under the detection limit. We also performed Northern blotting to confirm expression at the transcript level, and while hcp2 mRNA was clearly visible, hcp1 mRNA could not be detected, suggesting that no hcp1 transcript was present in P. syringae pv. tomato DC3000 cells (data not shown). Thus, hcp1 may be a silent gene or it is expressed under as-yet-uncharacterized conditions. Hence, P. syringae pv. tomato DC3000 differs from the more complex pattern discovered in Pectobacterium atrosepticum, which was previously found to secrete four different Hcp proteins into the culture medium (27). In vitro, P. syringae pv. tomato DC3000 hcp2 expression was enhanced by culture aging, nutrient depletion, and growth on a solid surface, and thus, Hcp2 function could be associated with bacterial growth in a nutrient-poor environment. Induction of hcp2 by quorum sensing could not be verified in liquid culture, possibly because the cell densities were not high enough. When the bacteria were inoculated into host plants, hcp2 expression in P. syringae pv. tomato was not upregulated, which also suggests that T6SS is regulated differently from T3SS and thus may be expressed at a different stage of the P. syringae pv. tomato life cycle. Interestingly, the hcp2 promoter contains an 18-nucleotide-long perfect inverted repeat (see Fig. S1 in the supplemental material). Such a long palindromic structure implies a role as a target sequence for a dimeric regulator protein. Only one “arm” of this repeat sequence is found in the hcp1 promoter, and apart from this motif, the promoter regions of the hcp1 and hcp2 genes show no sequence homology.
When the virulence and fitness of the hcp deletion mutants of P. syringae pv. tomato DC3000 were tested on host plants, none of the mutants were found to be significantly affected in colonization. This result is in agreement with the previous finding of Records and Gross (40) that the P. syringae pv. syringae B728a clpV (T6SS) mutant multiplied in planta and produced disease symptoms similar to those caused by the wild-type strain. In contrast to P. syringae pv. tomato DC3000, which multiplies mainly inside the host plant leaf tissues (5), P. syringae pv. syringae B728a resides primarily as an epiphyte on bean plants. The P. syringae pv. syringae B728a genome was shown to encode a functional T6SS and secrete Hcp under regulation by the sensor kinases RetS, LadS, and GacS (40). Records (39) proposed that the lack of an obvious phenotype in planta may indicate that the T6SS functions in other aspects of P. syringae pv. syringae B728a fitness, possibly in host specificity or intermicrobial interactions. This could include competition with other bacteria for space and nutrients, as well as acting as an antipredation mechanism. In Vibrio cholerae V52, a constitutively active T6SS has been shown to confer virulence toward phagocytic eukaryote cells, including the social amoeba Dictyostelium discoideum and murine macrophages (29, 38). We found evidence to suggest that the Hcp protein(s) of P. syringae pv. tomato DC3000 also plays a role in defense against predation by amoebae. However, the growth phase-dependent expression pattern of P. syringae pv. tomato hcp2 suggests a regulation different from that previously observed for T6SS genes in V. cholerae, Pseudomonas aeruginosa, and P. atrosepticum (18, 21, 23, 27). In P. syringae pv. tomato DC3000, hcp2 expression reached the highest level in the stationary growth phase, suggesting that the function could be important in the late stages of plant infection. In a heavily infected plant, secondary infections by yeasts and molds occur in the damaged plant tissues. Supporting this hypothesis, we found that the T6SS-associated hcp genes of P. syringae are required for competition against another type of eukaryotic organism, i.e., yeasts. Various species of yeast are common in soil and on plant surfaces, and some of them are parasites (56) or symbionts (9) while others live in a more intimate relationship with plants as endophytes (7). Among others, Cryptococcus and Rhodotorula species grow well on plant surfaces, including tomato plants, the host of P. syringae pv. tomato DC3000 (11). P. syringae pv. tomato, as well as other plant-associated bacteria, may have to compete with yeasts for sugar and other nutrients available in plants. Our results, associating hcp2 function with survival in a nutrient-limited environment and yeast growth suppression, suggest that T6SS might be the decisive component to win the battle for nutrients against yeasts. Sessitsch et al. (48) detected a high abundance of T6SS-encoding genes in the metagenome of a rice endophytic bacterial community and estimated that every endophytic bacterium would harbor T6SS (hcp) genes. At present, the molecular mechanism of yeast growth suppression by the T6SS of P. syringae pv. tomato is still unclear. T6SS could secrete toxins that kill the yeast or somehow deplete the environment of a growth factor essential for yeast cells.
When we tested the competitive fitness of P. syringae pv. tomato in cocultures with different bacteria, we found that P. syringae pv. tomato was able to suppress the growth of enterobacteria in a mixed culture and that the survival of P. syringae pv. tomato in competition with enterobacteria was completely dependent on a functional hcp2 gene. Of the bacterial species with which P. syringae pv. tomato was found to be able to compete, E. coli and P. vulgaris are common in surface waters and soil and P. carotovorum is well known to cause soft rot in numerous plants, including tomato plants. Thus, it is likely that P. syringae pv. tomato encounters these bacteria in nature at different stages of its life cycle. In vitro, this competitive activity against enterobacteria was detected only on solid medium and not in a liquid culture with shaking, which is similar to the result previously obtained with P. aeruginosa in a coculture with E. coli (16). Altogether, our findings on interbacterial competition suggest that the observed suppression of E. coli growth in a mixed culture with P. syringae pv. tomato DC3000 probably is not caused by a diffusible compound secreted into the culture medium by P. syringae pv. tomato but rather is a result of a more intimate interaction between the living cells of the two species. Also, the fact that no T6SS-dependent proteins other than Hcp2 were found in the secretome analysis gives support to the idea that these proteins, e.g., VgrG, could be directly translocated into the cells of the competing microorganism. Russell et al. (42) presented evidence of direct cell contact-dependent delivery of T6SS-secreted bacteriolytic effector proteins from P. aeruginosa to other bacteria. However, despite the similarity of P. syringae pv. tomato DC3000 and P. aeruginosa T6SS gene clusters (44), BLAST searches of the genomes of P. syringae strains revealed no sequences clearly homologous to the T6SS-secreted P. aeruginosa effectors Tse2 and Tse3, showing toxin and muramidase activities, respectively (16, 42). The third identified T6SS-secreted effector, Tse1, an amidase-cleaving peptidoglycan (42), shows only partial homology to the NlpC/P60 family bacterial cell wall hydrolase also found in P. syringae. Thus, although the secretion machinery seems to be highly conserved, the effectors P. syringae secretes to survive interbacterial competition are probably different from P. aeruginosa effectors. Burkholderia thailandensis T6SS-1 (47) and Serratia marcescens T6SS (32) were also found to have an essential role in fitness for competition against several other Gram-negative species in a mixed culture, suggesting that this function mediated by T6SS is both important and common.
On the basis of our results, it is clear that none of the hcp deletion mutants of P. syringae pv. tomato DC3000 has any discernible loss of fitness or pathogenicity in plants. Instead, the Hcp function is required for full fitness for competition against other Gram-negative bacteria, including Pectobacterium species, the opportunistic, soft-rot-causing plant pathogens, and against yeasts and amoebae, under biofilm-like conditions on a solid surface. Only hcp2 is required for interbacterial competition, but in competition with yeast, hcp1 also seems to play a minor role. As the two T6SSs of P. syringae pv. tomato DC3000 seem to be differently regulated, they might be specialized in interactions with different organisms. However, we cannot rule out the possibility that HSI-I is a silent operon, dominated by HSI-II. Functional divergence of different T6SS clusters in a bacterial species was discovered in Burkholderia (47), with one cluster specialized in interaction with the eukaryotic host and another cluster required for interbacterial competition. Moreover, in V. cholerae V52, which carries only one T6SS cluster, different T6SS-secreted VgrG proteins have different impacts on antibacterial and antiamoeba functions (57). In the context of interaction with plants and plant-associated microbes, we can postulate that the T6SS of P. syringae pv. tomato DC3000 functions to protect the bacterium from a range of competitors and predators. Further identification of T6SS effectors and mechanisms underlying this competitive fitness of P. syringae pv. tomato DC3000 should help us understand why P. syringae pv. tomato DC3000 possesses two divergent T6SS clusters. It will be of particular interest to determine whether the T6SS enhances bacterial survival in soil or leaf litter or prevents competitor ingress at sites of infection.
Supplementary Material
ACKNOWLEDGMENTS
We warmly thank Matti Korhola at the University of Helsinki for sharing his knowledge of yeasts.
For financial support, we thank the Academy of Finland, Centre of Excellence Program 2006-2011 project 129628, Comparative Proteomics of Plant Pathogenic Bacteria project 206219, and the Frontier and Innovative Research Program of National Taiwan University (99R70443, awarded to N.-C. Lin).
Footnotes
Published ahead of print 29 June 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Arnold DL, Godfrey SAC, Jackson RW. 2009. Pseudomonas syringae genomics provides important insights to secretion systems, effector genes and the evolution of virulence, p 203–226 In Jackson RW. (ed), Plant pathogenic bacteria: genomics and molecular biology. Academic Press, London, United Kingdom [Google Scholar]
- 2. Bais HP, Fall R, Vivanco JM. 2004. Biocontrol of Bacillus subtilis against infection of Arabidopsis roots by Pseudomonas syringae is facilitated by biofilm formation and surfactin production. Plant Physiol. 134:307–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Basler M, Pilhofer M, Henderson GP, Jensen GJ, Mekalanos JJ. 2012. Type VI secretion requires a dynamic contractile phage tail-like structure. Nature 483:182–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bingle LE, Bailey CM, Pallen MJ. 2008. Type VI secretion: a beginner's guide. Curr. Opin. Microbiol. 11:3–8 [DOI] [PubMed] [Google Scholar]
- 5. Boureau T, Routtu J, Roine E, Taira S, Romantschuk M. 2002. Localization of hrpA-induced Pseudomonas syringae pv. tomato DC3000 in infected tomato leaves. Mol. Plant Pathol. 3:451–460 [DOI] [PubMed] [Google Scholar]
- 6. Boyer F, Fichant G, Berthod J, Vandenbrouvk Y, Attree I. 2009. Dissecting the bacterial type VI secretion system by a genome wide in silico analysis: what can be learned from available microbial genomic resources? BMC Genomics 10:104–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Camatti-Sartori V, et al. 2005. Endophytic yeasts and filamentous fungi associated with southern Brazilian apple (Malus domestica) orchards subjected to conventional, integrated or organic cultivation. J. Basic Microbiol. 45:397–402 [DOI] [PubMed] [Google Scholar]
- 8. Cascales E. 2008. The type VI secretion toolkit. EMBO Rep. 9:735–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cloete KJ, Valentine AJ, Botha A. 2010. Effect of the soil yeast Cryptococcus laurentii on the photosynthetic water and nutrient-use efficiency and respiratory carbon costs of a Mediterranean sclerophyll, Agathosma betulina (Berg.) Pillans. Symbiosis 51:245–248 [Google Scholar]
- 10. Clos J, Brandau S. 1994. pJC20 and pJC40—two high-copy-number vectors for T7 RNA polymerase-dependent expression of recombinant genes in Escherichia coli. Protein Expr. Purif. 5:133–137 [DOI] [PubMed] [Google Scholar]
- 11. Elad Y, Köhl J, Fokkema NJ. 1994. Control of infection and sporulation of Botrytis cinerea on bean and tomato by saprophytic yeasts. Phytopathology 84:1193–1200 [Google Scholar]
- 12. Fronzes R, Christie PJ, Waksman G. 2009. The structural biology of type IV secretion systems. Nat. Rev. Microbiol. 7:703–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guo M, Tian F, Wamboldt Y, Alfano JR. 2009. The majority of the type III effector inventory of Pseudomonas syringae pv. tomato DC3000 can suppress plant immunity. Mol. Plant Microbe Interact. 22:1069–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haapalainen M, Karp M, Metzler MC. 1996. Isolation of strong promoters from Clavibacter xyli subsp. cynodontis using a promoter probe plasmid. Biochim. Biophys. Acta 1305:130–134 [DOI] [PubMed] [Google Scholar]
- 15. Haapalainen M, Van Gestel K, Pirhonen M, Taira S. 2009. Soluble plant cell signals induce the expression of the type III secretion system of Pseudomonas syringae and upregulate the production of pilus protein HrpA. Mol. Plant Microbe Interact. 22:282–290 [DOI] [PubMed] [Google Scholar]
- 16. Hood RD, et al. 2010. A type VI secretion system of Pseudomonas aeruginosa targets a toxin to bacteria. Cell Host Microbe 7:25–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huynh TV, Dahlbeck D, Staskawicz BJ. 1989. Bacterial blight of soybean: regulation of a pathogen gene determining host cultivar specificity. Science 245:1374–1377 [DOI] [PubMed] [Google Scholar]
- 18. Ishikawa T, Rompikuntal PK, Lindmark B, Milton DL, Wai SN. 2009. Quorum sensing regulation of the two hcp alleles in Vibrio cholerae O1 strains. PLoS One 4:e6734 doi:10.1371/journal.pone.0006734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. King EO, Ward MK, Raney DE. 1954. Two simple media for the demonstration of pyocyanin and fluorescin. J. Lab. Clin. Med. 44:301–307 [PubMed] [Google Scholar]
- 20. Leiman PG, et al. 2009. Type VI secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin. Proc. Natl. Acad. Sci. U. S. A. 106:4154–4159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lesic B, Starkey M, He J, Hazan R, Rahme GL. 2009. Quorum sensing differentially regulates Pseudomonas aeruginosa type VI secretion locus I and homologous loci II and III, which are required for pathogenesis. Microbiology 155:2845–2855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin NC, Martin GB. 2005. An avrPto/avrPtoB mutant of Pseudomonas syringae pv. tomato DC3000 does not elicit Pto-mediated resistance and is less virulent on tomato. Mol. Plant Microbe Interact. 18:43–51 [DOI] [PubMed] [Google Scholar]
- 23. Liu H, et al. 2008. Quorum sensing coordinates brute force and stealth modes of infection in the plant pathogen Pectobacterium atrosepticum. PLoS Pathog. 4:e1000093 doi:10.1371/journal.ppat.1000093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma L-S, Lin J-S, Lai E-M. 2009. An IcmF family protein, ImpLM, is an integral inner membrane protein interacting with ImpKL, and its Walker A motif is required for type VI secretion system-mediated Hcp secretion in Agrobacterium tumefaciens. J. Bacteriol. 191:4316–4329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Macho AP, Zumaquero A, Ortiz-Martín I, Beuzón CR. 2007. Competitive index in mixed infections: a sensitive and accurate assay for the genetic analysis of Pseudomonas syringae-plant interactions. Mol. Plant Pathol. 8:437–450 [DOI] [PubMed] [Google Scholar]
- 26. MacIntyre DL, Miyata ST, Kitaoka M, Pukatzki S. 2010. The Vibrio cholerae type VI secretion system displays antimicrobial properties. Proc. Natl. Acad. Sci. U. S. A. 107:19520–19524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mattinen L, Nissinen R, Riipi T, Kalkkinen N, Pirhonen M. 2007. Host-extract induced changes in the secretome of the plant pathogenic bacterium Pectobacterium atrosepticum. Proteomics 7:3527–3537 [DOI] [PubMed] [Google Scholar]
- 28. Mattinen L, et al. 2008. Microarray profiling of host-extract-induced genes and characterization of the type VI secretion cluster in the potato pathogen Pectobacterium atrosepticum. Microbiology 154:2387–2396 [DOI] [PubMed] [Google Scholar]
- 29. Miyata ST, Kitaoka M, Brooks TM, McAuley SB, Pukatzki S. 2011. Vibrio cholerae requires the type VI secretion system virulence factor VasX to kill Dictyostelium discoideum. Infect. Immun. 79:2941–2949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morris CE, et al. 2008. The life history of the plant pathogen Pseudomonas syringae is linked to the water cycle. ISME J. 2:321–334 [DOI] [PubMed] [Google Scholar]
- 31. Mougous JD, et al. 2006. A virulence locus of Pseudomonas aeruginosa encodes a protein secretion apparatus. Science 312:1526–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murdoch SL, et al. 2011. The opportunistic pathogen Serratia marcescens utilises type VI secretion to target bacterial competitors. J. Bacteriol. 193:6057–6069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nissinen R, Ytterberg AJ, Bogdanove AJ, van Wijk KJ, Beer SV. 2007. Analyses of the secretomes of Erwinia amylovora and selected hrp mutants reveal novel type III secreted proteins and an effect of HrpJ on extracellular harpin levels. Mol. Plant Pathol. 8:55–67 [DOI] [PubMed] [Google Scholar]
- 34. Pell LG, Kanelis V, Donaldson LW, Howell PL, Davidson AR. 2009. The phage major tail protein structure reveals a common evolution for long-tailed phages and the type VI bacterial secretion system. Proc. Natl. Acad. Sci. U. S. A. 106:4160–4165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pirhonen M, Heino P, Helander I, Harju P, Palva ET. 1988. Bacteriophage T4 resistant mutants of the plant pathogen Erwinia carotovora. Microb. Pathog. 4:359–367 [DOI] [PubMed] [Google Scholar]
- 36. Preston GM, Studholme DJ, Caldelari I. 2005. Profiling the secretomes of plant pathogenic proteobacteria. FEMS Microbiol. Rev. 29:331–360 [DOI] [PubMed] [Google Scholar]
- 37. Pukatzki S, Ma AT, Revel AT, Sturtevant D, Mekalanos JJ. 2007. Type VI secretion system translocates a phage tail spike-like protein into target cells where it cross-links actin. Proc. Natl. Acad. Sci. U. S. A. 104:15508–15513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pukatzki S, et al. 2006. Identification of a conserved bacterial protein secretion system in Vibrio cholerae using the Dictyostelium host model system. Proc. Natl. Acad. Sci. U. S. A. 103:1528–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Records AR. 2011. The type VI secretion system: a multipurpose delivery system with a phage-like machinery. Mol. Plant Microbe Interact. 24:751–757 [DOI] [PubMed] [Google Scholar]
- 40. Records AR, Gross DC. 2010. Sensor kinases RetS and LadS regulate Pseudomonas syringae type VI secretion and virulence factors. J. Bacteriol. 192:3584–3596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Roine E, et al. 1997. Hrp pilus: an hrp-dependent bacterial surface appendage produced by Pseudomonas syringae pv. tomato DC3000. Proc. Natl. Acad. Sci. U. S. A. 94:3459–3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Russell AB, et al. 2011. Type VI secretion delivers bacteriolytic effectors to target cells. Nature 475:343–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saarilahti HT, Palva ET. 1986. Major outer membrane proteins in the phytopathogenic bacteria Erwinia carotovora subsp. carotovora and subsp. atroseptica. FEMS Microbiol. Lett. 35:267–270 [Google Scholar]
- 44. Sarris PF, Skandalis N, Kokkinidis M, Panopoulos NJ. 2010. In silico analysis reveals multiple putative type VI secretion systems and effector proteins in Pseudomonas syringae pathovars. Mol. Plant Pathol. 11:795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schäfer A, Tauch A, Jäger Kalinowski WJ, Thierbach G, Pühler A. 1994. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene 145:69–73 [DOI] [PubMed] [Google Scholar]
- 46. Schell MA, et al. 2007. Type VI secretion is a major virulence determinant in Burkholderia mallei. Mol. Microbiol. 64:1466–1485 [DOI] [PubMed] [Google Scholar]
- 47. Schwarz S, et al. 2010. Burkholderia type VI secretion systems have distinct roles in eukaryotic and bacterial cell interactions. PLoS Pathog. 6:e1001068 doi:10.1371/journal.ppat.1001068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sessitsch A, et al. 2012. Functional characteristics of an endophyte community colonizing rice roots as revealed by metagenomic analysis. Mol. Plant Microbe Interact. 25:28–36 [DOI] [PubMed] [Google Scholar]
- 49. Simon R, Priefer U, Pühler A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Nat. Biotechnol. 1:784–791 [Google Scholar]
- 50. Suarez G, et al. 2010. A type VI secretion system effector protein, VgrG1, from Aeromonas hydrophila that induces host cell toxicity by ADP ribosylation of actin. J. Bacteriol. 192:155–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Tseng TT, Tyler BM, Setubal JC. 2009. Protein secretion systems in bacterial-host associations, and their description in the gene ontology. BMC Microbiol. 9(Suppl 1):S2 doi:10.1186/1471-2180-9-S1-S2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Veesler D, Cambillau C. 2011. A common evolutionary origin for tailed-bacteriophage functional modules and bacterial machineries. Microbiol. Mol. Biol. Rev. 75:423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wei CF, et al. 2007. A Pseudomonas syringae pv. tomato DC3000 mutant lacking the type III effector HopQ1-1 is able to cause disease in the model plant Nicotiana benthamiana. Plant J. 51:32–46 [DOI] [PubMed] [Google Scholar]
- 54. Wu H-Y, Chung P-C, Shih H-W, Wen S-R, Lai E-M. 2008. Secretome analysis uncovers an Hcp-family protein secreted via a type VI secretion system in Agrobacterium tumefaciens. J. Bacteriol. 190:2841–2850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xiao Y, Heu S, Yi J, Lu Y, Hutcheson SW. 1994. Identification of a putative alternate sigma factor and characterization of a multicomponent regulatory cascade controlling the expression of Pseudomonas syringae pv. syringae Pss61 hrp and hrmA genes. J. Bacteriol. 176:1025–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xue C, Tada Y, Dong X, Heitman J. 2007. The human fungal pathogen Cryptococcus can complete its sexual cycle during a pathogenic association with plants. Cell Host Microbe 1:263–273 [DOI] [PubMed] [Google Scholar]
- 57. Zheng J, Ho B, Mekalanos JJ. 2011. Genetic analysis of anti-amoebae and anti-bacterial activities of the type VI secretion system in Vibrio cholerae. PLoS One 6:e23876 doi:10.1371/journal.pone.0023876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zheng J, Leung KY. 2007. Dissection of a type VI secretion system in Edwardsiella tarda. Mol. Microbiol. 66:1192–1206 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.