Abstract
The organic hydroperoxide resistance protein Ohr has been identified in numerous bacteria where it functions in the detoxification of organic hydroperoxides, and expression of ohr is often regulated by a MarR-type regulator called OhrR. The genes annotated as BAB2_0350 and BAB2_0351 in the Brucella abortus 2308 genome sequence are predicted to encode OhrR and Ohr orthologs, respectively. Using isogenic ohr and ohrR mutants and lacZ promoter fusions, it was determined that Ohr contributes to resistance to organic hydroperoxide, but not hydrogen peroxide, in B. abortus 2308 and that OhrR represses the transcription of both ohr and ohrR in this strain. Moreover, electrophoretic mobility shift assays and DNase I footprinting revealed that OhrR binds directly to a specific region in the intergenic region between ohr and ohrR that shares extensive nucleotide sequence similarity with so-called “OhrR boxes” described in other bacteria. While Ohr plays a prominent role in protecting B. abortus 2308 from organic hydroperoxide stress in in vitro assays, this protein is not required for the wild-type virulence of this strain in cultured murine macrophages or experimentally infected mice.
INTRODUCTION
Brucella spp. are Gram-negative bacteria that belong to the α2 subclass of proteobacteria (30), and these bacteria infect a variety of wild and domestic animals, including cattle, sheep and goats, and swine, leading to abortions in pregnant females and sterility in males. Some Brucella strains also produce a zoonotic, debilitating febrile illness in humans (31). In areas of the world where brucellosis is endemic in food animals, including regions of Asia, Africa, and the Middle East, Brucella strains represent a major public health concern (1).
Within their mammalian hosts, the brucellae infect and reside within cells of the reticuloendothelial system, particularly macrophages (5). In these cells, the brucellae encounter a variety of harsh conditions, including nutrient deprivation, decreased pH, and exposure to reactive oxygen and nitrogen species (ROS and RNS, respectively) (33). ROS, most notably O2− and H2O2, are produced by macrophages as a defense mechanism against invading pathogens (12), and these molecules can cause DNA damage in the bacterium by participating in Fenton chemistry, leading to death of the bacterium (17, 18). ROS and RNS can also be harmful by causing the peroxidation of membrane lipids, leading to decreased membrane fluidity and subsequent dysfunction of membrane proteins (33).
Pathogenic bacteria, including Brucella strains, have evolved multiple strategies to resist the microbicidal activities of ROS and RNS produced by professional phagocytes in the host. Although the brucellae produce many enzymes capable of directly or indirectly detoxifying ROS and RNS (19, 35), only the periplasmic Cu,Zn superoxide dismutase SodC has been shown to play a role in protecting the intracellular brucellae from the oxidative killing pathways of host phagocytes (15).
The organic hydroperoxide resistance protein Ohr has been identified and characterized in several bacteria, including Actinobacillus pleuropneumoniae (37), Agrobacterium tumefaciens (6), Bacillus subtilis (14), Mycoplasma genitalium (36), Pseudomonas aeruginosa (2, 23), Francisella tularensis (24), and Sinorhizobium meliloti (11). These proteins belong to a family of peroxiredoxins that detoxify organic peroxides such as tert-butyl hydroperoxide or cumene hydroperoxide but are incapable of detoxifying H2O2 (7, 26). Organic peroxides are found widely in plants, where resistance to these compounds has been proposed to be an important determinant of the successful interactions between bacterial pathogens and symbionts and their plant hosts (20). In contrast, lipid peroxides formed by oxidative damage to host cell fatty acids have been proposed to be the only biologically relevant type of organic peroxides found in mammalian cells (16), and the importance of being resistant to organic peroxides for bacteria that are mammalian pathogens is unresolved (2, 8, 37).
Expression of bacterial ohr genes is typically controlled at the transcriptional level by the MarR-type regulator OhrR (9, 28). Under uninduced conditions, OhrR is in a reduced form that tightly binds to the ohr promoter, inhibiting ohr transcription (14). Upon exposure to organic peroxides, a conserved cysteine residue in OhrR becomes oxidized, and this transcriptional repressor loses its DNA binding activity, leading to elevated ohr transcription (10, 13). In many bacteria, OhrR also binds to the ohrR promoter region, leading to an autoregulatory circuit (2, 6, 14, 29).
The genes annotated as BAB2_0350 and BAB2_0351 in the B. abortus 2308 genome sequence are predicted to encode OhrR and Ohr orthologs, respectively. The studies described in this report were carried out to confirm the biological functions of these proteins and determine to what extent, if any, they contribute to the virulence of B. abortus 2308 in the mouse model of infection.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Brucella abortus 2308 and derivative strains were routinely grown on Schaedler blood agar (SBA; BD, Franklin Lakes, NJ) containing 5% defibrinated bovine blood (Quad Five, Ryegate, MT) or in brucella broth (BD). For cloning and recombinant protein expression, Escherichia coli strains (DH5α and BL21) were grown on tryptic soy agar (BD) or in Luria-Bertani (LB) broth. When appropriate, growth media were supplemented with ampicillin (100 μg/ml) or kanamycin (45 μl/ml).
Construction and genetic complementation of B. abortus ohr and ohrR mutants.
The ohrR and ohr genes (BAB2_0350 and BAB2_0351, respectively) in Brucella abortus 2308 were mutated using a nonpolar, unmarked gene excision strategy. An approximately 1-kb fragment of the upstream region of each gene to the second codon of the coding region was amplified by PCR using primers Δohr-Up-For and Δohr-Up-Rev (ohr mutant) or ΔohrR-Up-For and ΔohrR-Up-Rev (ohrR mutant) and genomic DNA from Brucella abortus 2308 as a template. Similarly, a fragment containing the last two codons of the coding region to approximately 1 kb downstream of each open reading frame (ORF) was amplified with primers Δohr-Down-For and Δohr-Down-Rev (ohr mutant) or ΔohrR-Down-For and ΔohrR-Down-Rev (ohrR mutant). The sequences of all oligonucleotide primers used in this study can be found in Table S1 in the supplemental material. The upstream fragment was digested with BamHI, while the downstream fragment was digested with PstI, and both fragments were treated with polynucleotide kinase in the presence of ATP. Both of the DNA fragments were included in a single ligation mix with BamHI/PstI-digested pNTPS138 (38), which contains an nptI-type kanamycin resistance gene and sacB for counterselection with sucrose. The resulting plasmids (pC3015 [Δohr] and pC3016 [ΔohrR]) (Table 1) were introduced into B. abortus 2308, and merodiploid transformants were obtained by selection on SBA plus kanamycin. Kanamycin-resistant transformants were grown for 6 to 8 h in brucella broth and then plated onto SBA containing 10% sucrose. Genomic DNA from sucrose-resistant, kanamycin-sensitive colonies was isolated and screened by PCR for loss of the ohr or ohrR gene. The isogenic ohr mutant derived from B. abortus 2308 was named JB15, and the isogenic ohrR mutant derived from B. abortus 2308 was named JB16. The genotypes of the mutant strains were verified by DNA sequence analyses and confirmed by Southern hybridizations.
Table 1.
Plasmids used in this study
| Plasmid name | Description | Source or reference |
|---|---|---|
| pBBR1MCS-4 | Broad-host-range cloning vector; Ampr | 21 |
| pNPTS138 | Cloning vector; contains sacB gene; Kanr | 38 |
| pMR15 | Broad-host-range vector containing a promoterless lacZ gene; Kanr | 3 |
| pC3015 | In-frame deletion of ohr plus 1 kb of each flanking region in pNPTS138 | This study |
| pC3016 | In-frame deletion of ohrR plus 1 kb of each flanking region in pNPTS138 | This study |
| pC3018 | ohr locus including the entire promoter region in pBBR1MCS-4 | This study |
| pC3019 | ohrR locus including the entire promoter region in pBBR1MCS-4 | This study |
| pC3020 | ohr promoter region cloned into pMR15 | This study |
| pC3021 | ohrR promoter region cloned into pMR15 | This study |
Plasmid pC3015 was also introduced into the previously described B. abortus ahpC mutant KH40 (39) by electroporation, and selection for an ahpC ohr double mutant was performed as described above. Following confirmation of its genotype by DNA sequence analysis and Southern hybridization, the resulting B. abortus ahpC ohr double mutant was named CC104.
Genetic complementation of the B. abortus ohr and ohrR mutants was achieved by in trans complementation using pBBR1MCS-4 (21). The ohr gene, along with the native ohr promoter, was amplified by PCR using primers ohr-comp-For and ohr-comp-Rev (see Table S1 in the supplemental material). The resulting DNA fragment was digested with PstI, followed by treatment with polynucleotide kinase, and the digested/treated fragment was ligated into PstI/HincII-digested pBBR1MCS-4, yielding the plasmid pC3017. The ohrR gene, along with the native ohrR promoter, was amplified by PCR using primers ohrR-comp-For and ohrR-comp-Rev (see Table S1), and this fragment was cloned into pBBR1MCS-4, producing pC3018. These plasmids were introduced into the corresponding B. abortus ohr and ohrR mutants by electroporation.
Construction of ohr-lacZ and ohrR-lacZ transcriptional fusions and β-galactosidase assays.
The promoter regions of ohr and ohrR were fused to a lacZ reporter as transcriptional fusions. For the ohr promoter fusion, approximately 200 bp of the ohr upstream region was amplified by PCR using primers ohr-lacZ-For and ohr-lacZ-Rev (see Table S1 in the supplemental material) and Brucella abortus 2308 genomic DNA as a template. For the ohrR promoter fusion, approximately 200 bp of the ohrR upstream region was amplified by PCR using primers ohrR-lacZ-For and ohrR-lacZ-Rev (see Table S1) and Brucella abortus 2308 genomic DNA as a template. The amplified DNA fragments were sequentially digested with BamHI and HindIII and subsequently ligated into BamHI/HindIII-digested pMR15 (3), which contains a promoterless lacZ gene. The resulting plasmids, pC3020 and pC3021, carrying the ohr-lacZ and ohrR-lacZ transcriptional fusions, respectively, were introduced into B. abortus 2308 and the isogenic ohr and ohrR mutants by electroporation. β-Galactosidase production by the Brucella strains carrying the ohr-lacZ and ohrR-lacZ fusions was determined using the methods described by Miller (25).
Real-time reverse transcriptase PCR (RT-PCR).
Brucella abortus 2308 was grown in brucella broth to late exponential phase (∼109 CFU/ml), and the cultures were treated with 5 mM H2O2, 5 mM cumene hydroperoxide, and 5 mM tert-buytl hydroperoxide or left untreated for 20 min with constant shaking at 37°C. Total RNA was isolated from the cultures, and genomic DNA was removed as described previously (4). cDNA was generated from the final RNA preparation using the SuperScript III cDNA synthesis system (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol, and this cDNA was used for real-time PCR employing a SYBR green PCR supermix (Roche, Mannheim, Germany). For these experiments, primers for 16S rRNA were used as a control, while gene-specific primers were used for evaluating relative levels of ohr and ohrR mRNA (see Table S1 in the supplemental material). Parameters for PCR included a single denaturing step for 5 min at 95°C, followed by 40 cycles (denature for 15 s at 95°C, anneal for 15 s at 50°C, and extend for 15 s at 72°C) of amplification. Fluorescence from SYBR green incorporation into double-stranded DNA was measured with an iCycler machine (Bio-Rad), and the relative abundance of mRNA was determined using the Pfaffl equation (32).
Expression and purification of recombinant OhrR.
The Strep-tag II system (IBA, Göttingen, Germany) was used to produce a recombinant version of the Brucella OhrR protein (rOhrR) in E. coli strain BL21. The coding region of the ohrR gene (BAB2_0350) was amplified using the primers rOhrR-For and rOhrR-Rev (see Table S1 in the supplemental material), B. abortus 2308 chromosomal DNA as a template, and Taq polymerase. The amplified DNA fragment was digested with BbsI and ligated into BsaI-digested pASK-IBA6, which is designed to produce an amino-terminal Strep-tag II “tagged” version of the protein of interest. The resulting plasmid, prOhrR, was transformed into E. coli strain BL21, and the strain harboring the recombinant protein expression plasmid was grown to an optical density at 600 nm (OD600) of approximately 0.6 in LB broth before production of the recombinant protein was initiated by the addition of anhydrotetracycline (final concentration, 200 μg/ml) to the growth medium. Following further incubation for 2 h at 37°C, the bacterial cells were collected by centrifugation (4,200 × g for 10 min at 4°C) and lysed by treatment with CelLytic B (Sigma, St. Louis, MO) in the presence of the protease inhibitor phenylmethylsulfonyl fluoride. The supernatant from the suspension of lysed cells was cleared by centrifugation (14,000 × g for 10 min at 4°C), and the clarified supernatant was passed through an affinity column packed with Strep-Tactin Sepharose. The column was washed extensively with buffer W (100 mM Tris-HCl, 300 mM NaCl, 1 mM EDTA [pH 8.0]), and recombinant protein was eluted with 2.5 mM desthiobiotin in buffer W. The degree of purity of recombinant OhrR was high as judged by visualization of a single major band on SDS-PAGE.
Determination of the transcriptional start site for ohr by primer extension.
Total RNA was extracted from B. abortus grown in brucella broth to late exponential phase as described previously (4). The oligonucleotide primer ohr-PE was end labeled with [γ-32P]ATP (PerkinElmer, San Jose, CA) and polynucleotide kinase (Promega, Madison, WI), and the labeled primer was annealed to 50 μg of Brucella RNA. Primer extension analysis was carried out using the AMV RT primer extension system (Promega, Madison, WI) according to the manufacturer's instructions. DNA sequence analysis was performed using the same radiolabeled primer and the SequiTherm EXCEL II DNA sequencing kit (Epicentre, Madison, WI) by following the manufacturer's protocol. Primer extension products and DNA sequencing reaction products were separated on 6% denaturing polyacrylamide gels containing 7 M urea and visualized by autoradiography.
Determination of the transcriptional start site for ohrR by 5′ rapid amplification of cDNA ends (5′-RACE).
5′-RACE was carried out using the FirstChoice RLM-RACE kit (Ambion, Austin, TX) according to the manufacturer's instructions. Brucella abortus 2308 was grown in brucella broth to late exponential phase, and RNA was isolated and treated with DNase I as described previously (4). The ohrR gene-specific primer ohrR-RACE (see Table S1 in the supplemental material) was used in the PCR steps of the 5′-RACE protocol where Taq polymerase was also employed. The ohrR 5′-RACE product was gel purified and cloned into pGEM-T Easy. Plasmid DNA was purified from E. coli transformants, and DNA sequencing was performed to identify the ohrR transcriptional start site.
EMSAs.
All rOhrR electrophoretic mobility shift assay (EMSA) experiments were carried out in a 20-μl total reaction volume containing binding buffer composed of 10 mM Tris-HCl (pH 7.4), 50 mM KCl, 1 mM dithiothreitol, 6% glycerol, 0.5 mM EDTA, 50 μg/ml bovine serum albumin, and 50 μg/ml salmon sperm DNA. A 205-bp DNA fragment corresponding to the ohrR-ohr intergenic region was amplified by PCR from Brucella abortus 2308 chromosomal DNA using primers ΔohrR-Down-For and Δohr-Up-Rev (see Table S1 in the supplemental material) and chromosomal DNA from B. abortus 2308 as a template. The amplified DNA fragment was purified by agarose gel electrophoresis, and the fragment was end labeled with [γ-32P]ATP (PerkinElmer, San Jose, CA) and polynucleotide kinase (Promega, Madison, WI). Increasing amounts of recombinant OhrR were mixed with radiolabeled ohr-ohrR intergenic region DNA in binding buffer, and the reaction mixtures were incubated at room temperature for 20 min. As controls, 50× molar concentrations of nonradiolabeled ohr-ohrR intergenic region DNA (specific competitor) or nonradiolabeled ohrR coding region DNA (nonspecific competitor) were added to some reaction mixtures. The binding reaction mixtures were subjected to electrophoresis on 6% native polyacrylamide gels in 0.5× Tris-borate-EDTA (TBE) running buffer for approximately 1 h at 4°C. Following electrophoresis, gels were dried onto Whatman 3MM filter paper using a vacuum gel dryer system and visualized by autoradiography.
DNase I footprint analysis.
A DNA fragment corresponding to the ohr-ohrR intergenic region was used for DNase I footprint analysis. Oligonucleotide primers (ΔohrR-Down-For and Δohr-Up-Rev) were individually end labeled with [γ-32P]ATP (PerkinElmer, San Jose, CA) and polynucleotide kinase (Promega, Madison, WI), and the primers were used in individual PCRs with Taq polymerase to generate DNA fragments with a radiolabeled plus or minus strand. Following gel purification, approximately 10,000 cpm of each labeled DNA fragment was incubated separately in EMSA binding buffer (excluding EDTA) containing increasing concentrations of recombinant OhrR protein (total reaction volume, 20 μl). Following incubation at room temperature for 20 min, the binding reaction mixtures were treated with 0.25 U of RQ1 DNase I (Promega, Madison, WI) for 2 min at room temperature. The DNase I reactions were terminated by the addition of 0.5 M EDTA and incubation at 90°C for 5 min. The reaction mixtures were then cleaned by phenol-chloroform extraction, and following ethanol precipitation, the reaction mixture contents were suspended in formamide loading dye (STR 2× loading solution from Promega). DNA sequence analysis was performed using the same radiolabeled primers and the SequiTherm EXCEL II DNA sequencing kit (Epicentre, Madison, WI) by following the manufacturer's protocol. The digested DNA fragments and DNA sequencing reaction products were separated on 8% denaturing polyacrylamide gels containing 7 M urea and visualized by autoradiography.
Sensitivity of B. abortus strains to hydrogen peroxide and cumene hydroperoxide in a disk assay.
Brucella strains were grown on Schaedler blood agar at 37°C under 5% CO2 for 48 to 72 h, and the bacterial cells were harvested in phosphate-buffered saline (PBS) and suspended at a concentration of ∼3.33 × 107 CFU/ml in brucella broth containing 0.5% agar (maintained at 55°C). Three milliliters of this suspension was overlaid onto brucella agar plates, and after solidification of the overlay, a sterile 7-mm Whatman disk was placed in the center of each plate. Seven microliters of a 30% H2O2 or 0.5 M cumene hydroperoxide solution was applied to each filter disk, and the plates were incubated at 37°C with 5% CO2 for 72 h. The zone of inhibition around each disk was then measured in millimeters.
Virulence of Brucella strains in cultured murine macrophages and experimentally infected mice.
Experiments to test the virulence of Brucella strains in primary, murine peritoneal macrophages were carried out as described previously (15). Briefly, resident peritoneal macrophages were isolated from mice and seeded into 96-well plates in Dulbecco's modified Eagle's medium with 5% fetal bovine serum, and the following day, the macrophages were infected with opsonized brucellae at a multiplicity of infection of 50:1. After 2 h of infection, extracellular bacteria were killed by treatment with gentamicin (50 μg/ml). For the 2-hour time point, the macrophages were then lysed with 0.1% deoxycholate in PBS, and serial dilutions were plated on Schaedler blood agar (SBA). For the 24- and 48-hour time points, the cells were washed with PBS following gentamicin treatment, and fresh cell culture medium containing gentamicin (20 μg/ml) was added to the monolayer. At the indicated time point, the macrophages were lysed, and serial dilutions were plated on SBA. Triplicate wells were used for each Brucella strain tested.
The infection and colonization of mice by Brucella strains was as described previously (15, 34). C57BL/6 mice (5 per Brucella strain) were infected intraperitoneally with ∼5 × 104 CFU of each Brucella strain in sterile PBS. The mice were sacrificed at 1 and 4 weeks postinfection, and serial dilutions of spleen homogenates were plated on SBA.
RESULTS AND DISCUSSION
B. abortus ohr and ohrR mutants exhibit divergent sensitivities to cumene hydroperoxide.
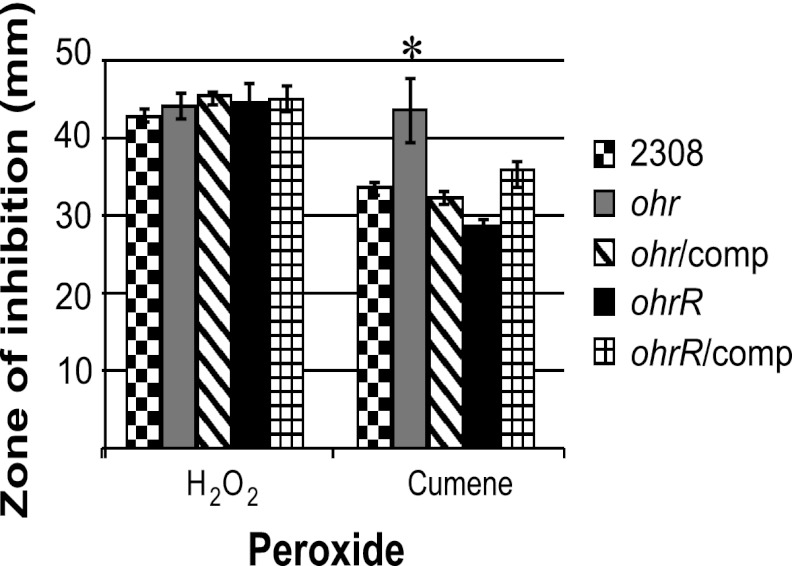
To determine the roles of Ohr and OhrR in the response of Brucella abortus 2308 to different types of peroxide stress, isogenic ohr and ohrR mutants were constructed, and the sensitivities of these strains to hydrogen peroxide (H2O2) and cumene hydroperoxide (CuOOH), an organic hydroperoxide, were assessed. There was no significant difference in the zones of inhibition exhibited by these strains when they were exposed to H2O2 in a disk diffusion assay (Fig. 1). In contrast, the B. abortus ohr mutant JB15 exhibited a significantly larger zone of inhibition when exposed to CuOOH than the parental strain 2308, and this increased susceptibility to CuOOH was alleviated in a derivative of JB15 carrying the ohr gene on a pBBR1MCS-based plasmid. These data indicate that, similar to its orthologs in other bacteria, Ohr in Brucella abortus 2308 functions as a detoxifier of organic hydroperoxides but is not responsible for the detoxification of H2O2.
Fig 1.
Ohr and OhrR are important determinants of resistance to organic hydroperoxide stress in Brucella abortus 2308. Isogenic ohr and ohrR mutants were constructed in Brucella abortus 2308, and each mutant strain was complemented with a plasmid-borne copy of the corresponding gene. These strains were tested in a disk diffusion assay for their comparative susceptibilities to hydrogen peroxide (H2O2) and the organic peroxide cumene hydroperoxide. The results are plotted as the average diameter (± standard deviation) of the zone of inhibition around a disk containing the indicated peroxide, and the results are from single experiment that was repeated in triplicate. An asterisk denotes a statistically significant difference (P < 0.05) between a given strain and the parental strain 2308.
The sensitivity of the B. abortus ohrR mutant to organic peroxide was also assessed in the disk diffusion assay, and in contrast to the phenotype displayed by the isogenic ohr mutant, the ohrR mutant JB16 exhibited a decreased zone of inhibition around CuOOH in the disk assay compared to the parental 2308 strain (Fig. 1). Importantly, the decreased susceptibility of the B. abortus ohrR mutant to CuOOH was reversed when a plasmid-borne copy of ohrR was introduced into this strain. In other bacteria, OhrR has been shown to be a repressor of ohr transcription, and therefore, in the absence of OhrR (i.e., the ohrR mutant), levels of Ohr would be increased, even in a situation where no organic hydroperoxide stress is present. Consequently, the phenotype exhibited by the B. abortus ohrR mutant in this assay supports the proposition that OhrR represses ohr transcription in B. abortus 2308.
A B. abortus ohrR mutant exhibits elevated expression of both ohr and ohrR.
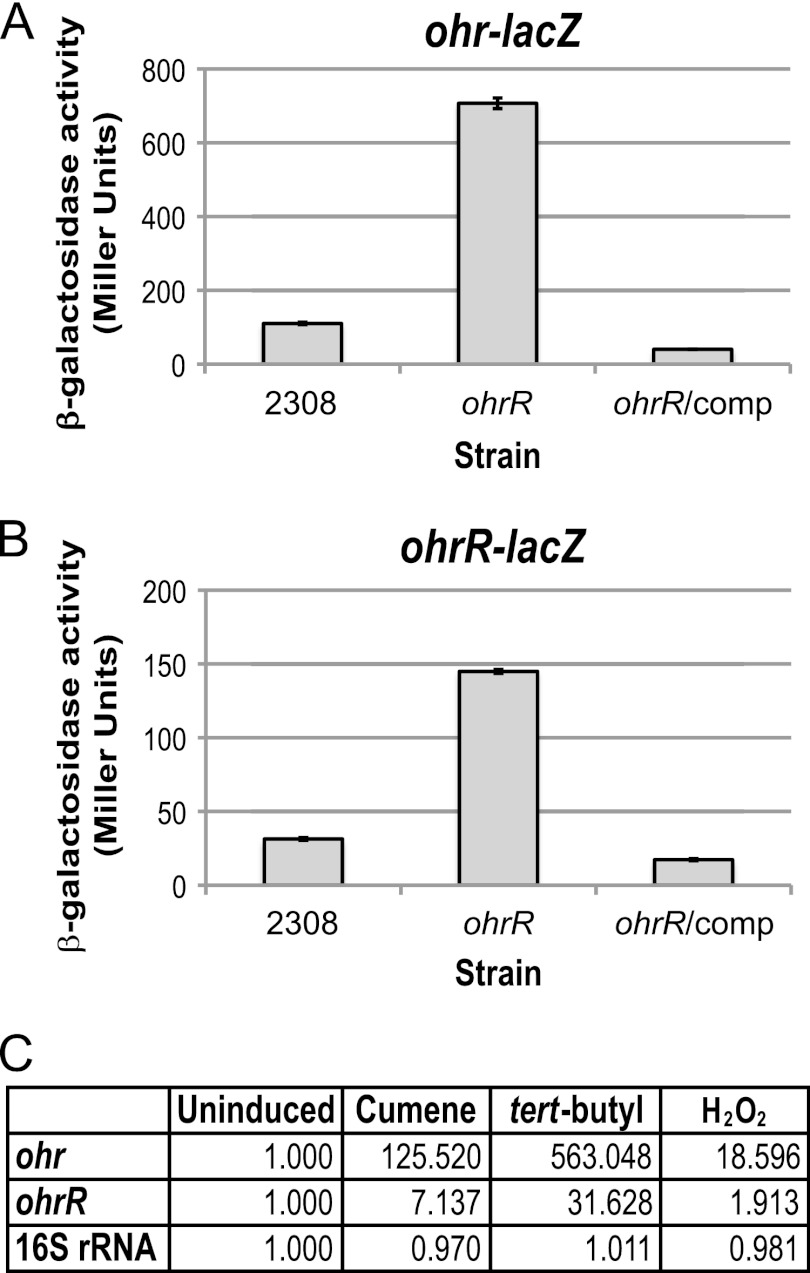
Studies of the genetic loci encoding ohr and ohrR in other bacteria have shown that OhrR serves as a repressor for both ohr and its own gene, ohrR (6, 9, 14, 28). To test the hypothesis that OhrR controls the expression of these divergently transcribed genes in B. abortus 2308, the levels of β-galactosidase activities produced by derivatives of B. abortus 2308 and the isogenic ohrR mutant JB16 carrying ohr-lacZ and ohrR-lacZ transcriptional fusions were determined. As shown in Fig. 2, increased expression of both the ohr-lacZ and ohrR-lacZ fusions was observed in the B. abortus ohrR mutant compared to the parental 2308 strain, and in both cases, introduction of a plasmid-borne copy of ohrR into the ohrR mutant restored the activities of the ohr-lacZ and ohrR-lacZ transcriptional fusions in this strain to parental levels (Fig. 2). Additionally, it was found that the relative abundance of ohr transcripts is ∼385-fold higher in the B. abortus ohrR mutant JB16 than it is in strain 2308 when total RNA from these strains was evaluated by real-time RT-PCR (data not shown), which provides an independent verification of the role of OhrR as a repressor of ohr expression in Brucella.
Fig 2.
OhrR is a transcriptional repressor of ohr and ohrR, and ohr and ohrR expression is induced by organic hydroperoxide stress in B. abortus 2308. The promoter region of the ohr or ohrR gene was cloned as a transcriptional reporter fused to lacZ, and the activities of these fusions were tested in B. abortus 2308, the isogenic ohrR mutant JB16, and JB16 expressing ohrR from a plasmid. The Brucella strains harboring the promoter fusion plasmids were grown in brucella broth to late exponential phase. β-Galactosidase activity is shown as average Miller units ± the standard deviation, and the results shown are from a single experiment that was repeated in triplicate. (A) β-Galactosidase activity of the ohr-lacZ transcriptional fusion. (B) β-Galactosidase activity of the ohrR-lacZ transcriptional fusion. (C) Real-time RT-PCR analysis of ohr and ohrR expression in response to peroxide treatment. Brucella abortus 2308 was grown in liquid media, and cultures were treated with 5 mM H2O2 cumene hydroperoxide or tert-butyl hydroperoxide. An untreated culture (uninduced) was included as a control. Oligonucleotide primers specific for ohr, ohrR, or 16S rRNA were used to amplify the target genes by PCR, and quantification of the amplified DNA fragments was performed using SYBR green incorporation. The values represent the abundances of specific mRNAs relative to the level of mRNA in the uninduced culture (1.000).
The expression of ohr and ohrR is induced by organic hydroperoxide stress in other bacteria, including, Agrobacterium tumefaciens (6), Bacillus subtilis (14), and Pseudomonas aeruginosa (2). The ability of peroxide treatment to induce the expression of ohr and ohrR in Brucella abortus 2308 was tested by treating cultures with 5 mM tert-butyl hydroperoxide, cumene hydroperoxide, or H2O2, and the levels of ohr and ohrR mRNA were assessed by real-time RT-PCR (Fig. 2C). Compared to untreated cultures, the expression of ohr was increased ∼125-fold and ∼563-fold by treatment with cumene hydroperoxide and tert-butyl hydroperoxide, respectively, and ohrR expression was elevated ∼7-fold and ∼31-fold under the same conditions. While exposure to H2O2 does not usually result in the induction of ohr and ohrR expression in other bacteria, there were slight elevations in ohr (∼18-fold) and ohrR (∼2-fold) mRNAs when B. abortus 2308 cultures were treated with H2O2. Additionally, elevation of ohr by H2O2 has also been observed by microarray analysis of H2O2-treated B. abortus 2308 cultures (data not shown). However, the increases in gene expression by organic hydroperoxides are substantially greater than the modest elevations observed during H2O2 stress, indicating that organic hydroperoxides are much more efficient at activating ohr and ohrR transcription than is H2O2. In all, these experiments show that, similar to the case with other bacteria, organic hydroperoxide stress induces the expression of ohr and ohrR in B. abortus 2308.
OhrR binds to an “OhrR box” in the ohr-ohrR intergenic region in B. abortus 2308.
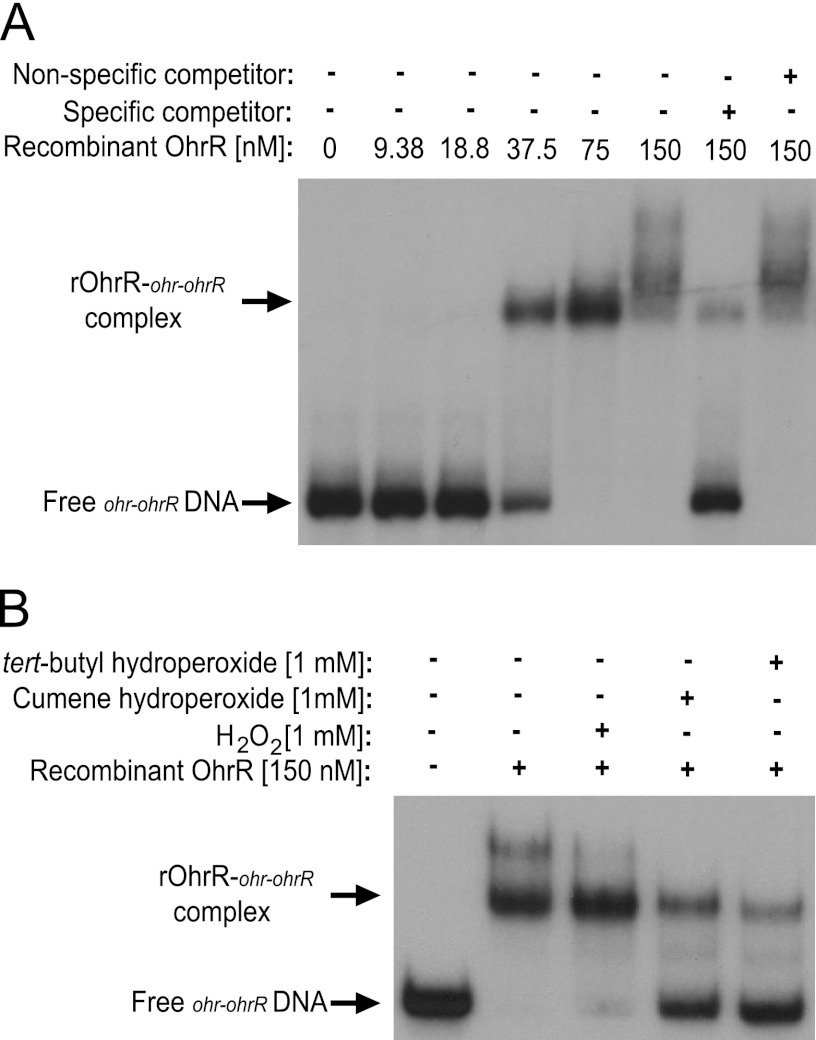
In order to determine if OhrR binds directly to the ohr and ohrR promoter regions to mediate transcriptional repression, electrophoretic mobility shift assays (EMSAs) were performed using purified OhrR and a DNA fragment corresponding to the ohr-ohrR intergenic region (Fig. 3). Recombinant OhrR protein bound to the ohr-ohrR DNA in a concentration-dependent manner, with significant binding observed at a 37.5 nM concentration of OhrR protein (Fig. 3A). The binding of OhrR to ohr-ohrR DNA was specific, because an excess of unlabeled ohr-ohrR DNA was able to compete for binding by OhrR; however, a nonspecific DNA fragment (the ohrR coding region) was not able to inhibit the binding between OhrR and ohr-ohrR intergenic region DNA. Notably, the addition of organic hydroperoxides (i.e., cumene and tert-butyl hydroperoxide), but not hydrogen peroxide (H2O2), to the EMSA reaction mixtures caused a significant inhibition of OhrR binding to the ohr-ohrR intergenic region (Fig. 3B). These experimental findings are consistent with the Brucella OhrR protein performing the same functions as its orthologs in other bacteria; that is, it serves as a transcriptional regulator that binds to the promoters of the ohr and ohrR genes and allows the maximum expression of these genes in response to exposure to organic peroxides but not hydrogen peroxide (2, 6, 14, 29).
Fig 3.
OhrR binds with high affinity to the Brucella ohr-ohrR intergenic region. (A) Recombinant OhrR (rOhrR) protein was tested for binding to the ohr-ohrR intergenic region using an electrophoretic mobility shift assay (EMSA). Increasing concentrations of rOhrR were incubated with radiolabeled ohr-ohrR intergenic region DNA, and in some binding reaction mixtures, unlabeled specific (ohr-ohrR intergenic region DNA) and nonspecific (ohrR coding sequence DNA) competitor DNA fragments were included as controls. The plus sign represents the inclusion of the specific component listed on the left side of the figure, and the minus sign indicates that the given component was not included in the binding reaction mixture. (B) EMSAs were performed with the ohr-ohrR intergenic region and 150 nM rOhrR protein in the presence and absence of different peroxides. A 1 mM final concentration of hydrogen peroxide (H2O2), tert-butyl hydroperoxide, or cumene hydroperoxide was included in some of the binding reaction mixtures.
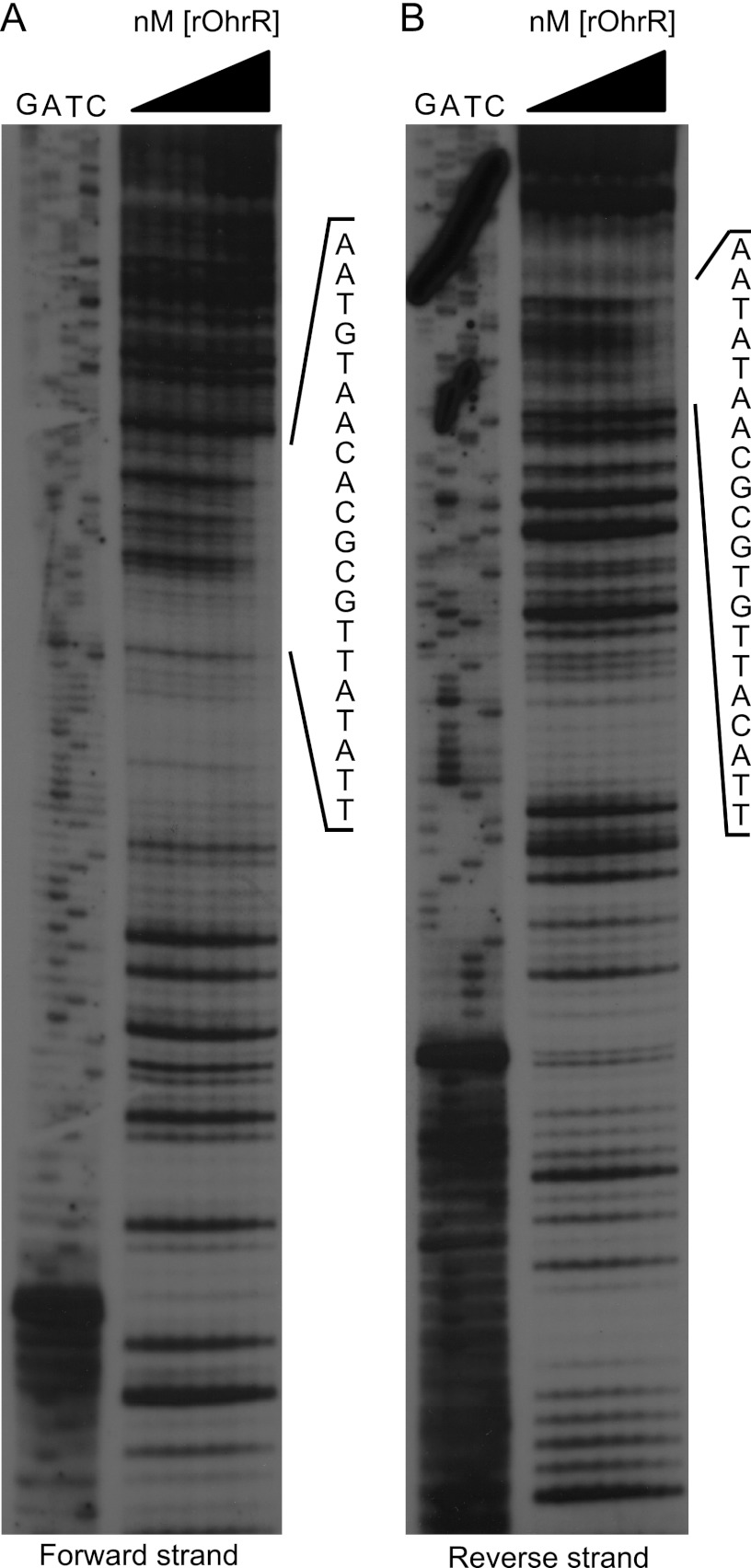
DNase I footprinting was subsequently employed to identify the specific site within the ohr-ohrR intergenic region that is bound by OhrR (Fig. 4). These experiments revealed that OhrR binds to the 20-nucleotide sequence TTATATTGCGCACAATGTAA. This sequence is similar to OhrR boxes described in other bacteria (6, 9), where the consensus OhrR binding motif is made up of two AT-rich palindromic sequences of approximately 7 nucleotides separated by a variable-length spacer sequence consisting of 1 to 17 nucleotides, depending on the bacterial strain in question. The OhrR binding region in the ohrR-ohr intergenic region in B. abortus 2308 is similarly comprised of two 7-nucleotide palindromic sequences with a 6-nucleotide spacer region, and it is notable that the OhrR box is found only in the ohr-ohrR intergenic region and in no other place in the genome sequence of this strain. This suggests that ohr and ohrR may be the only Brucella genes regulated by OhrR.
Fig 4.
OhrR binds to an OhrR box in the ohr-ohrR intergenic region of B. abortus 2308. DNase I footprint analysis was employed to determine the exact nucleotides bound by OhrR in the ohr-ohrR intergenic region. The forward or reverse strands of ohr-ohrR intergenic region DNA were individually radiolabeled, and the labeled DNA fragments were incubated with increasing concentration of rOhrR protein. Following treatment with DNase I, the digested DNA fragments, along with the corresponding DNA sequencing reaction products (shown as G, A, T, or C along the top of the gel), were separated on 8% denaturing polyacrylamide gels and visualized by autoradiography. The region of the DNA fragment protected by rOhrR is listed vertically on the right side of each gel.
Definition of the ohr and ohrR promoter elements and regulatory regions.
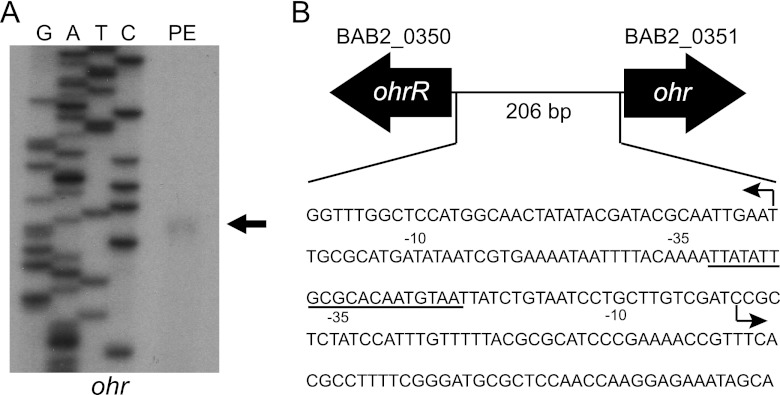
In B. abortus 2308, ohr and ohrR are divergently oriented on chromosome 2 and separated by a 206-bp intergenic region (Fig. 5B), and a survey of the currently available Brucella genome sequences determined that this genetic organization of ohr and ohrR is a common feature among Brucella strains. In fact, ohr and ohrR are found intact (i.e., they do not appear to be pseudogenes) in all publicly available sequenced Brucella genomes, including B. ovis strains, which have undergone significant genome degradation compared to other Brucella strains (41). In order to gain additional insight into the regulation of ohr and ohrR expression by OhrR, it was important to define the promoters of ohr and ohrR. To do this, the transcriptional start site of the ohr mRNA was determined by primer extension (Fig. 5A). The ohr mRNA initiates 87 nucleotides upstream of the predicted ohr start codon, and importantly, the OhrR box overlaps the −35 element of the ohr promoter (Fig. 5B). Repeated attempts at defining the ohrR transcriptional start site by primer extension failed. Therefore, 5′-RACE was employed as an alternative approach, revealing that the ohrR transcript is initiated 41 nucleotides upstream of the putative ohrR coding region (Fig. 5B). The OhrR binding site also overlaps a portion of the −35 region of the ohrR promoter. Taken together with the data presented earlier in this report, this suggests that, by binding to a single site in the ohr-ohrR intergenic region, the Brucella OhrR protein can repress the expression of both of these genes until its DNA binding activity is alleviated by exposure to organic peroxides. This genetic organization of ohr and ohrR divergently transcribed and regulated by a single OhrR binding site has been observed in other bacteria, including the close phylogenetic relative of Brucella, Agrobacterium tumefaciens (6).
Fig 5.
Organization of the ohr-ohrR locus in Brucella abortus 2308. (A) Primer extension analysis of the ohr mRNA was employed to determine the start site of transcription. The primer extension product, along with the corresponding DNA sequencing reaction products, was separated on 6% denaturing polyacrylamide gels and visualized by autoradiography. (B) Schematic representation of the ohr-ohrR locus. The transcriptional start sites of the ohr and ohrR mRNAs are denoted with an arrow, and the −10 and −35 elements of the promoters are shown. The region of the ohr-ohrR intergenic region that is bound by OhrR is underlined.
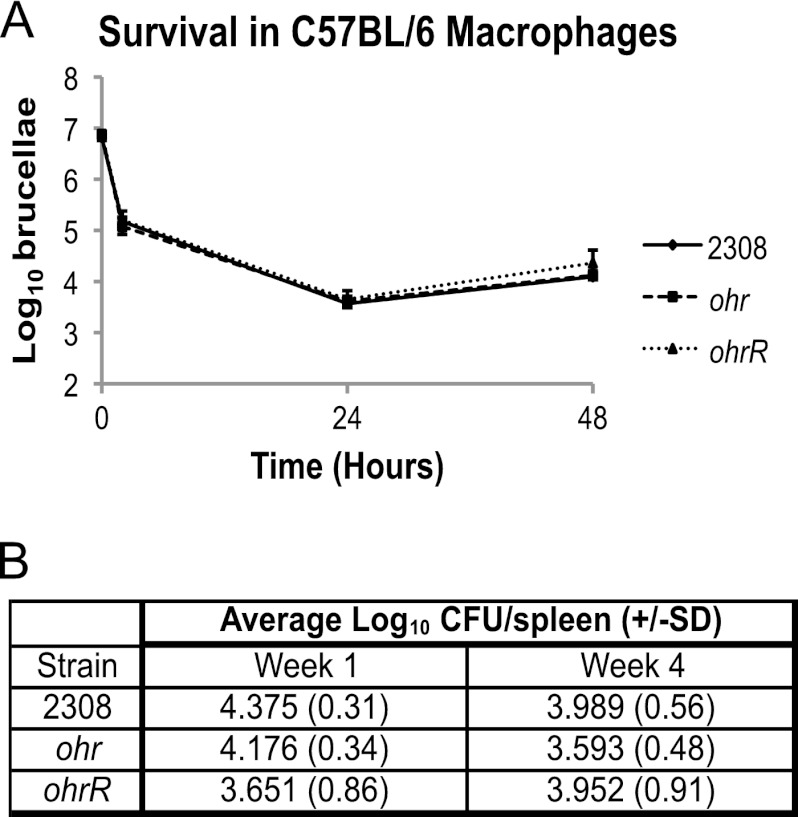
Neither the Brucella ohr mutant nor the Brucella ohrR mutant exhibits significant attenuation in the mouse model of infection.
The experimental results reported so far demonstrate that Ohr plays an important role in protecting B. abortus 2308 from organic peroxides and that OhrR regulates the expression of the ohr gene in response to exposure to these compounds, but as noted in the introduction, it is unclear what role resistance to organic peroxides plays in the virulence of mammalian pathogens. To address this issue, the virulence properties of the B. abortus ohr and ohrR mutants were assessed in murine peritoneal macrophages and experimentally infected mice. The B. abortus ohr and ohrR mutants displayed the same intracellular survival and replication profiles in macrophages as that exhibited by the parental 2308 strain (Fig. 6A). Additionally, activation of the macrophages with gamma interferon did not result in differences in intracellular survival and replication of the ohr and ohrR mutant strains compared to 2308 (data not shown). Although the B. abortus ohrR mutant displayed considerable variability in its spleen colonization profiles in individual mice at 4 weeks postinfection compared to those exhibited by 2308 and the isogenic ohr mutant (Fig. 6B), statistical analysis failed to yield a significant difference between the groups of mice infected with B. abortus 2308, JB15, or JB16. Based on these findings, we conclude that neither Ohr nor OhrR plays an indispensable role in the virulence of the parent strain in mice. However, it is interesting to note that the levels of Ohr protein are significantly decreased during trafficking and residence of B. abortus 2308 in macrophages (22), suggesting that, while deletion of ohr does not result in overt attenuation, Ohr levels are responsive to intracellular signals encountered by the brucellae during the natural course of macrophage infection.
Fig 6.
Virulence of B. abortus 2308 and the isogenic ohr and ohrR mutants in murine peritoneal macrophages and mice. (A) Macrophage survival and replication experiments. Cultured resident peritoneal macrophages from C57BL/6 mice were infected with B. abortus 2308, JB15 (2308 ohr), and JB16 (2308 ohrR). At the indicated times postinfection, the macrophages were lysed, and the number of intracellular brucellae present in these phagocytes was determined by serial dilution, plating, and bacteriologic culture. (B) Mouse infection experiments. C57BL/6 mice (5 per strain) were infected intraperitoneally with B. abortus 2308, JB15 (2308 ohr), and JB16 (2308 ohrR). Mice were sacrificed at weeks 1 and 4 postinfection, and the number of brucellae colonizing the spleens was determined. The data are represented as the average number of CFU ± the standard deviation (SD) from the 5 mice colonized with a specific Brucella strain at a specific time point.
The data presented here show a clear role for Ohr in the biology of Brucella abortus 2308; however, several important questions remain unanswered. The most apparent question is where the brucellae would encounter organic hydroperoxides. Organic hydroperoxides are commonly produced by plants as a defense mechanism (27). Therefore, plant-associated bacteria, such as Agrobacterium tumefaciens, would benefit from a system to inactivate organic hydroperoxides. In mammalian pathogens, such as the brucellae, it is not clear why this type of system evolved, and the results of these studies do not support a role for Ohr in virulence in a mouse model of infection. It is possible that the ohr-ohrR locus is an evolutionary remnant retained from a plant-associated alphaproteobacterial ancestor, but it is also conceivable that Ohr contributes to a phase of the life-style of the brucellae that is not simulated in the mouse model, such as an acute infection of a pregnant host animal (e.g., cattle, goats, or swine). Moreover, as discussed earlier, the ohr ohrR system is found intact in all Brucella strains whose genome sequences are publicly available, and from an evolutionary standpoint, this system should be retained only if it is beneficial for the bacterium. Therefore, it stands to reason that the genomic preservation of ohr and ohrR is advantageous to the life of the brucellae, but future work examining the role of Ohr and OhrR during the infection of natural host animals is needed in order to fully understand how these gene products benefit Brucella.
In regard to the conservation of the ohr ohrR system, these genes are also present in many other closely related members of the Alphaproteobacteria. In fact, the ohr ohrR systems of the alphaproteobacteria Agrobacterium tumefaciens (6) and Sinorhizobium meliloti (11) have been characterized and reported in the literature. In addition to these published examples, many other alphaproteobacteria possess the ohr and ohrR genes as determined from searches of publicly available genome sequence data. Using the online software available at http://www.microbesonline.org/, alignments of the ohr and ohrR loci were generated from Brucella strains and several other phylogenetically related alphaproteobacteria, including Ochrobactrum spp., Bradyrhizobium spp., Sinorhizobium spp., Rhizobium spp., and Agrobacterium spp. Among these alphaproteobacteria, all encode Ohr and OhrR, and the genetic organization of the ohr and ohrR genes is conserved and similar to that of Brucella strains; that is, ohr and ohrR are divergently oriented at a single genetic locus. However, the genomic context of the genes surrounding ohr and ohrR is strikingly different among the alphaproteobacteria, but the evolutionary implications of this variation remain unknown.
AhpC does not play a role in the detoxification of organic hydroperoxides in B. abortus 2308.
The peroxiredoxin AhpC was originally identified based on its role in protecting Salmonella enterica serovar Typhimurium and Escherichia coli from organic peroxides (40). Subsequent studies with other bacterial AhpC orthologs have shown that, while some of these proteins detoxify both organic peroxides and hydrogen peroxide, others have specificity for one or the other of these compounds (9). It was noted in a previous study that a B. abortus ahpC mutant did not exhibit increased sensitivity to cumene hydroperoxide or tert-butyl hydroperoxide compared to the parental strain (39), and the B. abortus ahpC ohr double mutant constructed for the study described here showed the same level of susceptibility to cumene hydroperoxide in disk assays as the ohr mutant JB15 from which it was derived (data not shown). These experimental findings indicate that, like its counterpart in Agrobacterium tumefaciens (6), Brucella AhpC has specific activity for H2O2 and does not contribute to resistance to organic peroxides. Thus, there is a theme emerging that Ohr plays the role of the primary detoxifier of organic hydroperoxides in the alphaproteobacteria; however, further studies are needed to determine if Ohr is in fact the sole detoxifier of organic hydroperoxides in Brucella strains or if other gene products also contribute to organic hydroperoxide defense in these bacteria.
Supplementary Material
ACKNOWLEDGMENT
This study was supported by grants (AI48499 and AI63516) from NIAID to R.M.R.
Footnotes
Published ahead of print 20 July 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Ariza J, et al. 2007. Perspectives for the treatment of brucellosis in the 21st century: the Ioannina recommendations. PLoS Med. 4:e317 doi:10.1371/journal.pmed.0040317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Atichartpongkul S, Fuangthong M, Vattanaviboon P, Mongkolsuk S. 2010. Analyses of the regulatory mechanism and physiological roles of Pseudomonas aeruginosa OhrR, a transcription regulator and a sensor of organic hydroperoxides. J. Bacteriol. 192:2093–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bellaire BH, Elzer PH, Baldwin CL, Roop RM., II 2003. Production of the siderophore 2,3-dihydroxybenzoic acid is required for wild-type growth of Brucella abortus in the presence of erythritol under low-iron conditions in vitro. Infect. Immun. 71:2927–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Caswell CC, Gaines JM, Roop RM., II 2012. The RNA chaperone Hfq independently coordinates expression of the VirB type IV secretion system and the LuxR-type regulator BabR in Brucella abortus 2308. J. Bacteriol. 194:3–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Celli J, Grovel JP. 2004. Organelle robbery: Brucella interactions with the endoplasmic reticulum. Curr. Opin. Microbiol. 7:93–97 [DOI] [PubMed] [Google Scholar]
- 6. Chuchue T, et al. 2006. ohrR and ohr are the primary sensor/regulator and protective genes against organic hydroperoxide stress in Agrobacterium tumefaciens. J. Bacteriol. 188:842–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cussiol JR, Alves SV, de Oliveira MA, Netto LE. 2003. Organic hydroperoxide resistance gene encodes a thiol-dependent peroxidase. J. Biol. Chem. 278:11570–11578 [DOI] [PubMed] [Google Scholar]
- 8. Dorsey CW, Tomaras AP, Actis LA. 2006. Sequence and organization of pMAC, an Acinetobacter baumannii plasmid harboring genes involved in organic peroxide resistance. Plasmid 56:112–123 [DOI] [PubMed] [Google Scholar]
- 9. Dubbs JM, Mongkolsuk S. 2007. Peroxiredoxins in bacterial antioxidant defense. Subcell. Biochem. 44:143–193 [DOI] [PubMed] [Google Scholar]
- 10. Eiamphungporn W, Soonsanga S, Lee J-W, Helmann JD. 2009. Oxidation of a single active site suffices for the functional inactivation of the dimeric Bacillus subtilis OhrR repressor in vitro. Nucleic Acids Res. 37:1174–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fontenelle C, Blanco C, Arrieta M, Dufour V, Trautwetter A. 2011. Resistance to organic hydroperoxides requires ohr and ohrR genes in Sinorhizobium meliloti. BMC Microbiol. 11:100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Forman HJ, Thomas MJ. 1986. Oxidant production and bactericidal activity of phagocytes. Annu. Rev. Physiol. 48:669–680 [DOI] [PubMed] [Google Scholar]
- 13. Fuangthong M, Helmann JD. 2002. The OhrR repressor senses organic hydroperoxides by reversible formation of a cysteine-sulfenic acid derivative. Proc. Natl. Acad. Sci. U. S. A. 99:6690–6695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fuangthong M, Atichartpongkul S, Mongkolsuk S, Helmann JD. 2001. OhrR is a repressor of ohrA, a key organic hydroperoxide resistance determinant in Bacillus subtilis. J. Bacteriol. 183:4134–4141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gee JM, et al. 2005. The Brucella abortus Cu,Zn superoxide dismutase is required for optimal resistance to oxidative killing by murine macrophages and wild-type virulence in experimentally infected mice. Infect. Immun. 73:2873–2880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Halliwell B, Chirico S. 1993. Lipid peroxidation: its mechanism, measurement, and significance. Am. J. Clin. Nutr. 57:715S–725S [DOI] [PubMed] [Google Scholar]
- 17. Imlay JA, Linn S. 1988. DNA damage and oxygen radical toxicity. Science 240:1302–1309 [DOI] [PubMed] [Google Scholar]
- 18. Imlay JA, Chin SM, Linn S. 1988. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science 240:640–642 [DOI] [PubMed] [Google Scholar]
- 19. Kim JA, Sha Z, Mayfield JE. 2000. Regulation of Brucella abortus catalase. Infect. Immun. 68:3861–3866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Klomsiri C, Panmanee W, Dharmsthiti S, Vattanaviboon P, Mongkolsuk S. 2005. Novel roles of ohrR-ohr in Xanthomonas sensing, metabolism, and physiological adaptive response to lipid hydroperoxide. J. Bacteriol. 187:3277–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kovach ME, et al. 1995. Four new derivatives of the broad-host-range cloning vector pBBR1MCS, carrying different antibiotic-resistance cassettes. Gene 166:175–176 [DOI] [PubMed] [Google Scholar]
- 22. Lamontagne J, et al. 2009. Intracellular adaptation of Brucella abortus. J. Proteome Res. 8:1594–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lesniak J, Barton WA, Nikolov DB. 2002. Structural and functional characterization of the Pseudomonas hydroperoxide resistance protein Ohr. EMBO J. 21:6649–6659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Llewellyn AC, Jones CL, Napier BA, Bina JE, Weiss DS. 2011. Macrophage replication screen identifies a novel Francisella hydroperoxide resistance protein involved in virulence. PLoS One 6:e24201 doi:10.1371/journal.pone.0024201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Miller JH. 1972. Experiments in molecular genetics, p 352–355 Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]
- 26. Mongkolsuk S, Praituan W, Loprasert S, Fuangthong M, Chamnongpol S. 1998. Identification and characterization of a new organic hydroperoxide resistance (ohr) gene with a novel pattern of oxidative stress regulation from Xanthomonas campestris pv. phaseoli. J. Bacteriol. 180:2636–2643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Noordermeer MA, Veldink GA, Vliegenthart JF. 2001. Fatty acid hydroperoxide lyase: a plant cytochrome p450 enzyme involved in wound healing and pest resistance. Chembiochem 2:494–504 [DOI] [PubMed] [Google Scholar]
- 28. Panmanee W, Vattanaviboon P, Poole LB, Mongkolsuk S. 2006. Novel organic hydroperoxide-sensing and responding mechanisms for OhrR, a major bacterial sensor and regulator of organic hydroperoxide stress. J. Bacteriol. 188:1389–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Panmanee W, et al. 2002. OhrR, a transcription repressor that senses and responds to changes in organic peroxide levels in Xanthomonas campestris pv. phaseoli. Mol. Microbiol. 45:1647–1654 [DOI] [PubMed] [Google Scholar]
- 30. Pappas G, Akritidis N, Bosilkovski M, Tsianos E. 2005. Brucellosis. N. Engl. J. Med. 352:2325–2336 [DOI] [PubMed] [Google Scholar]
- 31. Pappas G, Papadimitriou P, Akritidis N, Christou L, Tsianos EV. 2006. The new global map of human brucellosis. Lancet Infect. Dis. 6:91–99 [DOI] [PubMed] [Google Scholar]
- 32. Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:2002–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Richter C. 1987. Biophysical consequences of lipid peroxidation in membranes. Chem. Phys. Lipids 44:175–189 [DOI] [PubMed] [Google Scholar]
- 34. Robertson GT, Roop RM., II 1999. The Brucella abortus host factor I (HF-I) protein contributes to stress resistance during stationary phase and is a major determinant of virulence in mice. Mol. Microbiol. 34:690–700 [DOI] [PubMed] [Google Scholar]
- 35. Roop RM, II, Gaines JM, Anderson ES, Caswell CC, Martin DW. 2009. Survival of the fittest: how Brucella strains adapt to their intracellular niche in the host. Med. Microbiol. Immunol. 198:221–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saikolappan S, Sasindran SJ, Yu HD, Baseman JB, Dhandayuthapani S. 2009. The Mycoplasma genitalium MG_454 gene product resists killing by organic hydroperoxides. J. Bacteriol. 191:6675–6682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shea RJ, Mulks MH. 2002. ohr, encoding an organic hydroperoxide reductase, is an in vivo-induced gene in Actinobacillus pleuropneumoniae. Infect. Immun. 70:794–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Spratt BG, Hedge PJ, te Heesen S, Edelman A, Broome-Smith JK. 1986. Kanamycin-resistant vectors that are analogues of plasmids pUC8, pUC9, pEMBL8 and pEMBL9. Gene 41:337–342 [DOI] [PubMed] [Google Scholar]
- 39. Steele KH, Baumgartner JE, Valderas MW, Roop RM., II 2010. Comparative study of the roles of AhpC and KatE as respiratory antioxidants in Brucella abortus 2308. J. Bacteriol. 192:4912–4922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Storz G, et al. 1989. An alkyl hydroperoxide reductase induced by oxidative stress in Salmonella typhimurium and Escherichia coli: genetic characterization and cloning of ahp. J. Bacteriol. 171:2049–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Tsolis RM, et al. 2009. Genome degradation in Brucella ovis corresponds with narrowing of its host range and tissue tropism. PLoS One 4:e5519 doi:10.1371/journal.pone.0005519 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.