Abstract
N-glycosylation, a posttranslational modification required for the accurate folding and stability of many proteins, has been observed in organisms of all domains of life. Although the haloarchaeal S-layer glycoprotein was the first prokaryotic glycoprotein identified, little is known about the glycosylation of other haloarchaeal proteins. We demonstrate here that the glycosylation of Haloferax volcanii flagellins requires archaeal glycosylation (Agl) components involved in S-layer glycosylation and that the deletion of any Hfx. volcanii agl gene impairs its swimming motility to various extents. A comparison of proteins in CsCl density gradient centrifugation fractions from supernatants of wild-type Hfx. volcanii and deletion mutants lacking the oligosaccharyltransferase AglB suggests that when the Agl glycosylation pathway is disrupted, cells lack stable flagella, which purification studies indicate consist of a major flagellin, FlgA1, and a minor flagellin, FlgA2. Mass spectrometric analyses of FlgA1 confirm that its three predicted N-glycosylation sites are modified with covalently linked pentasaccharides having the same mass as that modifying its S-layer glycoprotein. Finally, the replacement of any of three predicted N-glycosylated asparagines of FlgA1 renders cells nonmotile, providing direct evidence for the first time that the N-glycosylation of archaeal flagellins is critical for motility. These results provide insight into the role that glycosylation plays in the assembly and function of Hfx. volcanii flagella and demonstrate that Hfx. volcanii flagellins are excellent reporter proteins for the study of haloarchaeal glycosylation processes.
INTRODUCTION
Despite their relatively recent discovery, prokaryotic glycoproteins have already been implicated in a wide range of functions, including pathogenesis, cell protection, motility, transport, and signaling (8, 18, 19, 25, 32, 34–36, 40). As in eukaryotes, examples of both O-linked and N-linked protein glycosylation have been identified in archaea and bacteria (30, 37). However, while researchers have studied the mechanisms of protein glycosylation in bacteria extensively, with a primary focus on O-linked glycosylation, prevalent in bacteria, much less is known about the glycosylation process in archaea. However, this paradigm is now rapidly changing with the elucidation of various archaeal N-glycosylation systems (11, 19, 29).
The surface (S)-layer glycoprotein of the haloarchaeon Halobacterium salinarum was the first noneukaryotic N-glycosylated protein to be reported (28), and subsequent efforts revealed that Hbt. salinarum flagellins are similarly modified (44). However, details of the molecular aspects of archaeal glycosylation did not surface until years later, with the publication of N-glycosylation studies of methanogenic archaea. Those studies revealed a set of archaeal glycosylation (agl) genes encoding enzymes that catalyze the assembly and attachment of N-linked glycans to target proteins (for reviews, see references 11 and 19). AglB, the most conserved of the agl-encoded proteins, is an oligosaccharyltransferase (OST), an enzyme that covalently links a fully assembled oligosaccharide to the appropriate asparagine residue of a target protein in the final step of glycan synthesis. Apart from the AglB homologs, little to no homology exists among the Agl components. This lack of homology is easily understood given the extremely diverse nature of the glycans found on glycoproteins produced by very closely related archaeal species. For instance, while the S-layer glycoprotein and flagella of Methanococcus voltae are found to be adorned with a trisaccharide [β-ManpNAcA6Thr-(1–4)-β-GlcpNAc3NAcA-(1–3)-β-GlcpNAc-Asn, added sequentially by AglH, AglK/C, and AglA, respectively] (43), the closely related methanogen Methanococcus maripaludis has flagellins that are decorated with a tetrasaccharide moiety [Sug-4-β-ManpNAcA6Thr-(1–4)-β-GlcpNAc3NAcA-(1–3)-β-GlcpNAc-Asn, added sequentially by an undefined glycosyltransferase, AglO, AglA, and AglL, respectively] (22). Furthermore, a rudimentary analysis of the S-layer glycoprotein of Hbt. salinarum revealed its modification by the addition of two vastly different N-linked polysaccharide moieties, along with an O-linked moiety, suggesting that multiple systems of glycosylation can sometimes exist even within the same organism (27).
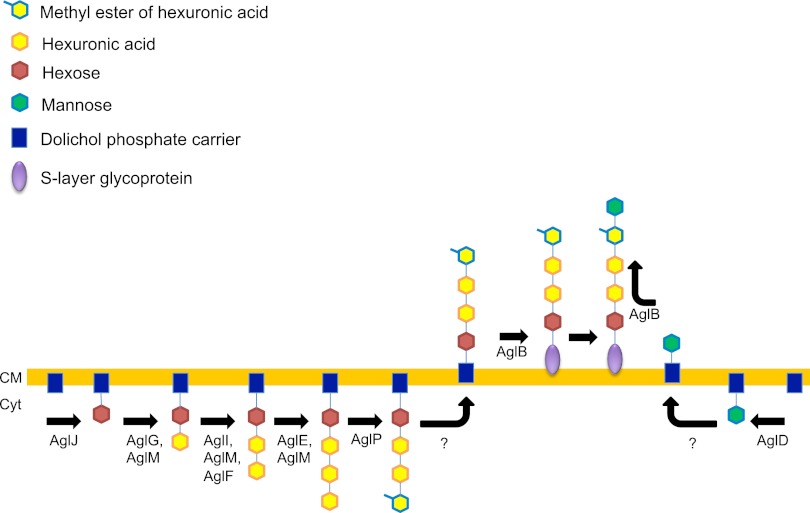
In the model haloarchaeon Hfx. volcanii, 11 Agl components involved in the assembly and attachment of an N-linked pentasaccharide to the Hfx. volcanii S-layer glycoprotein have been identified thus far (11). Of these, only the haloarchaeal oligosaccharyltransferase AglB shows significant homology to nonhaloarchaeal Agl components. The characterization of the other Agl proteins has also shed some light on the basic structures of the individual sugars within the Hfx. volcanii pentasaccharide, although the identities of the first four sugars in this glycan chain have not been directly identified (3, 4, 21, 26, 46, 47). Like the glycosyltransferases found in other archaeal species, all of these Agl enzymes function through an indirect method of synthesis, adding sugar residues sequentially to a transient lipid carrier (dolichol monophosphate in archaea rather than dolichol diphosphate in eukaryotes) before the transfer of the completed glycan by AglB to its final protein target. For a more detailed look at this process, refer to Fig. 1.
Fig 1.
Hfx. volcanii S-layer glycoprotein N-glycosylation model. The glycosylation mechanism in Hfx. volcanii can be described as two distinct processes. The initial process involves the synthesis and transfer of the first four sugar residues in the pentasaccharide. AglJ is the glycosyltransferase responsible for the addition of the hexose at position 1 in the pentasaccharide (21). The addition of the second sugar, a hexuronic acid, at position 2 is mediated by the glycosyltransferase AglG (46). Another hexuronic acid is added at position 3 with the help of AglI. AglM appears to be a UDP-glucose dehydrogenase involved in the conversion of UDP-glucose to UDP-glucuronic acid during the syntheses of the three hexuronic acids at positions 2, 3, and 4. AglF is a glucose-1-phosphate uridyltransferase that acts in conjunction with AglM to assemble the hexuronic acid at position 3 but plays no role in the addition of hexuronic acid at position 2 or 4 (47). AglE adds another hexuronic acid to position 4 with the help of AglM (2). This hexuronic acid is modified by a methyltransferase, AglP, to a methyl ester (26). This tetrasaccharide serves as the substrate for an unknown flippase that flips the glycan and its dolichol monophosphate carrier from the cytoplasmic leaflet of the cell membrane to the extracellular leaflet. The tetrasaccharide is transferred to its final destination on the S-layer glycoprotein by AglB. A dolichol phosphate mannose synthase, AglD, transfers the mannose, which it synthesizes to a dolichol monophosphate carrier that is separate from the one that holds the tetrasaccharide. Like the tetrasaccharide, this mannose is flipped across the cell membrane by an unknown flippase and then added directly to the tetrasaccharide moiety that had already been attached to the S-layer glycoprotein via the first process described above. AglB is also believed to be involved in the transfer of the final mannose (17). Cyt, cytoplasm. CM, cytoplasmic membrane.
Despite the strides made toward a comprehensive understanding of the Agl-mediated glycosylation of the S-layer glycoprotein in Hfx. volcanii, this cell wall glycoprotein is large and lacks a testable function, hence limiting the analyses that can be performed. Studies of methanogens have shown that the biosynthesis of their flagella requires the modification of flagellar subunits by the addition of sugar moieties and have indicated that archaeal flagella are a good system with which to study the effects that glycosylation can have on the structure and function of a protein (41). Our preliminary studies have also strongly suggested that the subunits of Hfx. volcanii flagella, the flagellins FlgA1 and FlgA2, are glycosylated, as each of these proteins contains predicted glycosylation sites, and deletion mutants lacking AglB are nonmotile (39).
In this study, we investigated the effects of various agl gene deletions on the glycosylation of Hfx. volcanii flagella. Our results indicate that the same Agl components are involved in the biosynthesis of the pentasaccharide that decorates both the S-layer and the flagella of Hfx. volcanii and that Hfx. volcanii swimming motility requires that its flagellins be decorated at the targeted sites by a polysaccharide containing at least a subset of the sugars found in the wild-type glycan chain, as has also been observed for the methanogens. We have also shown that a replacement of the asparagine in any of three predicted N-linked glycosylation sites with a glutamine prevents FlgA1 expressed in trans from complementing a chromosomal flgA1 gene deletion. By expressing these replacement mutants in a strain where all other glycoproteins are properly glycosylated, we were able to demonstrate definitively that the swimming motility and flagellar stability defects observed for agl deletion mutants are not due solely to a defective glycosylation of proteins other than the flagellins.
MATERIALS AND METHODS
Reagents.
All enzymes used for standard molecular biology procedures were purchased from New England BioLabs, except for iProof High-Fidelity DNA polymerase, which was purchased from Bio-Rad. The ECL Plus Western blotting system detection and horseradish peroxidase-linked sheep anti-mouse antibodies were purchased from Amersham Biosciences. The polyvinylidene difluoride membrane, MF membrane filters (0.025 μm), and Ultracel-3K membrane were purchased from Millipore. DNA and plasmid purification kits and anti-His antibodies were purchased from Qiagen. NuPAGE gels, buffers, reagents, and the Pro-Q Emerald 300 glycoprotein stain kit were purchased from Invitrogen. Difco agar and Bacto yeast extract were purchased from Becton, Dickinson, and Company. Peptone was purchased from Oxoid. 5-Fluoroorotic acid (5-FOA) was purchased from Toronto Research Biochemicals. All other chemicals and reagents were purchased from either Fisher or Sigma.
Strains and growth conditions.
The plasmids and strains used in this study are listed in Table 1. Hfx. volcanii strains were grown at 45°C in liquid or on solid complex (MGM) (13) or defined (CA) (13) medium. Plates contained 1.5% agar, unless mentioned otherwise. To ensure equal concentrations of agar in all plates, the agar was completely dissolved prior to autoclaving, and the autoclaved medium was stirred before plates were poured. Hfx. volcanii strain H53 (7) was grown in CA medium supplemented with tryptophan and uracil (50-μg/ml final concentration) or in MGM medium without any supplements. For the selection of the ΔflgA1 (Hvo_1210) deletion mutant (see below), 5-FOA was added at a final concentration of 150 μg/ml in CA medium, and uracil was added at one-fifth its normal concentration, i.e., a 10-μg/ml final concentration, during 5-FOA selection. Hfx. volcanii strain H53 and the ΔflgA1 and Δagl mutants transformed with pRV1-ptna (24) were grown in medium supplemented with novobiocin (2-μg/ml final concentration in plates and 1-μg/ml final concentration in liquid). To induce expression from plasmid pRV1-ptna, tryptophan was added to the medium (100-μg/ml final concentration) approximately 1 h before the cells were harvested. The same strains transformed with pTA963 were grown in CA medium supplemented only with tryptophan. Escherichia coli strains were grown at 37°C in NZCYM medium supplemented with ampicillin (200 μg/ml), when necessary (9).
Table 1.
Strains and plasmids
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| Plasmids | ||
| pTA131 | Ampr; pBluescript II with BamHI-XbaI fragment from pGB70 harboring pfdx-pyrE2 | 7 |
| pRV1-ptna | Ampr Novr; inducible ptna promoter | 24 |
| pTA963 | Ampr, pyrE2 and hdrB markers, inducible ptna promoter | 5 |
| pUC19 | Ampr | 45 |
| pMT7 | pRV1-ptna carrying flgA1-His | This study |
| pMT8 | pRV1-ptna carrying flgA2-His | This study |
| pMT9 | pTA963 carrying flgA1-His | This study |
| pJY1 | pTA131 carrying chromosomal flanking regions of flgA1 | This study |
| pST1 | pTA963 carrying flgA1-His with an N70Q mutation | This study |
| pST2 | pTA963 carrying flgA1-His with an N115Q mutation | This study |
| pST3 | pTA963 carrying flgA1-His with an N172Q mutation | This study |
| pST4 | pTA963 carrying flgA1-His with N70Q, N115Q and N172Q mutations | This study |
| Strains | ||
| DH5α | E. coli F− ϕ80dlacZΔM15 (lacZYA-argF)U169 recA1 endA1 hsdR17(rK− mK−) phoA supE44 thi-1 gyrA96 relA1 | Invitrogen |
| DL739 | E. coli MC4100 recA dam-13::Tn9 | 10 |
| H53 | Hfx. volcanii ΔpyrE2 Δtrp | 7 |
| MT2 | H53 ΔflgA1 ΔflgA2 | 39 |
| MT13 | H53 containing pTA963 | This study |
| MT14 | H53 ΔflgA1 | This study |
| MT15 | MT14 containing pTA963 | This study |
| MT16 | MT14 containing pMT9 | This study |
| ΔaglB | H53 ΔaglB | 1 |
| ΔaglJ | H53 ΔaglJ | 33 |
| ΔaglG | H53 ΔaglG | 46 |
| ΔaglM | H53 ΔaglM | 47 |
| ΔaglF | H53 ΔaglF | 46 |
| ΔaglI | H53 ΔaglI | 46 |
| ΔaglE | H53 ΔaglE | 3 |
| ΔaglP | H53 ΔaglP | 26 |
| ΔaglD | H53 ΔaglD | 1 |
| JY1 | H53 ΔaglB containing pMT7 | This study |
| JY2 | H53 ΔaglJ containing pMT7 | This study |
| JY3 | H53 ΔaglG containing pMT7 | This study |
| JY4 | H53 ΔaglM containing pMT7 | This study |
| JY5 | H53 ΔaglF containing pMT7 | This study |
| JY6 | H53 ΔaglI containing pMT7 | This study |
| JY7 | H53 ΔaglE containing pMT7 | This study |
| JY8 | H53 ΔaglP containing pMT7 | This study |
| JY9 | H53 ΔaglD containing pMT7 | This study |
| JY10 | H53 ΔaglB containing pMT8 | This study |
| JY11 | H53 ΔaglJ containing pMT8 | This study |
| JY12 | H53 ΔaglG containing pMT8 | This study |
| JY13 | H53 ΔaglM containing pMT8 | This study |
| JY14 | H53 ΔaglF containing pMT8 | This study |
| JY15 | H53 ΔaglI containing pMT8 | This study |
| JY16 | H53 ΔaglE containing pMT8 | This study |
| JY17 | H53 ΔaglP containing pMT8 | This study |
| JY18 | H53 ΔaglD containing pMT8 | This study |
| ST1 | MT14 containing pST1 | This study |
| ST2 | MT14 containing pST2 | This study |
| ST3 | MT14 containing pST3 | This study |
| ST4 | MT14 containing pST4 | This study |
Generation of a chromosomal flgA1 deletion.
Chromosomal deletions were generated by using a homologous recombination (pop-in pop-out) method previously described by Allers and Ngo (6). Plasmid constructs for use in the pop-in pop-out knockout process were generated by using overlap PCR as described previously by Tripepi et al. (39) (primers used are listed in Table S1 in the supplemental material). To confirm the chromosomal replacement event at the proper location on the chromosome, colonies derived from these techniques were screened by PCR (primers used are listed in Table S1 in the supplemental material). The identities of the PCR products were verified by sequencing using the same primers. The flgA1 deletion mutant generated in strain H53 was designated MT14.
Motility assay.
The motility assay was performed on motility plates (0.3% agar in MGM or CA medium). Agar media were supplemented with tryptophan, uracil, and/or novobiocin, depending on the strain being assayed. A toothpick was used to stab inoculate the agar, and halo sizes around the inoculation site were measured.
Construction of expression vectors encoding proteins with C-terminal His tags.
Gene fusions that encode flagellins containing a C-terminal His tag were created; the inducible tryptophanase promoter (tna) (24) drove the expression of these gene fusions. The genes encoding FlgA1-His and FlgA2-His were cloned in a manner similar to that used for FlgA1/2-His, which was described previously by Tripepi et al. (39). Primers used specifically for the construction of pMT7 and pMT8 (pRV1-ptna expressing C-terminally His-tagged FlgA1 and FlgA2, respectively) are listed in Table S1 in the supplemental material.
pMT7 and pMT8 were isolated from E. coli DH5α and transformed into E. coli DL739 (Table 1). Using the standard polyethylene glycol (PEG) (13), nonmethylated plasmid DNA isolated from E. coli DL739 was used to transform Hfx. volcanii strain H53.
Site-directed mutagenesis of the glycosylation sites of FlgA1.
To generate point mutations in flgA1, encoding the FlgA1 protein, the QuikChange site-directed mutagenesis protocol (Stratagene) was used with modifications that are described below. The fragment encoding FlgA1-His was excised from pMT7 and cloned into pUC19 (45). The plasmid was then amplified by using three sets of oligonucleotide primers, each containing a point mutation that resulted in the replacement of an asparagine with a glutamine at positions N70Q, N115Q, and N172Q, respectively (see Table S1 in the supplemental material). We also generated a plasmid containing all three mutations using a series of PCRs on the mutated templates. PCR amplification of the pUC19 insert containing the sequence encoding the FlgA1-His fragment was performed by using Phusion high-fidelity DNA polymerase, which has a non-strand-displacing action and incorporates the mutagenic primers, resulting in a nicked circular strand. The methylated nonmutated parental DNA template was digested by using the restriction enzyme DpnI, upon which the remaining mutated DNA was transformed into E. coli DH5α. Plasmid DNA was then isolated from transformed cells and sequenced to test for the presence of the point mutations. The mutated fragments were then excised from pUC19, cloned into pTA963 (5), and transformed into E. coli DH5α strains. The plasmids were isolated from the DH5α strains, transformed into E. coli DL739 strains, and finally isolated from strain DL739 and transformed into Hfx. volcanii H53.
Growth curves.
Growth curves were generated by using a Biotek Power Wave X2 microplate spectrophotometer. Hfx. volcanii wild-type strain H53 and the agl deletion strains were incubated in MGM medium with continuous shaking at 45°C. Approximately 6 μl of each culture (adjusted slightly for optical density [OD] differences) was transferred into 194 μl of fresh medium and grown to the stationary phase, and OD recordings were taken every 30 min. The growth curves represent an average of cell densities determined in four independent experiments. Each experiment was performed on 10 different strains with eight replicates each, for a final total of 32 replicates per strain.
Protein extraction, LDS-PAGE, and Western blotting.
Liquid cultures were grown until the mid-log phase (OD at 600 nm [OD600] of ∼0.5). Subsequently, cells were collected by centrifugation at 4,300 × g for 10 min at 4°C. Cell pellets were resuspended and lysed in 1% NuPAGE lithium dodecyl sulfate (LDS) supplemented with 50 mM dithiothreitol (DTT) at −20°C. The supernatants of relevant strains were recentrifuged at 4,300 × g for 10 min to remove cellular contamination, secreted proteins were precipitated with cold trichloroacetic acid (TCA) (10%, vol/vol), and pellets were washed twice with cold acetone (80%, vol/vol) and then resuspended in 1% LDS buffer supplemented with 50 mM DTT at −20°C. The electrophoresis of the protein samples was performed with Bis-Tris NuPAGE gels (Invitrogen) under denaturing conditions by using morpholinepropanesulfonic acid (MOPS) at pH 7.7. Proteins were transferred from the gel onto a polyvinylidene difluoride membrane by using a Bio-Rad Transblot-SD semidry transfer cell at 15 V for 30 min. Western blots of whole-cell lysates of Hfx. volcanii strains expressing His-tagged constructs were probed with an anti-His antibody at a dilution of 1:1,000, followed by a secondary anti-mouse antibody at a dilution of 1:10,000. Antibody-labeled protein bands were identified by using the Amersham ECL Plus Western blotting detection system.
Isolation and purification of flagella.
The isolation of flagella was performed as described previously by Fedorov et al. (14), with modifications described below. To select for motile cells, colonies from a solid-agar plate were stab inoculated twice onto motility plates and then inoculated into 5 ml MGM liquid medium. Two liters of MGM medium were inoculated with this 5-ml culture, and the cultures were harvested at an OD600 of approximately 0.2 by centrifugation at 8,700 rpm (JA-10 rotor; Beckman) for 30 min. The supernatant was centrifuged again (8,700 rpm for 30 min) and incubated at room temperature with 4% (wt/vol) PEG 6000 for 1 h. The PEG-precipitated proteins were then centrifuged at 16,000 rpm (JLA-16.250 rotor; Beckman) for 50 min at 4°C, and the flagella were purified by cesium chloride (CsCl) density gradient centrifugation (overnight centrifugation at 50,000 rpm) (VTI-65.1 rotor; Beckman). CsCl was dissolved in a 3 M NaCl saline solution to a final density of 1.37 g/cm. For analysis by LDS-polyacrylamide gel electrophoresis (PAGE) and Coomassie brilliant blue staining, CsCl gradient fractions were dialyzed against water. Glycosylation was tested by using the Pro-Q Emerald 300 glycoprotein gel and blot stain kit (Invitrogen).
Mass spectrometry analysis.
The chosen protein bands were manually excised from the gels and subjected to in-gel digestion with either trypsin or the endoproteinase GluC or double digestion with both enzymes. Tryptic peptide extracts were analyzed by using a Thermo LCQ Deca XP Plus tandem mass spectrometer coupled to an LC Packings Ultimate Nano liquid chromatography system controlled by Thermo Xcalibur 2.0 software. Nano-liquid chromatography-tandem mass spectrometry (LC-MS/MS), autosampling, and chromatography were performed essentially as described previously (31). SEQUEST searches against the Hfx. volcanii protein database (NCBI) were accomplished by using Thermo Bioworks 3.3 software, and results were filtered by using standard values for Xcorr (cross-correlation score) and ΔCN (delta-correlation score) for the preliminary assignment (31). Three missed cleavages were allowed. Following automatic assignment, results were manually validated to assess whether the theoretical y- and b-ion profiles bore a high degree of similarity to the experimental MS/MS spectrum. Those that lacked pairs of corresponding y or b ions were assigned as false positives. Modified peptides were manually inspected.
Electron microscopy.
Fifteen-microliter samples of CsCl gradient fractions were applied onto copper grids coated with carbon-Formvar. The grids were left for 30 min at room temperature, washed with water, blotted with a filter paper, and stained with 2% uranyl acetate for 1 min. Grids were then analyzed by using a Philips Tecnai 12 instrument operating at 120 kV and a Gatan US1000 2K-by-2K (2,024- by 2,024-pixel) camera.
RESULTS
Hfx. volcanii motility is compromised in Δagl strains.
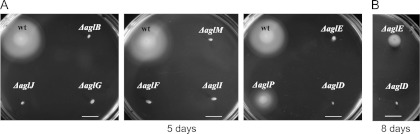
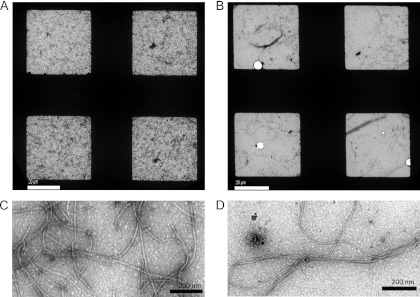
We previously showed that an Hfx. volcanii strain lacking the oligosaccharyltransferase AglB is nonmotile (39). To determine whether the eight other Agl components known to be involved in the glycosylation of the Hfx. volcanii S-layer glycoprotein (11) (Fig. 1) are also required for Hfx. volcanii swimming motility, we assayed the motilities of ΔaglJ, ΔaglG, ΔaglM, ΔaglF, ΔaglI, ΔaglE, ΔaglP, and ΔaglD strains. As shown previously, when H53 (the parent strain) is stab inoculated, robust halos form on MGM–0.3% agar motility plates after 5 days of incubation at 45°C, while ΔaglB cells are nonmotile, with growth being restricted to a small dense region at the stab site (Fig. 2A). We determined that all eight additional agl mutant strains examined in this study exhibited defective or limited motility. Specifically, motility halos were not observed for stab-inoculated ΔaglJ, ΔaglG, ΔaglM, ΔaglF, or ΔaglI strains, even after 10 days of incubation, indicating that the glycosylation of the flagellins and/or other glycoproteins required for flagellum biosynthesis with moieties containing only one or two sugars is not sufficient to produce functional flagella (Fig. 2A). Conversely, although the Hfx. volcanii ΔaglE strain exhibited little motility after 5 days of incubation (Fig. 2A), a notable halo was observed after an additional 3 days (i.e., 8 days total) (Fig. 2B), suggesting that the addition of moieties containing the first three sugars of the pentasaccharide to the flagellins and/or flagellar biosynthesis proteins is sufficient for the assembly of functional flagella. Even more striking, ΔaglP cells generated motility halos with diameters that were at least half of those of H53 halos. As the methylation of the fourth sugar by the methyltransferase AglP is essential to the addition of the fifth and final sugar to the polysaccharide moiety (see below) (26), it appears that this mannose is not required for Hfx. volcanii motility. However, the ΔaglD strain, in which the fourth sugar of the moiety modifying the flagellins is presumably methylated, is surprisingly nonmotile (Fig. 2A). Perhaps, altering the conformation of the structure associated with this tetrasaccharide, via the methylation of the fourth sugar, inhibits the formation of functional flagella.
Fig 2.
Deletion of any of the known Hfx. volcanii agl genes interferes with swimming motility. (A) Hfx. volcanii H53 (wild type [wt]) and Δagl strains were stab inoculated into 0.3% agar in complex medium (MGM) and incubated at 45°C for 5 days. (B) For the Hfx. volcanii ΔaglE strain, a motility halo is clearly visible after 8 days of incubation. A plate lacking the wild-type stab was used for this longer incubation, as the wild-type motility halo would have migrated across much of the plate at this time (horizontal bar, 1 cm). All inoculations were repeated at least three times for the verification of the phenotype.
All deletion strains remained viable through 10 days of incubation on motility plates, as determined by streaking them onto new agar plates, ruling out cell death as the cause of the motility defects, and none of the agl deletion mutants displayed an obvious growth defect when streaked onto solid medium (see Fig. S1A in the supplemental material). However, since the motility assays were carried out with semisolid media, we also compared the growth rates of the agl deletion strains and the H53 parent strain in liquid media. Interestingly, while all nonmotile agl mutants, except for ΔaglD, have doubling times in MGM liquid medium that are similar to that of H53, the ΔaglE and ΔaglP strains exhibited growth defects (see Fig. S1B in the supplemental material).
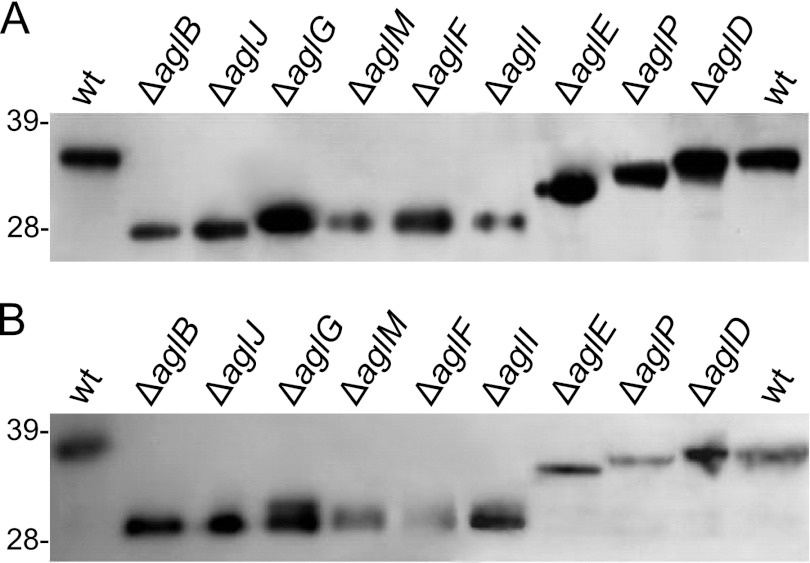
Flagellins expressed in most Δagl strains migrate faster than those expressed in H53 under PAGE.
To obtain evidence for the correlation between Hfx. volcanii swimming motility and flagellin glycosylation, we investigated the glycosylation status of flagellins expressed in the various Δagl strains based on their migration rates under LDS-PAGE. Although the expected molecular masses of His-tagged FlgA1 (FlgA1-His) and FlgA2 (FlgA2-His) are approximately 25 kDa and 24 kDa, respectively, the LDS-PAGE migration of these proteins corresponded to apparent masses of 36 kDa and 38 kDa, respectively (39) (Fig. 3). These flagellins are composed of a high percentage of negatively charged amino acids compared to their nonhalophilic counterparts, perhaps partially explaining the slower-than-expected migration of these proteins (15, 23). However, the unusually large shift in the expected migration rates of these two proteins is also consistent with glycosylation at their potential N-glycosylation sites (39).
Fig 3.
Flagellins expressed in Hfx. volcanii Δagl strains other than the ΔaglD strain migrate faster than when expressed in the wild type. Western blot analysis was performed on protein extracts from Hfx. volcanii H53 (wild type) or Δagl strains grown to the mid-log phase in MGM medium and expressing either FlgA1-His (A) or FlgA2-His (B), encoded by genes expressed under the regulation of a trp-inducible promoter. His-tagged proteins were detected by using anti-His antibodies. Comparable amounts of protein were loaded into each lane of panel A as well as panel B. There was twice as much sample loaded for expression in panel B as in panel A, since FlgA1-His appears to be more abundant than FlgA2-His in the cell extracts. The migration of molecular mass standards is indicated on the left (in kDa).
Indeed, Western blot analysis revealed that FlgA1-His and FlgA2-His isolated from an Hfx. volcanii ΔaglB mutant migrated significantly faster than the same proteins expressed in H53 (FlgA1-His and FlgA2-His isolated from the ΔaglB strain migrate at close to 28 kDa and 30 kDa, respectively) (Fig. 3).
We also determined the migration rates of FlgA1-His isolated from other Δagl strains. Western blot analysis of proteins isolated from these strains following separation by LDS-PAGE showed that the FlgA1-His expressed in all but the ΔaglD mutant strain migrates faster than when expressed in the wild-type strain. For example, FlgA1 expressed in a strain that lacks AglJ, which catalyzes the addition of the first sugar of the pentasaccharide, migrates at the same rate as that of the flagellin expressed in the ΔaglB strain, suggesting that the lack of the first sugar prevents the addition of the others, as was observed for the glycosylation of the S-layer glycoprotein (Fig. 3A) (21). The migration rate of FlgA1 does not appear to increase linearly as the number of sugars added increases (Fig. 3A). For example, a small decrease in the LDS-PAGE migration rate of FlgA1-His expressed in the ΔaglG, ΔaglM, ΔaglF, and ΔaglI strains was observed compared to the migration of the same protein isolated from the ΔaglJ and ΔaglB strains. Conversely, FlgA1-His expressed in either the Hfx. volcanii ΔaglE or ΔaglP strain migrated significantly more slowly than the same protein isolated from the ΔaglG, ΔaglM, ΔaglF, and ΔaglI strains. This, as well as the lack of a size shift of flagellins expressed in the aglD deletion mutant, may be due to factors other than size, such as the pI of the protein. It should be noted that the anti-His antibody detected two protein bands, migrating as a doublet, in extracts isolated from several agl strains expressing either FlgA1-His or FlgA2-His in trans, suggesting that the flagellins may undergo a second type of modification in addition to N-glycosylation.
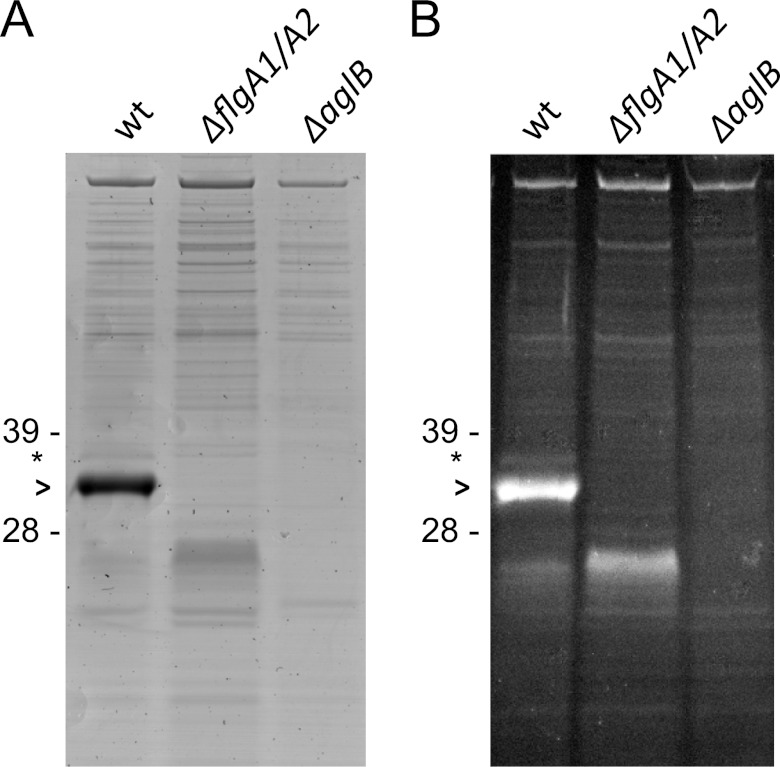
While the data presented above suggest that FlgA1 and FlgA2 contain the same pentasaccharide as the S-layer glycoprotein and that the components involved in the addition of the pentasaccharide to S-layer glycosylation are also required for flagellin glycosylation, it is possible that FlgA1 and FlgA2 contain additional sugars that are not added to the S-layer glycoprotein. Therefore, we set out to identify a protocol that would allow us to purify Hfx. volcanii flagella for mass spectrometry analyses. Two-liter cultures of a wild-type strain and a strain lacking the coregulated genes encoding the two Hfx. volcanii flagellins, flgA1 and fglA2, were each spun down, and the supernatants, upon PEG precipitation, were separated by using cesium chloride (CsCl) gradient centrifugation. Samples of seven CsCl gradient fractions were collected, separated by gel electrophoresis, and stained with Coomassie brilliant blue. Fraction 4 of the wild-type sample contained a prominent protein band migrating at an apparent molecular mass of approximately 35 kDa that was not observed in any of the fractions generated from the ΔflgA1 ΔflgA2 strain (Fig. 4A). Mass spectrometry confirmed that this 35-kDa band, which was also stained with the Pro-Q Emerald 300 glycoprotein gel and blot stain kit (Fig. 4B), contained mostly FlgA1 (see Fig. S2 and Table S2 in the supplemental material). In the same CsCl fractions, an additional faint band was observed to migrate at a rate consistent with a protein having a molecular mass of 37 kDa, the same molecular mass as that of FlgA2. Mass spectrometry confirmed that this band contained FlgA2, and in agreement with Coomassie blue staining, a comparison of the number of MS/MS spectra of the 35-kDa and 37-kDa bands strongly supports that the Hfx. volcanii flagellum is composed of a major flagellin, FlgA1, as well as a minor flagellin, FlgA2.
Fig 4.
AglB is required for the biosynthesis of stable flagella. CsCl-purified samples of strain H53 (wild type) and the ΔflgA1 ΔflgA2 and ΔaglB mutant strains stained with Coomassie brilliant blue (A) and Pro-Q Emerald glycoprotein stain (B) show the presence of glycosylated protein bands corresponding to molecular masses of FlgA1 (35 kDa) and FlgA2 (37 kDa) in the wild-type fraction, which were absent in both the ΔflgA1 ΔflgA2 and ΔaglB mutant strains. The migration of molecular mass standards is indicated on the left (in kDa). >, FlgA1; ∗, FlgA2.
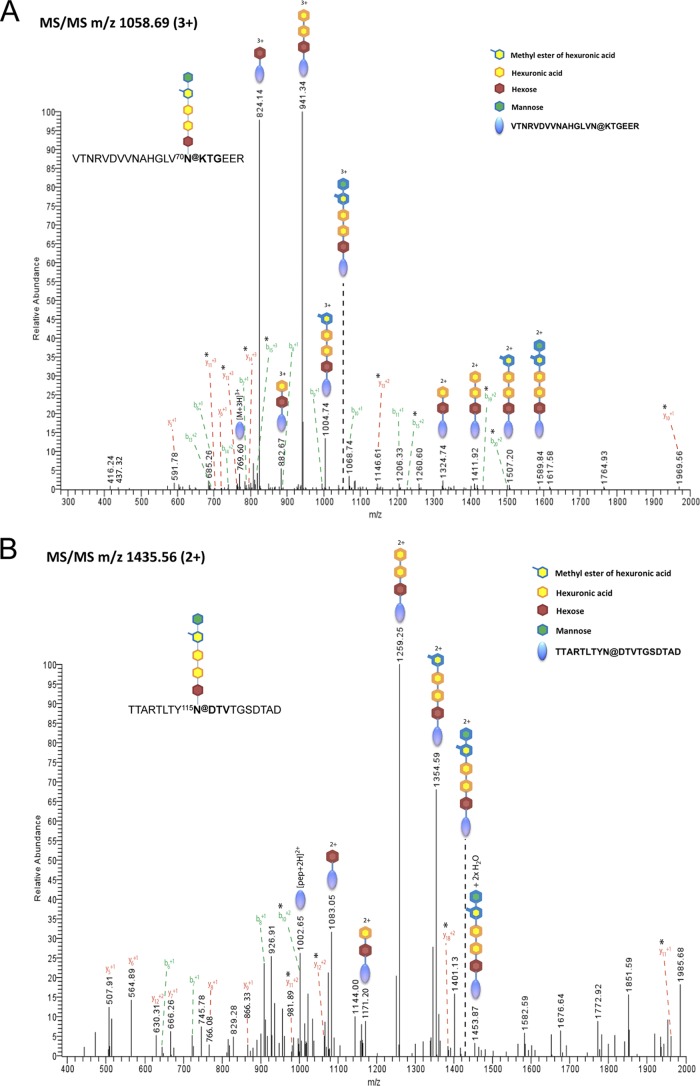
MS/MS of FlgA1 glycoprotein-derived proteolytic peptides revealed the presence of three peptides containing the predicted glycosylation sites modified by the pentasaccharide moiety (m/z 1,058.69 [3+], 1,230.27 [2+], and 1,435.56 [2+]), as identified for the S-layer glycoprotein (Fig. 5A to C). These data not only confirm that the same five sugars modify the flagellins and S-layer glycoprotein but also indicate that no additional sugars absent from the S layer are added to the flagellins. The identified glycosylation sites are consistent with three predicted N-glycosylation sites in the FlgA1 protein sequence (NetNGlyc 1.0) (Fig. 5D). The peptides containing three additional sequons were likely too large (or too small in the double-digested samples) to be detected by LC-MS/MS.
Fig 5.
Three predicted FlgA1 N-glycosylation sites are modified with the same pentasaccharides as its S-layer glycoprotein. Three distinct ion trap MS/MS spectra show all observed fragment ions that arose from backbone cleavage and fragmentation of the glycan moiety of intact peptides. The y- and b-ion fragments indicated by asterisks allowed the localization of the modification at the Asn residues in each peptide. (A) MS/MS of the precursor ion of m/z 1,058.69 (3+) corresponding to the glycopeptide VTNRVDVVNAHGLVN@KTGEER (@ represents the glycosylated Asn residue). (B) MS/MS of the precursor ion of m/z 1,230.27 (2+) corresponding to the glycopeptide GDNTN@GTATGQTVKLD. (C) MS/MS of the precursor ion of m/z 1,435.56 (2+) corresponding to the glycopeptide TTARTLTYN@DTVTGSDTAD. Peptides containing the glycan (a pentasaccharide) are indicated at the top left of each panel. The identities of the glycan components are presented with color coding in the inset at the top right of panel A (the complete pentasaccharide is mannose-MethHexUA-HexUA-HexUA-hexose-Asn, where HexUA is hexuronic acid and MethHexUA is the methyl ester of hexuronic acid). (D) Amino acid sequences of Hfx. volcanii FlgA1 with Asn-Xaa-Ser/Thr sequons are shown in boldface type. The three sequons predicted by NetNGlyc 1.0. were identified as being modified by MS/MS. Peptides containing sugar modifications shown in panels A, B, and C are boxed.
Glycosylation appears to be required for biosynthesis of stable flagella.
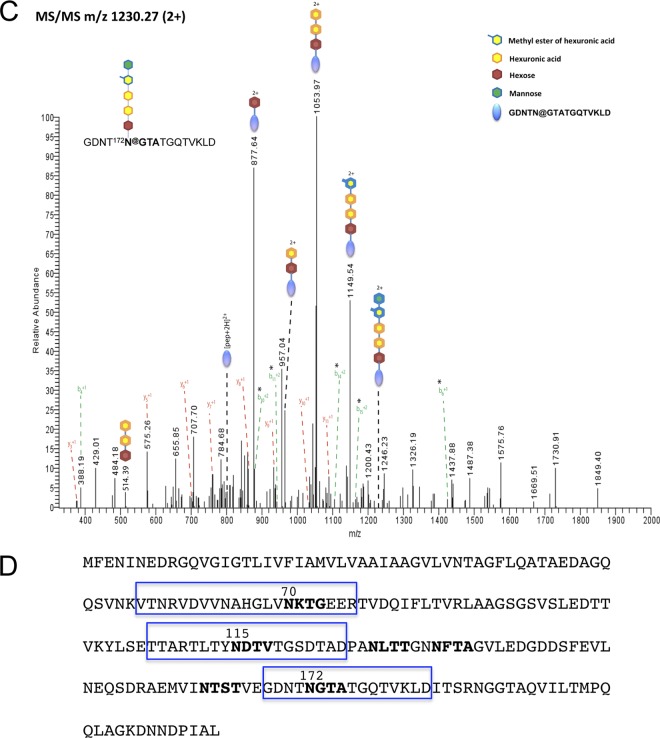
Nonglycosylated flagellins appear to be stable in a ΔaglB strain, based on the Western blot analyses presented above (Fig. 3). Therefore, it is possible that the swimming motility defect caused by the lack of flagellin glycosylation is due to the disruption of flagellum assembly or, alternatively, an inhibited function of assembled flagella. Hence, we used the CsCl density gradient fractionation protocol to determine whether assembled flagella can be isolated from a ΔaglB strain. Gel electrophoresis was employed to separate proteins isolated from CsCl density gradient fraction 4 of the ΔaglB culture supernatant, followed by Coomassie brilliant blue staining of the gel. This stain did not detect either a protein migrating at a rate corresponding to FlgA1 or a faster-migrating band that might contain nonglycosylated flagellins, consistent with the absence of stable flagella in the ΔaglB strain (Fig. 4A). Consistent with it containing purified flagella rather than flagellin subunits released into the supernatant, CsCl density gradient fraction 4 was enriched for filaments having diameters of 12 to 14 nm, as determined by electron microscopy (Fig. 6A). This fraction must also contain type IV pilus-like structures that are not flagella, as electron microscopy showed that, albeit to a significantly lesser extent, a ΔaglB strain produces filamentous structures (Fig. 6B). Consistent with these results, mass spectrometric analyses showed that CsCl density gradient fractions 4 for the wild-type and ΔaglB strains all contain peptides of proteins predicted to be pilins, while flagellins were identified only in the wild-type fraction (38; data not shown). Taken together, the Coomassie brilliant blue staining, mass spectrometry, and electron microscopy data presented above suggest that glycosylation is necessary for flagellins to form a stable flagellum in Hfx. volcanii.
Fig 6.
Hfx. volcanii contains AglB-independent type IV pilus-like structures. Shown are transmission electron microscopy images of Hfx. volcanii strain H53 (wild type) and ΔaglB CsCl fraction 4 stained with uranyl acetate. (A and B) While the abundance of observed surface filaments (diameter of between 12 and 14 nm) was highest in the wild-type fraction (A), a significant number of filaments of similar diameters were identified in CsCl fraction 4 of the ΔaglB strain (B). (C and D) Magnification of filaments observed for the wild-type (C) and ΔaglB (D) strains.
Predicted FlgA1 glycosylation sites are critical for flagellum function.
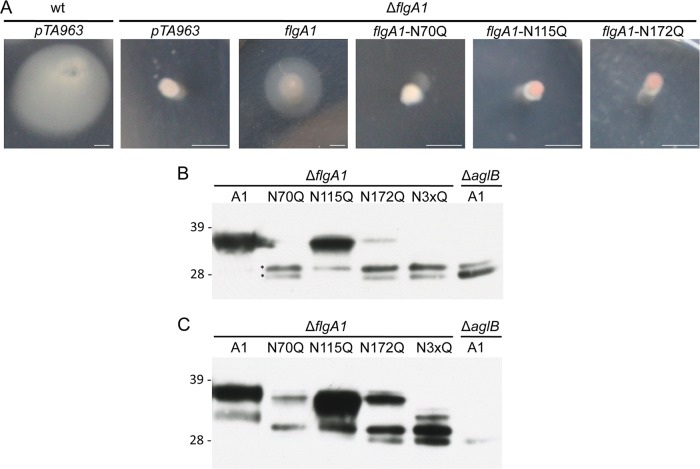
The mass spectrometry data presented above strongly support the modification of at least three NetNGlyc-predicted peptides by the pentasaccharide moiety (Fig. 5; see also Fig. S2 in the supplemental material). Here we analyzed the importance of the glycosylation of each of these three glycosylation sites. While we previously showed that the deletion of FlgA1 and FlgA2 rendered cells nonmotile, in this study, we showed that the deletion of FlgA1 alone can render cells nonmotile (Fig. 7 and 8A). The motility of this mutant can be rescued, albeit not to wild-type levels, by the expression of flgA1-His from a plasmid under the control of the inducible ptna promoter (Fig. 8A). We took advantage of this observation to test the importance of each of the N-glycosylation modifications of FlgA1 by expressing mutant proteins in which one of the three identified FlgA1 glycosylation sites was replaced with a glutamine in an Hfx. volcanii ΔflgA1 strain. Unlike wild-type FlgA1-His, the replacement mutant constructs did not rescue the swimming motility of an Hfx. volcanii ΔflgA1 strain when expressed in trans (Fig. 8A). Western blot analyses of the cell fractions for cells expressing the His-tagged flagellin constructs revealed that the mutant constructs were expressed. However, the intensities of the protein bands detected for FlgA1N70Q-His and FlgA1N172Q-His were considerably lower than the intensities of bands containing the FlgA1-His or FlgA1N115Q-His proteins (Fig. 8B). In fact, only protein bands migrating at the same rate as that of FlgA1-His expressed in the ΔaglB strain were detected in protein extracts isolated from the ΔflgA1 strain expressing FlgA1N70Q-His. Possibly, the glycosylation of this site in FlgA1 affects the modification of the other targeted sites in the same protein. Interestingly, while FlgA1-His can barely be detected in the supernatant fraction of the ΔaglB strain, consistent with the CsCl density gradient centrifugation results, all replacement mutants expressed in the ΔflgA1 strain are clearly present in the supernatant fractions. In future structure-function studies, we will determine whether these nonfunctional flagellin replacement mutants can form stable flagella.
Fig 7.
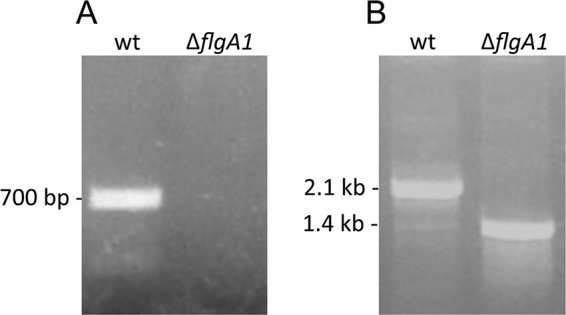
Confirmation of the deletion of the flgA1 locus in Hfx. volcanii. PCRs were performed using primers (see Table S1 in the supplemental material) against the flgA1 gene (A) and flanking regions approximately 700 nucleotides upstream and downstream of the flgA1 locus (B).
Fig 8.
Predicted N-glycosylation sites are crucial for Hfx. volcanii motility. (A) Hfx. volcanii H53 (wild type) or the ΔflgA1 strain transformed with expression vector pTA963 or pTA963 expressing either FlgA1-His or FlgA1-His constructs in which a predicted N-glycosylation site asparagine (N) is replaced with a glutamine (Q) under the control of an inducible trp promoter. Strains stab inoculated into complex medium (MGM) with 0.3% agar were incubated at 45°C for 5 days. Motility halos indicate the presence of functional flagella. All stabs and incubations were repeated at least three times for the verification of the phenotype. Horizontal bars, 0.5 cm. (B and C) Western blot analysis of cell extracts (B) and TCA-precipitated supernatant fractions (C) from the Hfx. volcanii ΔflgA1 strain transformed with pTA963 expressing either FlgA1-His (wild type) or FlgA1N-Q-His constructs and the Hfx. volcanii ΔaglB strain transformed with pTA963 expressing FlgA1-His. His-tagged proteins were detected by using anti-His antibodies. Comparable amounts of protein were loaded into each lane. There was twice as much protein loaded for samples in panel C as for samples in panel B, to show the mutated flagellins in the supernatant fractions. While slower-migrating bands (*) were detected in all N-Q-His samples, only proteins from the wild type and the N115Q mutant consistently contained a band that migrated at the expected molecular mass in the cell fraction. The migration of molecular mass standards is indicated on the left (in kDa).
Finally, while the FlgA1 replacement mutants each migrate slightly faster than wild-type flagellin, which is consistent with the lack of a pentasaccharide, FlgA1 lacking all three glycosylation sites migrates significantly faster than the wild-type proteins does. However, even FlgA1 lacking all three predicted glycosylation sites migrates significantly more slowly than FlgA1 produced by the ΔaglB strain, suggesting that, consistent with the additional three predicted sequons (Fig. 5), additional N-glycosylation occurs in these strains (Fig. 8).
DISCUSSION
Studies of the N-glycosylation of S-layer glycoproteins in two methanogens, a Sulfolobus strain, and two haloarchaeal species have clearly demonstrated that the details of the glycosylation process vary significantly across archaeal species, even among closely related organisms (11, 19, 29). Moreover, there can be important differences in how various sites within the same protein are glycosylated (28). The glycosylation of certain sites within the protein can even depend upon the specific environmental conditions under which the organism is grown, as was demonstrated recently for Hfx. volcanii (16). However, although glycosylation may stabilize the S layer, the lack of a well-defined functional assay makes assessing the importance of the pentasaccharide moieties, or any derivatives, that decorate the S-layer glycoprotein difficult. Further complicating matters, the S-layer glycoprotein is also O-glycosylated, making functional analyses even more complex. These limitations can be avoided by studying the effects of the disruption of the glycosylation of archaeal flagellum subunits, as was demonstrated previously for the methanogens M. maripaludis and M. voltae, since they are relatively small, do not appear to be O-glycosylated, and have a function that can be easily assayed (19).
Using CsCl density gradient centrifugation, we have developed a method to purify flagella, allowing us to demonstrate that FlgA1 is the major Hfx. volcanii flagellin and that FlgA2 is a minor flagellin. In addition to providing an important insight into the biosynthesis of Hfx. volcanii flagella, this has allowed us to accumulate enough Hfx. volcanii FlgA1 to complete a thorough biochemical analysis of its glycosylation. Taking advantage of this, we developed several lines of evidence indicating that Hfx. volcanii flagellins are glycosylated and that this glycosylation is required for the formation of a functional flagellum.
Using agl deletion mutants known to inhibit S-layer glycoprotein glycosylation in Hfx. volcanii, we determined that flagellins expressed in all mutants but one (ΔaglD) migrate faster than flagellins expressed in the wild type, consistent with flagellum subunits lacking sugars having lower molecular weights. Moreover, these data provide direct evidence that flagellins are glycosylated through a process that employs a set of components similar to that involved in the glycosylation of the S-layer glycoprotein. While these mobility shifts are reminiscent of size shifts observed for Methanococcus S-layer glycoproteins and flagellins in agl mutant strains, it is interesting that the Hfx. volcanii S-layer glycoprotein mobility shifts do not correlate with the number of glycans added (4, 21). It should be noted that in the aglG, aglI, aglF, and aglM deletion strains, AglB may transfer fewer polysaccharide chains to some targeted substrates. Such a substrate specificity may explain why, in these particular agl deletion strains, AglB might not attach a polysaccharide at each N-linked site predicted for FlgA1, which may account for the higher-than-expected electrophoretic mobility of FlgA1 proteins in these mutants than that of FlgA1 made in strains lacking AglP and AglE (Fig. 3).
Mass spectrometry analyses not only confirmed the modification of the three asparagines predicted to be glycosylated by NetNGlyc but also revealed that FlgA1 is modified by the same sugar moieties as the S-layer glycoprotein. This is consistent with observations of methanogens, where, although the sugars added to the structures in each species are distinct, within an organism the cell wall and flagellar subunits do appear to be modified by the same saccharides (12). Similarly, as was shown previously for M. maripaludis motility, it appears that, at the very least, a minimal-length glycan attached to the flagellins is required for functional flagella and that cells lacking AglB cannot produce stable flagella (20, 41). Finally, our preliminary data suggesting the presence of pili in the ΔaglB strain are also consistent with data from an analysis of an M. maripaludis strain lacking the acetyltransferase required for the proper assembly of flagella and pili, which revealed that although flagella were absent, pili were found in the supernatant fractions of cultures of this mutant strain (42). Studies are under way to determine whether the filaments enriched for in CsCl density gradient fraction 4 of the ΔaglB strain are indeed pili, as suggested by mass spectrometric analyses, which identified peptides of a predicted type IV pilin-like protein in fraction 4 for the ΔaglB culture supernatant. Thus far, the failure to raise antibodies against Hfx. volcanii flagellins, possibly due to the extensive glycosylation of these proteins, has prevented us from distinguishing flagella from non-flagellum type IV pilus-like structures and hence from excluding the possibility that a subset of these surface filaments are nonglycosylated flagella. Comparing and contrasting pilus and flagellum biosynthesis in Hfx. volcanii may yield some interesting insights, especially considering that glycosylation is apparently not required for the assembly of at least a subset of pili.
While the data described above clearly showed that, as in other archaeal systems studied, the subunits of the S-layer glycoprotein and flagella are glycosylated in a similar manner, it has not been determined whether the lack of glycosylation directly or indirectly affects flagellum motility and biosynthesis. A lack of glycosylation could interfere with flagella anchoring to the cell surface, possibly due to an absence of N-glycosylation of the S-layer glycoprotein, hence resulting in the degradation of the protein in the supernatant. Having identified three glycosylation sites in the major flagellin by mass spectrometry and having demonstrated that FlgA1 expressed from a plasmid can complement an flgA1 deletion strain, we showed that the specific amino acid residues targeted for glycosylation in the flagellins are absolutely required for the flagellum to function properly. We demonstrated this by showing that the expression of site-directed flgA1 mutants lacking one of the three identified glycosylation sites of FlgA1 does not rescue swimming motility. Moreover, while the N-glycosylation of each of these three predicted glycosylation sites is critical to motility, Western blot analysis of proteins isolated from cells and supernatant fractions of cells expressing the His-tagged replacement mutant flagellin constructs indicated that glycosylation differentially affects the stability of FlgA1, depending upon which of the glycosylation sites in the flagellin are modified by the addition of a polysaccharide moiety. Why this is so is one of many questions that can be addressed now that we have purified the glycosylated form of FlgA1, the major flagellin, and generated an flgA1 deletion mutant in which altered FlgA1 constructs can be expressed in trans. In future experiments, combined characterizations of flg and agl mutants using electron microscopy, high-resolution mass spectrometry, and functional assays will allow us to clarify the role that glycosylation plays in the proper assembly and function of Hfx. volcanii cell surface structures.
We can now begin to determine the importance of specific sugars to the structure and function of the Hfx. volcanii flagellum and the significance of the modifications of specific sugars, such as why the fourth sugar must be methylated prior to the addition of the mannose cap in the flagellar pentasaccharide and why, when the fifth sugar is absent, the methylation of the fourth sugar renders the cells nonmotile. Interestingly, the agl deletion mutants in which swimming motility was least affected are those that exhibit growth defects, suggesting that the various steps in the glycosylation process affect the functions of Hfx. volcanii glycoproteins to various degrees. Whether this is due to differences in the way different protein complexes form or because specific protein functions are differentially affected is unknown.
Supplementary Material
ACKNOWLEDGMENTS
J.Y. was supported by National Science Foundation grant MCB02. M.P., S.T., and M.T. were supported by National Aeronautics and Space Administration grant NNX10AR84G. D.B. and Ö.Ö. were supported by National Institutes of Health grant AI076342.
We thank Tanya Svitkina and Dewight Williams for advice on microscopy, Dieter Schifferli and Fevzi Daldal for helpful discussions, and Jerry Eichler for providing us with the Hfx. volcanii agl mutant strains.
Footnotes
Published ahead of print 22 June 2012
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1. Abu-Qarn M, Eichler J. 2006. Protein N-glycosylation in Archaea: defining Haloferax volcanii genes involved in S-layer glycoprotein glycosylation. Mol. Microbiol. 61:511–525 [DOI] [PubMed] [Google Scholar]
- 2. Abu-Qarn M, Eichler J, Sharon N. 2008. Not just for Eukarya anymore: protein glycosylation in Bacteria and Archaea. Curr. Opin. Struct. Biol. 18:544–550 [DOI] [PubMed] [Google Scholar]
- 3. Abu-Qarn M, et al. 2008. Identification of AglE, a second glycosyltransferase involved in N glycosylation of the Haloferax volcanii S-layer glycoprotein. J. Bacteriol. 190:3140–3146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Abu-Qarn M, et al. 2007. Haloferax volcanii AglB and AglD are involved in N-glycosylation of the S-layer glycoprotein and proper assembly of the surface layer. J. Mol. Biol. 374:1224–1236 [DOI] [PubMed] [Google Scholar]
- 5. Allers T, Barak S, Liddell S, Wardell K, Mevarech M. 2010. Improved strains and plasmid vectors for conditional overexpression of His-tagged proteins in Haloferax volcanii. Appl. Environ. Microbiol. 76:1759–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allers T, Ngo HP. 2003. Genetic analysis of homologous recombination in Archaea: Haloferax volcanii as a model organism. Biochem. Soc. Trans. 31:706–710 [DOI] [PubMed] [Google Scholar]
- 7. Allers T, Ngo HP, Mevarech M, Lloyd RG. 2004. Development of additional selectable markers for the halophilic archaeon Haloferax volcanii based on the leuB and trpA genes. Appl. Environ. Microbiol. 70:943–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benz I, Schmidt MA. 2002. Never say never again: protein glycosylation in pathogenic bacteria. Mol. Microbiol. 45:267–276 [DOI] [PubMed] [Google Scholar]
- 9. Blattner FR, et al. 1977. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science 196:161–169 [DOI] [PubMed] [Google Scholar]
- 10. Blyn LB, Braaten BA, Low DA. 1990. Regulation of pap pilin phase variation by a mechanism involving differential dam methylation states. EMBO J. 9:4045–4054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Calo D, Kaminski L, Eichler J. 2010. Protein glycosylation in Archaea: sweet and extreme. Glycobiology 20:1065–1076 [DOI] [PubMed] [Google Scholar]
- 12. Chaban B, Logan SM, Kelly JF, Jarrell KF. 2009. AglC and AglK are involved in biosynthesis and attachment of diacetylated glucuronic acid to the N-glycan in Methanococcus voltae. J. Bacteriol. 191:187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dyall-Smith M. 2004. The halohandbook—protocols for haloarchaeal genetics. University of Melbourne, Victoria, Australia: http://www.haloarchaea.com/resources/halohandbook/index.html [Google Scholar]
- 14. Fedorov OV, Pyatibratov MG, Kostyukova AS, Osina NK, Tarasov VY. 1994. Protofilament as a structural element of flagella of haloalkalophilic archaebacteria. Can. J. Microbiol. 40:45–53 [Google Scholar]
- 15. Frolow F, Harel M, Sussman JL, Mevarech M, Shoham M. 1996. Insights into protein adaptation to a saturated salt environment from the crystal structure of a halophilic 2Fe-2S ferredoxin. Nat. Struct. Biol. 3:452–458 [DOI] [PubMed] [Google Scholar]
- 16. Guan Z, Naparstek S, Calo D, Eichler J. 2012. Protein glycosylation as an adaptive response in Archaea: growth at different salt concentrations leads to alterations in Haloferax volcanii S-layer glycoprotein N-glycosylation. Environ. Microbiol. 14:743–753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guan Z, Naparstek S, Kaminski L, Konrad Z, Eichler J. 2010. Distinct glycan-charged phosphodolichol carriers are required for the assembly of the pentasaccharide N-linked to the Haloferax volcanii S-layer glycoprotein. Mol. Microbiol. 78:1294–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Horzempa J, Dean CR, Goldberg JB, Castric P. 2006. Pseudomonas aeruginosa 1244 pilin glycosylation: glycan substrate recognition. J. Bacteriol. 188:4244–4252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jarrell KF, Jones GM, Nair DB. 2010. Biosynthesis and role of N-linked glycosylation in cell surface structures of archaea with a focus on flagella and S layers. Int. J. Microbiol. 2010:470138 doi:10.1155/2010/470138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jones GM, et al. 2012. Identification of genes involved in the acetamidino group modification of the flagellin N-linked glycan of Methanococcus maripaludis. J. Bacteriol. 194:2693–2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kaminski L, et al. 2010. AglJ adds the first sugar of the N-linked pentasaccharide decorating the Haloferax volcanii S-layer glycoprotein. J. Bacteriol. 192:5572–5579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kelly J, Logan SM, Jarrell KF, VanDyke DJ, Vinogradov E. 2009. A novel N-linked flagellar glycan from Methanococcus maripaludis. Carbohydr. Res. 344:648–653 [DOI] [PubMed] [Google Scholar]
- 23. Kennedy SP, Ng WV, Salzberg SL, Hood L, DasSarma S. 2001. Understanding the adaptation of Halobacterium species NRC-1 to its extreme environment through computational analysis of its genome sequence. Genome Res. 11:1641–1650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Large A, et al. 2007. Characterization of a tightly controlled promoter of the halophilic archaeon Haloferax volcanii and its use in the analysis of the essential cct1 gene. Mol. Microbiol. 66:1092–1106 [DOI] [PubMed] [Google Scholar]
- 25. Live DH, Kumar RA, Beebe X, Danishefsky SJ. 1996. Conformational influences of glycosylation of a peptide: a possible model for the effect of glycosylation on the rate of protein folding. Proc. Natl. Acad. Sci. U. S. A. 93:12759–12761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Magidovich H, et al. 2010. AglP is a S-adenosyl-L-methionine-dependent methyltransferase that participates in the N-glycosylation pathway of Haloferax volcanii. Mol. Microbiol. 76:190–199 [DOI] [PubMed] [Google Scholar]
- 27. Mescher MF, Hansen U, Strominger JL. 1976. Formation of lipid-linked sugar compounds in Halobacterium salinarium. Presumed intermediates in glycoprotein synthesis. J. Biol. Chem. 251:7289–7294 [PubMed] [Google Scholar]
- 28. Mescher MF, Strominger JL. 1978. Glycosylation of the surface glycoprotein of Halobacterium salinarium via a cyclic pathway of lipid-linked intermediates. FEBS Lett. 89:37–41 [DOI] [PubMed] [Google Scholar]
- 29. Meyer BH, et al. 2011. Sulfoquinovose synthase—an important enzyme in the N-glycosylation pathway of Sulfolobus acidocaldarius. Mol. Microbiol. 82:1150–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nothaft H, Szymanski CM. 2010. Protein glycosylation in bacteria: sweeter than ever. Nat. Rev. Microbiol. 8:765–778 [DOI] [PubMed] [Google Scholar]
- 31. Onder O, Turkarslan S, Sun D, Daldal F. 2008. Overproduction or absence of the periplasmic protease DegP severely compromises bacterial growth in the absence of the dithiol:disulfide oxidoreductase DsbA. Mol. Cell. Proteomics 7:875–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peters J, et al. 1995. Tetrabrachion: a filamentous archaebacterial surface protein assembly of unusual structure and extreme stability. J. Mol. Biol. 245:385–401 [DOI] [PubMed] [Google Scholar]
- 33. Plavner N, Eichler J. 2008. Defining the topology of the N-glycosylation pathway in the halophilic archaeon Haloferax volcanii. J. Bacteriol. 190:8045–8052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rangarajan ES, et al. 2007. Structural context for protein N-glycosylation in bacteria: the structure of PEB3, an adhesin from Campylobacter jejuni. Protein Sci. 16:990–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shen A, Kamp HD, Grundling A, Higgins DE. 2006. A bifunctional O-GlcNAc transferase governs flagellar motility through anti-repression. Genes Dev. 20:3283–3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shental-Bechor D, Levy Y. 2008. Effect of glycosylation on protein folding: a close look at thermodynamic stabilization. Proc. Natl. Acad. Sci. U. S. A. 105:8256–8261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sumper M, Berg E, Mengele R, Strobel I. 1990. Primary structure and glycosylation of the S-layer protein of Haloferax volcanii. J. Bacteriol. 172:7111–7118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Szabo Z, et al. 2007. Identification of diverse archaeal proteins with class III signal peptides cleaved by distinct archaeal prepilin peptidases. J. Bacteriol. 189:772–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tripepi M, Imam S, Pohlschroder M. 2010. Haloferax volcanii flagella are required for motility but are not involved in PibD-dependent surface adhesion. J. Bacteriol. 192:3093–3102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Twine SM, et al. 2009. Motility and flagellar glycosylation in Clostridium difficile. J. Bacteriol. 191:7050–7062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. VanDyke DJ, et al. 2009. Identification of genes involved in the assembly and attachment of a novel flagellin N-linked tetrasaccharide important for motility in the archaeon Methanococcus maripaludis. Mol. Microbiol. 72:633–644 [DOI] [PubMed] [Google Scholar]
- 42. VanDyke DJ, et al. 2008. Identification of a putative acetyltransferase gene, MMP0350, which affects proper assembly of both flagella and pili in the archaeon Methanococcus maripaludis. J. Bacteriol. 190:5300–5307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Voisin S, et al. 2005. Identification and characterization of the unique N-linked glycan common to the flagellins and S-layer glycoprotein of Methanococcus voltae. J. Biol. Chem. 280:16586–16593 [DOI] [PubMed] [Google Scholar]
- 44. Wieland F, Paul G, Sumper M. 1985. Halobacterial flagellins are sulfated glycoproteins. J. Biol. Chem. 260:15180–15185 [PubMed] [Google Scholar]
- 45. Yanisch-Perron C, Vieira J, Messing J. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119 [DOI] [PubMed] [Google Scholar]
- 46. Yurist-Doutsch S, et al. 2008. aglF, aglG and aglI, novel members of a gene island involved in the N-glycosylation of the Haloferax volcanii S-layer glycoprotein. Mol. Microbiol. 69:1234–1245 [DOI] [PubMed] [Google Scholar]
- 47. Yurist-Doutsch S, et al. 2010. N-glycosylation in Archaea: on the coordinated actions of Haloferax volcanii AglF and AglM. Mol. Microbiol. 75:1047–1058 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.