Abstract
A phage moron is a DNA element inserted between a pair of genes in one phage genome that are adjacent in other related phage genomes. Phage morons are commonly found within phage genomes, and in a number of cases, they have been shown to mediate phenotypic changes in the bacterial host. The temperate phage HK97 encodes a moron element, gp15, within its tail morphogenesis region that is absent in most closely related phages. We show that gp15 is actively expressed from the HK97 prophage and is responsible for providing the host cell with resistance to infection by phages HK97 and HK75, independent of repressor immunity. To identify the target(s) of this gp15-mediated resistance, we created a hybrid of HK97 and the related phage HK022. This hybrid phage revealed that the tail tube or tape measure proteins likely mediate the susceptibility of HK97 to inhibition by gp15. The N terminus of gp15 is predicted with high probability to contain a single membrane-spanning helix by several transmembrane prediction programs. Consistent with this putative membrane localization, gp15 acts to prevent the entry of phage DNA into the cytoplasm, acting in a manner reminiscent of those of several previously characterized superinfection exclusion proteins. The N terminus of gp15 and its phage homologues bear sequence similarity to YebO proteins, a family of proteins of unknown function found ubiquitously in enterobacteria. The divergence of their C termini suggests that phages have co-opted this bacterial protein and subverted its activity to their advantage.
INTRODUCTION
Bacteriophages are the most abundant biological entities on earth, and they play significant roles in bacterial survival, physiology, and virulence (6–8). Phages exist in nature as either free virions or prophages, a form in which their genomes are maintained as an autonomously replicating plasmid or are integrated into the bacterial chromosome and passively replicated. Prophages are a major contributor to genomic diversity within bacterial species, and the average bacterial genome contains three prophages; prophage DNA can comprise up to 10% of a bacterial genome (8). It has been commonly thought that prophage genes, aside from those required for maintaining lysogeny, are predominantly silent and functionally unimportant. However, it has become increasingly clear in recent years that many genes expressed from prophages can provide the host cell with increased fitness and thus provide a selective advantage for prophage maintenance. The phenotypes arising from the integration of prophages include protection against infection by other phages, increased virulence of the host, or an increase in fitness of the host for a specific environment (for review, see the work of Brüssow et al. [6]).
The ability of prophages to mediate phenotypic changes in their bacterial hosts is sometimes conferred by moron elements. These elements contain one or more genes flanked by a promoter and a transcriptional terminator. They are thought to be inserted into phage genomes through horizontal transfer. These elements were first described by Juhala et al. (18) when annotating the genomes of Escherichia coli siphophages HK97 and HK022, two related phages belonging to the λ-like group. The conserved gene order in their morphogenetic regions allowed the identification of segments of DNA that were present in HK97 or HK022 and absent in phage λ. These segments were called morons because there is “more DNA” than there is without the element. Genes within morons were predicted to be transcribed autonomously, even from within a repressed prophage (18).
While morons are common in temperate phage genomes, functional information is available for only a small number of them (6, 14). It has been proposed that morons increase host fitness by encoding novel genes that make the bacteria more competitive in an old niche or allow them to exploit a new niche. A number of morons have been shown to play important roles in bacterial survival. For example, the presence of a λ prophage in E. coli leads to increased binding to mammalian host cells, as well as increased resistance to killing by the mammalian immune system (3, 4). These fitness factors are provided by morons containing the lom and bor genes. Morons in Salmonella phages Fels-2 and GIFSY-2 encode superoxide dismutases, which confer resistance against reactive oxygen species produced by the mammalian immune system (12).
Proteins expressed from moron elements can also provide resistance to phage infection. For example, the Cor protein from the ϕ80 prophage inactivates the cell surface receptor FhuA (37), resulting in the inability of ϕ80 itself to infect the host. Other phages that require this receptor (e.g., phages T1, T5, HK022, and N15), as well as toxins that are imported through it (e.g., colicin M), are likewise unable to infect cells that express the Cor protein (13, 15). Other proteins expressed from morons, such as Salmonella phage P22 SieA (33), E. coli phage T4 Sp and Imm (25, 26), and Lactococcus lactis phage Tuc2009 Sie2009 (27), prevent entry of phage DNA at a point downstream of the initial receptor binding event. Expression of moron-encoded proteins can also lead to abortive infections, as is the case with SieB of Salmonella phage P22. This gene aborts the infection of some superinfecting lytic phages, such as Salmonella phage L, leading to death of a cell infected by both phages before the superinfecting phage can produce progeny (16, 34). These mechanisms protect the lysogenized host population from death by invading phages or other bacteriocins.
The experiments described in this work were prompted by our serendipitous discovery that the protein product of HK97 gene 15 (gp15), which lies within a moron element, can mediate strong resistance to phage HK97 and its close relative, HK75. We have characterized the mechanism of action of gp15 and identified homologues in both phage and bacterial genomes. We provide evidence that gp15 represents a new class of phage-encoded superinfection exclusion proteins that appear to have been derived from a large family of enterobacterial proteins of unknown function.
MATERIALS AND METHODS
Expression plasmid construction.
The HK97 gene 15 open reading frame (ORF) was cloned into a pAD100 (10) plasmid along with 57 bp of upstream sequence and 43 bp of downstream sequence that contained the putative promoter and terminator regions (pEx15). It was also cloned into pAD100 with the 57-bp promoter region and a C-terminal FLAG epitope (pEx15-FLAG) (17) and no putative terminator region. A version with a nonsense mutation introduced at position Leu4 (p15am-FLAG) was also made via site-directed mutagenesis. The HK022 gene 20 open reading frame was cloned into the same plasmid along with 57 bp upstream containing its putative promoter region and a C-terminal FLAG tag (pEx20-FLAG).
In vivo assay for moron activity.
E. coli 594 cells containing either plasmid pEx15, pEx15-FLAG, pEx15am-FLAG, or pEx20-FLAG were suspended in molten 0.7% top agar and plated on LB agar. Serial dilutions of E. coli phages were spotted in 4-μl aliquots on top of the cells, and the degree of activity was scored by observing plaque formation after incubation overnight at 37°C. The phages tested include HK97, HK75, HK022, λ, HK243, HK140, HK225, HK446, HK542, HK544, HK578, P1, P2, T2, T3, T5, T6, T7, Mu, and ϕ80.
The ability of HK97 to form lysogens was assayed using 594 cells containing plasmid pEx15 or pEx15am-FLAG. Cells were grown in LB-Lennox medium supplemented with 10 mM MgSO4 and 0.2% maltose to an optical density at 600 nm (OD600) of 0.5. Ten microliters of HK97 (109 PFU/ml) was added to 100 μl of cells, the cultures were incubated for 60 min at 37°C to allow adsorption, 1 ml of fresh LB medium was added, and the cultures were incubated for 60 min at 37°C. Following incubation, the cells were diluted 1,000-fold and 100 μl was spread on LB agar plates and incubated overnight at 37°C. Resulting colonies were then cross-streaked against λcI857 to determine the number of lysogens formed.
Construction and characterization of an HK97 lysogen containing a gene 15 amber mutation.
The region of the HK97 genome from 100 nucleotides upstream of the start of gene 13 through to 100 nucleotides into gene 16 (bp 7372 to 8535) was first cloned into pAD100 (pNC1). This sequence encompassed the entire tail assembly chaperone (gp13/gp14) as well as the gene 15 upstream and downstream regions. The coding region of genes 13 and 14 were then replaced in this construct by a kanamycin resistance (Kanr) cassette amplified from a Keio Collection single gene knockout (2, 9). This plasmid was used to transform 594 cells harboring an HK97 lysogen, and phage were induced by growing the lysogen in the presence of mitomycin C at 37°C. Following lysis, the cell debris was collected by centrifugation, and the resulting phage lysate was used to infect fresh 594 cells. These cells were plated on LB agar supplemented with kanamycin (50 μg/ml) to select for HK97 lysogens in which genes 13 and 14 had been replaced with the Kanr cassette. We then used PCR-based mutagenesis to introduce an amber substitution at position Leu4 in gene 15 in pNC1, and the plasmid (pNC1-15am) was transformed into 594 cells carrying the HK97-Kanr lysogen and selected for by plating the transformant on ampicillin (100 μg/ml). The lysogen was induced by mitomycin C in the presence of pNC1-15am, and the resulting phage lysate was plated on QD5003 cells. Only phage that recombined with the plasmid produced plaques as genes 13 and 14 are essential. Lysogens were then streaked out from the resulting plaques, and the 15am substitution was confirmed by DNA sequencing. Cells containing an HK97 15am lysogen were grown at 37°C to an OD600 of 0.5 and then induced with mitomycin C (1 μg/ml). Samples were taken at 30-min intervals following induction and lysed with chloroform, and progeny phage were enumerated by plating on 594 cells. The timing of lysis of the 15am phage was monitored by measuring the OD600 every 30 min over the course of the induction.
Potassium efflux assays.
Bacterial cultures were grown to an OD600 of 0.5 at 37°C in LB-Lennox broth supplemented with 0.2% maltose. Cells from a 5-ml aliquot of the culture were collected by centrifugation, washed once in suspension medium (SM; 100 mM NaCl, 10 mM MgSO4, and 50 mM Tris at pH 7.5), and suspended in a final volume of 5 ml of SM. The cells were incubated at 37°C, and a baseline reading of potassium was collected using an Orion ionplus potassium electrode (Thermo Scientific). Following a 5-min baseline monitoring period, 150 μl of cesium chloride-banded phage (1 × 1012 PFU/ml) was added to the sample and potassium levels were monitored for 20 min. Potassium released by phage infection was then expressed as a fraction of total potassium content remaining in the cells as described by Boulanger and Letellier (5).
Construction of HK97/HK022 phage hybrid.
The HK022 genome sequence, bounded by nucleotides 4028 to 13630 (genes 5 to 21), was amplified by PCR and cloned into pAD100. This plasmid was transformed into cells harboring an HK97 lysogen in which gene 16, which encodes the tape measure protein, was replaced by a kanamycin resistance cassette as was described above for gp13/gp14 (2, 9). Mitomycin C was used to induce the lysogen in the presence of the plasmid bearing the tail genes of HK022, and recombinants were selected by plating on E. coli. As gene 16 is essential for phage particle formation, only recombinants could produce viable phage. The titer of phage recovered from this experiment was ∼105 PFU/ml, which is similar to the value obtained when crossing in the wild-type copy of the HK97 tape measure protein.
Analysis of protein expression from pAD100 vectors.
Fresh overnight cultures of cells transformed with plasmid pEx15-FLAG or pEx20-FLAG were used to inoculate 3 ml of LB-Lennox medium and grown for 5 h. The cells were collected by centrifugation, and the pellets resuspended in 200 μl of 2× SDS loading dye (0.125 M Tris-HCl, 4% SDS, 20% glycerol, 0.005% bromophenol blue, 2.5% 2-mercaptoethanol [pH 6.8]) and boiled for 10 min. Western blotting was performed as previously described (30).
Bioinformatic characterization of membrane localization.
The sequence of gp15 was analyzed using the algorithms HMMTOP (36), TOPPRED (38), and TMHMM (21). The putative membrane-spanning helices defined by these programs were compared, and the consensus was used to define the N-terminal helix boundaries.
RESULTS
HK97 gp15 strongly inhibits HK97 plaque formation.
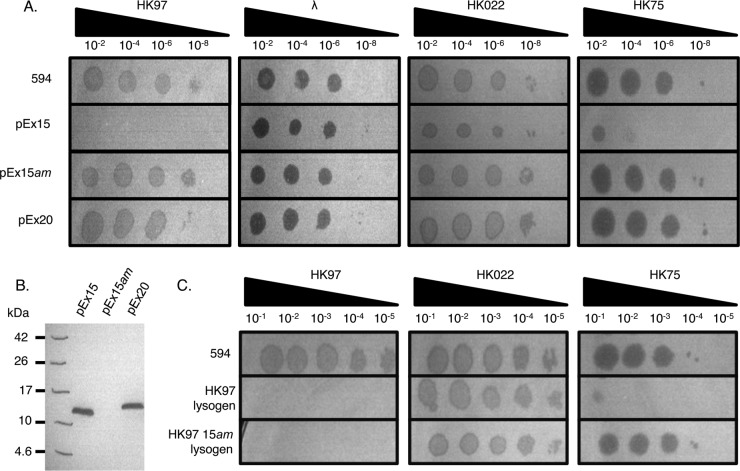
While investigating the tail genes of phage HK97, we were surprised to discover that expression plasmids containing HK97 genes 13 to 16 prevented plaque formation by wild-type HK97. This region contains genes encoding the tail assembly chaperones (gp13 and gp14), gp15 (expressed from the moron element), and the tail tape measure protein (gp16). By subcloning various regions of this original construct, we determined that expression of gp15 alone was the cause of this phenotype. Cells containing a plasmid (pEx15) containing only gene 15 with its endogenous promoter and terminator regions displayed a >108-fold inhibition of plaque formation by HK97 and a 104-fold inhibition of the closely related phage, HK75 (Fig. 1A). The plaquing efficiency of none of 18 other diverse coliphages tested was affected by the presence of gp15. These included phages HK022 and λ, which both possess some genomic regions that are very similar or identical to HK97.
Fig 1.
Expression of HK97 gp15 inhibits phage infection. (A) Plating of serial dilutions of phages HK97, λ, HK022, and HK75 on cells alone (594 cells) or transformed with plasmids expressing the moron elements from HK97 (pEx15; gp15) and HK022 (pEx20; gp20) reveals the inhibition of HK97 and HK75 by gp15. The introduction of a nonsense mutation in pEx15 abrogates this inhibition (pEx15am). (B) Western blot analysis probing for the FLAG epitope fused to the C terminus of gp15 and gp20 reveals that the proteins are expressed in E. coli and that the detectable expression of gp15 is correlated with the inhibition phenotype. (C) Expression of gp15 from an HK97 lysogen provides resistance to HK97 and HK75 that is independent of the repressor protein.
To assess the level of gp15 expression required for HK97 inhibition, a plasmid expressing gp15 with a FLAG epitope appended to its C terminus (pEx15-FLAG) was constructed. Expression levels were relatively low, with no protein band corresponding to gp15 being visible in a Coomassie-stained SDS-PAGE gel. However, Western blot analyses performed on lysates of cells containing this plasmid displayed a single band of 13 kDa, approximately twice the molecular mass of gp15 (Fig. 1B). Despite this low expression level, cells containing pEx15-FLAG were still completely insensitive to HK97 infection (Fig. 1A). In contrast, a version of pEx15-FLAG bearing an amber nonsense codon at the Leu4 position (pEx15am-FLAG) did not mediate inhibition of HK97, and no band corresponding to gp15 was observed in a Western blot (Fig. 1B).
In an attempt to increase the production of gp15, we expressed gp15-FLAG from a pET-based plasmid under the control of the T7 promoter using the strong translation initiation site contained within this vector. However, induced gp15 expression from this vector was toxic to the cells. Expression of gp15-FLAG from the weaker Tac promoter contained within a different construct did result in a level of HK97 inhibition similar to that seen with pEx15-FLAG in both the presence and absence of the inducer (isopropyl-β-d-thiogalactopyranoside [IPTG]), but addition of the inducer also resulted in poor cell growth (data not shown).
To determine whether the prophage-expressed gp15 was sufficient to fully block phage plaque formation, we tested the plating efficiency of phage HK75 on an HK97 lysogen. Although HK75 is closely related to HK97 across the morphogenetic region and possesses a gp15 homologue, its immunity region is divergent from that of HK97, more closely resembling that of HK022 (11). Despite their different immunities, HK75 was unable to form plaques on a wild-type HK97 lysogen but was able to plaque normally on the HK97 15am lysogen (Fig. 1C). Thus, the expression of gp15 mediates resistance of the wild-type HK97 lysogen to phage HK75.
The phage HK022 homologue of gp15, gp20, does not inhibit phage infection.
Both the head and tail tip genes of phage HK97 and HK022 are very similar, as is the overall order of the morphogenetic genes in these two phages (Fig. 2). HK022 possesses no gene 15 homologue at the corresponding position in its genome, but its gene 20 encodes a protein that is 39% identical to gp15 (Fig. 3). HK97 gp20 is identical to HK022 gp20, but it is truncated by a nonsense mutation at the codon corresponding to residue 33. To determine whether HK022 gp20 is able to inhibit phage infection, a plasmid similar to pEx15-FLAG was constructed that expressed gp20-FLAG. Although lysates of cells containing this construct accumulated levels of gp20-FLAG similar to those observed for gp15-FLAG (Fig. 1B), we observed no inhibition of any phages plated on cells containing this plasmid (Fig. 1A). While it is possible that HK022 gp20 may have an inhibitory effect on phages that we have not yet tested, these experiments demonstrate that its activity is not the same as that of gp15.
Fig 2.
Genome map of the morphogenetic regions of phages HK97 and HK022. Gene coding regions are indicated by boxes, and gene names are noted above the boxes. Shading of the boxes indicates the percent identity between the two phage homologues, with dark gray (■) indicating >95%, medium gray  75 to 95%, and white (□) <35%. The morons, gp15 and gp20, are boxed. The sites of recombination in the HK97/022 hybrid phage are shown with arrows.
75 to 95%, and white (□) <35%. The morons, gp15 and gp20, are boxed. The sites of recombination in the HK97/022 hybrid phage are shown with arrows.
Fig 3.
Sequence comparison of gp15, gp20, and the YebO family of bacterial proteins. The upper group of sequences is encoded by phages, and the lower group consists of the YebO family of proteins encoded within bacterial genomes. The percent identity of each sequence to gp15 is shown in the column marked “% ID.” Unnamed protein sequences are designated by their GI numbers. Three highly conserved hydrophobic positions and two conserved Arg residues are indicated by arrows, and the N-terminal putative membrane helix is indicated above the sequences.
HK97 gp15 is a predicted transmembrane protein with putative homologues found throughout the Enterobacteriaceae family.
We conducted iterative PSI-BLAST (1) searches with HK97 gp15 and identified 98 significantly similar sequences (Fig. 3). These proteins are all classified as members of the YebO family (pfam identifier [ID], PF13974), named after an E. coli protein of unknown function. These proteins are found exclusively in genera of the Enterobacteriaceae family, such as Escherichia, Yersinia, Salmonella, and Shigella, and three of their associated phages. E. coli phage proteins gp13 of HK75, gp20 of HK022 (discussed above), and gp18 of Yersinia phage PY54 display 98%, 39%, and 19% sequence identity with HK97 gp15, respectively. Each of these phage proteins is found within the gene cluster that encodes the proteins required for tail morphogenesis. Like gp15, HK75 gp13 is located between the predicted tail assembly chaperone and tape measure proteins, both of which have 99% sequence identity to their HK97 counterparts. HK022 gp20 is located between predicted tail tip proteins gp19 and gp21, and PY54 gp18 is located in a position similar to that of gp20, in that is it between the tape measure and tail fiber proteins near a series of ORFs of which some, based on genomic position, likely encode tail tip proteins.
Each of the 95 putative gp15 homologues identified in bacterial genomes was examined to determine whether it is located in a prophage-related region. Three proteins encoded upstream and downstream of the gene encoding the gp15 homologue were used to initiate PSI-BLAST searches to detect sequence similarity to phage proteins. Using this strategy, we identified only two sequences that were located in prophage elements. Salmonella enterica subsp. enterica serovar Uganda strain R8-3404 encodes a gp15 homologue (GI 353656277) with 97% sequence identity to gp15. It is flanked by tape measure and tail assembly chaperone protein homologues that are 77% and 56% identical, respectively, to their HK97 counterparts. This positioning between the tail assembly chaperone and tape measure proteins is the same as that of HK97 gp15. A more distantly related putative homologue that is 23% identical to gp15 was found in an HK97-like prophage of Xenorhabdus bovienii. The genomic position of the gene encoding this protein is similar to that of HK022 gene 20.
The bacterial sequence homologues that are not encoded in prophage-derived regions are more closely related to each other than they are to the phage sequences, with an average pairwise identity of 48%, compared to ∼30% when they are aligned with the phage sequences. All of the sequence similarity among the bacterial and phage proteins is found within the first 50 amino acids of these proteins; the C-terminal regions of the phage-derived proteins are completely divergent from the bacterial proteins in both length and sequence (Fig. 3). Residues 4 to 24 of gp15 and the corresponding residues in the putative homologues are predicted to form a membrane-spanning helix. In addition to the conserved membrane-spanning region, these proteins all display Arg residues at positions 25 and 29, and most have a Glu residue at position 32. Highly conserved hydrophobic positions are seen outside the membrane-spanning region at positions 26, 36, and 37.
HK97 gp15 inhibits DNA entry into the cytoplasm.
Since gp15 is predicted to possess a helical transmembrane domain, it is likely to localize to the inner membrane. Thus, we postulated that it might inhibit phage infection by preventing the entry of phage DNA into the cytoplasm. Since phage DNA entry is correlated with a transient efflux of K+ ions from the cell interior to the surrounding medium, monitoring K+ efflux during phage infection using an ion selective electrode attached to a pH meter provides a simple assay for this process (5, 24). Cells bearing pEx15-FLAG or pEx15am-FLAG were infected with HK97 or λ phage, and K+ efflux was monitored (Fig. 4A). Strikingly, cells containing full-length gp15 expressed from pEx15-FLAG displayed no K+ efflux when infected with HK97, yet the same cells showed robust levels of efflux when infected with λ. In contrast, cells containing pEx15am-FLAG evinced K+ efflux when infected by λ or HK97, demonstrating that the presence of full-length gp15 specifically prevents DNA entry only during infection by HK97. To confirm that the observed K+ efflux required adsorption of phage to cells, we also showed that K+ efflux was not induced by infection with either λ or HK97 when mutant cells lacking the LamB receptor were used. It should be noted that K+ efflux observed upon infection of permissive cells with HK97 always occurred more slowly than efflux from cells infected with λ. This appears to be an inherent property of these phages. To provide additional evidence that the potassium efflux observed was correlated with DNA entry, we examined the ability of HK97 DNA to enter cells and form lysogens in the presence and absence of gp15. Infection of 594 cells alone or transformed with pEx15am-FLAG with HK97 resulted in the formation of lysogens in 100% of the colonies tested (8/8 for each). When gp15 was expressed from pEx15, none of the colonies (0/8) tested harbored an HK97 lysogen.
Fig 4.
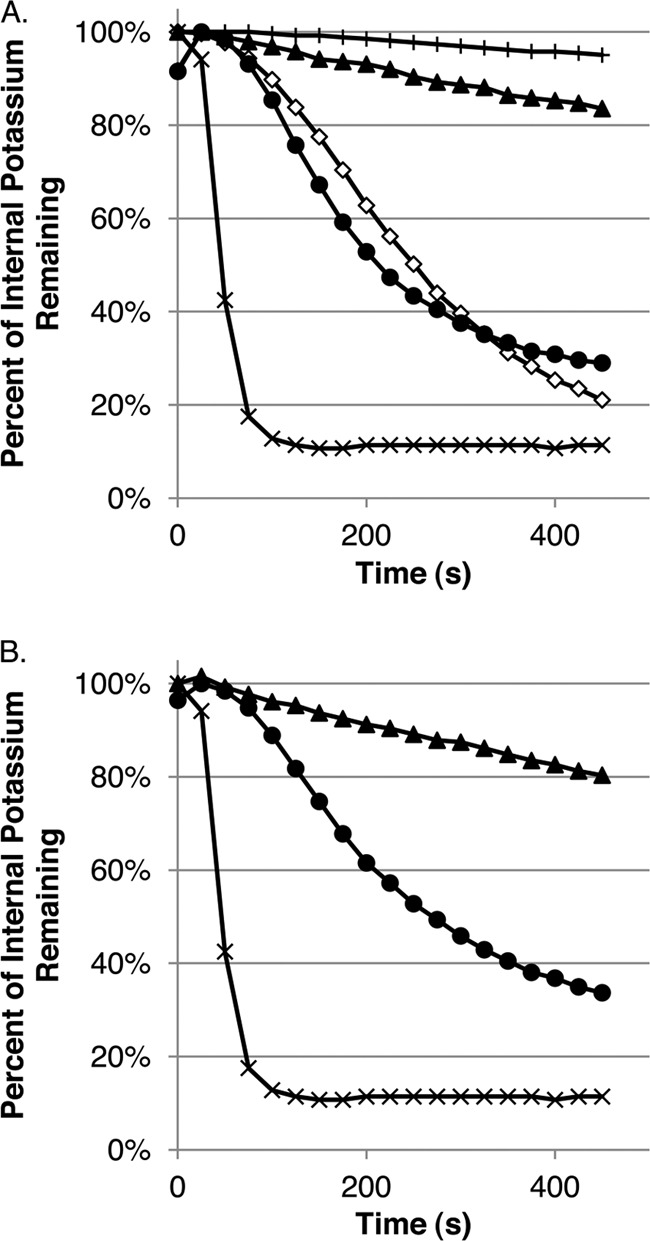
Potassium efflux induced by phages HK97 and λ as measured by a potassium-selective electrode. (A) Phage genome injection into wild-type E. coli by HK97 (♢) leads to efflux of potassium out of the cell and into the surrounding medium. This effect is abrogated in cells lacking the HK97 receptor (lamB) on their surface (+). The expression of pEx15-FLAG is able to block the entry of the HK97 (▲) genome but not the λ (×) genome. This effect is alleviated when pEx15-FLAG carries a nonsense mutation in gp15 (●). (B) The resistance to phage DNA injection is provided by gp15 expressed from an HK97 prophage. The presence of a wild-type HK97 lysogen in E. coli blocks the injection of the HK97 (▲) genome but not λ (×). The introduction of a nonsense mutation into gp15 relieves the inhibition for HK97 DNA injection (●).
To determine whether gp15 blocks DNA entry when expressed from a repressed prophage, K+ efflux was monitored in a wild-type HK97 lysogen. As can be seen in Fig. 4B, infection of this lysogen with HK97 resulted in no K+ efflux. Infection with phage λ, on the other hand, caused robust efflux even though this phage cannot form plaques on this strain because λ and HK97 possess identical cI repressor proteins. When we performed the same experiment with a lysogen of HK97 bearing an amber mutation in gene 15, infection with HK97 resulted in robust K+ efflux. These experiments demonstrate that expression of gp15 from the prophage-borne gene 15 is sufficient to mediate resistance to HK97 infection. Therefore, the HK97 prophage actually resists superinfection by HK97 through two distinct mechanisms: one is dependent on the gp15 protein, and the other is dependent on the cI repressor.
HK97 gp15 does not inhibit lytic phage growth and is not required for lytic growth.
To assess the possible effects of gp15 overexpression on the lytic growth cycle of HK97, we transformed a wild-type HK97 lysogen with pEx15-FLAG. The prophage within these cells was then induced by the addition of mitomycin C. As shown in Fig. 5A, the presence of gp15 did not cause a delay in cell lysis after prophage induction, nor did it reduce the yield of phage particles compared to induction in the absence of gp15 (Fig. 5B). Thus, overexpression of gp15 does not interfere with the lytic growth cycle of HK97 when induced from a lysogen.
Fig 5.
Gp15 does not affect the lytic growth of HK97. (A) Mitomycin induction of a wild-type HK97 prophage (●), a prophage with pEx15-FLAG (○), and a prophage bearing a nonsense mutation in gp15  where lysis of the culture was followed by monitoring the OD600. (B) Samples taken at various time points following mitomycin induction in panel A were lysed with chloroform. The resulting phage lysates were top-plated on E. coli and plaques enumerated, revealing no difference in the number of phage particles produced by the wild-type lysogen (□) compared to a lysogen with pEx15-FLAG (■) and a lysogen with a nonsense mutation in gp15
where lysis of the culture was followed by monitoring the OD600. (B) Samples taken at various time points following mitomycin induction in panel A were lysed with chloroform. The resulting phage lysates were top-plated on E. coli and plaques enumerated, revealing no difference in the number of phage particles produced by the wild-type lysogen (□) compared to a lysogen with pEx15-FLAG (■) and a lysogen with a nonsense mutation in gp15  .
.
Since gp15 and its phage homologues are located in the tail morphogenetic region, it seemed feasible that gp15 might play a role in tail morphogenesis. To test this idea, we induced wild-type HK97 and HK97 15am lysogens using mitomycin C. The numbers of PFU produced from the two strains were equal (Fig. 5B), and cell lysis occurred at the same time (Fig. 5A), demonstrating that gp15 is not required for virion morphogenesis or release. In addition, plating the phage particles produced by the HK97 15am induction on gp15-expressing cells revealed that they were unable to form plaques. This illustrates that the presence of gp15 in the virion is not a requirement for exclusion by cells expressing gp15.
Inhibition by gp15 is mediated through the phage tail but not through the LamB receptor.
Although phages HK022 and HK97 possess identical proteins in their heads, and the proteins in their tail tips are greater than 70% identical (Fig. 2), phage HK022 growth is not inhibited by gp15. However, these phages display highly divergent tail tube and tape measure proteins, and mutational alterations of both of these proteins have been shown to affect the injection process of phage λ (31, 39). We therefore reasoned that the differences in these proteins could account for the contrasting behaviors of these phages in the presence of gp15. To test this idea, we used in vivo homologous recombination (see Materials and Methods) to generate an HK97/022 hybrid phage in which the DNA segment carrying HK97 genes 7 (encoding a head-tail connector protein) to 18 (encoding the putative tail tip protein) was replaced by the homologous segment from HK022 (Fig. 2). Like for wild-type HK022, the plating of this hybrid phage was not inhibited by gp15. This suggests that the sensitivity of HK97 to inhibition by gp15 is likely the result of features of its tail tube (gp12) or tape measure (gp16) protein, since the HK022 proteins are highly diverged from their HK97 counterparts. While we cannot rule out amino acid differences in gp17 and gp18 to account for the distinct behavior of the hybrid phage, this is a less likely scenario given that the sequence identity of these proteins in the two phages is greater than 75%. The HK97/022 hybrid also differs from HK97 in proteins positioned in the vicinity of the head-tail connector, but these proteins are not known to be involved in the DNA injection process.
In addition to overcoming the inhibition provided by gp15, the HK97/022 hybrid phage still required the presence of the LamB receptor, as does HK97. In contrast, the parent phage HK022 employs FhuA as its outer membrane receptor. This demonstrates that inhibition by gp15 is not mediated through the LamB receptor. This conclusion is corroborated by our observation that phage HK75, which utilizes the FhuA receptor like HK022, is inhibited by gp15 (Fig. 1A).
DISCUSSION
In this study, we demonstrated that gp15 prevents infection by phages HK97 and HK75. We showed that gp15 blocks the HK97 life cycle at the DNA entry step by the use of K+ efflux assays. Phages λ and HK97/022 hybrid are not subjected to the inhibitory effect of gp15, although they utilize the same outer membrane receptor as HK97 (LamB). This shows that gp15 does not function by inactivating this receptor. The most likely targets of the HK97 gp15 inhibition are the HK97 tail tube and/or tape measure proteins. These findings are reminiscent of experiments showing that changes in the same proteins of phage λ allowed growth on Pel− E. coli strains (31). The mutations in these E. coli strains prevent λ DNA injection without affecting phage adsorption, a phenotype similar to that of cells containing gp15.
The ability of gp15 to prevent DNA entry when expressed from a prophage and its possession of a putative membrane-spanning domain classify it as a member of the superinfection exclusion (Sie) family of proteins (22). The Sie proteins are postulated to act either by masking host factors that are required for injection of phage DNA or through a direct interaction with a structural element of the adsorbed phage. One example of the former is the phage ϕ80 Cor protein; it binds specifically to the FhuA outer membrane protein and thereby inhibits infection by phages utilizing this receptor (e.g., ϕ80, HK022, and T1). The mechanism of action of gp15 is clearly different because it inhibits infection by phages that use either the FhuA receptor (e.g., HK97/022 hybrid and HK75) or the LamB receptor (e.g., HK97). Furthermore, phages such as λ and HK243, which also utilize the LamB receptor, are not inhibited by gp15. The mechanism of gp15 action appears to resemble that of Sie proteins that block DNA entry downstream of receptor binding. For example, Salmonella phage P22 encodes an inner membrane protein, SieA, that prevents infection by phages L, MG178, and MG40 (16, 33). SieA blocks the transfer of phage DNA across the inner membrane into the cytoplasm, likely by interacting with unidentified components of the DNA transfer system (33, 35). The E. coli phage T4 imm protein is associated with specific sites in the plasma membrane, where it is thought to prevent DNA entry by changing the conformation of the injection site (25, 26). In each of these cases, the prophage-encoded membrane protein is believed to displace a required component of the DNA transfer system. A second type of Sie protein is represented by the T4 protein species, which is membrane associated and prevents DNA entry by inhibiting the activity of gp5, a component of the T4 tail baseplate that possesses lysozyme activity (19, 20). Although gp15 may function analogously to these Sie proteins, we can detect no sequence similarity between gp15 and other Sie proteins, and it is uncertain whether gp15 functions through a similar or distinctive mechanism.
HK97 gp15 and its putative phage homologues resemble the YebO family of proteins that are found ubiquitously in the Enterobacteriaceae family. A marked distinction of the phage proteins, however, is that their C-terminal regions are completely divergent both from one another and from the bacterial proteins. In contrast, the bacterial YebO homologues display a highly conserved motif at their C termini. We suspect that the phage gp15-like proteins have arisen, possibly through separate events, by co-opting the YebO N-terminal region and attaching a unique C-terminal region with functions specific to a given set of phages (e.g., HK97 and HK75). We entertained the hypothesis that gp15 could be a dominant negative inhibitor of E. coli YebO and that YebO might be required for HK97 DNA entry. However, we found that HK97 is able to form plaques normally on a strain bearing a deletion of the yebO gene (data not shown). In other experiments, we also determined that the inhibition of HK97 by gp15 was not dependent on the presence of YebO (data not shown), arguing against a model whereby gp15 would subvert the normal activity of YebO and cause it to inhibit phage infection. At this juncture, we speculate that the YebO-like N-terminal region of gp15 and its phage relatives serves as an appropriately localized membrane-bound anchor allowing the C-terminal domain to inhibit either a specific phage tail protein or bacterial protein required for DNA entry.
In conclusion, we have shown that the phage HK97 moron-encoded gp15 is an inhibitor of DNA entry specific to HK97 and the closely related phage, HK75. Since moron-encoded proteins similar in sequence to gp15 were identified in other phages, we suspect that these proteins might also inhibit phage infection; however, we were unable to identify a phage inhibited by the putative gp15 homologue in HK022, gp20. Our work reinforces the growing realization that moron-encoded proteins often confer phage resistance to lysogenic strains, providing a rationale for the horizontal spread of these elements among diverse phages. In recent years, many striking examples have been uncovered in which bacteria have incorporated prophage-derived elements into their own physiology in order to derive a competitive advantage. Examples of this include the type VI secretion system (23), gene transfer agents (32), R- and F-pyocins (28), and Staphylococcus aureus pathogenicity islands (29). HK97 gp15 and its related phage-encoded proteins appear to provide an interesting counterexample in which phages have co-opted a bacterial function and turned it to their own advantage.
ACKNOWLEDGMENTS
We thank Paul Sadowski for critical reading of the manuscript as well as Rodney King for the kind gift of the HK phages.
This work was supported by Operating Grants from the Canadian Institutes for Health Research to K.L.M. and A.M.E. (Fund No. MOP-6279) and A.R.D. (Fund No. MOP-77680). N.C. was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) CGS-D scholarship.
Footnotes
Published ahead of print 13 July 2012
REFERENCES
- 1. Altschul SF, et al. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baba T, et al. 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2:2006.0008 doi:10.1038/msb4100050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barondess JJ, Beckwith J. 1990. A bacterial virulence determinant encoded by lysogenic coliphage lambda. Nature 346:871–874 [DOI] [PubMed] [Google Scholar]
- 4. Barondess JJ, Beckwith J. 1995. bor gene of phage λ, involved in serum resistance, encodes a widely conserved outer membrane lipoprotein. J. Bacteriol. 177:1247–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boulanger P, Letellier L. 1992. Ion channels are likely to be involved in the two steps of phage T5 DNA penetration into Escherichia coli cells. J. Biol. Chem. 267:3168–3172 [PubMed] [Google Scholar]
- 6. Brüssow H, Canchaya C, Hardt WD. 2004. Phages and the evolution of bacterial pathogens: from genomic rearrangements to lysogenic conversion. Microbiol. Mol. Biol. Rev. 68:560–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Canchaya C, Fournous G, Chibani-Chennoufi S, Dillmann ML, Brussow H. 2003. Phage as agents of lateral gene transfer. Curr. Opin. Microbiol. 6:417–424 [DOI] [PubMed] [Google Scholar]
- 8. Casjens S. 2003. Prophages and bacterial genomics: what have we learned so far? Mol. Microbiol. 49:277–300 [DOI] [PubMed] [Google Scholar]
- 9. Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. U. S. A. 97:6640–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davidson AR, Sauer RT. 1994. Folded proteins occur frequently in libraries of random amino acid sequences. Proc. Natl. Acad. Sci. U. S. A. 91:2146–2150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dhillon TS, Dhillon EK, Chau HC, Li WK, Tsang AH. 1976. Studies on bacteriophage distribution: virulent and temperate bacteriophage content of mammalian feces. Appl. Environ. Microbiol. 32:68–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Figueroa-Bossi N, Bossi L. 1999. Inducible prophages contribute to Salmonella virulence in mice. Mol. Microbiol. 33:167–176 [DOI] [PubMed] [Google Scholar]
- 13. Harkness RE, Olschlager T. 1991. The biology of colicin M. FEMS Microbiol. Rev. 8:27–41 [DOI] [PubMed] [Google Scholar]
- 14. Hendrix RW, Lawrence JG, Hatfull GF, Casjens S. 2000. The origins and ongoing evolution of viruses. Trends Microbiol. 8:504–508 [DOI] [PubMed] [Google Scholar]
- 15. Hernández-Sánchez J, et al. 2008. Analysis of some phenotypic traits of feces-borne temperate lambdoid bacteriophages from different immunity groups: a high incidence of cor+, FhuA-dependent phages. Arch. Virol. 153:1271–1280 [DOI] [PubMed] [Google Scholar]
- 16. Hofer B, Ruge M, Dreiseikelmann B. 1995. The superinfection exclusion gene (sieA) of bacteriophage P22: identification and overexpression of the gene and localization of the gene product. J. Bacteriol. 177:3080–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hopp TP, et al. 1988. A short polypeptide marker sequence useful for recombinant protein identification and purification. Biotechnology (NY) 6:1204–1210 [Google Scholar]
- 18. Juhala RJ, et al. 2000. Genomic sequences of bacteriophages HK97 and HK022: pervasive genetic mosaicism in the lambdoid bacteriophages. J. Mol. Biol. 299:27–51 [DOI] [PubMed] [Google Scholar]
- 19. Kao SH, McClain WH. 1980. Baseplate protein of bacteriophage T4 with both structural and lytic functions. J. Virol. 34:95–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kao SH, McClain WH. 1980. Roles of bacteriophage T4 gene 5 and gene s products in cell lysis. J. Virol. 34:104–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krogh A, Larsson B, von Heijne G, Sonnhammer EL. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305:567–580 [DOI] [PubMed] [Google Scholar]
- 22. Labrie SJ, Samson JE, Moineau S. 2010. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 8:317–327 [DOI] [PubMed] [Google Scholar]
- 23. Leiman PG, et al. 2009. Type VI secretion apparatus and phage tail-associated protein complexes share a common evolutionary origin. Proc. Natl. Acad. Sci. U. S. A. 106:4154–4159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Letellier L, Plancon L, Bonhivers M, Boulanger P. 1999. Phage DNA transport across membranes. Res. Microbiol. 150:499–505 [DOI] [PubMed] [Google Scholar]
- 25. Lu MJ, Henning U. 1994. Superinfection exclusion by T-even-type coliphages. Trends Microbiol. 2:137–139 [DOI] [PubMed] [Google Scholar]
- 26. Lu MJ, Stierhof YD, Henning U. 1993. Location and unusual membrane topology of the immunity protein of the Escherichia coli phage T4. J. Virol. 67:4905–4913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mahony J, McGrath S, Fitzgerald GF, van Sinderen D. 2008. Identification and characterization of lactococcal-prophage-carried superinfection exclusion genes. Appl. Environ. Microbiol. 74:6206–6215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakayama K, et al. 2000. The R-type pyocin of Pseudomonas aeruginosa is related to P2 phage, and the F-type is related to lambda phage. Mol. Microbiol. 38:213–231 [DOI] [PubMed] [Google Scholar]
- 29. Novick RP, Christie GE, Penades JR. 2010. The phage-related chromosomal islands of Gram-positive bacteria. Nat. Rev. Microbiol. 8:541–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pell LG, et al. 2010. The solution structure of the C-terminal Ig-like domain of the bacteriophage lambda tail tube protein. J. Mol. Biol. 403:468–479 [DOI] [PubMed] [Google Scholar]
- 31. Scandella D, Arber W. 1976. Phage λDNA injection into Escherichia coli pel− mutants is restored by mutations in phage genes V or H. Virology 69:206–215 [DOI] [PubMed] [Google Scholar]
- 32. Stanton TB. 2007. Prophage-like gene transfer agents—novel mechanisms of gene exchange for Methanococcus, Desulfovibrio, Brachyspira, and Rhodobacter species. Anaerobe 13:43–49 [DOI] [PubMed] [Google Scholar]
- 33. Susskind MM, Botstein D, Wright A. 1974. Superinfection exclusion by P22 prophage in lysogens of Salmonella typhimurium. III. Failure of superinfecting phage DNA to enter sieA+ lysogens. Virology 62:350–366 [DOI] [PubMed] [Google Scholar]
- 34. Susskind MM, Wright A, Botstein D. 1974. Superinfection exclusion by P22 prophage in lysogens of Salmonella typhimurium. IV. Genetics and physiology of sieB exclusion. Virology 62:367–384 [DOI] [PubMed] [Google Scholar]
- 35. Taneja SK, Chakravorty M. 1978. Superinfection exclusion and changes in cellular transport processes in phage infected Salmonella typhimurium. Mol. Gen. Genet. 159:293–296 [DOI] [PubMed] [Google Scholar]
- 36. Tusnády GE, Simon I. 2001. The HMMTOP transmembrane topology prediction server. Bioinformatics 17:849–850 [DOI] [PubMed] [Google Scholar]
- 37. Uc-Mass A, et al. 2004. An orthologue of the cor gene is involved in the exclusion of temperate lambdoid phages. Evidence that Cor inactivates FhuA receptor functions. Virology 329:425–433 [DOI] [PubMed] [Google Scholar]
- 38. von Heijne G. 1992. Membrane protein structure prediction. Hydrophobicity analysis and the positive-inside rule. J. Mol. Biol. 225:487–494 [DOI] [PubMed] [Google Scholar]
- 39. Williams N, Fox DK, Shea C, Roseman S. 1986. Pel, the protein that permits lambda DNA penetration of Escherichia coli, is encoded by a gene in ptsM and is required for mannose utilization by the phosphotransferase system. Proc. Natl. Acad. Sci. U. S. A. 83:8934–8938 [DOI] [PMC free article] [PubMed] [Google Scholar]