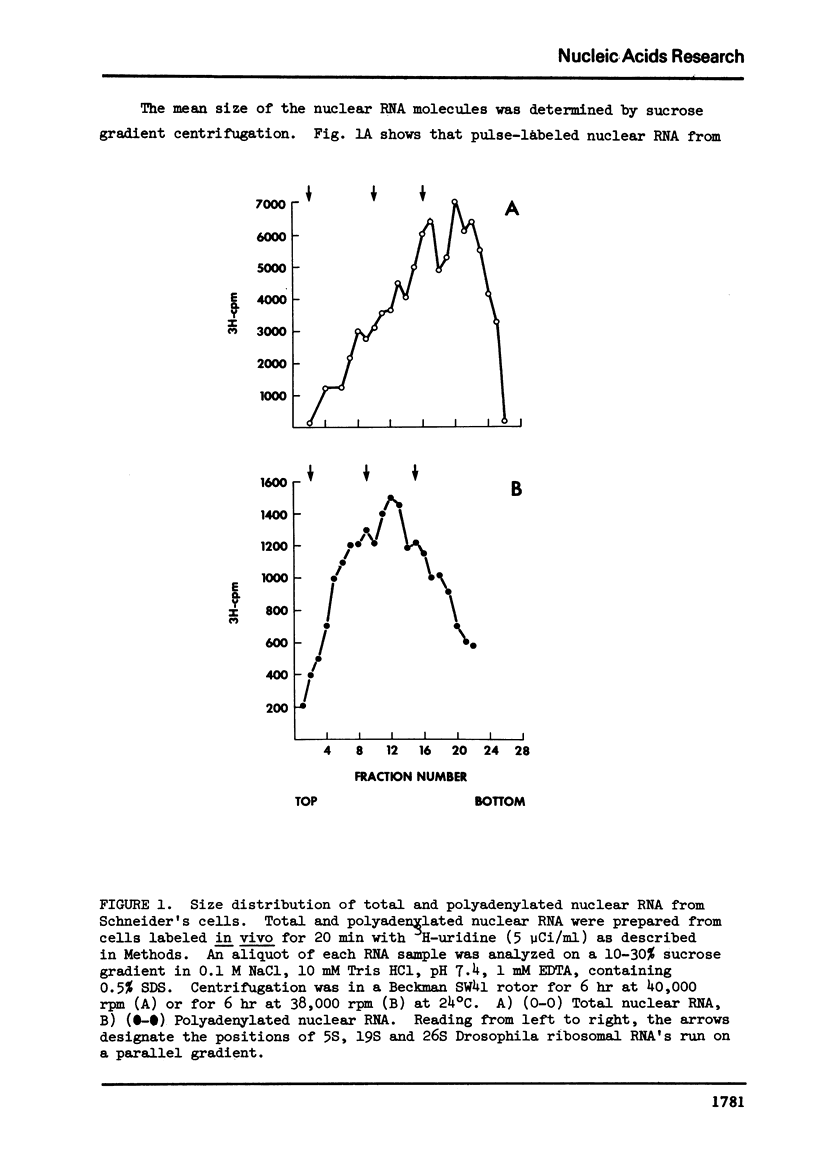
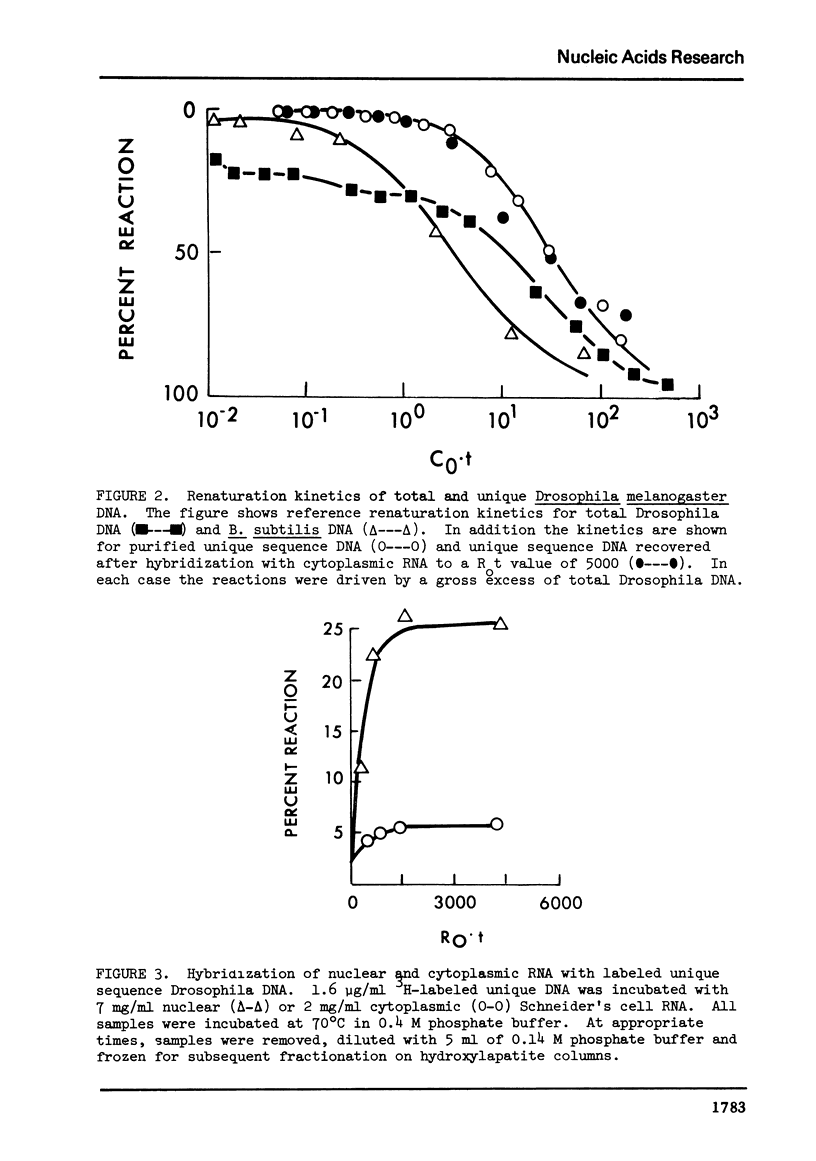
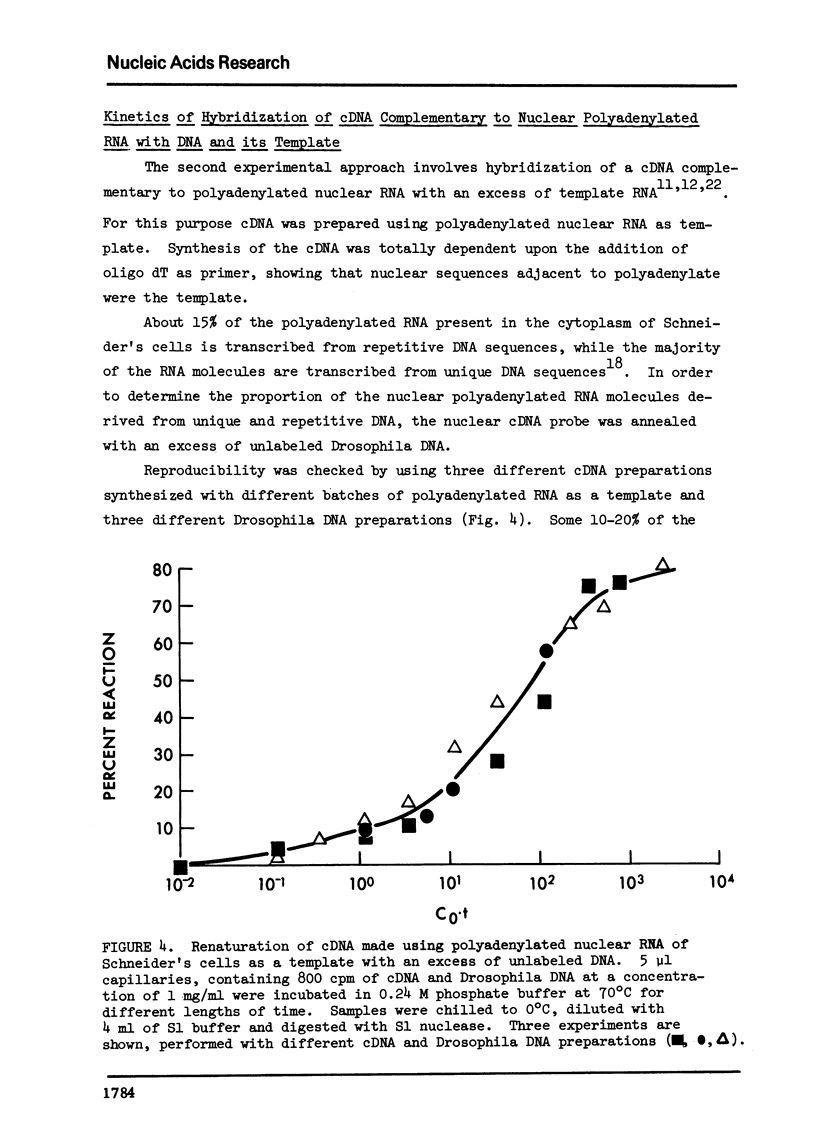
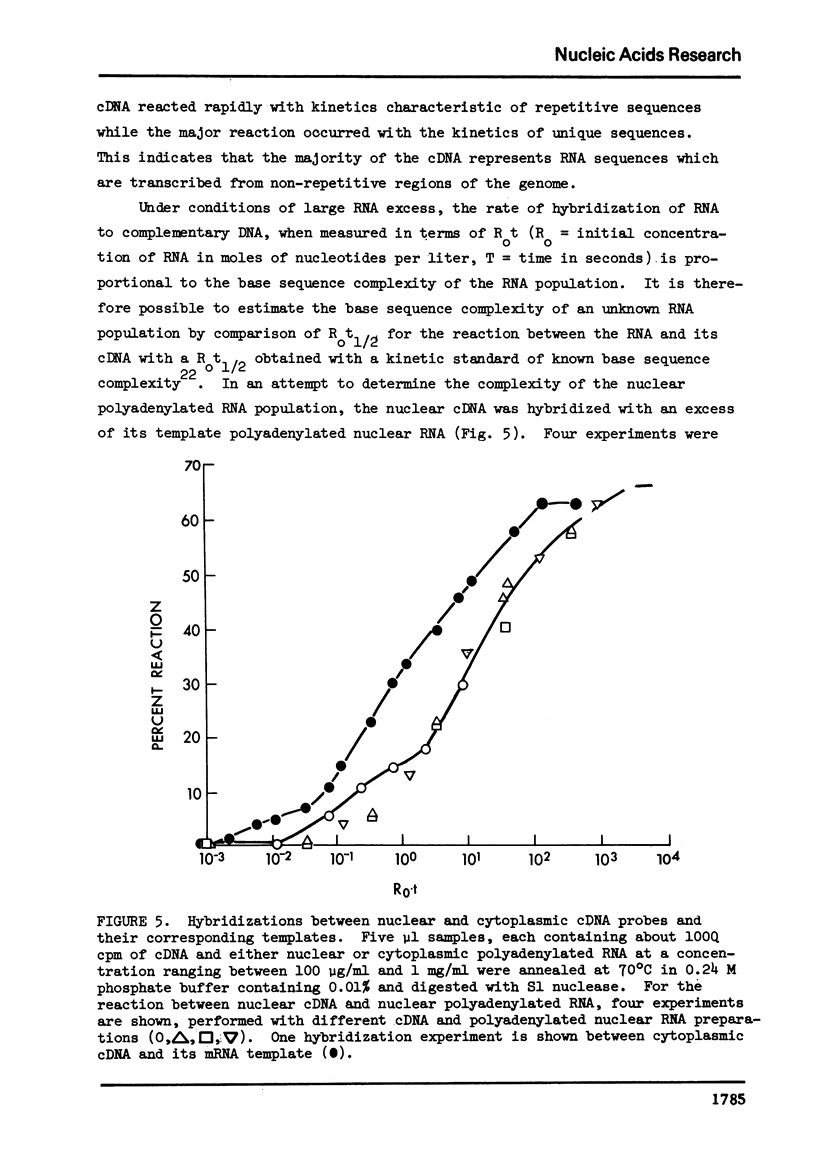
Abstract
Complementary DNA was synthesized using polyadenylated nuclear RNA of cultured Drosophila cells as template. The kinetics of hybridization of this cDNA with nuclear RNA indicated that the complexity of this RNA population is five to ten times greater than that of cytoplasmic mRNA. The same difference in the fraction of DNA represented was obtained when nuclear and cytoplasmic RNA were hybridized with labeled unique sequence DNA. The fraction of the DNA sequences represented in total number of polyadenylated nuclear RNA is much higher than that represented in cytoplasmic RNA.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beatriz L. W., McCarthy B. J. Messenger RNA complexity in Drosophila melanogaster. Biochemistry. 1975 Jun 3;14(11):2440–2446. doi: 10.1021/bi00682a026. [DOI] [PubMed] [Google Scholar]
- Birnie G. D., MacPhail E., Young B. D., Getz M. J., Paul J. The diversity of the messenger RNA population in growing Friend cells. Cell Differ. 1974 Nov;3(4):221–232. doi: 10.1016/0045-6039(74)90005-0. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Morton J. G., Rosbash M., Richardson M. Three abundance classes in HeLa cell messenger RNA. Nature. 1974 Jul 19;250(463):199–204. doi: 10.1038/250199a0. [DOI] [PubMed] [Google Scholar]
- Galau G. A., Britten R. J., Davidson E. H. A measurement of the sequence complexity of polysomal messenger RNA in sea urchin embryos. Cell. 1974 May;2(1):9–20. doi: 10.1016/0092-8674(74)90003-8. [DOI] [PubMed] [Google Scholar]
- Getz M. J., Birnie G. D., Young B. D., MacPhail E., Paul J. A kinetic estimation of base sequence complexity of nuclear poly(A)-containing RNA in mouse Friend cells. Cell. 1975 Feb;4(2):121–129. doi: 10.1016/0092-8674(75)90118-x. [DOI] [PubMed] [Google Scholar]
- Hough B. R., Smith M. J., Britten R. J., Davidson E. H. Sequence complexity of heterogeneous nuclear RNA in sea urchin embryos. Cell. 1975 Jul;5(3):291–299. doi: 10.1016/0092-8674(75)90104-x. [DOI] [PubMed] [Google Scholar]
- Imaizumi T., Diggelmann H., Scherrer K. Demonstration of globin messenger sequences in giant nuclear precursors of messenger RNA of avian erythroblasts. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1122–1126. doi: 10.1073/pnas.70.4.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelinek W., Adesnik M., Salditt M., Sheiness D., Wall R., Molloy G., Philipson L., Darnell J. E. Further evidence on the nuclear origin and transfer to the cytoplasm of polyadenylic acid sequences in mammalian cell RNA. J Mol Biol. 1973 Apr 15;75(3):515–532. doi: 10.1016/0022-2836(73)90458-0. [DOI] [PubMed] [Google Scholar]
- Lamb M. M., Laird C. D. The size of poly(A)-containing RNAs in Drosophila melanogaster embryos. Biochem Genet. 1976 Apr;14(3-4):357–371. doi: 10.1007/BF00484774. [DOI] [PubMed] [Google Scholar]
- Lee S. Y., Mendecki J., Brawerman G. A polynucleotide segment rich in adenylic acid in the rapidly-labeled polyribosomal RNA component of mouse sarcoma 180 ascites cells. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1331–1335. doi: 10.1073/pnas.68.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengyel J., Penman S. hnRNA size and processing as related to different DNA content in two dipterans: Drosophila and Aedes. Cell. 1975 Jul;5(3):281–290. doi: 10.1016/0092-8674(75)90103-8. [DOI] [PubMed] [Google Scholar]
- Leong J. A., Garapin A. C., Jackson N., Fanshier L., Levinson W., Bishop J. M. Virus-specific ribonucleic acid in cells producing rous sarcoma virus: detection and characterization. J Virol. 1972 Jun;9(6):891–902. doi: 10.1128/jvi.9.6.891-902.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F., Firtel R. A., Jacobson A. Transcription and structure of the genome of the cellular slime mold Dictyostelium discoideum. Cold Spring Harb Symp Quant Biol. 1974;38:899–914. doi: 10.1101/sqb.1974.038.01.092. [DOI] [PubMed] [Google Scholar]
- MCCARTHY B. J., BOLTON E. T. INTERACTION OF COMPLEMENTARY RNA AND DNA. J Mol Biol. 1964 Feb;8:184–200. doi: 10.1016/s0022-2836(64)80128-5. [DOI] [PubMed] [Google Scholar]
- McCarthy B. J., Nishiura J. T., Doenecke D., Nasser D. S., Johnson C. B. Transcription and chromatin structure. Cold Spring Harb Symp Quant Biol. 1974;38:763–771. doi: 10.1101/sqb.1974.038.01.081. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Inhibition of RNA synthesis by actinomycin D: characteristic dose-response of different RNA species. J Cell Physiol. 1970 Oct;76(2):127–139. doi: 10.1002/jcp.1040760202. [DOI] [PubMed] [Google Scholar]
- Ryffel G. U., McCarthy B. J. Complexity of cytoplasmic RNA in different mouse tissues measured by hybridization of polyadenylated RNA to complementary DNA. Biochemistry. 1975 Apr 8;14(7):1379–1385. doi: 10.1021/bi00678a006. [DOI] [PubMed] [Google Scholar]
- Schneider I. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol. 1972 Apr;27(2):353–365. [PubMed] [Google Scholar]
- Shearer R. W., McCarthy B. J. Evidence for ribonucleic acid molecules restricted to the cell nucleus. Biochemistry. 1967 Jan;6(1):283–289. doi: 10.1021/bi00853a044. [DOI] [PubMed] [Google Scholar]
- Turner S. H., Laird C. D. Diversity of RNA sequences in Drosophila melanogaster. Biochem Genet. 1973 Nov;10(3):263–274. doi: 10.1007/BF00485704. [DOI] [PubMed] [Google Scholar]



