Abstract
We have previously shown that hypoxia results in increased activation of caspase-9 in the cerebral cortex of newborn piglets. The present study tests the hypothesis that the increased activation of caspase-9 during hypoxia is mediated by Src kinase. To test this hypothesis a highly selective Src kinase inhibitor PP2 [IC50 5nm] was administered to prevent caspase-9 activation during hypoxia. Cytosolic fraction from the cerebral cortical tissue was isolated and the activation of caspase-9 was documented by the expression of active caspase-9 and the activity of caspase-9 and caspase-3. Piglets were divided into: normoxic (Nx, n=5), hypoxic (Hx, n=5) and hypoxic-treated with Src inhibitor (Hx-PP2). Hypoxia was induced by decreasing FiO2to 0.07 for 60 min. PP2 was administered (0.4 mg/kg, i.v.) 30 min prior to hypoxia. ATP and phosphocreatine (PCr) levels were determined to document cerebral tissue hypoxia. Activity of caspase-9 and caspase-3 were determined spectrofluorometrically using specific fluorogenic substrates. Expression of active caspase-9 was determined by Western blot using active caspase-9 antibody. Caspase-9 activity (nmoles/mg protein/hr) was 1.40± 0.12 in Nx, 2.12±0.11 in Hx (p< 0.05 vs Nx) and 1.61±0.14 in Hx-PP2 (p<0.05 vs Hx). Active caspase-9 expression (OD x mm2) was 42.3±8.3 in Nx, 78.9±11.0 in Hx (p<0.05 vs Nx) and 41.2±7.6 in Hx-PP2 (p<0.05 vs Hx). Caspase-3 activity (nmoles/mg protein/hr) was 4.11±0.1 in Nx, 6.51±.1 in Hx (p<0.05 vs Nx) and 4.57±0.7 in Hx+PP2 (p<0.05 vs Hx). Active caspase-3 expression (OD x mm2) was 392.1±23.1 in Nx, 645.0±90.3 in Hx (p<0.05 vs Nx) and 329.7±51.5 in Hx-PP2 (p<0.05 vs Hx). The data show that pretreatment with Src kinase inhibitor prevents the hypoxia-induced increased expression of active caspase-9 and the activity of caspase-9. Src kinase inhibitor also prevented the hypoxia-induced increased activation of caspase-3, a consequence of caspase-9 activation. We conclude that the hypoxia-induced activation of caspase-9 is mediated by Src kinase. We propose Src kinase-dependent tyrosine phosphorylation (Tyr154) in the active site domain of caspase-9 is a potential mechanism of caspase-9 activation in the hypoxic brain.
Keywords: Caspase-9, Caspase-3, Src kinase, PP2, Hypoxia, Newborn
The activation of procaspase-9 to active caspase-9 is a key step that initiates execution of programmed cell death in brain following hypoxia, however, the mechanism of activation is not understood. Previous studies have determined the expression of pro- and anti-apoptotic proteins and their ratios. However, recent studies demonstrate that post-translational modification may alter the function of these proteins including that of procaspase-9. We have shown that hypoxia results in increased tyrosine phosphorylation of procaspase-9 and apoptotic protease activating factor-1(Apaf-1) [19]. Caspases play an important role in the initiation and execution of programmed cell death [13, 26, 29]. Caspases are a group of cysteine proteases that are essential for initiating and executing programmed cell death [9,12]. Studies on the nematode Caenorhbditis elegans (C. elegans) have demonstrated that an aspartate specific cysteine protease is essential for programmed cell death during development [7, 30].
In C. elegans, a group of genes including egl-1(egl, egg-laying defective), ced-3 (cell death abnormal), ced-4 and ced-9 are at the core of programmed cell death. Three protein components (Ced-3, Ced-4 and Egl-1) are required for cell death. These code for a caspase (Ced-3), an adopter protein (Ced-4) and a proapoptotic member of the Bcl-2 family of proteins (Egl-1). The Bcl-2 homolog Ced-9 is needed for cell survival. Protein – protein interactions between Ced -3, Ced-4, Ced-9 and Egl-1 provide a direct link between caspases and Bcl-2 family of proteins [2, 3, 25]. In mammalian cells, the adaptor protein comparable to Ced-4 in C.elegans is apoptotic protease activating factor-1(Apaf-1). [31, 32, 5] Antiapoptotic proteins Bcl-2 and Bcl-xl bind to Apaf-1 and this. binding is essential for the antiapoptotic function of Bcl-2 family proteins [31, 32]. Apaf-1 acts upstream of caspases, and that Ced-9 or the antiapoptotic proteins Bcl-2 or Bcl-xl act as inhibitors of Apaf-1. Ced-4 or Apaf-1 can simultaneously bind to procaspase-9 (Ced-3 homolog), as well as the apoptotic proteins (Ced-9 homologs) [4, 9]. In brief, the genetic components of programmed cell death have been identified, with a possible activation sequence of these components as follows: In C elegans: Egl-1→ Ced-9→Ced-4→apoptosis; In mammals: Bax→Bcl-2/Bcl-xl→ Apaf-1→ procaspase-9→caspase-9→procaspase-3→caspase-3→apoptosis. However, the mechanism of activation of procaspase-9 during hypoxia that may initiate programmed cell death in the mammalian brain tissue is not known.
In vitro studies have indicated that the apoptotic caspase cascade is activated by cytochrome c and ATP. In vitro studies using 100,000 g supernatant (S-100) extracts of HeLa 60 cells demonstrated that incubation with dATP or ATP (1–2 mM) and cytochrome c (10μM) together for 1 hr at 37°C resulted in cleaved products of poly-(ADP-ribose)polymerase (PARP) indicating activation of caspase-3 [15, 16]. Cleaved active caspase-9 and caspase-3 were also demonstrated. In caspase-9 deficient mice, it was shown that caspase-9 is needed for caspase-3 activation [12]. On the basis of these studies it was generally accepted that ATP and cytochrome c together activate caspase-9. There are several studies, however, not in agreement with this general idea regarding the role of cytochrome c in programmed cell death [8] and have raised questions regarding the appropriateness of the concentrations of ATP and cytochrome c and apoptosome formation, as well as caspase-9 activation [10, 20].
Apoptosome is assembled in response to several cellular stresses (i.e., hypoxia, DNA damage, oncogene activation, etc.). Activation by these signals finally leads to caspase activation via the intrinsic mitochondrial pathway resulting in apoptotic cell death. Apaf-1 knockout mice showed severe defects in the apoptotic response to hypoxic stimulation[ 28]. This finding demonstrates Apaf-1 as an essential component of the apoptotic response to hypoxia in vitro. Understanding the pathways that lead to apoptosis and identifying strategies to regulate this pathway may have important clinical implications. In stress-induced apoptosis, mitochondria releases apoptogenic factors such as cytochrome c. Pro-apoptotic members of the Bcl-2 family such as Bax and Bid induce the release of apoptogenic factors, whereas anti-apoptotic members such as Bcl-2 or Bcl-XL prevent their releases. Cytochrome c binds to Apaf-1 and, when dATP is added, the oligomeric complex of Apaf-1 is formed which recruits procaspase-9 to form apoptosome and results in activation of procaspase-9[27].
In our previous studies, we have shown that cerebral hypoxia results in increased activity of caspase-9 and nNOS inhibition prevented the hypoxia-induced increased activity of caspase-9 indicating that the hypoxia-induced increase in caspase-9 activity is mediated by NO [6, 11, 22]. We have also shown that hypoxia results in increased tyrosine phosphorylation of procapsase-9 and Apaf-1 [19]. The present study was specifically designed to test the hypothesis that the hypoxia-induced increased activation of caspase-9 is mediated by Src kinase, a protein tyrosine kinase. A highly selective inhibitor of Src kinase, PP2[IC505nm], was administered prior to the hypoxic exposure. The results of the present study provide evidence that hypoxia-induced increased activation of caspase-9 is mediated by Src kinase.
The experimental protocol was approved by the Institutional Animal Care and Use Committee of Drexel University. Studies were conducted on anesthetized, ventilated and instrumented 3–5 days old newborn piglets. Fifteen piglets were divided into three groups: normoxic (Nx, n=5), hypoxic (Hx, n=5) and hypoxic and hypoxic treated with Src kinase inhibitor, PP2, (Hx+PP2, 0.4 mg/Kg, i.v., 30 min prior to hypoxia). Anesthesia was induced with 4% halothane and lowered to 1% during surgery while allowing the animals to breathe spontaneously. Lidocaine 2% was injected locally before instrumentation for endotracheal tube insertion and femoral arterial and venous catheter insertion. After instrumentation, the use of halothane was discontinued, and anesthesia was maintained with nitrous oxide 79%, oxygen 21% and Fentanyl (50 μg/kg ) throughout the experiment. Tubocurarine (0.3 mg/kg) was administered after placing the animal on a volume ventilator. Arterial blood gases, heart rate and blood pressure were monitored in all animals throughout the study. Core body temperature was maintained at 38.5–39°C with a warming blanket. Baseline measurements were obtained in both groups for one hour after surgery to ensure normal arterial pressures and blood gas values. After stabilization following surgery, the piglets assigned to the hypoxic group were exposed to hypoxia (FiO2 = 0.06) for 1hr, while the piglets assigned to the normoxic group were ventilated at FiO2 of 0.21 for 1 hr. At the end of the experiment, the cortical brain tissue was removed and placed in buffer for the preparation of cytosolic fractions (100,000 g supernatant) or frozen in liquid nitrogen within 5–7 sec for the analysis of high energy phosphates, ATP and phosphocreatine (PCr), to document cerebral tissue hypoxia.
Cerebral tissue ATP and phosphocreatine (PCr) concentrations were determined spectrophotometrically by the method of Lamprecht et al. [14] and calculated from the increase in the absorbance at 340 nm.
Cerebral cortical cytosolic fraction was isolated in a buffer containing 50 mM Tris-HCl, (pH 7.4), 1 mM EDTA, 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 1 mM PMSF, 1 mM Na3VO4, 1 mM NaF and 1 μg/ml each of aprotinin, leupeptin and pepstatin. Equal amounts (20μg) of each protein sample was separated using 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE), transferred onto nitrocellulose membrane and incubated with polyclonal anti-active caspase-9 antibody (Santa Cruz). Subsequently, the nitrocellulose membrane was washed and incubated with horseradish peroxidase conjugated secondary antibody (Rockland, Gilbertsville, PA, USA) in 3% milk for 1.5 h at room temperature with constant agitation. Following washings with dH2O and PBS–0.05% Tween 20, the specific complexes were detected using ECL reagents for 2–3 min. Protein bands were analyzed using imaging densitometry. The density of proteins was expressed as absorbance (OD mm2).
The activity of caspase-9 and caspase-3 was determined spectrofluorometrically at 37°C using specific fluorogenic substrates for caspase-9 (Ac-LEHD-AMC) and caspase-3 (Ac-DEVD-AMC) obtained from Enzo Life Sciences. Continuous recording was performed at excitation 360nm/emission 435nm wave lengths. The activity was determined from the initial slope where the rate of reaction is linear and calculated using the amino-methyl-coumarin (AMC) as standard. The activity was expressed as nmoles/mg protein/hr.
The statistical analysis of the data on ATP, PCr, active caspase-9, the activity of caspase-9 and caspase-3 were performed using one way analysis of variance (ANOVA). A p value <0.05 was considered significant. Pair-wise comparison between the groups was done by Tukey-test.
The levels of tissue high energy phosphates in the cerebral cortex of normoxic, hypoxic and hypoxic treated with PP2 piglets were determined. Cerebral tissue hypoxia was documented by decreases in the levels of high energy phosphates, ATP and phosphocreatine (PCr). ATP levels (μmoles/g brain) were 4.35±0.21 in the Nx and 1.43±0.28 in the Hx (p<0.05 vs Nx) and 1.73± 0.33 in the Hx +PP2 (p<0.05 vs Nx, and p=NS vs. Hx) groups. PCr levels (μmoles/g brain) were 3.80±0.26 in the Nx and 0.96±0.20 in the Hx (p<0.05 vs Nx) and 1.09± 0.39 in the Hx+PP2 (p<0.05 vs Nx, and p=NS vs. Hx) group. These results demonstrate that cerebral tissue hypoxia was achieved in the hypoxic group and the degree of hypoxia in the cerebral cortex of hypoxic and hypoxic with the Src kinase inhibitor groups was comparable.
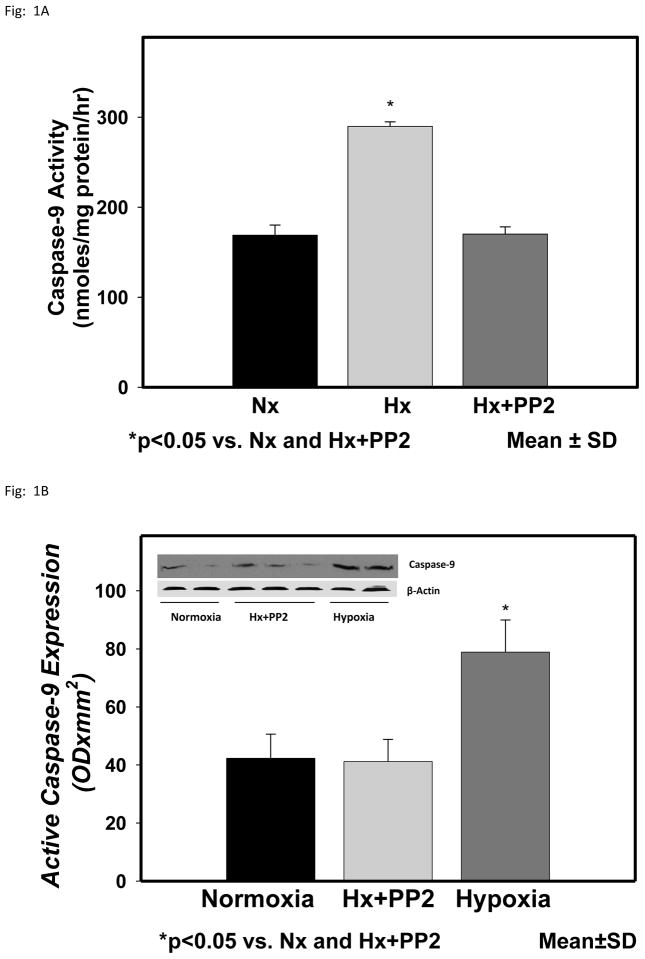
The activity of caspase-9 in the cytosolic fraction of the cerebral cortex of normoxic, hypoxic and hypoxic with Src inhibitor PP2 are shown in Fig 1. Caspase-9 activity (nmoles/mg protein/hr) was 1.40± 0.12 in Nx, 2.12±0.11 in Hx (p< 0.05 vs Nx) and 1.61±0.14 in Hx+PP2 (p<0.05 vs Hx). The results show that hypoxia results in increased activity of caspase-9 in the cytosolic fraction of the cerebral cortex of newborn piglets. Pretreatment with Src kinase inhibitor PP2 prevented the hypoxia-induced increased activity of caspase-9. The results indicate that the hypoxia-induced increased activation of caspase-9 is Src kinase mediated.
Figure 1.
Caspase-9 activity in the cytosolic fraction of the cerebral cortex of normoxic, hypoxic and hypoxic pretreated with Src kinase inhibitor, PP2, groups of newborn piglets. The activity is presented as nmoles/mg protein/hr. Activecaspase-9 protein expression in the cytosolic fraction of the cerebral cortex of normoxic, hypoxic and hypoxic pretreated with Src kinase inhibitor, PP2, groups of newborn piglets. The protein density is presented as absorbance (OD x mm2 ). The data is presented as mean ± standard deviation. *p<0.05 vs Nx and Hx+PP2.
The representative expression of active caspase-9 protein in the cytosolic fraction of the cerebral cortex of normoxic, hypoxic and hypoxic with Src inhibitor PP2 groups of piglets is also shown in Fig 1. The tyrosine phosphorylated protein density data from all the animals is shown as bar graph. The density of active caspase-9 (ODx mm2) was 42.3±8.3 in Nx, 78.9±11.0 in Hx (p<0.05 vs Nx) and 41.2±7.6 in Hx-PP2 (p<0.05 vs Hx) group. These results demonstrate that cerebral tissue hypoxia results in increased levels of active caspase-9 in the hypoxic group. Pretreatment with the Src ikinase inhibitor PP2 prevented the hypoxia-induced increased active caspase-9 indicating that the increased activation of caspase-9 during hypoxia is mediated by Src kinase.
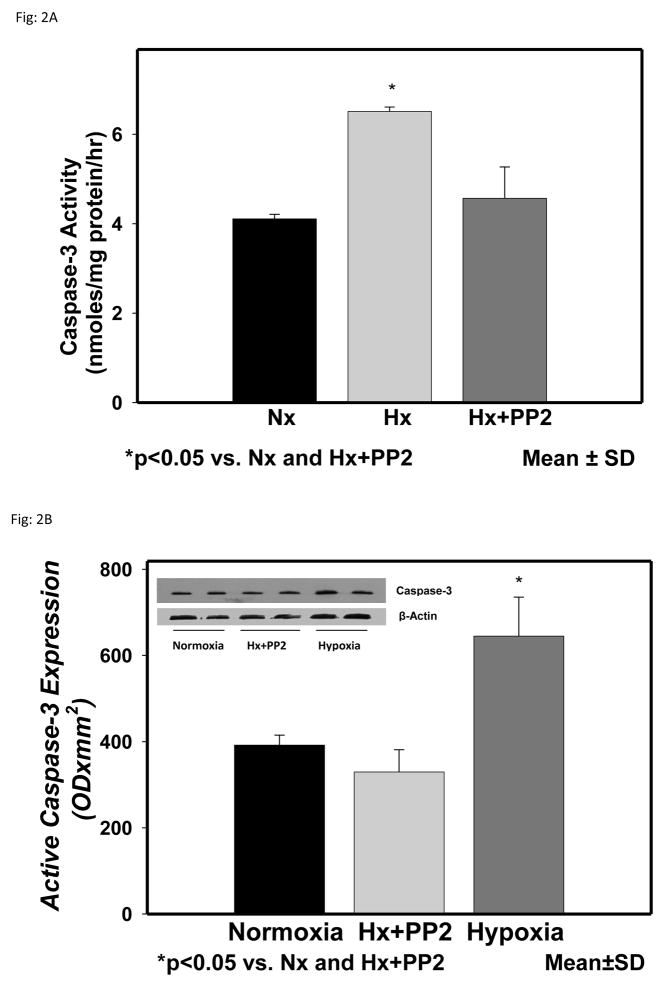
The activity of caspase-3 in the cytosolic fraction of the cerebral cortex of normoxic, hypoxic and hypoxic with Src inhibitor PP2 are shown in Fig 2. Caspase-3 activity (nmoles/mg protein/hr) was 4.11±0.1 in Nx, 6.51±.1 in Hx (p<0.05 vs Nx) and 4.57±0.7 in Hx+PP2 (p<0.05 vs Hx). The results show that hypoxia results in increased activity of caspase-3 in the cytosolic fraction of the cerebral cortex of newborn piglets. Pretreatment with Src kinase inhibitor PP2 prevented the hypoxia-induced increased activity of caspase-3. These results indicate that caspase-3 activation, a consequence of caspase-9 activation, is mediated by Src kinase.
Figure 2.
Caspase-3 activity in the cytosolic fraction of the cerebral cortex of normoxic, hypoxic and hypoxic pretreated with Src kinase inhibitor, PP2, groups of newborn piglets. The activity is presented as nmoles/mg protein/hr. Active caspase-3 protein expression in cytosolic fraction of the cerebral cortex of normoxic, hypoxic and hypoxic pretreated with nNOS inhibitor groups of newborn piglets. The protein density is presented as absorbance (OD x mm2 ). The data is presented as mean ± standard deviation. *p<0.05 vs Nx and Hx+PP2.
These results further confirm that caspase-9 activation during hypoxia is Src kinase mediated. The representative expression of active caspase-3 protein in the cytosolic fraction of the cerebral cortex of normoxic, hypoxic and hypoxic with Src inhibitor PP2 groups of piglets is shown in Fig 2. The tyrosine phosphorylated protein density data from all the animals is shown as bar graph. The density of active caspase-3 (ODx mm2) was 392.1±23.1 in Nx, 645.0±90.3 in Hx (p<0.05 vs Nx) and 329.7±51.5 in Hx-PP2 (p<0.05 vs Hx) group. These results demonstrate that cerebral tissue hypoxia results in increased levels of active caspase-3 in the hypoxic group. Pretreatment with the Src ikinase inhibitor PP2 prevented the hypoxia-induced increased active caspase-3 indicating that the increased activation of caspase-3 during hypoxia is mediated by Src kinase. These results further indicate that caspase-3 activation, a consequence of caspase-9 activation, is mediated by Src kinase.
In previous studies we demonstrated that hypoxia results in increased activation of Src kinase. We have also shown that hypoxia results in increased tyrosine phosphorylation of procaspase-9 and Apaf-1 in the cytosolic compartment of the cerebral cortex of newborn piglets. The increased tyrosine phosphorylation of procaspase-9 and Apaf-1 may lead to increased interaction between the two molecules leading to increased activation of procaspase-9 to caspase-9 that initiates cascade of programmed cell death in the hypoxic brain. Thus Src kinase activation may lead to increased activation of caspase-9. The results of the present study confirms this proposed mechanism and demonstrated that the increased activation of caspase-9 during hypoxia is Src kinase mediated.
In previous studies we have demonstrated that hypoxia results in increased generation of nitric oxide free radicals in the cerebral cortical tissue[17]. NO produced during hypoxia results in inactivation of protein tyrosine phosphatase (PTP, SH-PTP1 and SH-PTP2) activity that leads to increased tyrosine phosphorylation of a number of proteins. We have shown that cerebral hypoxia results in increased tyrosine phosphorylation of Src at its active site Tyr416 (an index of Src kinase activation) and increased activity of Src kinase in the cerebral cortex of newborn piglets and the increased activation of Src kinase during hypoxia is NO-mediated[18]. Therefore, we propose that NO generated during hypoxia leads to increased activation of Src kinase which subsequently phosphorylates tyrosine residue at the active site domain of procaspase-9 and results in its increased activation during hypoxia. We have shown that hypoxia results in inhibition of protein tyrosine phosphatase activity and increase in protein tyrosine kinase (PTK, Src kinase) activity, and the administration of a nitric oxide synthase inhibitor prevented both the decrease in PTP (SH-PTP1 and SH-PTP2) activity and the increase in PTK (Src kinase) activity [1, 21]. Therefore, NO produced during hypoxia in the cerebral cortex of newborn piglets mediates tyrosine phosphorylation of procaspase-9 which initiate cascade of neuronal death by a transcription-independent mechanism.
We have previously proposed that tyrosine phosphorylated procaspase-9 strongly binds the Apaf-1 molecule due to additional H-bondings between the phosphate group of tyrosine residue of procaspase-9 and the amino and imino groups of two arginine residues present in the CARD domain of Apaf-1, thus increasing interaction between the two molecules for activation of procaspase-9. Simultaneously, tyrosine phosphorylated Apaf-1 binds strongly with two arginine residues present in the CARD domain of procaspase-9 and results in further increasing the interaction between these two molecules, thereby leading to exceedingly strong interaction between procaspase-9 and Apaf-1 molecules, resulting in increased activation of procaspase-9.
Studies showed that the recognition of procaspase-9 by Apaf-1 is essential for formation of apoptosome and activation of procaspase-9 [23,24,5]. Binding between the procaspase-9 and Apaf-1 is due to the basic surface from one molecule to the acidic surface of the other through electrostatic interactions that results in 1:1 binding stoichiometry between procaspase-9 and Apaf-1. Mutational studies have shown that a mutation at Tyr24 with Ala decreased the ability of Apaf-1 CARD to precipitate procaspase-9 CARD indicating that mutation at Tyr24 is essential for Apaf-1 and procaspase-9 interaction. Mutation at Tyr 153 decreased the activation of procaspase-9. These studies provide structural basis for increased electrostatic charge-charge interaction between tyrosine phosphorylated procaspase-9 and Apaf-1 molecules leading to procaspase-9 activation.
In summary: we have shown that cerebral tissue hypoxia results in increased activation of caspase-9 as indicated by increased activity and increased density expression of active caspase-9 protein. Pretreatment with Src kinase inhibitor PP2 prevented the hypoxia-induced increased activation of caspase-9 in the cytosolic fraction of the cerebral cortex of newborn piglets. We conclude that the hypoxia-induced increased activation of caspase-9, which activates downstream caspase-3, is mediated by Src kinase. We propose that Src kinase mediated tyrosine phosphorylation of procaspase-9 results in strong binding with the Apaf-1 molecule between the phosphate group of tyrosine residue of procaspase-9 and the amino and imino groups of two arginine residues of the CARD domain of Apaf-1 results in increased activation of caspase-9.
Table 1.
Level of High Energy Phosphates During Hypoxia in Cerebral Cortex of Newborn Piglets
| Study Groups | ATP (μmoles/g brain) | Phosphocreatine (μmoles/g brain) |
|---|---|---|
| Normoxia | 4.35 ± 0.21 | 3.80 ± 0.26 |
| Hypoxia | 1.43 ± 0.28* | 0.96 ± 0.20* |
| Hypoxia + PP2 | 1.73 ± 0.33 | 1.09 ± 0.39 |
p<0.05 vs. Nx
Mean ± SD
p=NS vs. Hx+PP2
Highlights.
Src kinase inhibitor prevents the hypoxia-induced activation of caspase-9.
Src kinase inhibitor also prevented the hypoxia-induced activation of caspase-3.
Hypoxia-induced activation of caspase-9 is mediated by Src kinase.
Acknowledgments
This study was supported by grants from the National Institutes of Health, NIH-HD-20337.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ashraf QM, Haider HS, Katsetos CD, Delivoria-Papadopoulos M, Mishra OP. Nitric oxide mediated alterations of protein tyrosine phosphatase activity and expression during hypoxia in the cerebral cortex of newborn piglets. Neurosci Lett. 2004;362:108–112. doi: 10.1016/j.neulet.2004.02.069. [DOI] [PubMed] [Google Scholar]
- 2.Chang HY, Yang X. Proteases for cell suicide: Functions and regulation of caspases. Microbiol Mol Biol Rev. 2000;64:821–846. doi: 10.1128/mmbr.64.4.821-846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinnaiyan AM. The apoptosome: Heart and soul of the cell death machine. Neoplasia. 1999;1:5–15. doi: 10.1038/sj.neo.7900003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chinnaiyan AM, O’Rourke K, Lane BR, Dixit VM. Interaction of CED-4 with CED-3 and CED-9: a molecular framework for cell death. Science. 1997;275:1122–1126. doi: 10.1126/science.275.5303.1122. [DOI] [PubMed] [Google Scholar]
- 5.Day CL, Dupont C, Lackmann M, Vaux DL, Hinds MG. Solution structure and mutagenesis of the caspase recruitment domain (CARD) from Apaf-1. Cell death and Differentiation. 1999;6:1125–1132. doi: 10.1038/sj.cdd.4400584. [DOI] [PubMed] [Google Scholar]
- 6.Delivoria-Papadopoulos M, Mishra OP. Mechanism of Activation of Caspase-9 and Caspase-3 during hypoxia in the cerebral cortex of newborn piglets: The role of nuclear Ca2+ influx. Neurochem Res. 2007;32:401–405. doi: 10.1007/s11064-006-9229-1. [DOI] [PubMed] [Google Scholar]
- 7.Ellis RE, Yuan J, Horvitz HR. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- 8.Finkel E. The mitochrondion: is it central to apoptosis? Science. 2001;292:624–626. doi: 10.1126/science.292.5517.624. [DOI] [PubMed] [Google Scholar]
- 9.Grutter MG. Caspases: key players in Programmed cell death. Curr Opin Strl Biol. 2000;10:649–655. doi: 10.1016/s0959-440x(00)00146-9. [DOI] [PubMed] [Google Scholar]
- 10.Jiang X, Kim H-E, Shu H, et al. Distintive roles of PHAP proteins and prothymosin-α in a death regulatory pathway. Science. 2003;299:223–226. doi: 10.1126/science.1076807. [DOI] [PubMed] [Google Scholar]
- 11.Khurana P, Ashraf QM, Mishra OP, Delivoria-Papadopoulos M. Effect of hypoxia on caspase-3, -8 and –9 activity and expression in the cerebral cortex of newborn piglets. Neurochem Res. 2002;27:931–938. doi: 10.1023/a:1020347732741. [DOI] [PubMed] [Google Scholar]
- 12.Kuida K, Haydar TF, Kuan CY, Gu Y, Taya C, Karasuyama H, Su MSS, Rakic P, Flavell RA. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase-9. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- 13.Kumar S. Caspases and their many biological functions. Cell Death and Differentiation. 2007;14:1–2. doi: 10.1038/sj.cdd.4402060. [DOI] [PubMed] [Google Scholar]
- 14.Lamprecht W, Stein P, Heinz F, Weisser H. Creatine phosphate. In: Bergmeyer HU, editor. Methods of enzymatic analysis. Vol. 4. 1974. pp. 1777–1781. [Google Scholar]
- 15.Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri E, Wang X. Cytochrome c and dATP-dependent formation of Apaf-1/Caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 16.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 17.Mishra OP, Zanelli S, Ohnishi ST, Delivoria-Papadopoulos M. Hypoxia-induced generation of nitric oxide free radicals in cerebral cortex of newborn guinea pigs. Neurochem Res. 2002;25:1559–1565. doi: 10.1023/a:1026610301978. [DOI] [PubMed] [Google Scholar]
- 18.Mishra OP, Ashraf QM, Delivoria-Papadopoulos M. NO-mediated pactivation of Src kinase during hypoxia in cerebral cortex of newborn piglets. Neurosci Lett. 2009;460:61–65. doi: 10.1016/j.neulet.2009.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishra OP, Delivoria-Papadopoulos M. Mechanism of tyrosine phosphorylation of procaspase-9 and Apaf-1 in cytosolic fractions of the cerebral cortex of newborn piglets during hypoxia. Neurosci Lett. 2010;480:35–39. doi: 10.1016/j.neulet.2010.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mishra OP, Delivoria Papadopoulos M. ATP and cytochrome c –dependent inhibition of caspase-9 activity in the cerebral cortex of newborn piglets. Neurosci Lett. 2004;364:119–123. doi: 10.1016/j.neulet.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 21.Mishra OP, Delivoria-Papadopoulos M. Effect of hypoxia on protein tyrosine kinase activity in cortical membranes of newborn piglets- The role of nitric oxide. Neurosci Lett. 2004;372:114–118. doi: 10.1016/j.neulet.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 22.Mishra OP, Delivoria-Papadopoulos M. Effect of neuronal nitric oxide synthase inhibition of caspase-9 activity during hypoxia in the cerebral cortex of newborn piglets. Neuroscience Letters. 2006;1–2:81–85. doi: 10.1016/j.neulet.2006.02.070. [DOI] [PubMed] [Google Scholar]
- 23.Shi Y. Apoptosome: The cellular engine for activation of caspase-9. Structure. 2002;10:285–288. doi: 10.1016/s0969-2126(02)00732-3. [DOI] [PubMed] [Google Scholar]
- 24.Qin H, Srinivasula SM, Wu G, Alnemri TF, Alnemri ES, Shi Y. Structural basis of procaspase-9 recruitment by the apoptotic protease-activating factor. 1. Nature. 1999;399:549–557. doi: 10.1038/21124. [DOI] [PubMed] [Google Scholar]
- 25.Ranganath RM, Nagashree NR. Role of programmed cell death in development. Int Rev Cytol. 2001;202:59–242. doi: 10.1016/s0074-7696(01)02005-8. [DOI] [PubMed] [Google Scholar]
- 26.Riedl SJ, Salvesen GS. The apoptosome: signalling platform of cell death. Molecular Cell Biology. 2007;8:405–413. doi: 10.1038/nrm2153. [DOI] [PubMed] [Google Scholar]
- 27.Saleh A, Srinivasula SM, Acharya S, Fishel R, Alnemri ES. Cytochrome c and dATP-mediated oligomerization of Apaf-1 is a prerequisite for procaspase-9 activation. J Biol Chem. 1999;274:17941–17945. doi: 10.1074/jbc.274.25.17941. [DOI] [PubMed] [Google Scholar]
- 28.Soengas MS, Alarcon RM, Yoshida H, Giaccia AJ, Hakem R, Mak TW, Lowe SW. Apaf-1 and caspase-9 in p53-dependent apoptosis and tumor inhibition. Science. 1999;284:156–159. doi: 10.1126/science.284.5411.156. [DOI] [PubMed] [Google Scholar]
- 29.Timmer JC, Salvesen GS. Caspases substrates. Cell Death and Differentiation. 2007;14:66–72. doi: 10.1038/sj.cdd.4402059. [DOI] [PubMed] [Google Scholar]
- 30.Xue D, Shaham S, Horvitz HR. The Caenorhabditis elagans cell death protein CED-3 is a cysteine protease with substrate specificities similar to those of human CPP32 protease. Genes Dev. 1996;10:1073–1083. doi: 10.1101/gad.10.9.1073. [DOI] [PubMed] [Google Scholar]
- 31.Yuan JY, Horvitz RH. The C. elegans cell death gene ced-4 encodes a novel protein and is expressed during the period of extensive programmed cell death. Development. 1992;116:309–320. doi: 10.1242/dev.116.2.309. [DOI] [PubMed] [Google Scholar]
- 32.Zou H, Henzel WJ, Liu X, Lutschg A, Wang X. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]