Abstract
Bone substitute materials are required to support the remodeling process, which consists of osteoclastic resorption and osteoblastic synthesis. Osteoclasts, the bone resorbing cells, generate from differentiation of hemopoietic mononuclear cells. In the present study we have evaluated the effects of 1.0 wt% strontium (Sr) and 1.0 wt% magnesium (Mg) doping in beta-tricalcium phosphate (β-TCP) on the differentiation of mononuclear cells into osteoclast-like cells and its resorptive activity. In vitro osteoclast-like cell formation, adhesion, and resorption were studied using osteoclast precursor RAW 264.7 cell, supplemented with receptor activator of nuclear factor κβ ligand (RANKL). Osteoclast-like cell formation was noticed on pure and Sr doped β-TCP samples at day 8 which was absent on Mg doped β-TCP samples indicating decrease in initial osteoclast differentiation due to Mg doping. After 21 days of culture, osteoclast-like cell formation was evident on all samples with osteoclastic markers such as actin ring, multiple nuclei, and presence of vitronectin receptor αvβ3 integrin. After osteoclast differentiation, all substrates showed osteoclast-like cell mediated degradation, however; significantly restricted for Mg doped β-TCP samples. Our present results indicated substrate chemistry controlled osteoclast differentiation and resorptive activity which can be used in designing TCP based resorbable bone substitutes with controlled degradation properties.
Keywords: Beta-tricalcium phosphate, Dopants, Osteoclast-like cells, Osteoclastogenesis, Resorption lacunae
1. Introduction
Calcium phosphate (CaP) ceramics, especially β-TCP, found wide application in reconstructive bone surgery, such as spinal fusion and craniomaxillofacial applications due to its resorbable property and close proximity in chemical composition to natural bone [1–4]. In physiological conditions, β-TCP slowly degrades by both chemical dissolution and osteoclastic resorption, where the surrounding tissues proliferate on the diminishing surface to initiate the bone remodeling process [5]. Bone remodeling is a dynamic and coordinated biochemical process of osteoclastic resorption and osteoblastic synthesis of new bone. The balance of this bone remodeling can get disrupted by either increased osteoclastic activity (loss of bone density) or dysfunction of osteoclast (increase in bone density) or by both [6]. Therefore, bone replacement materials, such as β-TCP, must be able to provide support for both osteoblast and osteoclast functions in order to keep the dynamics of bone remodeling. Osteoclasts, the bone resorbing cells, generate from mononuclear cells and in regulation of cytokines and growth factors [6, 7]. Among the cytokines, RANKL is one of the primary factors for osteoclast formation, a process known as osteoclastogenesis [7].
Incorporating inorganic compounds in bone replacement materials, which are either essential elements of bone, or known to effectively influence bone development or regeneration is an attractive approach to effect mechanical and biological properties of these materials [8–11]. It was found that 1.5% Si substituted HA enhances the osteoclastic activity [12]. Zn substitution was found to increase the compressive strength of β-TCP with an inhibiting effect on osteoclast formation or resorption [13]. Incorporation of Co2+ in the CaP increased the osteoclast formation by 75% with a 2.3 to 2.7 fold increase in mineral resorption area [14]. In our previous studies, we have shown that SrO and MgO incorporated β-TCP enhanced in vitro cell attachment, growth and improved in vivo biocompatibility [10, 15]. We have also shown that incorporation of MgO in TCP ceramics can significantly enhance the osteoblast activity [16]. It has also been reported that Mg deficiency reduces bone density and toughness which increases the chance of bone fracture [17]. 1.0 wt% Sr doping in HA was found to increase osteoblast proliferation that reduces on increasing Sr content [18]. Studies concentrating on the effectiveness of these dopants are still ongoing and it is vitally important to understand the role of these substitutes in adjusting the bone remodeling process.
Considering the beneficial effects of Sr and Mg, it is believed that their presence in β-TCP will have an influence on osteoclastogenesis and its resorption activity. The objective of the present study is to understand the role of Sr and Mg substitution in β-TCP on the differentiation of mononuclear cells into osteoclast-like cells and their resorptive activity. The influence of Sr and Mg doping on osteoclastogenesis was studied in vitro, using osteoclast precursor cell RAW 264.7, supplemented with RANKL. The formation of osteoclast-like cells was confirmed by cellular morphology, acting ring formation and multiple nucleuses. In addition, resorption lacunae formation was studied to understand the osteoclast activity.
2. Materials and Methods
2.1 Materials processing
β-TCP powder (Berkeley Advanced Biomaterials Inc. Berkeley, CA, USA) with an average particle size of 550 nm were mixed with high purity SrO (99.9% purity, Aldrich, MO, USA) and MgO (99.998%, Alfa Aesar, MA, USA) for this study. Three compositions of β-TCP were prepared for this study: β-TCP, β-TCP doped with 1.0 wt% Mg (2.1 mole %) and β-TCP doped with 1.0 wt% Sr (0.57 mole %) (hereafter refereed as TCP, Mg-TCP and Sr-TCP respectively). The powder was ball milled in anhydrous ethanol, dried in an oven at 60 °C for 72 h, uniaxially pressed to disc (12 mm diameter and 2.5 mm thickness) shapes, cold isostatically pressed at 414 MPa for 5 min, and sintered at 1250 °C for 2 h in a muffle furnace. Apparent density of each composition was determined by the Archimede’s method and reported as relative density after normalizing to the theoretical density of β-TCP, i.e., 3.07 g/cm3. Average grain size was determined from FESEM images via a linear intercept method [10]. For each composition, 30 lines from 3 samples were used to calculate the grain size. The data is reported as mean± standard deviation. Siemens D500 Krystalloflex X-ray diffractometer using Cu Kα radiation at 35 kV and 30 mA at room temperature was used to determine different phases with a Ni-filter over the 2θ range between 20° and 45°, at a step size of 0.02° and a count time of 0.5 sec per step.
2.2 Contact angle measurement
Wettability of sample surfaces were investigated by determining contact angles of deionized distilled water (D.I.) and cell culture media. Contact angles were measured using the static sessile drop method using a face contact angle set-up equipped with a camera (VCA Optima, AST Products Inc., MA, USA) [19].
2.3 Osteoclast cell culture
The human monocyte-like cell line RAW 264.7 (ATCC, USA) was cultured in DMEM media (ATCC, USA) supplemented with 10 vol. % fetal bovine serum (FBS, Sigma, Germany) and 1 vol. % penicillin/streptomycin (Invitrogen, Germany) at 37 °C in an atmosphere of 5% CO2. β-TCP disks were autoclaved at 121 °C for 20 min and then RAW 264.7 cells were seeded at a concentration of 105 cells/ ml and incubated at 37 °C in an atmosphere of 5% CO2. At day 1, 50 ng/ml RANKL (Biolegend, CA, USA) was added to the culture media. After this period, cell culture media, containing 50 ng/ml RANKL, was changed every 2 days for rest of the experiment. For the control, TCP, Sr-TCP and Mg-TCP disks were also incubated with RAW 264.7 cells at 37 °C in an atmosphere of 5% CO2; however, media was not supplemented with RANKL. The control samples were denoted as TCP(c), Sr-TCP(c) and Mg-TCP(c).
2.4 Osteoclast cell morphology
Morphology of cells was assessed by FESEM (FEI 200F, FEI Inc., OR, USA). Samples were removed from culture after 3, 5 and 8 days of incubation and were rinsed with 0.1 M phosphate-buffered saline (PBS). Cells were subsequently fixed with 2% paraformaldehyde/2% glutaraldehyde in 0.1 M cacodylate buffer overnight at 4 °C. Following a rinse in 0.1 M cacodylate buffer, each sample was post-fixed in 2% osmium tetroxide (OsO4) for 2 h at room temperature. Fixed samples were then dehydrated in an ethanol series followed by hexamethyl-disililane (HMDS) drying. Dried samples were gold coated and observed under FESEM.
2.5 Immunofluorescence and confocal laser-microscopy
After specific culture days, osteoclast-like cells were fixed in 3.7% paraformaldehyde/ phosphate buffered solution, pH 7.4 and at room temperature for 10 min. After washing with PBS for 3 times (5 min each), the cells were permeabilized with 0.1% Triton X-100 (in PBS) for 4 min at room temperature. Samples were then washed with PBS and incubated in TBST-BSA (Tris-buffered saline tween with 1% bovine serum albumin, 250 mM NaCl, pH 8.3) blocking solution for 1h at room temperature. Actin staining was done by incubating the samples in Rhodamine-phalloidine (molecular probes, Invitrogen, USA), diluted in PBS 1:40 for 30 min in the dark. Samples were then rinsed in PBS and incubated in the primary antibody (mouse-anti-vitronectin receptor, abcam), with 1:50 in TBST solution for 2h and kept at 4 °C overnight. Samples were then washed with TBST for 10 min for 3 times. The secondary antibody (Alex Fluor 488 anti-mouse) was diluted 1:100 in TBST and was used to incubate the cells for 1h. After 2 × 5 min washing with TBST followed by 5 min washing with PBS, the samples [5] were then mounted on glass coverslips with Vectashield mounting medium (Vector Labs, Burlingame, CA) with 4′,6-diamidino-2-phenonylindole (DAPI) and kept at 4 °C for future imaging. The microscopical examinations were performed on a Zeiss 510 laser scanning microscope (LSM 510 META, Carl Zeiss MicroImaging, Inc., NY, USA).
Low magnification FESEM images and immunohistochemical analysis were used to calculate osteoclast-like cells density after 8 days of culture. The data were reported as mean ± standard deviation (n = 4). The statistical significance in osteoclast-like cells density among the three samples was determined by student’s “t” test.
2.6 Resorption pit assay
Osteoclast-like cells were removed from sample surface after 14, 21 and 28 days of culture. Samples were ultrasocicated in 1M NaCl solution with 0.2% Triton X-100 to remove the osteoclast-like cells and then washed with PBS [14]. After gold coating, samples were observed in FESEM for resorption lacuna. To ensure that the surface degradation of the TCP ceramics are related to osteoclast-like cell activity and not related to chemical degradation, TCP disks were kept in culture media without cells and were used as controls.
2.7 Metal ion release in the media
Release of Ca2+, Sr2+, Mg2+ ions from the pure and doped β-TCP substrates in the culture media was measured using a Shimadzu AA-6800 Atomic Absorption Spectrophotometer (Shimadzu, Kyoto, Japan). Ca2+ was measured in air-acetylene flame mode by diluting the media at 1:200 in deionized water. For the measurement of Sr2+ and Mg2+, culture media was diluted to 1:100 and 1:200, respectively, and measured in graphite furnace mode. The data were reported as mean ± standard deviation (n = 6).
3. Results
3.1. Physico-chemical characteristics
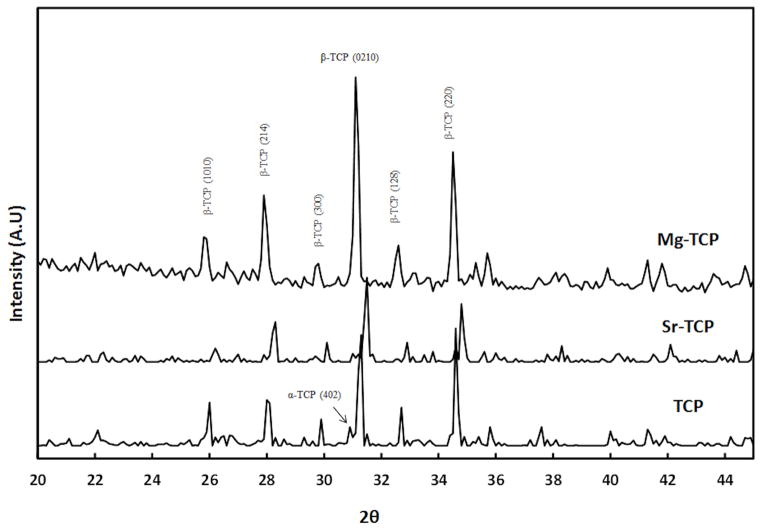
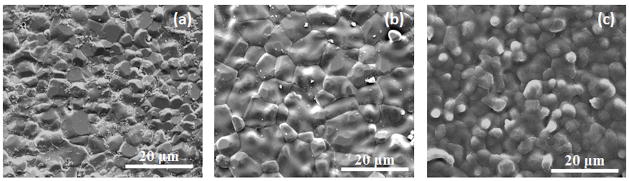
X-ray diffraction (XRD) pattern of the three different β-TCP ceramics, sintered at 1250 °C, is shown in Figure 1. Majority of the peaks can be attributed to β-TCP (JCPDS # 09-169) phase. Peaks related to α-TCP (JCPDS # 09-0348) phase were also identified, indicating β → α phase transition during densification. However, α-TCP peaks were not detected in Mg-TCP. The relative density of TCP, Sr-TCP and Mg-TCP were found to be 96.17 ±0.89%, 95.02 ±1.39%, and 98.61 ±0.97%, respectively. Addition of Mg resulted in a slight increase in density of Mg-TCP compacts. Influence of Sr and Mg dopants on grain size of sintered TCP compacts are shown in Figure 2 and summarized in Table 1. Figure 2C shows glassy phase in Mg-TCP samples. The grain size was found to reduce for Mg-TCP samples while it increased for Sr-TCP samples.
Figure 1.
X-ray powder diffraction patterns of pure β-TCP and Mg/Sr doped β-TCP.
Figure 2.
Effect of dopant on grain size of sintered β-TCP (a) TCP, (b) Sr-TCP, and (c) Mg-TCP.
Table 1.
Physical properties of TCP, Sr-TCP and Mg-TCP samples
| Density (%) | Grain size (μm) | Contact angle | ||
|---|---|---|---|---|
| DI water | Cell media | |||
| TCP | 96.17±0.89 | 4.09±0.69 | 57.83±7.44 | 45.41±4.38 |
| Sr-TCP | 95.02±1.39 | 4.86±0.84 | 52.35±2.65 | 47.10±4.63 |
| Mg-TCP | 98.61±0.97 | 2.91±0.79 | 55.72±2.9 | 50.61±5.3 |
Table 1 shows the measured contact angles of two different liquids (D.I. water and cell culture media) on TCP, Sr-TCP, and Mg-TCP samples. Contact angle found to decrease significantly for cell culture media compared to that of D.I. water. Differences in contact angles among the three different samples were not significant for D.I. water as well as cell culture media.
3.2. Osteoclast cell morphology
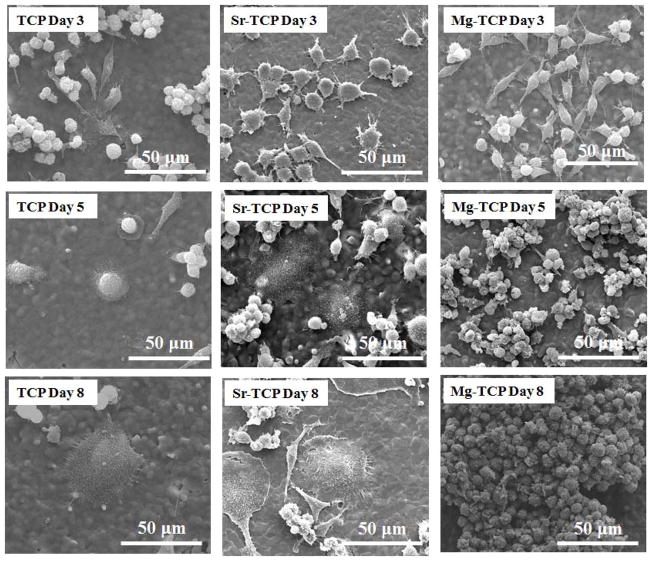
RAW 264.7 monocyte cell morphology and its interaction with the β-TCP substrates were analyzed using FESEM. Figure 3 shows the cell morphology on TCP, Sr-TCP, and Mg-TCP samples after 3, 5 and 8 days of culture in RANKL supplemented media. At day 3, the monocytes showed spontaneous adhesion to all the substrates with similar cellular microextensions. At day 5, attached monocytes on TCP and Sr-TCP had undergone a significant morphological change. Giant cells with round and flat morphology were noticed on these samples. In comparison, cellular agglomeration was found on Mg-TCP samples. After 8 days of culture, adhered cells on TCP and Sr-TCP samples increased in size with features resembling to morphology of osteoclasts. Highly oriented pseudopodia were found all around the cell membrane. Similar morphological features of osteoclast like cells on HA and TCP substrates have previously been reported [20]. Comparatively, monocytes extensively proliferated on Mg-TCP samples; however, with no indication of osteoclast-like cell formation. Figure 4 showed osteoclast-like cell adhesion on TCP, Sr-TCP and Mg-TCP samples. As characteristics of these cell lines, osteoclast-like cells showed highly oriented cell membrane on TCP and Sr-TCP surfaces with far-reaching pseudopodia extensions. Cells adhered to Mg-TCP samples did not show any such features.
Figure 3.
FESEM micrographs illustrating the RAW 264.7 cell morphologies after 3, 5 and 8 days of culture with RANKL.
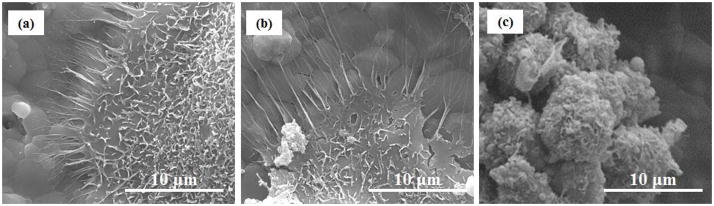
Figure 4.
FESEM micrographs of osteoclast-like cells on (a) TCP, (b) Sr-TCP and (c) Mg-TCP samples after 8 days of culture. Well defined pseudopodia can be noted around the cell membane attached to TCP and Sr-TCP.
3.3. Immunohistochemistry and actin ring formation
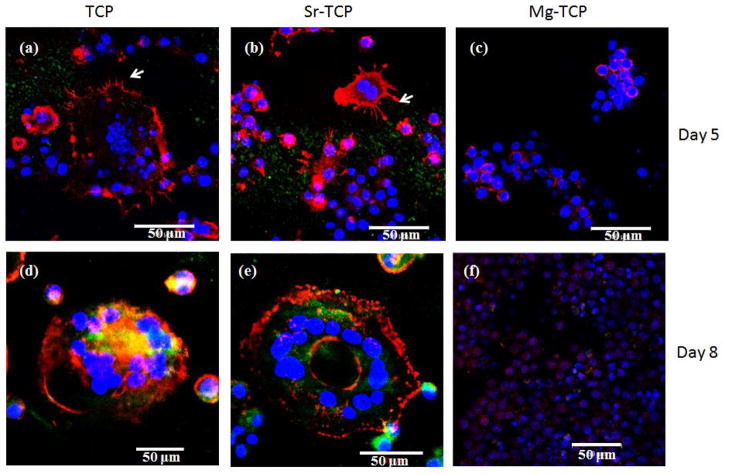
Along with morphology, osteoclast formation was also monitored by immunohistochemistry to analyze these cells for multinuclearity and actin ring formation. Figure 5 shows the fluorescence microscope images of RAW 264.7 cells cultured on doped or undoped TCP samples. After 5 days of culture, multinuclear cells with actin rings, typical characteristics of osteoclasts, were observed on both TCP and Sr-TCP sample surfaces, as shown in Figures 5a and b. Podosomes were clearly visible at the periphery of these cells. In comparison, multinuclearity and actin ring was not noticed in cells adhered to Mg-TCP samples. Instead, distinct colonies of mononuclear RAW 264.7 cells were found on the sample surface (Figure 5c). Phenotypic expression of the cells after 8 days of culture is shown in Figures 5d–f. Fully differentiated osteoclast-like cells with numerous nucleuses and localized actin ring was noticed on TCP and Sr-TCP samples. Cells on the Mg-TCP samples did not show any characteristics of osteoclast differentiation.
Figure 5.
Fluorescence microscopy images of cells cultured for 5 and 8 days on TCP, Sr-TCP and Mg-TCP samples. The red represents the actin cytoskeleton and the blue represents the nucleolus. The podosomes are represented by white arrow. Cells with multiple nucleuses and acting ring were identified as osteoclast-like cells.
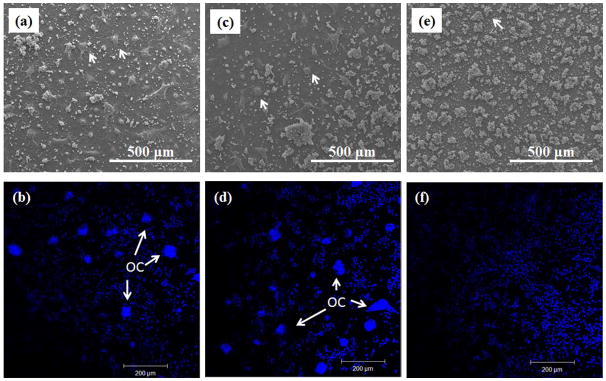
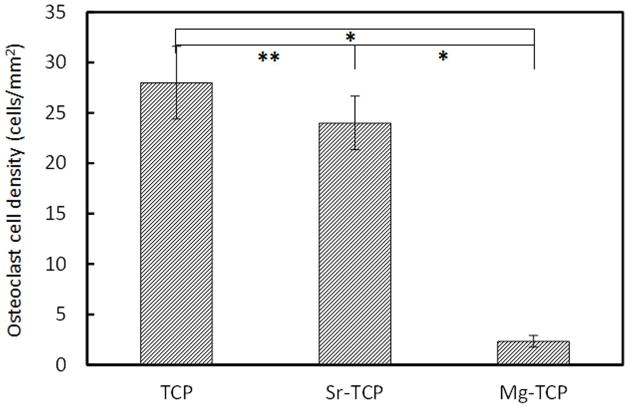
Osteoclast-like cell density, after 8 days of culture, was calculated using low magnification FESEM images and immunohistochemical analysis. Figure 6a, c and e shows the low magnification FESEM images of cultured cell on TCP, Sr-TCP and Mg-TCP. Giant cells with highly oriented cell membrane were counted as osteoclast-like cells (indicated by white arrow). Osteoclast-like cells were found in abundance on both TCP and Sr-TCP sample surfaces (Figure 6a and c) which almost grew to confluence. However, Mg-TCP sample surface was fully covered with colonies of monocytes. The results were also confirmed by immunohistochemical analysis, where nucleus of the cells was stained blue and is shown in Figure 6b, d and f. Cluster of nucleuses were considered as osteoclast-like cells and indicated by white arrow. Figure 7 shows the osteoclast-like cell density (cells/mm2) on TCP, Sr-TCP and Mg-TCP samples after 8 days of culture. Statistically significant difference in osteoclast-like cell density was not found between TCP and Sr-TCP samples. When compared with Mg-TCP samples, osteoclast-like cell number on TCP and Sr-TCP samples showed nearly 10-fold increase.
Figure 6.
FESEM micrographs of osteoclast-like cells on (a) TCP, (b) Sr-TCP and (c) Mg-TCP samples after 8 days of culture. (d), (e) and (f) represents fluorescence microscopy images of cells attached to TCP, Sr-TCP and Mg-TCP respectively. Blue represents the nucleolus. White arrows indicate osteoclast-like cells.
Figure 7.
Osteoclast-like cell density after 8 days of culture ( P < 0.05, *P > 0.05, n = 4).
Figure 8 shows the fluorescence microscopy images of osteoclast-like cells after 21 days of culture. Cells were found to be multinuclear with concentrated and continuous actin ring on all three of the TCP samples. The αvβ3 integrin, a vitronectin receptor protein, was highly expressed in the cells plasma membrane and cytoplasm [5]. Although osteoclast-like cell formation was scarce on Mg-TCP samples on 8th day of culture, multinucleated osteoclast-like cells were found after 21 days of culture on these sample surfaces. Similar matured osteoclast-like cells were also found in TCP and Sr-TCP samples.
Figure 8.
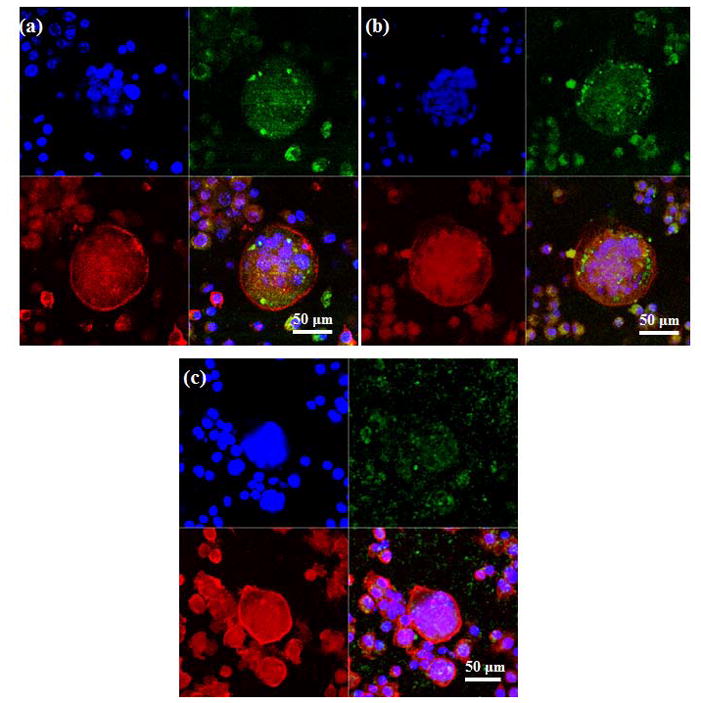
Fluorescence microscopy images of cells cultured for 21 days on (a) TCP, (b) Sr-TCP and (c) Mg-TCP samples. The red represents the actin cytoskeleton, green indicates vitronectin receptor αvβ3 integrin and the blue represents the nucleolus. Osteoclast-like cells were identified by the presence of multiple nucleuses, acting ring and were vitronectin receptor αvβ3 integrin.
3.4. Surface degradation
Surface degradation behavior of TCP, Sr-TCP and Mg-TCP samples were examined by lysing the osteoclast-like cells from the sample surfaces. Figure 9 shows the surface degradation of TCP samples. Resorption lacunae formation was not detected in control TCP substrates (Figure 9a– c); however, small etch pits were noticed after 21 and 28 days of culture. In RANKL treated cultures, impressions on the sample surfaces were noticed which resembled to the size and shape of the differentiated osteoclast-like cells at day 8 (Figure 9d and g). The resorption lacunae did not increase in numbers in the successive culture days; however, increased in depth after 21 days of culture, as shown in Figure 9h. A similar surface degradation behavior on both HA and TCP surfaces has been previously reported [20, 21]. At day 28, surface degradation was much more intense with more volume of the materials being removed from the substrate surface (Figure 9i).
Figure 9.
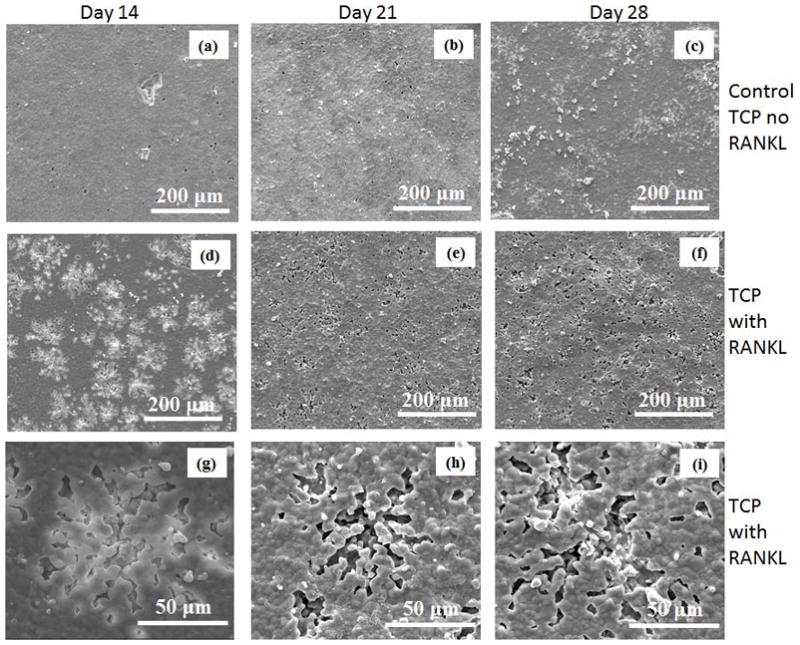
Surface morphology of TCP samples after culturing with RAW 264.7 cells at different days.
The FESEM micrographs of the Sr-TCP and Mg-TCP sample surfaces at different culture days are shown in Figure 10 and 11. Numerous lacunae were found on Sr-TCP samples in RANKL supplemented culture after 14 days, as shown in Figures 10d and g. When compared to pure TCP, lacunae formation was similar in nature in Sr-TCP samples however markedly different for Mg-TCP samples. Resorption lacunae were not noticed in Mg-TCP samples even when cultured with RANKL supplements for 14 and 21 days (Figures 11d and e). Few resorption lacunae were found on Mg-TCP sample surface at day 28, as shown in Figure 11i. High magnification image showed that the resorption areas on Sr-TCP samples were mostly surface phenomenon at day 14, which did not increase in depth at day 21, however, indicated bulk degradation after 28 days of culture.
Figure 10.
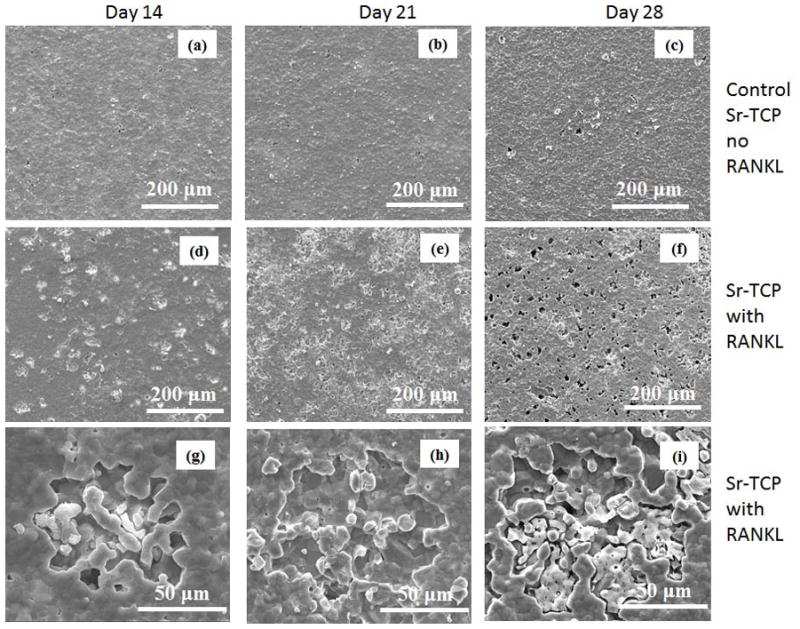
Surface morphology of Sr-TCP samples after culturing with RAW 264.7 cells at different days.
Figure 11.
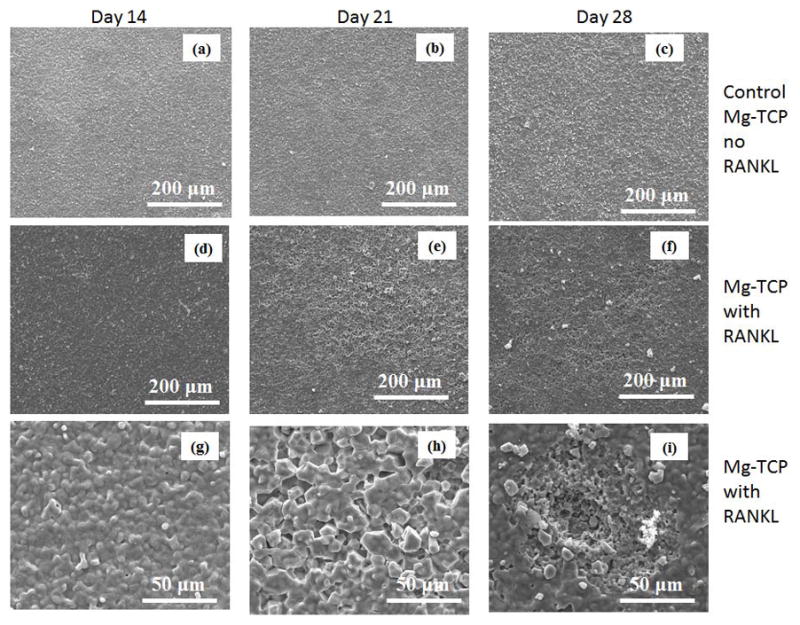
Surface morphology of Mg-TCP samples after culturing with RAW 264.7 cells at different days.
3.5. Metal ion concentration in the media
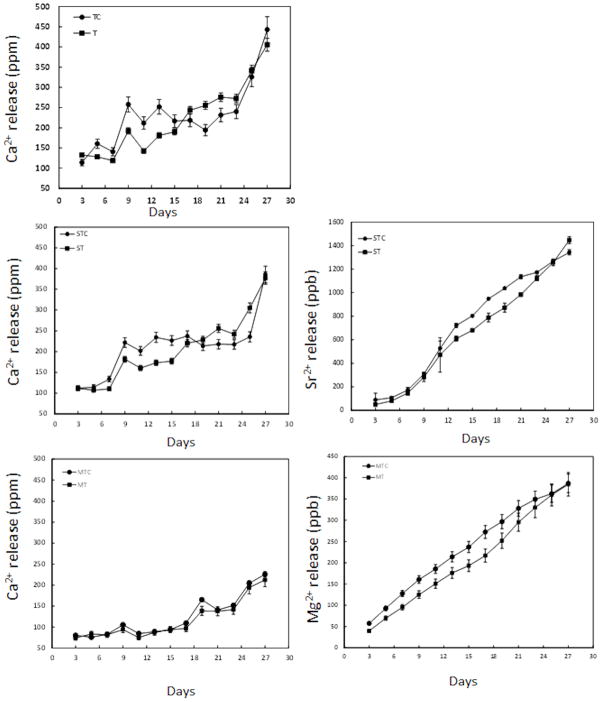
The release of metal ions in the culture medium was measured to corroborate the resorptive activity of osteoclast-like cells. Figure 12 shows the Ca2+, Sr2+, and Mg2+ concentrations in the culture media as a function of culture days and composition. The ionic concentrations for the control samples were also measured to distinguish the osteoclast-like cell mediated and cell independent (chemical dissolution) degradation of doped and undoped β-TCP samples. Higher released Ca2+ in the culture media was noticed for TCP(c) compared to TCP up to 18 days. However, after 18 days, the Ca2+ concentration in the media increased for TCP samples indicating that the osteoclast-like cells on TCP sample surfaces were active and resorbing cells. Similar behavior in ionic dissolution was noticed for Sr doped β-TCP samples. During initial incubation (up to 18 days), Ca2+ content in the media was higher for Sr-TCP(c) samples whereas increased Ca2+ concentration was noticed for Sr-TCP at longer culture. However, increased release of Sr2+ in the media for the Sr-TCP samples was noticed only after 24 days. Throughout the experiment, lowest release of Ca2+ in the culture media was recorded for Mg-TCP samples. The cumulative release of Mg2+ in the culture media was less than 0.5 ppm which indicated that Mg-TCP samples were chemically stable in cell culture condition. A higher initial release of Mg2+ was noticed for Mg-TCP(c) samples which become less significant after 21 days. Release of Ca2+ from the Mg-TCP samples was found to be similar for both Mg-TCP and Mg-TCP(c) samples.
Figure 12.
Ionic concentration in cell culture media at different time points. (C) represents control for the corresponding compositions (n=6).
4. Discussion
The present study reveals a systematic understanding of the differentiation of mononuclear RAW 264.7 cells into osteoclast-like cells and the influence of Sr and Mg ions. This study also unveils the osteoclast-like cell mediated degradation of β-TCP as a function of culture days and chemistry. In the present system, stabilization of β-phase is due to the presence of Sr and Mg. It has been shown that phase transition temperature of β to α in TCP can be increased by Sr and Mg doping [10, 22]. With minimum phase transition of β → α for Mg-TCP samples, the density increases significantly. Presence of glassy phase in the Mg-TCP samples indicates the samples went through liquid phase sintering which contributes to its increased density [16]. It may also be possible that MgO stayed at the grain boundaries and restricts the grain boundary movement that results in reduced grain size. Although Sr has a phase stabilization effect on β-TCP, it does not increase the density. It has been shown that Sr can easily substitute Ca ions in β-TCP structures [23]. Substitution of Ca2+ (0.99 Å) with larger sized Sr2+ (1.13 Å) leads to linear enlargement of unit cell volume that results in larger grain size and reduced density [10, 24]. In comparison, smaller sized magnesium (0.69 Å) preferentially substitute M(5) and at higher concentration substitute M(4) crystallographic location of Ca [25].
Osteoclast develops by the differentiation of hemopoietic monocytes at or near the bone surface with the use of many stimulatory factors, such as RANKL [6]. Therefore, formation of osteoclasts from the monocytes is an important step in overall bone remodeling. Attachment of monocytes to the substrate surface is the initial stage of osteoclast cell activity which is related to the external structures of the plasma membrane, such as filopodia. At initial culture days, presence of extensive cellular micro-extensions indicates good cellular adhesion on all of the sample surfaces. The morphological similarities of the monocytes and the absence of any preferential attachment on any of the sample surfaces, indicate that the doping have insignificant effect on monocyte cell attachment. The findings are also supported by contact angle measurement. Generally, cellular attachment enhances with decrease in contact angle [19]. Comaparble contact angles for TCP, Sr-TCP, and Mg-TCP samples elucidate similar monocyte attachment on all the substrates. Formation of flat, round and giant cells on TCP and Sr-TCP surface, after 5 and 8 days of culture, indicates the onset and maturation of osteoclast differentiation. Presence of multiple nucleuses in the cells along with actin ring formation indicates the cells to be osteoclast-like cells. Degradation of Sr-TCP samples does not affect the formation of osteoclast-like cells on Sr-TCP samples. It has been shown that a soluble salt concentration of 10 ppm Sr2+ does not alter osteoclast size or numbers [26]. In our study, the release of Sr2+ ion was less that 200 ppb after 8 days of culture. Therefore, dissolution of Sr-TCP samples does not influence osteoclastogenesis on Sr-TCP samples. Our findings on restricted osteoclastogenesis on Mg-TCP samples are in line with the recent findings by Janning et al. [27]. It has been shown that magnesium hydroxide temporarily (up to 4 weeks) reduce the osteoclast formation in the surrounding area of the implant. The presence of Mg2+ can locally restrict osteoclast proliferation and chemotaxis, which increases bone growth [27]. Our results on ionic analysis of culture media indicates that cumulatively, a total of 100 ppb Mg2+ ion releases in the culture media after 8 days of culture that can temporary reduce osteoclast differentiation. Interestingly, presence of Mg2+ ion does not inhibit osteoclast differentiation in long culture days. Similar results was also reported by by Jamming et al. showing osteoclast formation around magnesium hydroxyapatite implants only after 4 weeks of in vivo study [27]. It is also noteworthy that although Mg-TCP samples contain almost 4 times Mg ion (2.1 mol%) compare to that of Sr ion in Sr-TCP samples (0.57 mol%), dissolution of these samples results in less Mg2+ in the solution than Sr2+ as shown from AAS analysis. The results indicate that the Mg substitution reduces the dissolution of Mg-TCP samples in vitro. Restricted osteoclastogenesis can also be due to reduced Mg2+ in the culture media rather than relatively higher amount of Mg in Mg-TCP (compared to Sr in Sr-TCP samples) in the β-TCP samples. Therefore, our results indicate that the osteoclastogenesis is slowed down in Mg-TCP samples, however, is not completely restricted.
Once formed, osteoclasts start resorbing the substrate. Formation of round impressions on TCP and Sr-TCP substrates after 14 days of culture are due to the osteoclast-like cells that differentiated at 8th day of culture. The resorption lacunae are also similar in size to the matured osteoclast-like cells found at day 8 indicating that the osteoclast-like cells are active and resorbing. No osteoclastic resorption lacuna is noticed on Mg-TCP sample surface due to restricted osteoclastogenesis on these samples.
Pronounced resorption lacunae on TCP and Sr-TCP samples, after 21 days of culture, are due to the increased osteoclast-like cellular activity. Degradation of TCP ceramic is dependent on properties such as pH, grain size, crystallinity, presence of secondary phases etc. Higher initial dissolution of TCP(c) samples is due to the presence of higher amount of α-TCP phase. As Sr doping results in reduced α phase in the Sr-TCP samples, the dissolution of Sr-TCP(c) is lower than TCP(c) samples. Mg-TCP(c) samples owing to their smallest grain size and relatively stable β-TCP phase shows the lowest amount of dissolution [28].
Due to limited osteoclast-like cell differentiation, resorption lacunae are not found on Mg-TCP sample surfaces even after 21 days of culture. Formation of resorption lacuna on Mg-TCP samples after 28 days of culture are due to the osteoclast-like cells that are found at 21st day of culture. The increased depth of the resorption lacunae on TCP and Sr-TCP samples after 28 days confirms the continuous resorption activity of the osteoclast-like cells.
The osteoclast-like cellular degradation of doped and undoped TCP ceramics is expected to increase the Ca2+ concentration in the culture medium. In the present study, the osteoclast-like cell mediated surface degradation increases Ca2+ concentration in the culture medium, above that seen in control cultures, after 18 days for TCP and Sr-TCP samples. For Mg-TCP samples, both control and experimental groups have similar Ca2+ concentration in the media which is due to the restricted osteoclastogenesis on these samples. The continued osteoclast activity on TCP and Sr-TCP samples does not increase the Ca2+ concentration at longer culture days (27 days). One possible reason can be re-precipitation of calcium containing minerals on the sample surface [29]. The precipitation may happen due to increased Ca2+ concentration in the media. In physiological conditions, the bone resorption products, excreted from the basal plane of the osteoclasts, are carried away by the blood [30]. As the in vitro studies are done under static condition, the excreted calcium remains in the media and get deposited on the substrate surface.
This study shows that while strontium does not inhibit osteoclast-like cell formation, maturation or activation in vitro, osteoclastogenesis can be significantly reduced on TCP ceramics by Mg doping, even in the presence of sufficient amount of RANKL. The exact mechanism is still unknown and further studies are required to understand the signaling pathways that may be responsible for Mg related reduced osteoclastogenesis. A detailed in vivo analysis will verify and shed some light on the restricted osteoclastogenesis in Mg doped β-TCP ceramics. The results will be useful in designing TCP ceramics based resorbable bone substitutes with controlled degradation properties.
4. Conclusions
This study showed the effect of β-TCP surface chemistry on osteoclast-like cell formation and its activity. RAW 264.7 cells spontaneously adhered to β-TCP substrates and differentiated to osteoclast-like cells in the presence of RANKL. FESEM images revealed the osteoclast phenotype, such as formation of giant and flat cells with sealing zone on TCP and Sr-TCP samples. Immunohistochemical analysis confirmed the formation of osteoclast-like cells by expressing specific markers. Mg doping in β-TCP was found to be an effective way of slowing down osteoclastogenesis, however, not completely inhibited. Formation of resorption lacunae confirmed the osteoclast-like cellular degradation of the pure β-TCP and Sr-TCP ceramics, however slower for Mg doped samples. The present results can be used to modify osteoclast function in bone remodeling and thus adjust resorption kinetics of β-TCP towards bone graft application, based on specific application need.
Acknowledgments
Authors acknowledge financial support from the National Institute of Health (N1H-RO1-EB-007351), Office of Naval Research and the W. M. Keck Foundation to establish a Biomedical Materials Research Laboratory at WSU. Authors thank Dr. Ted S Gross and L. Worton from University of Washington for technical help and Franceschi Microscopy and Imaging Center, Washington State University for helpful assistance in the immunohistochemical and FESEM analyses.
References
- 1.Hong Y, Fan H, Li B, Guo B, Liu M, Zhang X. Fabrication, biological effects, and medical applications of calcium phosphate nanoceramics. Mater Sci Eng R. 2010;70:225–242. [Google Scholar]
- 2.Dorozhkin SV, Epple M. Biological and medical significance of calcium phosphates. Angew Chem Int Edit. 2002;41:3130–3146. doi: 10.1002/1521-3773(20020902)41:17<3130::AID-ANIE3130>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 3.Okuda T, Ioku K, Yonezawa I, Minagi H, Kawachi G, Gonda Y, Murayama H, Shibata Y, Minami S, Kamihira S, Kurosawa H, Ikeda T. The effect of the microstructure of β-tricalcium phosphate on the metabolism of subsequently formed bone tissue. Biomaterials. 2007;28:2612– 2621. doi: 10.1016/j.biomaterials.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 4.Seeley Z, Bandyopadhyay A, Bose S. Tricalcium Phosphate Based Resorbable Ceramics: Influence of NaF and CaO Addition. Mater Sci Eng C. 2008;28:11–17. [Google Scholar]
- 5.Schilling AF, Linhart W, Filke S, Gebauer M, Schinke T, Rueger JM, Amling M. Resorbability of bone substitute biomaterials by human osteoclasts. Biomaterials. 2004;25:3963–3972. doi: 10.1016/j.biomaterials.2003.10.079. [DOI] [PubMed] [Google Scholar]
- 6.Teitelbaum SL. Bone Resorption by Osteoclasts. Science. 2000;289:1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 7.Boyle WJ, Simonet WS, Lacey D. Osteoclast Differentiation and Activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 8.Yang L, Perez-Amodio S, Groot F, Everts V, Blitterswijk CA, Habibovic P. The effects of inorganic additives to calcium phosphate on in vitro behavior of osteoblasts and osteoclasts. Biomaterials. 2010;31:2976–2989. doi: 10.1016/j.biomaterials.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Bandyopadhyay A, Bernard S, Xue W, Bose S. Calcium Phosphate-Based Resorbable Ceramics: Influence of MgO, ZnO, and SiO2 dopants. J Am Ceram Soc. 2006;89:2675–2688. [Google Scholar]
- 10.Banerjee SS, Tarafder MS, Davies NM, Bandyopadhyay A, Bose S. Understanding the Influence of MgO and SrO Binary Doping on Mechanical and Biological Properties of β-TCP Ceramics. Acta Biomater. 2010;6:4167–4174. doi: 10.1016/j.actbio.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 11.Hoppe A, Güldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32:2757–2774. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 12.Botelho CM, Brooks RA, Spence G, McFarlane I, Lopes MA, Best SM, Santos JD, Rushton N, Bonfield W. Differentiation of mononuclear precursors into osteoclasts on the surface of Si-substituted hydroxyapatite. J Biomed Mater Res. 2006;78A:709–720. doi: 10.1002/jbm.a.30726. [DOI] [PubMed] [Google Scholar]
- 13.Yamada Y, Ito A, Kojima H, Sakane M, Miyakawa S, Uemura T, LeGeros RZ. Inhibitory effect of Zn2+ in zinc-containing β-tricalcium phosphate on resorbing activity of mature osteoclasts. J Biomed Mater Res. 2008;84A:344–352. doi: 10.1002/jbm.a.31265. [DOI] [PubMed] [Google Scholar]
- 14.Patntirapong S, Habibovic P, Hauschka PV. Effects of soluble cobalt and cobalt incorporated into calcium phosphate layers on osteoclast differentiation and activation. Biomaterials. 2009;30:548–555. doi: 10.1016/j.biomaterials.2008.09.062. [DOI] [PubMed] [Google Scholar]
- 15.Bose S, Tarafder S, Banerjee SS, Davies NM, Bandyopadhyay A. Understanding in vivo response and mechanical property variation in MgO, SrO and SiO2 doped β-TCP. Bone. 2011;48:1282–1290. doi: 10.1016/j.bone.2011.03.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xue W, Dahlquist K, Banerjee A, Bandyopadhyay A, Bose S. Synthesis and characterization of tricalcium phosphate with Zn and Mg based dopants. J Mater Sci Mater Med. 2008;19:2669–2677. doi: 10.1007/s10856-008-3395-4. [DOI] [PubMed] [Google Scholar]
- 17.Rude RK, Singer FR, Gruber HE. Skeletal and Hormonal Effects of Magnesium Deficiency. J Am Coll Nutr. 2009;28:131–141. doi: 10.1080/07315724.2009.10719764. [DOI] [PubMed] [Google Scholar]
- 18.Capuccini C, Torricelli P, Sima F, Boanini E, Ristoscu C, Bracci B, Socol G, Fini M, Mihailescu IN, Bigi A. Strontium-substituted hydroxyapatite coatings synthesized by pulsed-laser deposition: In vitro osteoblast and osteoclast response. Acta Biomater. 2008;4:1885–1893. doi: 10.1016/j.actbio.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Roy M, Krishna BV, Bose S, Bandyopadhyay A. Comparison of Tantalum and Hydroxyapatite Coatings on Titanium for Applications in Load Bearing Implants. Adv Biomater (Adv Eng Mater) 2010;12(11):B637–B641. [Google Scholar]
- 20.Detsch R, Mayr H, Ziehler G. Formation of osteoclast-Like Cells on HA and TCP Ceramics. Acta Biomater. 2008;4:139–148. doi: 10.1016/j.actbio.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Detsch R, Hagmeyer D, Neumann M, Schaefer S, Vortkamp A, Wuelling M, Ziegler G, Epple M. The resorption of nanocrystalline calcium phosphates by osteoclast-like cells. Acta Biomater. 2010;6:3223–3233. doi: 10.1016/j.actbio.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Enderle R, Götz-Neunhoeffer F, Göbbels M, Müller FA, Greil P. Influence of magnesium doping on the phase transformation temperature of β-TCP ceramics examined by Rietveld refinement. Biomaterials. 2005;26:3379–3384. doi: 10.1016/j.biomaterials.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 23.Kannan S, Goetz-Neunhoeffer F, Neubauer J, Pina S, Torres PMC, Ferreira JMF. Synthesis and structural characterization of strontium- and magnesium-cosubstituted β-tricalcium phosphate. Acta Biomater. 2010;6:571–576. doi: 10.1016/j.actbio.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Bigi A, Foresti E, Gandolfi M, Gazzano M, Roveri N. Isomorphous Substitutions in β-Tricalcium Phosphate: The Different Effects of Zinc and Strontium. J Inorg Biochem. 1997;66:259–265. [Google Scholar]
- 25.Enderle R, Götz-Neunhoeffer F, Göbbels M, Müller FA, Greil P. Influence of magnesium doping on the phase transformation temperature of b-TCP ceramics examined by Rietveld refinement. Biomaterials. 2005;26:3379–3384. doi: 10.1016/j.biomaterials.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 26.Rousselle AV, Heymann D, Demais V, Charrier C, Passuti N, Baslé MF. Influence of metal ion solutions on rabbit osteoclast activities in vitro. Histol Histopathol. 2002;17:1025–1032. doi: 10.14670/HH-17.1025. [DOI] [PubMed] [Google Scholar]
- 27.Janning C, Willbold E, Vogt C, Nellesen J, Meyer-Lindenberg A, Windhagen H, Thorey F, Witte F. Magnesium hydroxide temporarily enhancing osteoblast activity and decreasing the osteoclast number in peri-implant bone remodeling. Acta Biomater. 2010;6:1861–1868. doi: 10.1016/j.actbio.2009.12.037. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Ito A, Sogo Y, Wang X, LeGeros RZ. Solubility of Mg-containing β-tricalcium phosphate at 25 °C. Acta Biomater. 2009;5:508–517. doi: 10.1016/j.actbio.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia ZL, Groverb M, Huangc Y, Adamopoulosa IE, Gbureckd U, Triffitta JT, Sheltone RM, Barralet JE. In vitro biodegradation of three brushite calcium phosphate cements by a macrophage cell-line. Biomaterials. 2006;27:4557–4565. doi: 10.1016/j.biomaterials.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 30.Salo J, Lehenkari P, Mulari M, MetsikkÖ K, Väänänen HK. Removal of Osteoclast Bone Resorption Products by Transcytosis. Science. 1997;276:270–273. doi: 10.1126/science.276.5310.270. [DOI] [PubMed] [Google Scholar]