Abstract
Purpose
The Radiation Therapy Oncology Group (RTOG) multi-institutional Phase II study 98-12, evaluating paclitaxel and concurrent radiation (RT) for locally advanced pancreatic cancer, demonstrated a median survival of 11.3 months and a 1-year survival of 43%. The purpose of the randomized Phase II study by RTOG 0020 was to evaluate the addition of weekly low- dose gemcitabine with concurrent paclitaxel/RT and to evaluate the efficacy and safety of the farnesyl transferase inhibitor R115777 following chemoradiation.
Patients and methods
Patients with unresectable, nonmetastatic adenocarcinoma of the pancreas were eligible. Patients in Arm 1 received gemcitabine, 75 mg/m2/week, and paclitaxel, 40 mg/m2/week, for 6 weeks, with 50.4 Gy radiation (CXRT). Patients in Arm 2 received an identical chemoradiation regimen but then received maintenance R115777, 300 mg twice a day for 21 days every 28 days (CXRT+R115777), until disease progression or unacceptable toxicity.
Results
One hundred ninety-five patients were entered into this study, and 184 were analyzable. Grade 4 nonhematologic toxicities occurred in less than 5% of CXRT patients. The most common grade 3/4 toxicity from R115777 was myelosuppression; however, grade 3/4 hepatic, metabolic, musculoskeletal, and neurologic toxicities were also reported. The median survival time was 11.5 months and 8.9 months for the CXRT and CXRT+R115777 arms, respectively.
Conclusions
The CXRT arm achieved a median survival of almost 1-year, supporting chemoradiation as an important therapeutic modality for locally advanced pancreatic cancer. Maintenance R115777 is not effective and is associated with a broad range of toxicities. These findings provide clinical evidence that inhibition of farnesylation affects many metabolic pathways, underscoring the challenge of developing an effective K-ras inhibitor.
Keywords: pancreas cancer, paclitaxel, gemcitabine, irradiation
Introduction
The optimal treatment for locally advanced pancreatic cancer is controversial. Chemoradiation is a standard treatment.1 However, since systemic progression is common, it has been suggested that the focus of treatment should be with full-dose systemic chemotherapy.2 This controversy may intensify with the development of a more effective combination chemotherapy such as FOLFIRINOX, which is comprised of oxaliplatin, irinotecan, fluorouracil, and leucovorin.3
A continuous 5-fluorouracil (5-FU) infusion or capecitabine represent the most commonly utilized chemoradiation regimens.4–6 The Brown University Oncology Group developed paclitaxel as a radiation sensitizer for pancreatic cancer in Phase I/II studies.7–9 The Radiation Therapy Oncology Group (RTOG) conducted a multi-institutional Phase II study (RTOG 98-12) with paclitaxel and concurrent radiation for 110 patients that resulted in a median survival of 11.3 months and a 1-year survival of 43%.10 Gemcitabine is now widely accepted as one of the most active single agents for pancreatic cancer, and it is a powerful radiation sensitizer.11–13 Based on a Brown University Oncology Group Phase I study,14 the RTOG sought to investigate the regimen of low-dose weekly gemcitabine, paclitaxel, and radiation.
The RTOG hypothesized that if chemoradiation were effective in controlling locoregional disease, then a biologic agent that could interfere with the growth and development of distant metastases would be beneficial in the maintenance setting after chemoradiation.
K-ras mutations are demonstrated in approximately 70%–80% of pancreatic cancers.15 Farnesylation is a critical step in the membrane anchorage of ras proteins, required for ras activity. R115777 competitively inhibits the enzyme farnesyl protein transferase, which adds a 15-carbon farnesyl isoprenoid moiety to the cysteine residue of ras proteins. At the time this study was initiated, the inhibition of ras by blocking farnesyl transferase was a promising strategy in pancreatic cancer.16
The RTOG therefore initiated a randomized Phase II study to evaluate if the addition of gemcitabine radiosensitization improved survival, compared to RTOG 98-12, and to study whether the addition of maintenance R115777 could delay the development of distant metastases. This is the final report of the multi-institutional RTOG 0020 protocol.
Materials and methods
Eligibility
All patients had pathologically confirmed, unresectable, nonmetastatic adenocarcinoma of the pancreas deemed unresectable by extrapancreatic involvement, extensive peripancreatic lymphatic involvement, nodal involvement beyond the peripancreatic tissue, or encasement or direct invasion of the superior mesenteric vein, artery, inferior vena cava, aorta, or celiac plexus. Ineligible were those with metastatic disease to distant organs, ascites, or peritoneal implants and those who had received prior irradiation to the planned field or prior chemotherapy including gemcitabine or paclitaxel. Patients with biliary or gastroduodenal obstruction had drainage prior to chemoradiation. All malignant disease had to be encompassable within an irradiation field no greater than 15 cm × 15 cm. Patients were not permitted to have a malignancy within the past 2 years, except for nonmelanoma skin cancer or carcinoma in situ of the cervix, uterus, or bladder. Patients were to have radiographically assessable disease, a Zubrod performance status of 0 or 1, and have no significant infection or other coexistent uncontrolled medical condition.
Evaluation prior to treatment
A complete history and physical examination were performed on all patients before treatment. Height, weight, performance status, and tumor stage were recorded. Required staging studies included a chest radiograph and an abdominal computed tomographic scan. Patients were required to have the following laboratory values: granulocytes at ≥1800/μL, platelets at ≥100,000 μL, bilirubin at <2.0 mg/dL, alanine aminotransferase at <3 times upper limit of normal, and creatine at <3.0 mg/dL. The study was approved by the institutional review boards of all participating hospitals and complied with the tenets of the Declaration of Helsinki. All patients gave written informed consent according to federal and institutional guidelines.
Treatment
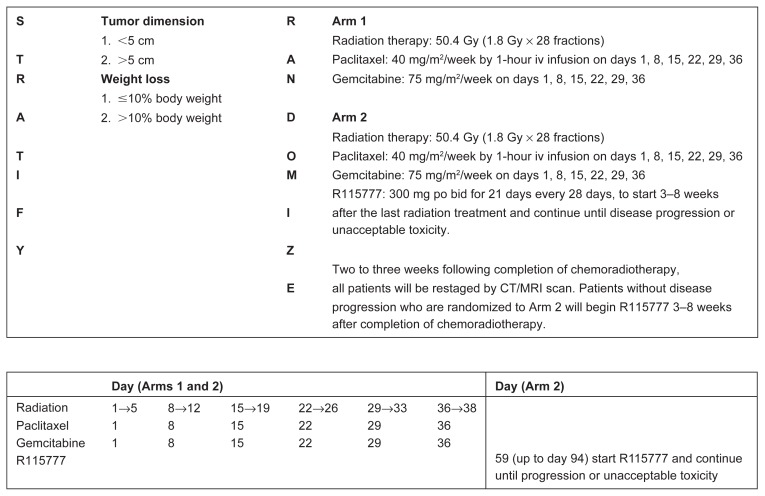
The structure of the protocol is illustrated in the treatment schema in Figure 1. Radiation therapy was delivered to the primary tumor and draining lymph nodes over 5.5 weeks with coplanar anterior-posterior and lateral ports using a ≥10 MV linear accelerator. The initial fields included the primary tumor plus the regional peripancreatic, celiac, and porta hepatis lymph nodes. A conedown field was used for the last three fractions to encompass the gross tumor volume with a 1–1.5 cm margin. Computed tomographic scans in the treatment position were used to identify appropriate anatomy. When available, three-dimensional treatment planning was performed. The spinal cord dose was maintained below 45 Gy. No more than 30% of the total kidney volume received 50% of the prescribed dose. Concurrent systemic chemotherapy included paclitaxel, 40 mg/m2, and gemcitabine, 75 mg/m2, weekly for 6 weeks, then R115777, 300 mg, twice a day for 21 days of a 28-day cycle, 3 to 8 weeks after completion of concurrent therapy.
Figure 1.
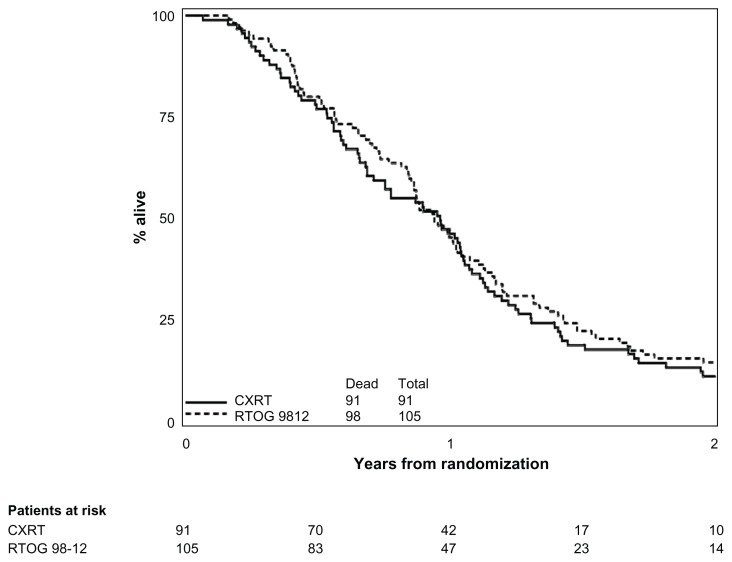
Overall survival.
Abbreviations: CXRT, concurrent radiation with gemcitabine and paclitaxel; RTOG 98-12, Radiation Therapy Oncology Group study 98-
Quality assurance
Radiotherapy quality assurance for this study included the central review of all diagnostic imaging and simulation films regarding field size, placement, tumor and lymph node margin coverage, and isodose distributions. Compliance with chemotherapy parameters was also evaluated.
Statistics
The primary endpoint of this protocol was to determine the 1-year survival rates of patients treated with paclitaxel, gemcitabine, and radiation with or without R115777. Secondary endpoints were to determine the toxicity and locoregional activity of paclitaxel, gemcitabine, and radiation, to determine the feasibility and toxicity of prolonged administration of R115777 after paclitaxel, gemcitabine, and radiation, and to evaluate whether R115777 could increase progression-free and overall survival after chemoradiation for locally advanced pancreatic cancer.
In the previous RTOG 98-12 protocol for unresectable pancreatic cancer, a 1-year survival rate of approximately 50% was observed. Using the method of Dixon and Simon,17 a sample size of 69 analyzable patients per arm followed over 12 months would ensure at least 80% probability of detecting a minimum of 15% improvement in the 1-year survival rate, compared to RTOG 98-12, at the 0.05 significance level (with a one-sided test). Adjusting this figure by 10% to allow for patient ineligibility or loss, a total sample size of at least 154 patients was required for this study. A secondary endpoint of this study was to estimate the difference in 1-year survival for the two treatment arms. Assuming a binomial distribution, the difference could be estimated with a 95% confidence interval (CI) with a margin of error of ≤17.2%.
Chi-squared tests were used to compare pretreatment characteristics between the treatment arms and historical control (RTOG 98-12). Overall and progression-free survival were estimated univariately with the Kaplan–Meier method,18 and treatment arms were compared using the log-rank test. Reported median survival and progression-free survival times were the times (in years) at which 50% of the patients had failed the respective endpoint. Multivariate analyses were performed with Cox proportional hazard models19 to test for treatment differences while adjusting for unbalanced pretreatment characteristics or time-dependent covariates. Multivariate analyses were done such that a hazard ratio greater than 1 implied an increased risk for the second level of the variable and a hazard ratio less than 1 implied an increased risk for the first level of the variable. All analyses were performed using SAS software (version 9.2; SAS Institute, Cary, NC).
Results
Patients
One hundred ninety-five patients from 71 institutions were enrolled in this study between October 2001 and December 2003. There were 185 analyzable patients for this report: 91 on the CXRT arm and 94 on the CXRT+R115777 arm. Six patients were ineligible; eligibility could not be confirmed for one patient, and three received no protocol therapy. Pretreatment characteristics for the two arms of RTOG 0020 are shown in Table 1 and compared to the historic control from RTOG 98-12.
Table 1.
Pretreatment characteristics of CXRT and CXRT+R115777 vs RTOG 98-12
| Characteristics | RTOG 98-12 (n = 105) |
CXRT (n = 91) |
P-valuea | CXRT+R115777 (n = 94) |
P-valuea | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Number | % | Number | % | Number | % | |||
| Age, years | ||||||||
| Median | 63 | 62 | – | 60 | – | |||
| Min–max | 29–84 | 40–82 | 43–82 | |||||
| Sex | ||||||||
| Male | 50 | 48 | 54 | 59 | 0.10 | 52 | 55 | 0.28 |
| Female | 55 | 52 | 37 | 41 | 42 | 45 | ||
| Zubrod | ||||||||
| 0 | 62 | 59 | 29 | 32 | 0.0001 | 39 | 41 | 0.013 |
| 1 | 43 | 41 | 62 | 68 | 55 | 59 | ||
| Weight loss (last 6 months) | ||||||||
| None | n/a | n/a | 4 | 4 | – | 5 | 5 | – |
| ≤10% | n/a | n/a | 30 | 33 | 33 | 35 | ||
| >10% | n/a | n/a | 57 | 63 | 56 | 60 | ||
| T-stage | ||||||||
| T1 | 3 | 3 | 0 | 0 | –b | 2 | 2 | –b |
| T2 | 16 | 15 | 10 | 11 | 14 | 15 | ||
| T3 | 48 | 46 | 17 | 19 | 12 | 13 | ||
| T4 | 36 | 34 | 64 | 70 | 66 | 70 | ||
| Tx | 2 | 2 | 0 | 0 | 0 | 0 | ||
| T-stage, dichotomized | ||||||||
| T1, T2, TX | 21 | 20 | 10 | 11 | 0.08 | 16 | 17 | 0.59 |
| T3, T4 | 84 | 80 | 81 | 89 | 78 | 83 | ||
| N-stage | ||||||||
| N0 | 70 | 67 | 46 | 51 | 0.04 | 59 | 63 | 0.61 |
| N1 | 24 | 23 | 36 | 40 | 27 | 29 | ||
| Nx | 11 | 10 | 9 | 10 | 8 | 9 | ||
| N-stage, dichotomized | ||||||||
| N0, NX | 81 | 77 | 55 | 60 | 0.01 | 67 | 71 | 0.34 |
| N1 | 24 | 23 | 36 | 40 | 27 | 29 | ||
| Maximum tumor size, cm | ||||||||
| Not measurable | n/a | n/a | 2 | 2 | – | 5 | 5 | – |
| <5 cm | n/a | n/a | 62 | 68 | 54 | 57 | ||
| ≥5 cm | n/a | n/a | 27 | 30 | 35 | 37 | ||
Notes:
From Chi-square test compared to RTOG 98-12;
Chi-square test not valid.
Abbreviations: CXRT, concurrent radiation with gemcitabine and paclitaxel; RTOG 98-12, Radiation Therapy Oncology Group study 98-12; R115777, farnesyl transferase inhibitor; n/a, not applicable.
The pretreatment characteristics were well balanced between the CXRT and CXRT+R115777 arms. Compared to RTOG 98-12, the RTOG 0020 CXRT arm had significantly more node-positive patients and a trend toward more higher-tumor stage patients than had RTOG 98-12 (P = 0.04 and P = 0.08, respectively), while there was a trend toward more females (P = 0.10) in RTOG 98-12. These differences were not seen in the CXRT+R115777 arm and RTOG 98-12 comparison. RTOG 98-12 had a statistically better Zubrod performance status than either Arm 1 or Arm 2 of RTOG 0020 (P = 0.0001 vs CXRT and P = 0.013 vs CXRT+R115777, respectively).
Quality assurance review of the radio- and chemotherapy treatment showed that 93% and 95%, and 87% and 87%, on the CXRT and CXRT+R115777 arms, respectively, were scored as per protocol or with acceptable variation.
Survival
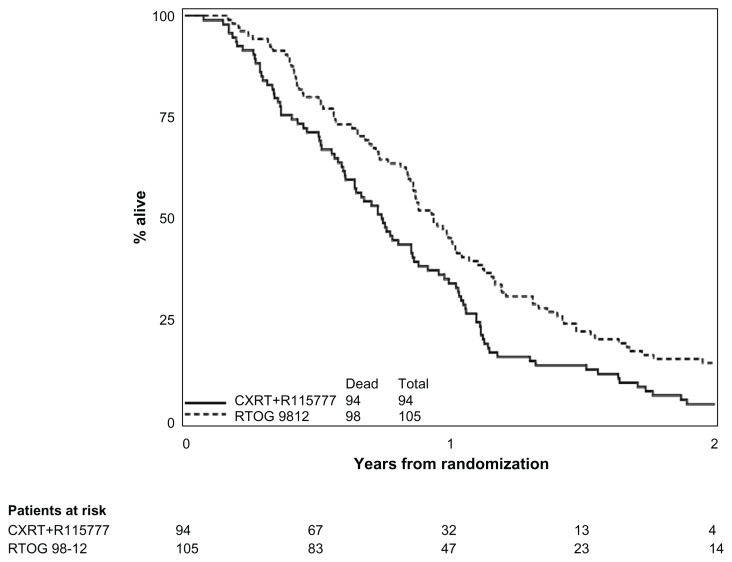
All RTOG 0020 patients included in this analysis have died. For RTOG 0020, the median survival time was 11.5 months (95% CI: 8.2–12.6) for the CXRT arm and 8.9 months (95% CI: 7.3–10.4) for the CXRT+R115777 arm. The cause of death was pancreas cancer-related in 90% and 93% of the patients on the CXRT and CXRT+R115777 arms, respectively. The respective estimated 1-year survival results for the CXRT and the CXRT+R115777 arms were 46.2% (95% CI: 35.7%–56.0%, respectively for Arms 1 and 2) and 34.0% (95% CI: 24.7%–43.6%, respectively), as compared to 45.3% (95% CI: 35.6%–54.5%) for RTOG 98-12.
Tables 2 and 3 show univariate and multivariate overall survival for RTOG 0020 and RTOG 98-12. Comparing survival between each arm of RTOG 0020 to patients treated on RTOG 98-12, neither regimen of RTOG 0020 was statistically superior (one-sided log-rank test: RTOG 0020 CXRT vs 98-12, P = 0.15; RTOG 0020 CXRT+R115777 vs 98-12, P > 0.99) to the historical control (Figures 2 and 3). Given the imbalance of sex, Zubrod, N-stage, and T-stage between RTOG 98-12 and the CXRT arm on RTOG 0020, and the imbalance of Zubrod between RTOG 98-12 and the CXRT+R115777 arm on RTOG 0020, multivariate analyses using a backward selection procedure were performed to evaluate treatment differences after adjusting for these unbalanced variables. After adjusting for the above-mentioned variables, overall survival was still not significantly improved for the CXRT arm, as compared to 98-12. However, the hazard ratio indicated worse survival for the CXRT+R115777 arm.
Table 2.
Overall survival comparisons
| Year | CXRT | CXRT+R115777 | RTOG 98-12 | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| % alive (95% CI) |
Cumulative deaths | Number at risk | % alive (95% CI) |
Cumulative deaths | Number at risk | % alive (95% CI) |
Cumulative deaths | Number at risk | |
| 0 | 100.0 | 0 | 91 | 100.0 | 0 | 94 | 100.0 | 0 | 105 |
| 0.5 | 76.9 | 21 | 70 | 71.3 | 27 | 67 | 80.0 | 21 | 83 |
| (66.8, 84.3) | (61.0, 79.3) | (71.0, 86.5) | |||||||
| 1.0 | 46.2 | 49 | 42 | 34.0 | 62 | 32 | 45.3 | 57 | 47 |
| (35.7, 56.0) | (24.7, 43.6) | (35.6, 54.5) | |||||||
| 1.5 | 18.7 | 74 | 17 | 13.8 | 81 | 13 | 22.2 | 81 | 23 |
| (11.5, 27.3) | (7.8, 21.6) | (14.8, 30.5) | |||||||
| 2.0 | 11.0 | 81 | 10 | 4.3 | 80 | 4 | 14.5 | 89 | 14 |
| (5.6, 18.4) | (1.4, 9.7) | (8.5, 21.9) | |||||||
| Total deaths | 91 | 94 | 98 | ||||||
| MST | 11.5 mos | 8.9 mos | 11.3 mos | ||||||
| 95% CI | (8.2, 12.6) | (7.3, 10.4) | (10.2, 12.6) | ||||||
Notes: Treatment comparisons vs historical control (one-sided log-rank test; RTOG 0020 testing better than RTOG 98-12 testing): CXRT vs RTOG 98-12, P = 0.1496; CXRT+R115777 vs RTOG 98-12, P > 0.99.
Abbreviations: CI, confidence interval; CXRT, concurrent radiation with gemcitabine and paclitaxel; R115777, farnesyl transferase inhibitor; MST, median survival time; mos, months; RTOG 0020 and 98-12, Radiation Therapy Oncology Group studies 0020 and 98-12.
Table 3.
Multivariate analyses of overall survival
| Covariate | Adjusted hazard ratioa | 95% CI | P-valueb |
|---|---|---|---|
| Model for RTOG 0020 CXRT and 98-12 | |||
| Treatment (0 = 98-12, 1 = 0020 CXRT) | 1.13 | (0.85, 1.51) | 0.15c |
| Treatment stage (0 = T1–2, Tx, 1 = T3–4) | 1.64 | (1.09, 2.46) | 0.02 |
| Model for RTOG 0020 CXRT+R115777 and 98-12 | |||
| Treatment (0 = 98-12, 1 = 0020 CXRT+R115777) | 1.54 | (1.16, 2.06) | >0.99c |
Notes:
Hazard ratio: an HR > 1 indicates an increased risk for the second level of the variable;
P-value from Cox proportional hazards model;
one-sided test per statistical design, testing RTOG 0020 better than RTOG 98-12.
Abbreviations: CI, confidence interval; CXRT, concurrent radiation with gemcitabine and paclitaxel; R115777, farnesyl transferase inhibitor; RTOG 0020 and 98-12, Radiation Therapy Oncology Group studies 0020 and 98-12.
Figure 2.
Overall survival comparison of RTOG 98-12 and experimental arm of RTOG 020 (online).
Abbreviations: CXRT, concurrent radiation with gemcitabine and paclitaxel; R115777, farnesyl transferase inhibitor; RTOG 98-12, Radiation Therapy Oncology Group study 98-12.
Figure 3.
Treatment schema (online).
Abbreviations: R115777, farnesyl transferase inhibitor; po bid, taken orally twice a day; CT, computed tomographic; MRI, magnetic resonance imaging.
Toxicity
Table 4 displays drug and acute radiotherapy toxicities in Arm 1 (CXRT) and Arm 2 (CXRT+R115777). Multiple toxicities are scored as separate events. In the group receiving chemoradiation alone, Grade 3/4 hematologic toxicity occurred in one-third of patients. However, grade 4 hematologic toxicity developed in only 4 of 91 patients (4%). Neutropenic fever developed in 5% of patients treated with CXRT. Grade 3 gastrointestinal toxicities, consisting mainly of nausea, vomiting, diarrhea, and dehydration, occurred in one-third of patients. However, only 2% had grade 4 gastrointestinal toxicities. There were no grade 5 treatment-related events.
Table 4.
Chemotherapy, inhibitor, and acute radiation toxicity
| Category | CXRT (n = 91) grade |
CXRT+R115777 (n = 94) grade |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| Allergy/immunology | 1 | 0 | 3 | 0 | 0 | 1 | 0 | 2 | 0 | 0 |
| Auditory/hearing | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Blood/bone marrow | 21 | 30 | 26 | 4 | 0 | 13 | 30 | 32 | 11 | 0 |
| Cardiovascular (arrhythmia) | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Cardiovascular (general) | 4 | 8 | 2 | 1 | 0 | 7 | 4 | 7 | 0 | 0 |
| Coagulation | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 |
| Constitutional symptoms | 25 | 29 | 8 | 0 | 0 | 23 | 33 | 13 | 1 | 0 |
| Dermatology/skin | 12 | 5 | 0 | 0 | 0 | 13 | 6 | 0 | 0 | 0 |
| Endocrine | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gastrointestinal | 18 | 30 | 30 | 2 | 0 | 9 | 37 | 33 | 4 | 0 |
| Hemorrhage | 2 | 0 | 0 | 0 | 0 | 4 | 0 | 1 | 1 | 0 |
| Hepatic | 14 | 12 | 3 | 0 | 0 | 17 | 22 | 10 | 2 | 0 |
| Infection febrile neutropenia | 0 | 3 | 5 | 0 | 0 | 0 | 7 | 8 | 0 | 0 |
| Metabolic/laboratory | 25 | 13 | 5 | 0 | 0 | 16 | 15 | 15 | 1 | 0 |
| Musculoskeletal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 |
| Neurology | 13 | 6 | 1 | 0 | 0 | 17 | 16 | 7 | 1 | 0 |
| Ocular/visual | 0 | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | 0 |
| Pain | 12 | 18 | 6 | 1 | 0 | 14 | 20 | 7 | 0 | 0 |
| Pulmonary | 4 | 5 | 1 | 0 | 0 | 1 | 9 | 1 | 1 | 0 |
| Renal/genitourinary | 6 | 0 | 0 | 0 | 0 | 11 | 2 | 0 | 0 | 0 |
| Sexual/reproductive function | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Worst nonhematologic | 16 (17.6%) | 24 (26.4%) | 45 (49.5%) | 4 (4.4%) | 0 (0%) | 4 (4.3%) | 24 (25.5%) | 52 (55.3%) | 7 (7.4%) | 0 (0%) |
| Worst GI and pulmonary | 18 (19.8%) | 30 (33.0%) | 31 (34.1%) | 2 (2.2%) | 0 (0%) | 8 (8.5%) | 36 (38.3%) | 33 (35.1%) | 5 (5.3%) | 0 (0%) |
| Worst overall | 5 (5.5%) | 25 (27.5%) | 54 (59.3%) | 7 (7.7%) | 0 (0%) | 3 (3.2%) | 16 (17.0%) | 54 (57.4%) | 17 (18.1%) | 0 (0%) |
Note: Toxicities were graded with CTC version 2.0.
Abbreviations: CTC, common toxicity criteria; CXRT, concurrent radiation with gemcitabine and paclitaxel; R115777, farnesyl transferase inhibitor; GI, gastrointestinal.
In contrast, Arm 2, which utilized maintenance R115777 after chemoradiation, was associated with grade 4 myelosuppression in 11% of patients. Grade 3/4 nonhematologic toxicities in the patients receiving maintenance R115777, as compared to no maintenance treatment, included fatigue (12 vs 6 patients), increase in alanine aminotransferase (15 patients vs 1 patient), hypocalcemia (3 vs 0 patients), hyperglycemia (7 vs 0 patients), hypermagnesemia (1 patient vs 0 patients), muscle weakness (1 patient vs 0 patients), myositis (1 patient vs 0 patients), ataxia (2 vs 0 patients), peripheral neuropathy (2 vs 0 patients), confusion (2 vs 0 patients), and myalgia (1 vs 0 patients). Table 5 shows late radiotherapy toxicities.
Table 5.
Late radiotherapy toxicity (online)
| CXRT (n = 80) grade |
CXRT+R115777 (n = 78) grade |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | |
| Small/large intestine | 2 | 3 | 0 | 0 | 0 | 4 | 0 | 1 | 0 | 0 |
| Skin | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Subcutaneous tissue | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Other | 2 | 0 | 4 | 0 | 0 | 0 | 1 | 2 | 0 | 0 |
| Worst late toxicity | 5 (6.3%) | 2 (2.5%) | 4 (5%) | 0 (0%) | 0 (0%) | 3 (3.8%) | 1 (1.3%) | 2 (2.6%) | 0 (0%) | 0 (0%) |
Note: Toxicities were graded with RTOG/EORTC late toxicity criteria.
Abbreviations: CXRT, concurrent radiation with gemcitabine and paclitaxel; R115777, farnesyl transferase inhibitor; EORTC, European Organization for Research and Treatment of Cancer; RTOG, Radiation Therapy Oncology Group.
Discussion
Improving the outcome of chemosensitized external beam irradiation for unresectable cancer of the pancreas has been a goal of national protocols for the past decade; RTOG study 98-1210,20 evaluated paclitaxel and concurrent radiation without postchemoradiation gemcitabine. This study added low-dose gemcitabine as an additional radiation sensitizer.
Paclitaxel is thought to be a synchronizer of cells at G2/M, a relatively radiosensitive phase of the cell cycle.8,20–23 In vitro studies suggest that paclitaxel enhances the cytotoxicity of radiation even in cancer cells resistant to paclitaxel as a single agent,4 whereas stem cells of the gastrointestinal mucosa are not substantially radiosensitized by paclitaxel.22
A critical event in gemcitabine-mediated radiosensitization is the inhibition of ribonucleotide reductase by one of its metabolites, difluorodeoxycytidine diphosphate, which in turn leads to depletion of deoxyadenosine triphosphate pools.24 The greatest radiosensitization effect is noticed in S-phase cells. Additional effects may be the result of the lowering of the threshold for radiation-induced apoptosis by gemcitabine.24 These differing and potentially complementary pathways of radiation sensitization by these two agents provide an attractive rationale for clinical testing.
Phase I/II studies of gemcitabine plus concurrent radiation showed the maximum tolerated dosage to depend on the gemcitabine dose, schedule, and volume of the radiation field.11 For example, Hoffman et al13 escalated gemcitabine from 300 mg/m2/week to 600 g/m2/week for 6 weeks, with concurrent radiation of 50.4 Gy in patients with potentially resectable pancreatic cancer, irradiating only the tumor, not nodal drainage areas. In contrast, Blackstock et al12 demonstrated that the maximum tolerated dose of twice- weekly gemcitabine was 40 mg/m2 with 50 Gy radiation and conventional radiation fields. The Cancer and Leukemia Group B25 performed a confirmatory Phase II trial of gemcitabine 40 mg/m2 twice weekly, with 50.4 Gy concurrent radiation in locally advanced pancreatic cancer, and found a median survival of 8.2 months. Small bowel and stomach toxicities reduced the maximum tolerated dose that could be achieved with gemcitabine and radiation, especially when relatively large radiation fields were used. Another approach has been reported by McGinn et al,26 who used full-dose gemcitabine (1000 mg/m2 weekly for 3 weeks), with radiation confined to the primary tumor. Among 33 patients with response-evaluable disease, ten (33%) experienced an objective response. The maximum tolerated dose was 36 Gy administered in 2.4 Gy fractions.
The combination of paclitaxel, gemcitabine, and external irradiation has been evaluated in a Phase I study by Brown University Oncology Group (BrUOG), showing that the limiting toxicities were gastrointestinal side effects such as nausea, diarrhea, and resultant dehydration, presumably from small bowel toxicity.14 The level of toxicity of this combination with large radiation fields (averaging 15 × 15 cm) explains the toxicity observed in our study, in which conventional radiation fields similar to those used in RTOG 98-12 were used. Based on these preliminary data, we chose to evaluate weekly gemcitabine at a dosage of 75 mg/m2 and a lower weekly paclitaxel dosage of 40 mg/m2 (20% below that used in RTOG 98-12), since the Phase I study suggested they could be logistically incorporated, with acceptable toxicity, into a multicenter treatment protocol.
Our data demonstrate that a dual radiation sensitizer combination produces a median survival of 1-year and a 1-year survival rate of 50%. While these results were not significantly better than paclitaxel and concurrent radiation in RTOG 98-12, they provide further validation of the importance of chemoradiation in locally advanced pancreatic cancer. Using only locoregional treatment, without systemic dosages of gemcitabine, a median 1-year survival of approximately 50% was achieved. In future, one could consider the use of higher radiation doses accompanied by single or multiple radiation sensitizers, especially now that highly conformal treatment can be delivered to well-defined tumor volumes, to determine benefit.
R115777 following chemoradiation was not effective and may have been harmful. Farnesyl transferase inhibitors have been developed to alter the activity of ras oncogenes and the proteins they encode, which are commonly found in pancreatic cancer, making inhibition of ras a rational target. R115777 has been shown in several preclinical tests to inhibit H-ras, K-ras, and N-ras activity in transformed tumors.27 Since the original design of RTOG 0020, results from two Phase II studies and a Phase III study failed to show single-agent activity for R115777. In a Southwest Oncology Group study (9924),28 48 previously untreated patients with metastatic and locally advanced pancreatic cancer were treated with R115777 (300 mg orally twice daily × 21 days of a 28-day cycle). The median survival was 3 months, with a time to treatment failure of 1 month. A Belgian study of R115777 for metastatic disease patients29 showed pharmacodynamic data demonstrating that the drug did indeed successfully inhibit farnesyl transferase activity and suppress farnesylation of a target protein in peripheral blood mononuclear cells. This confirmed mechanism of action did not translate into clinical efficacy, since no objective responses were observed, the median survival was 19.7 weeks, and the 6-month survival rate was 25%. A Phase III study of 688 patients with pancreatic cancer comparing gemcitabine to the combination of gemcitabine and R115777 did not demonstrate an improvement in outcome.30
Hematologic toxicity from R115777 has been reported in prior studies. The occurrences of neurologic, hepatic, musculoskeletal, cardiac, and metabolic toxicities were not appreciated in prior studies. Some of these toxicities may have been due to disease progression including edema (classified as a cardiac toxicity), hyperglycemia, and hypoalbuminemia. However, the elevation of hepatic transaminases in the absence of hyperbilirubinemia and the neurologic and musculoskeletal adverse events may have been due to the broad range of pathway inhibition by R115777. The appearance and recognition of these toxicities in RTOG 0020, as compared to other studies, may have been due to the effectiveness of the chemoradiation regimen, so that patients remained on R115777 for an extended period of time. The median treatment time was only 77 days in the Phase III study of R115777 in advanced colon cancer. In addition to the Ras family of proteins, R115777 inhibits farnesyl transferase in Rho-B and Rho-E, protein tyrosine phosphatase 4A (PTP4A)-1 and PTP4A-2, centromere-associated protein (CENP)-E and CENP-F, and lamins. This nonselectivity inhibiting multiple pathways may have contributed to the toxicities associated with R115777 maintenance.
In summary, the RTOG trial 0020 failed to demonstrate that the addition of a second radiation sensitizer, weekly gemcitabine, significantly improves survival for patients with locally advanced pancreatic cancer, as compared to RTOG 98-12. However, the 1-year survival of approximately 50% is encouraging and reaffirms the role of chemoradiation in locally advanced pancreatic cancer. In the future, more effective systemic agents, either chemotherapeutic or biologic, will be combined with chemoradiation to optimize locoregional and systemic control to ultimately improve survival.
Acknowledgments
Our research was supported by RTOG U10 CA21661, CCOP U10 CA37422, and Stat U10 CA32115 grants from the NCI. It was presented at the 2006 Gastrointestinal Cancers Symposium (Abstract 121).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Krzyzanowska MK, Weeks JC, Earle CC. Treatment of locally advanced pancreatic cancer in the real world: population-based practices and effectiveness. J Clin Oncol. 2003;21(18):3409–3414. doi: 10.1200/JCO.2003.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Huguet F, Girard N, Guerche CS, et al. Chemoradiotherapy in the management of locally advanced pancreatic carcinoma: a qualitative systematic review. J Clin Oncol. 2009;27(13):2269–2277. doi: 10.1200/JCO.2008.19.7921. [DOI] [PubMed] [Google Scholar]
- 3.Conroy T, Desseigne M, Ychou M, et al. Randomized Phase III trial comparing FOLFIRINOX (F: 5FU/leucovorin [LV], irinotecan [I], and oxaliplatin [O] versus gemcitabine [G] as first-line treatment for metastatic pancreatic adenocarcinoma (MPA): preplanned interim analysis results of the PRODIGE 4/ACCORD 11 trial. J Clin Oncol. 2010;28(Suppl, abstract 4010):S15. [Google Scholar]
- 4.Bo G, De Paoli A, Innocente R, et al. Radiotherapy and continuous infusion 5-fluorouracil in patients with nonresectable pancreatic carcinoma. Int J Radiat Oncol Biol Phys. 2001;51:736–740. doi: 10.1016/s0360-3016(01)01708-4. [DOI] [PubMed] [Google Scholar]
- 5.Crane CH, Winter K, Regine W, et al. Phase II study of bevacizumab with concurrent capecitabine and radiation followed by maintenance gemcitabine and bevacizumab for locally advanced pancreatic cancer: Radiation Therapy Oncology Group RTOG 0411. J Clin Oncol. 2009;27:4096–4102. doi: 10.1200/JCO.2009.21.8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaishampayan UN, Ben-Josef E, Philip PA, et al. A single-institution experience with concurrent capecitabine and radiation therapy in gastrointestinal malignancies. Int J Radiat Oncol Biol Phys. 2002;53:675–679. doi: 10.1016/s0360-3016(02)02772-4. [DOI] [PubMed] [Google Scholar]
- 7.Choy H, Rodriguez FF, Koester S, et al. Investigation of taxol as a potential radiation sensitizer. Cancer. 1993;71:3774–3778. doi: 10.1002/1097-0142(19930601)71:11<3774::aid-cncr2820711147>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Safran H, Moore T, Iannitti D, et al. Paclitaxel and concurrent radiation for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys. 2001;49:1275–1279. doi: 10.1016/s0360-3016(00)01527-3. [DOI] [PubMed] [Google Scholar]
- 9.Safran H, King TP, Choy H, et al. Paclitaxel and concurrent radiation for locally advanced pancreatic and gastric cancer: a Phase I study. J Clin Oncol. 1997;15:901–907. doi: 10.1200/JCO.1997.15.3.901. [DOI] [PubMed] [Google Scholar]
- 10.Rich TA, Harris J, Abrams R, et al. A Phase II study of external beam irradiation and weekly paclitaxel for non-metastatic, unresectable pancreatic cancer: RTOG 98-12. Am J Clin Oncol. 2007;27(1):51–56. doi: 10.1097/01.coc.0000046300.88847.bf. [DOI] [PubMed] [Google Scholar]
- 11.Burris HA, Moore MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 12.Blackstock AW, Lesser GJ, Fletcher-Steede J, et al. Phase I study of twice-weekly gemcitabine and concurrent thoracic radiation for patients with locally advanced non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2001;51:1281–1289. doi: 10.1016/s0360-3016(01)01732-1. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman JP, McGinn CJ, Szarka C, et al. A Phase I study of preoperative gemcitabine (GEM) with radiation therapy (RT) followed by postoperative GEM for patients with localized, resectable pancreatic adenocarcinoma (PAC) Proc Am Soc Clin Oncol. 2007;18:283a. [Google Scholar]
- 14.Safran H, Dipetrillo T, Iannitti D, et al. Gemcitabine, paclitaxel, and radiation for locally advanced pancreatic cancer: a Phase I trial. Int J Radiat Oncol Biol Phys. 2002;54:137–141. doi: 10.1016/s0360-3016(02)02902-4. [DOI] [PubMed] [Google Scholar]
- 15.Baselga J, Rosen N. Determinants of RASistance to anti-epidermal growth factor receptor agents. J Clin Oncol. 2008;26:1582–1584. doi: 10.1200/JCO.2007.15.3700. [DOI] [PubMed] [Google Scholar]
- 16.Rowinsky EK, Windle JJ, Von Hoff DD. Ras protein farnesyltransferase: a strategic target for anticancer therapeutic development. J Clin Oncol. 1999;17:3631–3652. doi: 10.1200/JCO.1999.17.11.3631. [DOI] [PubMed] [Google Scholar]
- 17.Dixon DO, Simon R. Sample size considerations for studies comparing survival curves using historical controls. J Clin Epidemiol. 1988;41:1209–1213. doi: 10.1016/0895-4356(88)90025-x. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Non-parametric estimation from incomplete observation. J Am Stat. 2007;53:457–481. [Google Scholar]
- 19.Cox DR. Regression models and life tables. J R Stat Soc. 2007;34:187–229. [Google Scholar]
- 20.Willett CG, Safran H, Abrams RA, et al. Clinical research in pancreatic cancer: the Radiation Therapy Oncology Group trials. Int J Radiat Oncol Biol Phys. 2003;56:31–37. doi: 10.1016/s0360-3016(03)00446-2. [DOI] [PubMed] [Google Scholar]
- 21.Milross CG, Mason KA, Hunter NR, et al. Enhanced radioresponse of paclitaxel-sensitive and -resistant tumours in vivo. Eur J Cancer. 1997;33:1299–1308. doi: 10.1016/s0959-8049(97)00107-x. [DOI] [PubMed] [Google Scholar]
- 22.Mason KA, Milas L, Peters LJ. Effect of paclitaxel (taxol) alone and in combination with radiation on the gastrointestinal mucosa. Int J Radiat Oncol Biol Phys. 1995;32:1381–1389. doi: 10.1016/0360-3016(95)00037-Y. [DOI] [PubMed] [Google Scholar]
- 23.Safran H, King T, Choy H, et al. p53 mutations do not predict response to paclitaxel/radiation for nonsmall cell lung carcinoma. Cancer. 1996;78:1203–1210. doi: 10.1002/(SICI)1097-0142(19960915)78:6<1203::AID-CNCR6>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence T. Gemcitabine as a radiation sensitizer. Semin Oncol. 2007;22:68–71. [Google Scholar]
- 25.Blackstock AW, Tepper JE, Niedwiecki D, et al. Cancer and Leukemia Group B (CALGB) 89805: Phase II chemoradiation trial using gemcitabine in patients with locoregional adenocarcinoma of the pancreas. Int J Gastrointest Cancer. 2003;34:107–116. doi: 10.1385/ijgc:34:2-3:107. [DOI] [PubMed] [Google Scholar]
- 26.McGinn CJ, Zalupski MM, Shureiqi I, et al. Phase I trial of radiation dose escalation with concurrent weekly full-dose gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2001;19:4202–4208. doi: 10.1200/JCO.2001.19.22.4202. [DOI] [PubMed] [Google Scholar]
- 27.Kohl NE, Wilson FR, Mosser SD, et al. Protein farnesyltransferase inhibitors block the growth of ras-dependent tumors in nude mice. Proc Natl Acad Sci U S A. 1994;91:9141–9145. doi: 10.1073/pnas.91.19.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macdonald JS, McCoy S, Whitehead RP, et al. A Phase II study of farnesyl transferase inhibitor R115777 in pancreatic cancer: a Southwest oncology group (SWOG 9924) study. Invest New Drugs. 2005;23:485–487. doi: 10.1007/s10637-005-2908-y. [DOI] [PubMed] [Google Scholar]
- 29.Van Cutsem E, Karasek P, Oettle H, et al. Phase III trial comparing gemcitabine + R115777 (Zarnestra) versus gemcitabine + placebo in advanced pancreatic cancer. Programs and abstracts of the 38th Annual Meeting of the American Society of Clinica. [Google Scholar]
- 30.Van Cutsem E, de Belde HV, Karasek P, et al. Phase III trial of gemcitabine plus tipifarnib compared with gemcitabine plus placebo in advanced pancreatic cancer. J Clin Oncol. 2004;22:1430–1438. doi: 10.1200/JCO.2004.10.112. [DOI] [PubMed] [Google Scholar]