Abstract
Objective
The objective of this study was to assess the efficacy and safety of SX (combination of yohimbine and L-arginine) in the treatment of erectile dysfunction (ED).
Methods
This trial was a 4-week, double blind study of parallel groups of patients with mild to moderate ED. Forty married male patients with ED of mild-to-moderate severity were screened for the study entry;among them, those aged 25–50 who reported a minimum of a- 3-month history of ED were eligible to enroll in this study. The severity of ED was based on EF domain scores on the international index of erectile function (IIEF). The scores of 15–25 was considered as mild to moderate ED. Patients were randomized to receive one capsule of SX or placebo on demand in a 1:1 ratio using a computer-generated code.
Results
The difference between the two groups was significant at week 4 (endpoint) (P=0.03). Four adverse events were observed over the study. The difference between the SX and placebo was not significant in the frequency of adverse events.
Conclusion
This study indicates that SX is safe and effective for the treatment of mild to moderate ED at least in the short-term.
Keywords: Erectile Dysfunction, L-Arginine, Yohimbine
Approximately 30 million American men are afflicted with Erectile Dysfunction (ED). Because of increasing life expectancies, the prevalence of ED is expected to increase by more than 30% over the next 25 years (1). The prevalence and severity of ED have been shown to increase with age. The causes of ED are divided into 3 broad categories: psychogenic, organic, and mixed. Psychogenic factors are involved alone or in combination with organic causes in a substantial number of ED cases (1–3).
Orally administered phosphodiesterase 5 (PDE5) inhibitors have become the first-line treatment option for ED. PDE5 inhibitors have been shown to enhance erectile response in patients with ED by mediating the relaxation of the vascular smooth muscle of the penis. Nevertheless, the available approved medications for ED are often unsatisfactory, and there may be a place for alternative medicines (2, 3).
Yohimbine (Yocon, Yohimex) is derived from an herbal remedy. It appears to boost erectile function by improving blood flow (4, 5). Studies have been inconclusive about its benefits, but a recent analysis of seven trials reported that between 34% and 75% of men achieved favorable results when taking 5 mg to 10 mg (4, 5). L-arginine, while not an herb, is a naturally occurring amino acid present in foods. Studies on L-arginine have shown mixed results (2, 3)
The objective of this study was to assess the efficacy and safety of SX (combination of yohimbine and L-arginine) in the treatment of ED.
Materials and Methods
This trial was a 4-week, double blind study of parallel groups of patients with mild to moderate ED and was undertaken in two centers from September 2009 to December 2009.
Participants
Forty married male patients with ED of mild-to-moderate severity were screened for the study entry. Those men aged 25–50 who reported a minimum of a -3-month history of ED were eligible to enroll in this study. The severity of ED was based on EF domain scores on the international index of erectile function (IIEF) (6). The scores of 15–25 were considered as mild to moderate ED (6). Patients were excluded from the study if they met any of the exclusion criteria specified in the protocol. These criteria included clinically significant penile deformities or penile implants; a recent history of stroke or spinal cord trauma; unstable cardiovascular status (eg, unstable angina, myocardial infarction, or myocardial revascularization within the prior 90 days); cancer chemotherapy, or antiandrogens; or failure to achieve any erection following radical prostatectomy or pelvic surgery. No other approved or experimental medications or treatments or devices to treat ED were allowed during the study. Written informed consent was obtained from all patients participating in this study before the performance of any protocol-specific procedure occurred.
Efficacy Variables
The primary efficacy measure was the EF domain score (questions 1–5 and 15) of the International Index of Erectile Function (IIEF) questionnaire. The additional secondary efficacy measure reported here was the response of those patients who completed week 4 of the treatment to the following Global Assessment Question (GAQ): (‘‘Have the treatment you have taken over the past 4 weeks improved your sexual function?’’).
Intervention
Patients were randomized to receive one capsule of SX (a product of nature Gift) or placebo on demand in a 1:1 ratio using a computer-generated code. No individual participant randomization code was revealed during the trial. Treatment codes were unblinded at the termination of the study after the database was locked. Placebo and SX were visually identical. In this double blind, multi center trial, patients were randomly assigned to receive SX (Group A) or placebo (Group B) for a 4-week study. All adverse events, reported or observed, were recorded at each visit. Routine physical examinations were also conducted at each visit.
Statistical Analysis
To compare the score of the IIEF at weeks 0 and 4 in the two groups, an unpaired two sided Student T test was used. Fisher's exact test was employed to compare the baseline data and frequency of adverse events between the protocols. Results are presented as mean (SD) and were considered significant at a probability (p) value of < 0.05.
Results
No difference was observed in the baseline characteristics including, age, times since diagnosis and weight (Table 1).
Table 1.
Characteristics of the patients
| Variable | SX Group | Placebo Group |
|---|---|---|
| Number | 20 | 20 |
| Age (Mean±SD) (year) | 38.16 ± 6.12 (year) | 38.45 ± 5.65 (year) |
| Weight (Mean±SD) (kg) | 82.46 ± 10.56 | 54.65 ± 12.45 |
| Time since diagnosis (year) | 2.45±0.65 | 2.18 ± 0.58 |
Efficacy measures
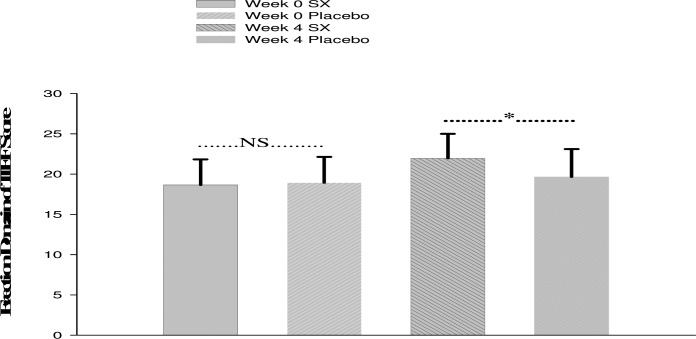
The mean ± SD scores of the two groups are presented in Figure 1. There were no significant differences between the two groups at week 0 (baseline) on the EF domain of IIEF (P=0.76).
Figure 1.
Comparison of baseline and endpoint of the two groups (week 4) based on erection domain of IIEF Sscore.NS: non significant and *=P<0.05.
The difference between the two groups was significant at week 4 (endpoint) (P=0.03).There was a significant difference between the two treatments in terms of the percentage of responders (Response to Global Assessment Question (GAQ): ‘‘Have the treatment you have taken over the past 4 weeks improved your sexual function?’’).
(SX group: 15 of 20 and placebo group: 6 of 20; P=0.01).
Safety
Four adverse events were observed over the study. The difference between the SX and placebo in the frequency of adverse events was not significant (Table 2). None of the adverse events were severe or caused a drop-out.
Table 2.
Clinical complications and side effects were reported as number per group.
| Side Effects | SX Group | Placebo Group | P |
|---|---|---|---|
| Itching | 3 (15%) | 1 (5%) | 0.60 |
| Hypertension | 1 (5%) | 1 (5%) | 1.00 |
| Headache | 5 (25%) | 1 (5%) | 0.18 |
| Insomnia | 3 (15%) | 2 (10%) | 1.00 |
Discussion
Male sexual arousal is a complex process that involves the brain, hormones, emotions, nerves, muscles and blood vessels; and erectile dysfunction can result from a problem with any of them (1). Likewise, stress and mental health problems can cause or worsen erectile dysfunction. Research on drugs for treating ED is expanding rapidly. Several alternative treatments are used to treat erectile dysfunction, but more studies are needed to ensure whether they are safe or effective (2, 3).
This study indicates that the SX is effective for the treatment of patients with mild to moderate ED as shown by improvements in both the IIEF and GAQ measures. This is the first study to evaluate SX in the treatment of patients with mild to moderate ED and so it is not possible to draw any comparisons with the results of others trials. However, there are a couple of published studies that have investigated the efficacy of combination of L-arginine and yohimbine (7, 8). Both studies reported that on-demand oral administration of the L-arginine and 6 mg yohimbine combination is effective in improving erectile function in patients with mild to moderate ED.
The results of the present study are in line with those of the conducted studies (7, 8). Adverse events were generally mild to moderate with no drop-out as a result of adverse events. The limitations of the present study include the small number of patients and a relatively short period of the follow-up. Therefore, further randomized controlled evaluation should be undertaken.
Conclusions
This study indicates that SX is safe and effective for the treatment of mild to moderate ED at least in the short-term.
Acknowledgment
This study was supported by a grant from Nature Gift.
References
- 1.Katz A, Katz A. Erectile dysfunction. CMAJ. 2010 Feb 8; doi: 10.1503/cmaj.090422. (Epub ahead of print)\. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eardley I, Donatucci C, Corbin J, El-Meliegy A, Hatzimouratidis K, McVary K, et al. Pharmacotherapy for erectile dysfunction. J Sex Med. 2010;7:524–540. doi: 10.1111/j.1743-6109.2009.01627.x. [DOI] [PubMed] [Google Scholar]
- 3.Heidelbaugh JJ. Management of erectile dysfunction. Am Fam Physician. 2010;81:305–312. [PubMed] [Google Scholar]
- 4.Morales A. Yohimbine in erectile dysfunction: the facts. Int J Impot Res. 2000;12:S70–74. [PubMed] [Google Scholar]
- 5.Witt DK. Yohimbine for erectile dysfunction. J Fam Pract. 1998;46:282–283. [PubMed] [Google Scholar]
- 6.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. TheInternational index of erectile function (IIEF): a multidimensional scale forAssessment of erectile dysfunction. Urology. 1997;49:822–830. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 7.Lebret T, Hervé JM, Gorny P, Worcel M, Botto H. Efficacy and safety of a novelcombination of L-arginine glutamate and yohimbine hydrochloride: a new oraltherapy for erectile dysfunction. Eur Urol. 2002;41:608–613. doi: 10.1016/s0302-2838(02)00175-6. [DOI] [PubMed] [Google Scholar]
- 8.Kernohan AF, McIntyre M, Hughes DM, Tam SW, Worcel M, Reid JL. An oralyohimbine/L-arginine combination (NMI 861) for the treatment of male erectiledysfunction: a pharmacokinetic, pharmacodynamic and interaction study withintravenous nitroglycerine in healthy male subjects. Br J Clin Pharmacol. 2005;59:85–93. doi: 10.1111/j.1365-2125.2004.02243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]