Abstract
The underlying mechanisms behind both low-dose hyper-radiosensitivity (HRS) and induced radioresistance (IRR), generally occurring at elevated radiation levels, remain unclear; however, elucidation of the relationship between cell cycle division 25 homolog c (Cdc25c) phosphatase and HRS/IRR may provide important insights into this process. Two cell lines with disparate HRS status, A549 and SiHa cells, were selected as cell models for comparison of dose-dependent Cdc25c phosphatase expression subsequent to low-dose irradiation. Knockdown of Cdc25c in A549 cells was mediated by transfection with a pGCsi-RAN-U6neo vector containing hairpin siRNA sequences. S216-phosphorylated Cdc25c protein [p-Cdc25c (Ser216)], cell survival and mitotic ratio were measured by western blot, colony-forming assay and histone H3 phosphorylation analysis. Variant p-Cdc25c (Ser216) expression was observed in the two cell lines after irradiation. The p-Cdc25c (Ser216) expression noted in SiHa cells after administration of 0–1 Gy radiation was similar to the radioresistance model; however, in A549 cells, the dose response for the phosphorylation of the Cdc25c Ser216 residue overlapped the level required to overcome the HRS response. Furthermore, Cdc25c repression prior to low-dose radiation induced more distinct HRS and prevented the development of IRR. The dose required to overcome the HRS response coincided with the effect of early G2-phase checkpoint arrest in A549 cells (approximately 0.3 Gy), and Cdc25c knockdown in A549 cells (approximately 0.5 Gy) corresponded to the phosphorylation of the Cdc25c Ser216 residue. Resultant data confirmed that dose-dependent Cdc25c phosphatase does effectively act as an early G2-phase checkpoint, thus indicating mechanistic importance in the HRS to IRR transition in A549 cells.
Keywords: low-dose irradiation, HRS/IRR, Cdc25c, cell cycle arrest
INTRODUCTION
Radiation therapy, often coupled with surgery and chemotherapy, is also one of the most important methods of contemporary cancer treatment [1]. More than 60% of all cancer patients will require radiotherapy at various stages over the course of their treatment. Though the technology for clinical administration of radiation therapy and radiation dose fractionation has greatly improved over the past decades, increasing application of these techniques warrants increasing concern as to the effects of low-dose radiation [2–5]. In addition to the previously considered roles of adaptive responses, bystander effects and genetic instability, the phenomenon of low-dose hyper-radiosensitivity (HRS) is one of the important biological effects of low-dose ionizing radiation, and HRS may play an as yet undefined role in the outcomes of cancer treatment [6–7]. It has been confirmed both in vitro and in vivo that numerous tumor types as well as several normal tissues show hypersensitive responses at very low radiation doses; however, the same tumors and tissues exhibit induced radioresistance (IRR) at elevated radiation levels [8]. Because of the important biological consequences of the HRS/IRR phenomenon, it is considered to be of clinical and therapeutic significance, playing a particularly notable role in the treatment of refractory cancers with conventional radiotherapy and reirradiation treatments.
Explanation of the mechanism of low-dose HRS has been recently attempted by several research groups, though IRR is rarely considered simultaneously. These studies indicate that dose-dependent low-dose irradiation induces early check point arrest at the G2/M phase of the cell cycle, indicating the likely predominant mechanism of both HRS and IRR [9]. Krueger et al. found that the dose-activation profiles for both ataxia-telangiectasia mutated (ATM) activity and the early occurrence of the G2/M checkpoint correspond to the transition in cell survival from low-dose HRS to IRR [10]. Furthermore, abrogation of the checkpoint by inhibition of checkpoint kinase 1 (Chk1) and checkpoint kinase 2 (Chk2) also increased low-dose radiosensitivity [11]. These results suggest that the ATM-Chk1/Chk2 checkpoint pathway is important in the transition from HRS to IRR. However, at the molecular level, multiple kinases contribute to the arrest of early G2/M checkpoints to ensure that the cell is prepared for normal mitosis [12, 13]. At present, the molecular mechanisms involved in many of the early G2/M checkpoints in HRS/IRR are still poorly defined.
DNA double-strand breaks (DSBs) are biologically significant lesions induced by low-dose ionizing radiation [14]. In response to DSBs, the ATM-Chk1/Chk2 checkpoint pathway is activated to prevent cell cycle progression. The mitotic inducer, Cdc25c phosphatase, is a downstream component of the ATM-Chk1/Chk2 checkpoint pathway that is activated by ionizing radiation in response to DSBs [15]. Phosphorylation of Serine 216 residues (S216) by Chk1 and Chk2 has been shown to inhibit Cdc25c activation, thus arresting cells in G2/M phase [16, 17]. Therefore, it was hypothesized that Cdc25c phosphatase was likely involved in the underlying control mechanisms behind the shift in cell behavior between HRS and IRR.
The role of Cdc25c phosphatase in the shifting cell behavior between HRS and IRR was examined in the current study using two distinct tumor cell lines of disparate HRS status. Cell lines A549 and SiHa, the two most commonly examined tumor cell lines in radiological biological research, were selected as models. The dose-dependent expression of Cdc25c protein and S216-phosphorylated Cdc25c protein [p-Cdc25c(Ser216)] were analyzed after low-dose irradiation of both cell lines. Moreover, the effects of Cdc25c down-regulation in A549 cells on HRS/IRR and G2/M arrest were examined. This study provides unique insight into the precise molecular mechanism underlying HRS/IRR.
MATERIALS AND METHODS
Cell culture
Human lung adenocarcinoma bronchioloalveolar carcinoma A549 and human cervical cancer SiHa cell lines were kindly provided by the Gastrointestinal Surgery Institute of Union Hospital in Tongji Medical College of Huazhong University of Science and Technology (Wuhan, China). Cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (GIBCO BRL, Eggenstein, Germany), 3 mmol/l glutamate (Gibco BRL), 100 U/ml penicillin and 100 µg/ml streptomycin (Gibco BRL) under standard incubator conditions (37°C, 5% CO2).
Irradiation
For cell survival assays, cells were irradiated using a Siemens Primus K X-ray unit (Primus, Siemens AG, Erlangen, Germany) with single doses administered at a dose rate of 0.8 Gy min−1. The cells were irradiated within a dose range of 0.1–2.0 Gy and then harvested at different times for cell extracts.
Generation of the Cdc25c shRNA plasmids
The small hairpin RNAs (shRNAs) targeting Cdc25c were commercially designed and synthesized by Genechem Co. Ltd (Shanghai, China). The shRNA expression cassette consisted of a 21-bp target gene specific sequence, a 9-bp loop and another 21-bp reverse complementary sequence, all influenced by a human U6 promoter. A termination sequence (TTTTT) was located immediately downstream of the second 21-bp reverse complementary sequence to terminate transcription by RNA Pol III. Target DNA fragments to the coding region were approximately 1907–1927 bp, 774–794 bp and 970–990 bp, respectively. Enzyme restriction sites of BamHI and HindIII were constructed into the extreme of the oligonucleotide fragments. The specificity of the constructed oligonucleotide fragments was analyzed by BLAST to ensure a match to the target gene. The sequences used were as follows:
shRNA1, 5′-GGATCCCGACAACACAATACCAGATAAATTCAGAGATTTATCTGGTATTGTGTTGTCTTTTTGGATAGCTT-3′; shRNA2, 5′-GGATCCCGTCCCATTACTACTGTTCCAATTCAAGAGATTGGAACAGTAGTAATGGGACTTTTTGGATAGCTT-3′; and shRNA3, 5′-GGATCCCAATCTCTATGCAACTCAAGGCTTCAAGAGAGCCTTGAGTTGCATAGAGATTTTTTTGGATAGCTT-3′.
A nonsense sequence shRNA was constructed as a positive control as follows: 5′-GGATCCCTTCTCCGAACGTGTCACGTTTCAAGAGACGTGACACGTTCGGAGAATTTTTGGATAGCTT-3′. After purification, the shRNA oligonucleotide fragments were ligated into the pGCsi-U6/Neo/DsRed vector (Genechem Co. Ltd, Shanghai, China). The Cdc25c shRNA recombinants were identified by enzyme digestion and sequencing (Genechem Co., Ltd, Shanghai, China). The red fluorescent colonies were selected after 2 weeks and then maintained in 600 µg/ml G418. Reverse transcription polymerase chain reaction (RT-PCR) and western blot were applied to analyze Cdc25c mRNA and protein levels, respectively.
Clonogenic cell survival assay
Immediately following irradiation, cells were trypsinized and diluted in phosphate-buffered saline (PBS). Flow cytometry (FACSVantage, BD Bioscience, San Jose, CA, USA) was used to precisely quantify cell numbers. Cells were plated at 200 cells per 60-mm culture dish. Three replicates were prepared for each data point. After 10–14 days of incubation, the cells were fixed in 95% methanol and stained with crystal violet (100% methanol solution). A population of >50 cells were counted as one survived colony. Assays were repeated three times.
Dose–survival curves were generated from the average survival levels of the three data points. The data were fitted to a modification of the linear-quadratic (LQ) model called the induced-repair (IR) model using non-linear least-squares regression. Survival fraction, measured at the tested doses, was fitted with the SAS JMP software (SAS Institute, Cary, NC, USA) to produce the best-fit parameters. The IR model is described as [18]:
Where αs is the low-dose value of α (derived from the response at very low doses), αr is the value extrapolated from the conventional high-dose response, D is the radiation dose, β is a constant (as in the linear-quadratic equation) and SF is the fraction of cells surviving at dose D. The presence of low-dose HRS was deduced from the values of αs and αr without overlap of confidence limits and from a Dc value (dose transition between hyper-radiosensitivity and increased resistance) significantly greater than zero.
RT-PCR
Total RNA was extracted from A549 and SiHa cells using Trizol reagent (Life Technologies Inc., Gaithersburg, MD, USA) according to the protocol provided by the manufacturer. In brief, cDNA was synthesized from 2 µg of total RNA using superscript reverse transcriptase (Life Technologies Inc). The glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene was used as an internal control. Primer sequences for Cdc25c detection were as follows: 5'-TTGTAGCACTCCGAATGGTT-3' (forward) and 5'- CTTCTGCCTGGTCTTCTCCT-3' (reverse). The amplification conditions were 94°C for 10 min followed by 30 cycles of 94°C for 30 s, 56.6°C for 30 s and 72°C for 30 s with an additional 10 min 72°C extension. PCR products were analyzed by 1.2% agarose gel electrophoresis with ethidium bromide using UV light transilluminator visualization.
Western blot analysis
Polyclonal antibodies against Cdc25c and phosphorylated Cdc25c at Ser216 were purchased from Cell Signaling Technology (Danvers, MA, USA). Confluent cells were lysed in lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM sodium chloride, 1 mM EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM h-glycerolphosphate, 1 mM sodium orthovanadate, 0.5 mM phenylmethylsulfonyl fluoride and 1 mg/ml leupeptin). Protein lysates (200 µg/lane) were subjected to 8% polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane. Non-specific binding sites were blocked by incubating the nitrocellulose membrane for 1 h at 37°C with 5% nonfat dried milk in Tris-buffered saline containing 0.05% Tween-20 (TBST). Membranes were incubated overnight at 4°C with primary antibodies (1:500 dilution). Then, membranes were additionally washed and incubated with horseradish peroxidase-conjugated secondary antibodies (1:1000 dilution). Bands were visualized using an enhanced chemiluminescence system (ECL, Pierce, Rockford, IL, USA).
Assessment of phosphorylated histone H3 by flow cytometry
Cells in the exponential phase of growth were irradiated at different doses (0 Gy, 0.3 Gy and 0.5 Gy) at 37°C and harvested after radiation exposure. These cells were then trypsinized, fixed in 2% paraformaldehyde, permeabilized with 1% Triton X-100 (Sigma) and stained with anti-phospho-histone 3 (Ser 10)-FITC (Cell Signaling Technology, Danvers, MA, USA) in a 1:2000 dilution at room temperature for 60 min. The samples were subjected to a fluorescence-activated cell sorter caliber flow cytometry and data analysis was completed using CellQuest Pro software (Becton Dickinson, Mississauga, CA, USA). The mitotic ratio was determined as the ratio of irradiated to unirradiated cells staining positive for phosphorylated histone H3 in matched cell cultures.
Statistical analysis
All values in the text and figures are expressed as the mean ± SEM. Statistical significance was determined by a Student's t-test using the software package SPSS (http://SPSS.com, Chicago, IL, USA) for Windows 11.5. A value of P < 0.05 was considered statistically significant.
RESULTS
HRS/IRR and Cdc25c activity in A549 and SiHa cells
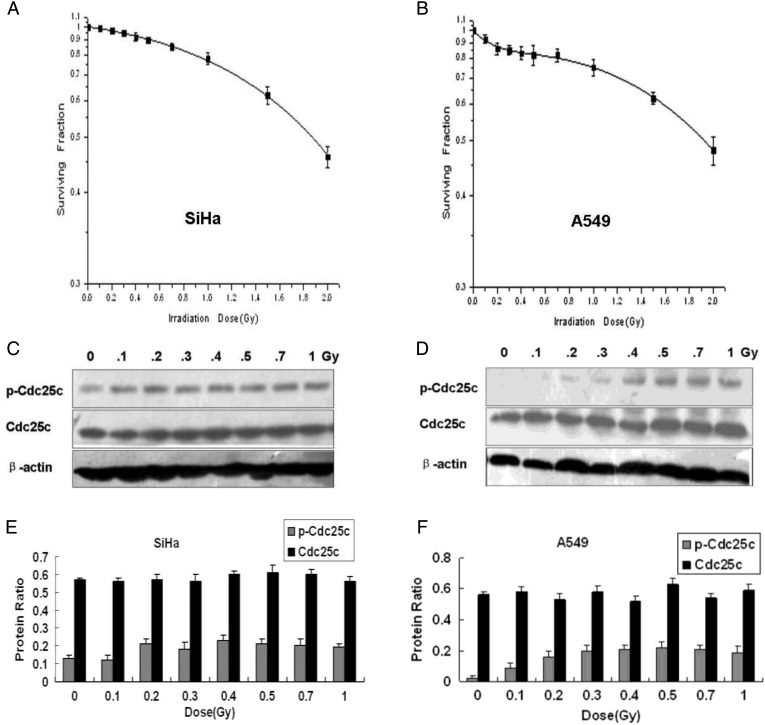
Previous studies indicated that the ATM-Chk1/Chk2 checkpoint pathway is important in the cellular transition from HRS to IRR; however, the effector checkpoint kinase Cdc25c located downstream from these proteins has never been studied in conjunction with HRS. The involvement of Cdc25c in HRS/IRR is, however, explored over the course of the current study. First, the low-dose cell survival of A549 and SiHa cells was measured. Survival of SiHa cells was decreased in a dose-dependent manner, and no evidence of HRS/IRR was exhibited (Fig. 1A). In A549 cells subjected to X-ray doses >0.3 Gy, the dose–survival relationship conformed to the conventional LQ model. When extrapolated backward below 0.3 Gy, a hypersensitive response to X-rays was noted (Fig. 1). The Dc value, marking the transition point in dosage at which cells were observed to switch between HRS and IRR, was 0.27 Gy (±0.08) (Table 1).
Figure 1:
Low-dose clonogenic survival and expression levels of cell division cycle 25C phosphatases in SiHa and A549 cells
(A, B) Clonogenic survival of SiHa cells (A) and A549 cells (B) by X-ray irradiation dose. HRS is observed in A549 cells but not in SiHa cells. (C, D) Cdc25c and p-Cdc25c(Ser216) expression by X-ray irradiation dose in SiHa cells (C) and A549 cells (D). (E, F) The levels of Cdc25c and p-Cdc25c(Ser216) expression observed in SiHa cells and A549 cells were quantified by band intensities and normalized to that of β-actin (mean ± standard error; n = 3), showing dose-dependent Cdc25c Ser216 phosphorylation from 0 Gy to 0.3 Gy in A549 cells but not in SiHa cells.
Table 1:
Mathematical modeling parameters obtained from the induced-repair model
| αs (mean ± SE) | αr (mean ± SE) | β (mean ± SE) | Dc (mean ± SE) | |
|---|---|---|---|---|
| A549 | 1.86 ± 0.05 | 0.18 ± 0.01 | 0.14 ± 0.04 | 0.27 ± 0.08 |
| SiHa | 0.14 ± 0.01 | –0.07 ± 0.01 | 0.12 ± 0.03 | 897.07 ± 0.01 |
| pGCsi-A549 | 1.14 ± 0.16 | 0.11 ± 0.03 | 1.13 ± 0.03 | 0.30 ± 0.05 |
| Cdc25c-shRNA-A549 | 1.09 ± 0.09 | 0.24 ± 0.07 | 0.09 ± 0.05 | 0.51 ± 0.13 |
Subsequently, p-Cdc25c(Ser216) and Cdc25c expression following low-dose X-ray irradiation of A549 and SiHa cells were measured by western blot analysis. A different p-Cdc25c(Ser216) expression was observed in the two cell lines after irradiation. In SiHa cells that did not exhibit the HRS/IRR phenomenon, a similar p-Cdc25c(Ser216) level occurred with irradiation of 0–1 Gy (Fig. 1C, E). In A549 cells, however, much lower p-Cdc25c(Ser216) levels were noted at <0.3 Gy, indicating a possible insufficient phosphorylation of the Ser216 of Cdc25c. An increase in phosphorylation at radiation levels between 0.3–0.5 Gy was subsequently observed, with little further increase as the dose exceeded 0.5 Gy (Fig. 1D, F). The dose required to overcome the HRS response (approximately 0.3 Gy) corresponded to the dose necessary for the phosphorylation of Ser216 in Cdc25c in A549 cells. These data established that the pattern of p-Cdc25c (Ser216) expression corresponds with the transition from HRS to IRR in A549 cells.
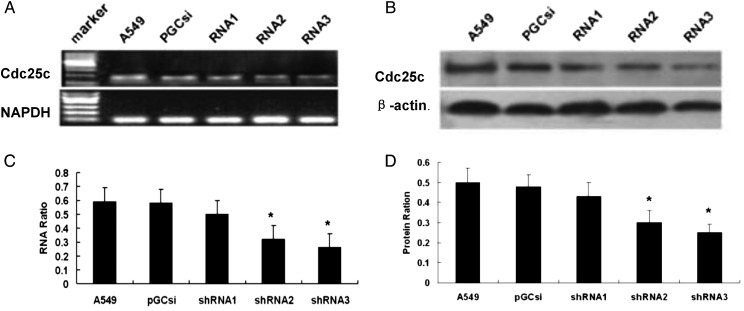
Knockdown of Cdc25c expression by Cdc25c shRNAs
A549 cells were individually transfected with three different Cdc25c-shRNAs and an empty plasmid pGCsi, and then the cells were stably screened by 600 µg/ml G418. A549 clones stably expressing Cdc25c shRNAs were selected, and Cdc25c expression was further identified by RT-PCR and western blot analyses. As shown in Fig. 2A and C, the transfection of A549 cells with Cdc25c-shRNA2 and Cdc25c-shRNA3 among the three Cdc25c-shRNAs resulted in a significant decrease in Cdc25c mRNA expression. The amount of Cdc25c in A549 cells transfected with Cdc25c-shRNA2 and Cdc25c-shRNA3 decreased by 66% and 74%, respectively, compared with the amounts observed in the pGCsi (control shRNA) and untreated A549 cells. No significant difference was observed in the mRNA expression level between A549 cells treated with Cdc25c-shRNA1, control shRNA and untreated A549 cells. As shown in Fig. 2B and D, a similar trend was observed in Cdc25c protein expression by western blot analyses. The figure showed a 65% and 75% reduction by Cdc25c-shRNA2 and Cdc25c-shRNA3, respectively, without any significant modulation of the amount of β-actin. Therefore, Cdc25c-shRNA3 (targeting approximately 970–990 bp) was selected to inhibit Cdc25c expression because of the relatively high inhibitory effectivity.
Figure 2:
Specific silencing of Cdc25c mediated by stable expression of Cdc25c shRNA in A549 cells
RT-PCR analysis of total RNA (A, C) and western blot analysis of whole-cell lysates (B, D) of A549 cells stably expressing Cdc25c shRNA, empty plasmid pGCsi and untreated A549 cells are shown. Decreased Cdc25c mRNA and protein expression by Cdc25c shRNA2 and shRNA3 were present compared with pGCsi-transfected cells. *P < 0.01 versus control cells (CON).
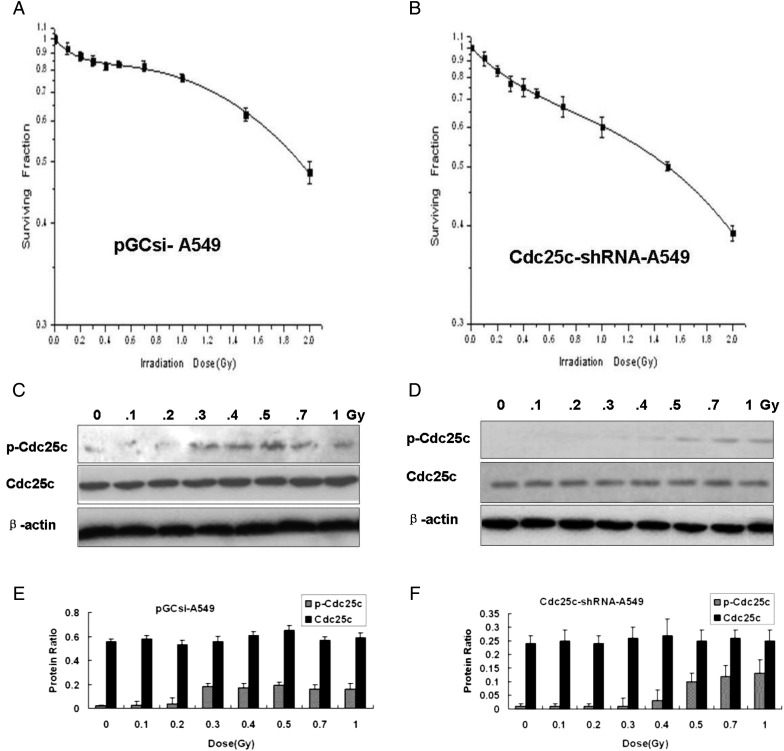
Measurement of low-dose HRS and Cdc25c phosphatase after Cdc25c knockdown in A549
In order to investigate the existence of HRS/IRR in A549 cells and the involvement of Cdc25c, clonogenic cell survival assays were performed in pGCsi-A549 cells and Cdc25c-shRNA-A549 cells. Compared with pGCsi-A549 cells, the knockdown of Cdc25c prior to low-dose radiation induced a more distinct HRS/IRR and prevented the development of IRR (Fig. 3A, B). The expression of p-Cdc25c (Ser216) after irradiation with variant X-ray doses was found to be much weaker in Cdc25c-shRNA3-A549 cells than in A549 and pGCsi-A549 cells (Fig. 3C, D, E and F). In Cdc25c-shRNA3-A549 cells, an obvious increase in p-Cdc25c (Ser216) expression was observed from 0.5 to 1 Gy of radiation compared with the levels observed below 0.5 Gy (Fig. 3D and F). Dose-dependent p-Cdc25c (Ser216) expression was found in both A549 cells and pGCsi-A549 control cells. The Dc value was 0.30 Gy (±0.05) in pGCsi-A549 cells and 0.51 Gy (±0.13) in Cdc25c-shRNA3-A549 cells (Table 1).
Figure 3:
Cdc25c-shRNA used to modulate HRS in A549 cells
(A, B) Clonogenic survival of stably expressing pGCsi-A549 cells (A) or Cdc25c-shRNA-A549 cells (B). Cdc25c-shRNA-A549 cells with stable expression, but not pGCsi-A549 cells, exhibited enhanced HRS as well as an amplified radioresistance response. (C, D) Western blotting for Cdc25c and p-Cdc25c(Ser216) expression 1 h after different doses of X-ray irradiation in pGCsi-A549 cells (C) and stable Cdc25c-shRNA-A549 cells (D). (E, F) The levels of Cdc25c and p-Cdc25c (Ser216) expression were quantified by band intensities and normalized to β-actin levels (mean ± standard error; n = 3).
The Cdc25c knockdown can be deduced to be the molecule that reduces p-Cdc25c (Ser216) levels in A549 cells, as seen in Fig. 3. The dose required to overcome the HRS response (approximately 0.5 Gy) closely corresponded to the dose necessary for Cdc25c phosphorylation in Cdc25c knockdown A549 cells. The data establish that the pattern of Cdc25c phosphorylation corresponds with the transition from HRS to IRR.
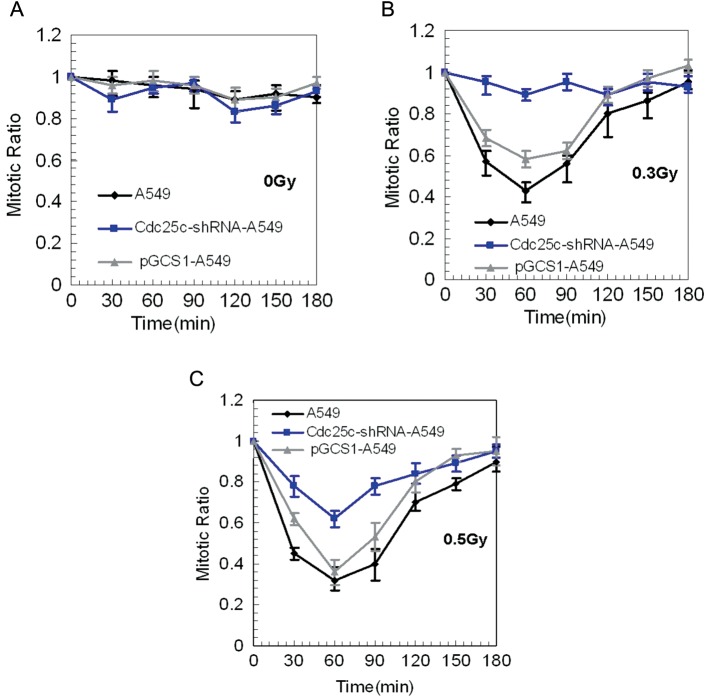
Measurement of low-dose radiation-induced early G2/M checkpoint response after Cdc25c knockdown
The effect of the irradiation-induced function of the early G2 checkpoint in A549 cells, pGCsi-A549 control cells and Cdc25c-shRNA-A549 cells was assessed by the progression of irradiated G2 cells into mitosis through the use of a mitosis-specific marker, phosphorylated histone H3. The observed decrease in the mitotic ratio indicated that radiation-induced G2-phase cells were arrested in mitosis. The baseline mitosis ratios of the three cell lines were similar, as shown in Fig. 4A. G2 arrest was evident up to 30 min after exposure to 0.3-Gy radiation in A549 cells and control cells, but not in Cdc25c knockdown A549 cells (Fig. 4B). These data indicate that the early G2 checkpoint plays a key role in Cdc25c knockdown of A549 cells at doses of 0.3 Gy. G2 arrest occurred, however, after exposure to 0.5-Gy radiation in both A549 cells and Cdc25c knockdown A549 cells (Fig. 4C). Based on these findings, the radiation dose at the early G2 phase checkpoint arrest is coincident with the dose necessary to overcome the HRS response and Cdc25c phosphorylation.
Figure 4:
Mitotic ratio quantified in A549, Cdc25c-shRNA-A549 and pGCsi-A549 cells after radiotherapy
(A) No significant differences were observed between A549, Cdc25c-shRNA-A549 and pGCsi-A549 cells after sham-irradiation (0 Gy) (mean ± standard error; n = 3). (B) A549 and pGCsi-A549 cells, but not Cdc25c knockdown A549 cells, exhibited a reduction in the mitotic ratio from 30 min to 120 min after 0.3-Gy irradiation. (C) A reduction in the mitotic ratio from 30 min to 120 min after 0.3-Gy irradiation occurred in each of the three cell sets.
DISCUSSION
The novel radiobiological phenomenon of HRS/IRR, an effect in which cell death is induced by excessive sensitivity to small single doses of ionizing radiation but resistance (per unit dose) to larger single doses increases, has been observed in cell lines of lung adenocarcinoma, colorectal carcinoma, bladder carcinoma, melanoma, prostate carcinoma, cervical squamous carcinoma, neuroblastoma and glioma, as well as in a single nonmalignant lung epithelial cell line and a single primary human fibroblast cell line [6]. Though HRS/IRR is a widely occurring phenomenon, the effect has been shown to be more prominent in proliferating malignant tissues than in quiescent normal tissues [19, 20]. In addition, tumorigenic cells with high metastatic potential have been demonstrated to preferentially exhibit HRS at a much greater level than that seen in poorly metastatic cells [21–22]. Examination of the mechanistic background of the HRS/IRR phenomenon in tumor cells may provide the possibility of designing new therapeutic options, lending significance to this field of research.
Over the past decade, numerous studies concerning the mechanisms involved with HRS/IRR have been documented. Although significant consideration has been given to the activity of DNA repair proteins, such as DNA-dependent protein kinase catalytic subunit (DNA-PKcs) [23], poly (ADP-ribose) polymerase (PARP) [24, 25] and P53 protein [26], these only partially account for HRS/IRR occurrence. Radiation-induced activation of ATM activity and early G2 checkpoint arrest are still the most widely accepted underlying mechanisms [10]. Irradiation-induced DNA damage will generally arrest cells at the G1/S or G2/M boundary. The p53 tumor suppressor gene plays a vital role in the arrest of DNA-damaged cells at the G1 checkpoint. Mutations of p53, a common occurrence in human cancers, possess the notable ability to abolish this response [27]. G2/M arrest, however, is triggered by activation of the ATM and Rad3-related (ATR) pathway. ATM responds primarily to DSBs, while ATR responds mainly to UV-induced single-strand breaks [28]. The central radiobiological effect of low-dose X-ray radiation involves the formation of ions, ultimately leading to DSBs [14]. Cdc25c is one of the ATM-Chk1/Chk2 downstream components phosphorylated by both Chk1 and Chk2. As a dual-specificity phosphatase, it controls entry into the mitotic phase of the cell cycle by dephosphorylation of Cdc2 at both the threonine 14 and tyrosine 15 positions. During the G2/M transition and later in prophase, Cdc2/cyclin B is translocated in the nucleus. Therein, the complex is fully activated by Cdc25c [29]. These findings provided the basis for the present study, providing evidence for the role of Cdc25c phosphatase in HRS/IRR. Additionally, the relationship between Cdc25c phosphatase and HRS/IRR is particularly important in overcoming HRS and establishing IRR in A549 tumor cell lines.
As a mitotic trigger, Cdc25c is highly regulated by multiple post-translational modifications and phosphorylation of a single inhibitory site, the Ser216 residue. These factors together determine the timing of Cdc25c activation and subsequent cell arrest in the G2 phase [16]. A comparison of the Cdc25c protein and p-Cdc25c (Ser216) protein expression was conducted in order to determine whether Cdc25c phosphatase was involved in HRS/IRR. The trend observed in the p-Cdc25c (Ser216) protein level interestingly coincided with the transition of the survival response from HRS to IRR in A549 cells (Fig. 1B, D and F). No association, however, was found between Cdc25c and p-Cdc25c (Ser216) protein expression when SiHa cells, a HRS/IRR-negative cell line, were subjected to various low doses of X-ray irradiation (Fig. 1A, C and D). This observation initially prompted exploration of the possible role of Cdc25c phosphatase in HRS/IRR in A549 cells. The knockdown of Cdc25c by shRNA subsequently resulted in repression of p-Cdc25c (Ser216) expression as shown in Fig. 3C and D, as well as in the trend of the p-Cdc25c (Ser216) expression level in Cdc25c-shRNA-A549 cells. This indicated that Cdc25c still coincided with the transition in the survival response from HRS to IRR (Fig. 3B, D and F). Furthermore, knockdown of Cdc25c induced a significantly more distinct HRS and prevented the development of IRR (Fig. 3B). The dose points at which G2 arrest and Cdc25c knockdown occurred in A549 cells were coincident with the doses needed to overcome the HRS response, suggesting a correlation (Fig. 4). Cdc25c serves as a factor controlling mitotic initiation, providing a link between overcoming HRS and G2 arrest in A549 cells. Thus, the dose-dependent Cdc25c phosphatase is an important regulator of the HRS/IRR transition.
In previous studies concerning HRS/IRR, a distinct pattern in ATM phosphorylation has been consistently observed [10, 11]. Although radiation-induced ATM Ser1981 phosphorylation has been shown to be the key determinant for overcoming HRS and establishing the IRR survival response, it is not the only factor involved in the transition. The present study demonstrates that a similar phosphorylation pattern of Cdc25c exists in HRS/IRR as in ATM, consistent with previous reports. Thus, it is likely that a newly described cell cycle checkpoint p-Cdc25c (Ser216) is also important in the transition from HRS to IRR in A549 cells. In light of the ATM-Chk1/Chk2-Cdc25c pathway's general activation by irradiation-induced DNA damage [28], activation of this pathway may additionally be involved in the HRS/IRR transition. Further research will be required to clarify the role of each protein involved in the ATM-Chk1/Chk2-Cdc25c pathway associated with HRS/IRR.
Non-small cell lung cancer (NSCLC) is among the most common malignancies in the world, and radiotherapy is one of the most effective nonsurgical treatments for patients suffering with this disease [30]. The potential for hyperfractionation to improve the therapeutic ratio of radiation treatments for NSCLC was recognized through failure of large-dose fractionation to improve local control and the observation that smaller fractions were associated with fewer effects in normal tissues [31]. Understanding the mechanism of HRS/IRR in lung cancer cells can aid in the optimization of the therapeutic ratio, allowing successful treatment with smaller dose fractions. The current findings suggest that Cdc25c may be a potentially useful target when multiple small-dose fractions of radiation are employed for the development of more effective radiation strategies. The first robust evidence for the role of Cdc25c phosphatase in HRS/IRR in A549 cell lines is contained in the current study, suggesting that Cdc25c may act as a promising new target for the development of effective radiation for NSCLC by hyperfractionation.
REFERENCES
- 1.Krause S, Debus J, Neuhof D. Radiotherapy. Recent Results Cancer Res. 2011;183:285–91. doi: 10.1007/978-3-540-85772-3_13. [DOI] [PubMed] [Google Scholar]
- 2.Honore HB, Bentzen SM. A modeling study of the potential influence of low dose hypersensitivity on radiation treatment planning. Radiother Oncol. 2006;79:115–21. doi: 10.1016/j.radonc.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Lin P-S, Wu A. Not all 2 Gray radiation prescriptions are equivalent: Cytotoxic effect depends on delivery sequences of partial fractionated doses. Int J Radiat Oncol Biol Phys. 2005;63:536–44. doi: 10.1016/j.ijrobp.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Richards GM, Tomé WA, Robins HI, et al. Pulsed reduced dose-rate radiotherapy: A novel locoregional retreatment strategy for breast cancer recurrence in the previously irradiated chest wall, axilla, or supraclavicular region. Breast Cancer Res Treat. 2009;114:307–13. doi: 10.1007/s10549-008-9995-3. [DOI] [PubMed] [Google Scholar]
- 5.Tome WA, Howard SP. On the possible increase in local tumour control probability for gliomas exhibiting low dose hyper-radiosensitivity using a pulsed schedule. Br J Radiol. 2007;80:32–7. doi: 10.1259/bjr/15764945. [DOI] [PubMed] [Google Scholar]
- 6.Joiner MC, Lambin P, Malaise EP, et al. Hypersensitivity to very-low single radiation doses: its relationship to the adaptive response and induced radioresistance. Mutat Res. 1996;358:171–83. doi: 10.1016/s0027-5107(96)00118-2. [DOI] [PubMed] [Google Scholar]
- 7.Kadhim MA, Moore SR, Goodwin EH. Interrelationships amongst radiation-induced genomic instability, bystander effects, and the adaptive response. Mutat Res. 2004;568:21–32. doi: 10.1016/j.mrfmmm.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 8.Joiner MC, Marples B, Lambin P, et al. Low-dose hypersensitivity: current status and possible mechanisms. Int J Radiat Oncol Biol Phys. 2001;49:379–89. doi: 10.1016/s0360-3016(00)01471-1. [DOI] [PubMed] [Google Scholar]
- 9.Marples B. Is low-dose hyper-radiosensitivity a measure of G2-phase cell radiosensitivity? Cancer Metastasis Rev. 2004;23:197–207. doi: 10.1023/B:CANC.0000031761.61361.2a. [DOI] [PubMed] [Google Scholar]
- 10.Krueger SA, Wilsom GD, Piasentin E, et al. The effects of G2-phase enrichment and checkpoint abrogation on low-dose hyper-radiosensitivity. Int J Radiat Oncol Biol Phys. 2010;77(5):1509–17. doi: 10.1016/j.ijrobp.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krueger SA, Collis SJ, Joiner MC, et al. Transition in survival from low-dose hyper-radiosensitivity to increased radioresistance is independent of activation of ATM Ser1981 activity. Int J Radiat Oncol Biol Phys. 2007;69:1262–71. doi: 10.1016/j.ijrobp.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 12.Kitagawa R, Kastan MB. The ATM-dependent DNA damage signaling pathway. Cold Spring Harb Symp Quant Biol. 2005;70:99–109. doi: 10.1101/sqb.2005.70.002. [DOI] [PubMed] [Google Scholar]
- 13.Fernet M, Mégnin-Chanet F, Hall J, et al. Control of the G2/M checkpoints after exposure to low doses of ionising radiation: Implications for hyper-radiosensitivity. DNA Repair (Amst) 2010;9:48–57. doi: 10.1016/j.dnarep.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Rothkamm K, Löbrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Natl Acad Sci USA. 2003;100:5057–62. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bucher N, Britten CD. G2 checkpoint abrogation and checkpoint kinase-1 targeting in the treatment of cancer. Br J Cancer. 2008;98(3):523–8. doi: 10.1038/sj.bjc.6604208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutchins JR, Clarke PR. Many fingers on the mitotic trigger: post-translational regulation of the Cdc25C phosphatase. Cell Cycle. 2004;3(1):41–5. [PubMed] [Google Scholar]
- 17.Peng C-Y, Graves PR, Thoma RS, et al. Mitotic and G2 checkpoint control: regulation of 14-3-3 protein binding by phosphorylation of Cdc25c on serine-216. Science. 1997;277:1501–5. doi: 10.1126/science.277.5331.1501. [DOI] [PubMed] [Google Scholar]
- 18.Marples B, Joiner MC. The elimination of low-dose hypersensitivity in Chinese hamster V79-379A cells by pretreatment with X rays or hydrogen peroxide. Radiat Res. 1995;141(2):160–9. [PubMed] [Google Scholar]
- 19.Slonina D, Biesaga B, Urbański K, et al. Low-dose radiation response of primary keratinocytes and fibroblasts from patients with cervix cancer. Radiat Res. 2007;167:251–9. doi: 10.1667/rr0649. [DOI] [PubMed] [Google Scholar]
- 20.Thomas C, Buronfosse A, Portoukalian J, et al. The gangliosides as a molecular coupling factor between the proportion of radiosensitive cells in vitro and the metastatic potential in vivo within a human melanoma cell line. Brit J Cancer. 1997;75:639–49. doi: 10.1038/bjc.1997.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harney J, Short SC, Shah N, et al. Low dose hyper-radiosensitivity in metastatic tumors. Int J Radiat Biol. 2004;59:1190–5. doi: 10.1016/j.ijrobp.2003.12.029. [DOI] [PubMed] [Google Scholar]
- 22.Thomas C, Fertil B, Foray N. Very low-dose hyperradiosensitivity: Impact for radiotherapy of micrometastases. Cancer/Radiotherapie. 2007;11:260–5. doi: 10.1016/j.canrad.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Marples B, Cann NE, Mitchell CR, et al. Evidence for the involvement of DNA-dependent protein kinase in the phenomena of low dose hyper-radiosensitivity and increased radioresistance. Int J Radiat Biol. 2002;78:1139–47. doi: 10.1080/09553000210166606. [DOI] [PubMed] [Google Scholar]
- 24.Chalmers A, Johnston P, Woodcock M, et al. PARP-1, PARP-2, and the cellular response to low doses of ionizing radiation. Int J Radiat Oncol Biol Phys. 2004;58:410–19. doi: 10.1016/j.ijrobp.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 25.Cheng G-H, Wu N, Jiang D-F, et al. Increased levels of p53 and PARP-1 in EL-4 cells probably related with the immune adaptive response induced by low dose ionizing radiation in vitro. Biomed Environ Sci. 2010;23:487–95. doi: 10.1016/S0895-3988(11)60012-3. [DOI] [PubMed] [Google Scholar]
- 26.Enns L, Bogen KT, Wizniak J, et al. Low-dose radiation hypersensitivity is associated with p53–dependent apoptosis. Mol Cancer Res. 2004;2:557–66. [PubMed] [Google Scholar]
- 27.Murnane JP. Cell cycle regulation in response to DNA damage in mammalian cells: a historical perspective. Cancer Metastasis Rev. 1995;14(1):17–29. doi: 10.1007/BF00690208. [DOI] [PubMed] [Google Scholar]
- 28.Sancar A, Lindsey-Boltz LA, Unsal-Kaçmaz K, et al. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 29.Perdiguero E, Nebreda AR. Regulation of Cdc25C activity during the meiotic G2/M transition. Cell Cycle. 2004;3:733–7. [PubMed] [Google Scholar]
- 30.Rivera S, Quéro L, Wong Hee Kam S, et al. Targeted therapies and radiation therapy in non-small cell lung cancer. Cancer Radiother. 2011;15(6–7):527–35. doi: 10.1016/j.canrad.2011.07.234. [DOI] [PubMed] [Google Scholar]
- 31.Jeremic B, Milicic B. From conventionally fractionated radiation therapy to hyperfractionated radiation therapy alone and with concurrent chemotherapy in patients with earlystage nonsmall cell lung cancer. Cancer. 2008;112(4):876–84. doi: 10.1002/cncr.23240. [DOI] [PubMed] [Google Scholar]