Abstract
External beam radiotherapy is a potential salvage or adjuvant therapy after radical prostatectomy (RP). The purpose of this study was to investigate the treatment outcome of salvage radiotherapy (RT) following RP for clinically localized prostate cancer and to identify factors that may predict the outcome of salvage RT. Between 2000 and 2006, 41 patients received salvage RT because of increasing prostate-specific antigen (PSA) levels following an RP for clinically localized prostate cancer. All the patients received conformal radiotherapy to the prostate bed. The prescribed radiation dose was 60–70 Gy in 26–35 fractions. The overall 5-year biochemical disease-free survival rate was 38%. A multivariate analysis showed that the following pathological findings of the surgical specimen were significantly associated with biochemical failure following salvage RT: a high Gleason score, a negative surgical margin, seminal vesicle invasion, lymphatic vessel invasion and negative vascular invasion. Among these factors, lymphatic vessel invasion was the strongest predictor. In conclusion, the pathological features affected the outcome of salvage RT following RP. Lymphatic vessel invasion was strongly associated with the risk of biochemical failure despite salvage RT. Meanwhile, vascular invasion was not a significant hazardous factor.
Keywords: prostatectomy, salvage radiotherapy, prostate cancer, prostate-specific antigen, pathological findings
INTRODUCTION
Prostate cancer is the most commonly diagnosed malignant tumor in men because of the widespread use of prostate-specific antigen (PSA) testing. Prostate cancer is also the second leading cause of death from cancer in the USA [1]. Many treatments have been developed for clinically localized prostate cancer, resulting in improved treatment outcomes; for example, radical prostatectomy (RP), external beam radiotherapy, brachytherapy, high-intensity focused ultrasound and cryotherapy have all been used to treat prostate cancer. RP is widely used as a primary treatment in patients with localized prostate cancer [2]. Despite the effectiveness of RP, approximately one-third of patients experience biochemical failure within 10 years of undergoing their surgery [3]. Biochemical failure is associated with increases in the rates of distant metastasis and death [3, 4]. The median intervals from biochemical failure to distant metastasis and from metastasis to death were reported to be 8 years and 5 years, respectively [3].
External beam radiotherapy (RT) is a potential salvage or adjuvant therapy after RP. The addition of RT and/or hormonal therapy for patients with biochemical failure after surgery has been reported to lower the risk of an adverse outcome [4]. Although salvage RT is effective for biochemical control, the 4- or 5-year biochemical disease-free survival (bDFS) rates after RT have been reported to be 46–60% [5–7]. Many previous studies have reported outcome predictors for salvage or adjuvant RT. Clinical factors, such as the interval from the salvage RT to biochemical failure, the PSA doubling time (PSA-DT), and the preradiotherapy PSA level, and pathologic findings from the surgical specimen, such as the Gleason score, the status of the surgical margins, seminal vesicle invasion, lymphovascular invasion (LVI) and perineural invasion, have all been suggested as predictive factors [3, 5, 7, 8–13]. A minimum total radiation dose of 64 Gy has been recommended [5, 6].
In this study, we evaluated the outcome of salvage RT following RP and identified predictive factors, focusing on the detailed pathological findings, radiation dose and size of the radiation field.
MATERIALS AND METHODS
Between May 2000 and October 2006, 41 patients were treated with external beam radiotherapy as salvage RT because of increasing PSA levels following an RP at our institution. Informed consent for treatment was obtained from all the patients. This study was approved by the university's research ethics committee.
The median age at the time of surgery was 64 years (range, 50–72 years). All the patients underwent a bone scintigraphy or computed tomography (CT) examination before the RP and were diagnosed as being free from metastasis. Sixteen patients (39%) received hormonal therapy prior to surgery. Pelvic lymph node dissection was performed in 36 patients (88%), and 11 patients (27%) had preserved neurovascular bundles. At our institute, most prostatectomy surgeries are performed using a minimum incision endoscopic surgical technique, in which the surgeons operate via a single incision just large enough to permit the extraction of the specimen; the surgery is performed using an endoscope without gas insufflations, any trocar ports or injury to the peritoneum [14].
Table 1 shows the clinical and pathological features of the enrolled patients. The pathologic features of the prostatectomy specimen were confirmed by at least two pathologists using light microscopy. Pathologic evaluation of specimen was based on Hematoxylin–Eosin staining and Elastica–Van-Gieson staining. In the cases where it was difficult to distinguish lymphatic vessel invasion (LI) from vascular invasion (VI), D2-40 immunostaining was supplementarily used to detect lymphatic vessel invasion. The pathological staging of the specimens according to the seventh Union for International Cancer Control – Tumor, Node, Metastasis classification was as follows: T2a, 3 patients; T2b, 6 patients; T2c, 9 patients; T3a, 16 patients; T3b, 6 patients; N0, 34 patients; N1, 2 patients; and NX, 5 patients. The Gleason scores of the excised specimens were as follows: 3 + 3, 7 patients; 3 + 4, 12 patients; 4 + 4, 6 patients; 4 + 4, 2 patients; 4 + 5, 3 patients; 5 + 4, 3 patients; and 5 + 5, 1 patient. One patient had no microscopic residual cancer cells because of preoperative hormonal therapy. The surgical margin was positive in 19 patients (46%) and negative in 22 patients (54%). Pathological tumor invasion was observed as follows: seminar vesicle invasion in 6 patients (15%); extracapsular invasion in 21 patients (51%); LI in 17 patients (41%); VI in 16 patients (39%); perineural invasions in 23 patients (56%), and urethral mucosa invasion in 1 patient (2%). Many previous reports have combined LI and VI as LVI, but we distinguished between LI and VI to enable a more detailed analysis. None of the patients had bladder or rectal wall invasion.
Table 1.
Clinical and pathological characteristics of 41 patients undergoing salvage radiotherapy
| Total number of patients | 41 | |
| Age at surgery, mean (range) (years) | 64 (50–72) | |
| Pathologic stage | pT2a | 3 |
| pT2b | 6 | |
| pT2c | 9 | |
| pT3a | 16 | |
| pT3b | 6 | |
| Positive lymph nodesa | 2 | |
| Gleason scoreb | 6 | 7 |
| 7 | 22 | |
| 8–10 | 12 | |
| Status of surgical margins | Positive | 19 |
| Negative | 22 | |
| Seminal vesicle invasion | Positive | 6 |
| Negative | 35 | |
| Extracapsular invasion | Positive | 21 |
| Negative | 20 | |
| Lymphatic vessel invasion | Positive | 17 |
| Negative | 24 | |
| Vascular invasion | Positive | 16 |
| Negative | 25 | |
| Perineural invasion | Positive | 23 |
| Negative | 18 | |
| PSAc doubling time, median (range) (months) | 4.80 (0.94–21.3) | |
| Preprostatectomy PSA level, median (range) (ng/ml) | 8.55 (3.9–40) | |
| Preradiotherapy PSA level, median (range) (ng/ml) | 0.43 (0.02–4.26) | |
| Radiation dose, median (range) (Gy) | 66 (60–70) | |
| Size of radiation field at gantry 0°, median (range) (cm2) | 27.20 (16.00–65.52) | |
| Time from RPd to biochemical failure after RP, median (range) (days) | 370 (81–2363) | |
| Time from RP to RTe, median (range) (days) | 438 (84–2342) | |
| Time from biochemical failure to RT, median (range) (days) | 27 (0–173) | |
| Time from RT to biochemical failure after RT, median (range) (days) | 213 (31–1095) | |
| Follow-up after RP, median (range) (months) | 72.8 (39.2–121.6) | |
| Follow-up after RT, median (range) (months) | 50.6 (2.4–107.8) | |
aPelvic lymph node dissection was performed in 36 patients
bno remnant malignant cells were found at the time of surgery in one patient who had received neoadjuvant hormonal therapy
cPSA = prostate-specific antigen
dRP = radical prostatectomy
eRT = radiotherapy.
Urologists were primarily responsible for monitoring a patient's condition after the RP and for making the decision to refer the patient to undergo salvage RT or to receive hormonal therapy. The follow-up evaluations after the RP consisted of a medical history, physical examination and PSA level measurement. Before April 2002, the conventional limit for PSA measurements was 0.2 ng/ml using a radioimmunoassay (RIA). After April 2002, high-sensitivity PSA measurements became possible using a chemiluminescent-enzyme immunoassay (CLEIA). The detection limits of the PSA measurements were 0.02 ng/ml (2002.4–2003.2), 0.004 ng/ml (2003.2–2006.3) and 0.008 ng/ml (2006.4–2006.7). After July 2006, the conventional PSA level was measured using a fluorescence-enzyme immunoassay (FEIA), and the detection limit was 0.01 ng/ml. The median interval between the RP and postoperative biochemical failure was 370 days (range, 81–2363 days). The median interval between postoperative biochemical failure and salvage RT was 27 days (range, 0–173 days). As a result, the median interval between the RP and the salvage RT was 438 days (range, 84–2342 days). The preradiotherapy PSA level ranged from 0.02 to 4.26 ng/ml (median, 0.43 ng/ml). The PSA doubling time (PSA-DT) was calculated using the log-linear regression method as previously described: PSA-DT = log(2) × T(log [final PSA] – log [initial PSA]) [15]. T is the interval between the initial and the final PSA level. Final PSA is the PSA level noted at the time of postoperative biochemical failure, and initial PSA is that noted at the time of the nadir for the PSA level. The median PSA-DT was 4.08 months (range, 0.94–21.3 months).
RT was performed using dynamic arc conformal radiotherapy using 10-MV X-ray photons. The clinical target volume (CTV) was the prostate bed, and an arc with an angle of 180° of rotation (from 270 to 180°) was used with dynamic conformal fitting of the multileaf collimator (MLC) to the CTV with a 1-cm margin. Additional bilateral ports with manually adjusted MLC were made to prevent the irradiation of the posterior rectal wall in three patients. The prescribed radiation dose ranged from 60 to 70 Gy (median, 66 Gy) in 26–35 fractions. Thirty-three patients were treated with five fractions of 2 Gy per week, and eight patients were treated with four fractions of 2.5 Gy per week. The size of the arc radiation field at gantry 0° ranged from 16.00 cm2 (transverse, 4.0 cm; longitudinal, 4.0 cm) to 65.52 cm2 (transverse, 8.4 cm; longitudinal, 7.8 cm), and the median size was 26.4 cm2 (transverse, 6.0 cm; longitudinal, 4.4 cm). Two patients received hormonal therapy prior to and during salvage RT.
The follow-up evaluations after salvage RT were performed by urologists and/or radiologists and consisted of a medical history, physical examination and PSA level measurement. When patients were suspected of having metastasis, they received a bone scintigraphy and/or CT examination. The judgement of biochemical failure was made based on the criteria of the American Society for Therapeutic Radiology and Oncology (ASTRO) Consensus Panel 1999 [6]. After RT, 76% of the patients achieved a PSA nadir of <0.2 ng/ml and 51% of the patients achieved a nadir of <0.05 ng/ml. The postradiotherapy PSA nadir ranged from 0.007 to 2.91 ng/ml (median, 0.05 ng/ml). The median interval from RT to postradiotherapy biochemical failure was 213 days (range, 31–1095 days). The median observation period was 52 months (range, 4–108 months).
The statistical analysis was performed using StatView 5.0 software (SAS Institute Inc., Cary, NC, USA). The survival rates were calculated using the Kaplan–Meier method, and the survival curves were compared using the log-rank test. In univariate and multivariate analyses, the prognostic factors were evaluated using the Cox-proportional hazards model. Relationships between the prognostic factors and the treatment outcome were analyzed using logistic regression models, and the models were compared using a likelihood-ratio test. A P value less than 0.05 was considered statistically significant. Acute and late toxicity were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events, version 4.0.
RESULTS
Efficacy
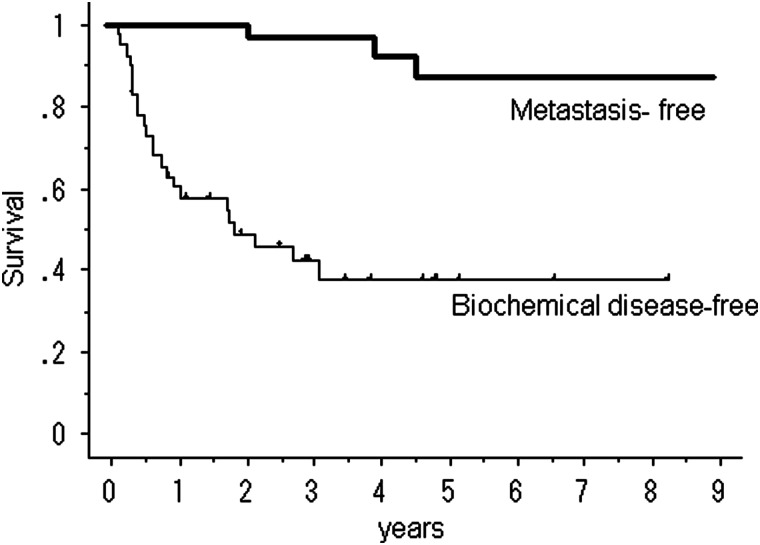
Within the first year following salvage RT, 18 patients experienced biochemical failure. During the entire observation period, biochemical failure occurred in 23 patients, local recurrence occurred in 1 patient, and distant metastasis to the thoracic or lumbar spine occurred in 2 patients. None of the patients died from prostate cancer or any other cause. Fifteen patients received salvage hormonal therapy after undergoing RT. The overall 5-year clinical relapse-free survival rate was 87%. The overall bDFS rate was 58% at 1 year, 38% at 3 years and 38% at 5 years (Fig. 1).
Fig. 1.
Biochemical disease-free survival and metastasis-free survival after salvage radiotherapy.
Toxicity
Acute Grade 1 or Grade 2 renal and urinary (RU) complications occurred in 13 cases (32%) and 4 cases (10%), respectively. Acute Grade 1 gastrointestinal (GI) complications occurred in 18 cases (44%). None of the patients experienced acute Grade 3/4/5 RU complications or Grade 3/4/5 GI complications. Late Grade 1 and Grade 2 RU complications occurred in 5 cases (12%) and 1 case (2%), respectively. Two patients experienced a Grade 3 RU complication consisting of a urinary tract obstruction that required operative intervention. No late GI complications were seen.
Univariate and multivariate analyses
Table 2 shows the univariate and multivariate analyses of possible prognostic factors for biochemical failure following salvage RT. We focused on the detailed pathological findings. Many previous reports combined LI and VI as LVI, but we distinguished LI from VI. In the multivariate analysis, the Gleason score (P = 0.030), negative surgical margin (P = 0.018), seminal vesicle invasion (P = 0.006), LI (P < 0.0001), negative VI (P = 0.011), preradiotherapy PSA level (P = 0.017), radiation dose (P = 0.010) and size of the radiation field (P = 0.047) significantly increased the risk of biochemical failure following salvage RT. The results of the univariate analyses supported the results of the multivariate analysis. The Gleason score (P = 0.013), seminal vesicle invasion (P = 0.003) and LI (P = 0.001) were significantly associated with biochemical failure.
Table 2:
Univariate and multivariate analysis for the prediction of salvage radiotherapy outcome
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Prognostic factors | Hazard ratio | 95% CId | P value | Hazard ratio | 95% CI | P value |
| Age | 1.01 | 0.95–1.08 | 0.832 | 0.90 | 0.80–1.02 | 0.083 |
| Pathologic stage | 1.59 | 1.06–2.40 | 0.028 | 0.54 | 0.13–2.30 | 0.398 |
| Gleason score | 1.62 | 1.11–2.36 | 0.013 | 1.80 | 1.06–3.07 | 0.030 |
| Negative surgical margins | 1.01 | 0.44–2.28 | 0.998 | 11.16 | 1.53–81.36 | 0.018 |
| Seminal vesicle invasion | 4.25 | 1.65–11.00 | 0.003 | 41.67 | 0.01–0.34 | 0.006 |
| Extracapsular invasion | 0.18 | 0.06–0.56 | 0.004 | 0.43 | 0.30–6.15 | 0.534 |
| Lymphatic vessel invasion | 4.35 | 1.86–10.18 | 0.001 | 500.00 | 24.39–12119.75 | <0.0001 |
| Negative vascular invasion | 0.59 | 0.26–1.34 | 0.203 | 8.04 | 1.62–40.03 | 0.011 |
| Perineural infiltration | 1.97 | 0.84–4.63 | 0.120 | 0.58 | 0.12–2.93 | 0.508 |
| PSAa doubling time | 0.97 | 0.88–1.06 | 0.461 | 1.31 | 0.92–1.87 | 0.136 |
| Preradiotherapy PSA level | 1.28 | 0.88–1.86 | 0.203 | 3.54 | 1.61–7.76 | 0.017 |
| Time from RPb to RTc | 1.00 | 1.00–1.01 | 0.962 | 1.00 | 1.00–1.01 | 0.671 |
| Time from biochemical failure to RT | 1.00 | 0.99–1.02 | 0.903 | 1.01 | 0.98–1.04 | 0.546 |
| Prescribed radiation dose | 0.98 | 0.93–1.02 | 0.250 | 0.85 | 0.75–0.96 | 0.010 |
| Size of radiation field at gantry 0° | 0.99 | 0.95–1.03 | 0.522 | 1.07 | 1.01–1.14 | 0.047 |
aPSA = prostate-specific antigen
bRP = radical prostatectomy
cRT = radiotherapy
dCI = confidence interval.
Comparison of bDFS curves
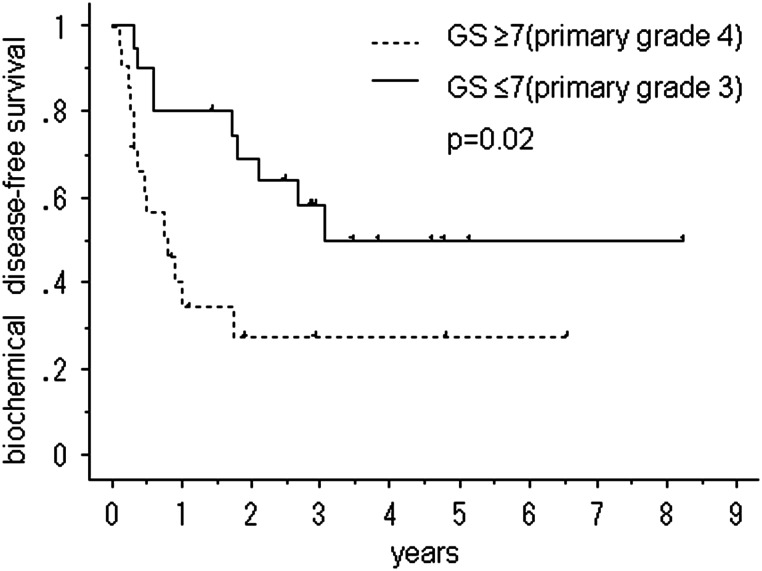
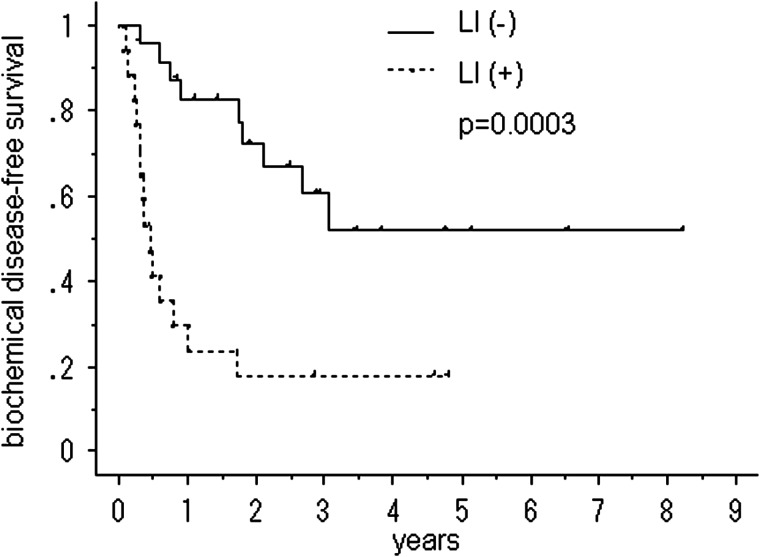
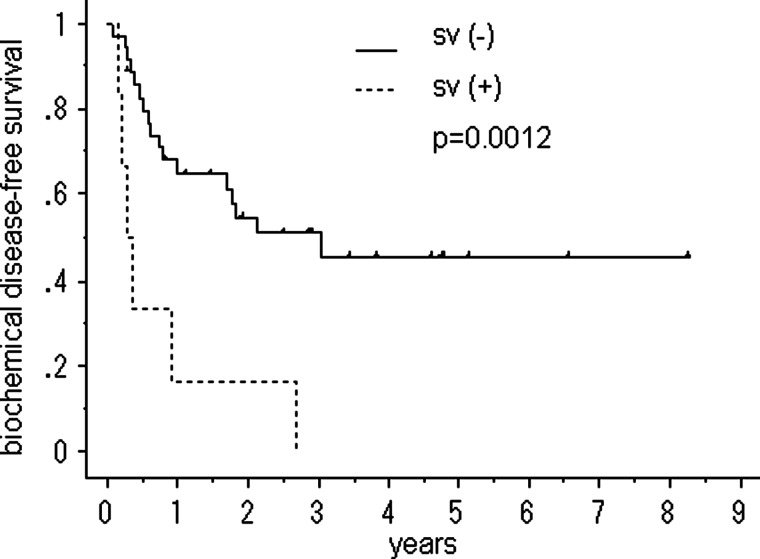
Figures 2–4 show the cumulative bDFS curves according to significant prognostic factors. The differences between the survival curves were statistically significant for a Gleason score of <3 + 4 vs. greater than 4 + 3 (50% vs. 28%, 5-year bDFS, P = 0.018; Fig. 2), the LI status (52% vs. 18%, P = 0.001; Fig. 3), the status of seminal vesicle invasion (45% vs. 0%, P = 0.002; Fig. 4) and a preradiotherapy PSA level of > 0.2 ng/ml vs. < 0.2 ng/ml (59% vs. 15%, P = 0.036). The survival curves for other factors were not significantly different: surgical margin status (P = 1.00); VI status (P = 0.20); radiation dose (P = 0.80) and size of radiation field (P = 0.49). Among the patients with LI, neither the radiation dose (P = 0.31) nor the size of the radiation field (P = 0.13) improved the bDFS.
Fig. 2.
Biochemical disease-free survival after salvage radiotherapy following a radical prostatectomy, stratified according to the Gleason score (GS).
Fig. 3.
Biochemical disease-free survival after salvage radiotherapy following a radical prostatectomy, stratified according to lymphatic vessel invasion (LI).
Fig. 4.
biochemical disease-free survival after salvage radiotherapy following a radical prostatectomy, stratified according to seminal vesicle invasion (sv).
After salvage RT, 76% patients achieved a PSA nadir of <0.2 ng/ml and 51% patients achieved a nadir of <0.05 ng/ml. A Kaplan–Meier analysis showed that the cumulative bDFS curves differed significantly between patients with a postradiotherapy PSA nadir of < 0.05 ng/ml and those with a nadir of > 0.05 ng/ml (75% vs. 10% for 5-year bDFS, P < 0.0001). In a logistic regression analysis, a negative surgical margin (P = 0.020), LI (P = 0.0001) and the preradiotherapy PSA level (P = 0.0002) were significantly associated with a postradiotherapy PSA nadir of >0.05 ng/ml.
DISCUSSION
The target of salvage RT following RP is to control local recurrence in the prostate bed leading to biochemical failure. However previous studies have reported that almost half of patients experience biochemical failure within 5 years [5–8]. Biochemical failure was found to increase the risks of distance metastasis and death [3, 4]. Biochemical failure after salvage RT suggests the presence of cancer cells beyond the radiation field or the presence of residual cancer cells within the radiation field. Patients with a rapid PSA-DT (<10 months), a high Gleason score (≧8), a short interval between prostatectomy and biochemical failure (1–2 years), lymph node metastasis and seminal vesicle invasion are more likely to develop distant metastasis than local recurrence [3, 11, 12]. In addition, previously reported prognostic factors for biochemical failure following salvage RT include the preprostatectomy PSA level, the preradiotherapy PSA level, the disease stage and grade, the Gleason score, the surgical margin status and the presence of seminal vesicle invasion, LVI, and perineural invasion as well as the radiation dose [5, 7, 8–10, 16].
The results of our study are almost identical to those of previous studies. The Gleason score, seminal vesicle invasion and LI were considered to be reliable predictors of biochemical failure following salvage RT, since the results of the univariate analysis, multivariate analysis and Kaplan–Meier analysis were consistent. In addition, a preradiotherapy PSA level and a negative surgical margin were independent predictors of biochemical failure following salvage RT in the multivariate analysis. The previous study already reported that negative surgical margin increase the risk of biochemical failure [8], because it suggested high risk of distant micrometastasis at the beginning of the period of salvage RT. On the other hand, PSA-DT and the interval from RP to postoperative biochemical failure were not predictors of the treatment outcome in the present series. The reason for this discrepancy may be related to the population that was used in the present study. Previous studies have reported that a shorter PSA-DT (<10 months) and the interval from RP to postoperative biochemical failure (1–2 years) were associated with the failure of salvage RT [3, 11, 12]. From this perspective, most of the patients in the present study had a high risk of biochemical failure, since the median PSA-DT was 4.8 months and the median interval from RP to postprostatectomy biochemical failure was 370 days. Furthermore, the characteristics of the enrolled patients were thought to be one of the reasons why the 5-year bDFS rate was 38% in our study, which was somewhat lower than in previous reports [5–7].
Some previous reports have examined the frequency of LVI and the association between LVI and the outcome of salvage RT. The reported frequency of LVI ranged widely from 5 to 53% [13, 17, 18]. Some authors reported that LVI was associated with disease progression and distant metastasis after RP [13, 16], whereas others did not [18]. However, these reports combined LI and VI as LVI. We distinguished between LI and VI and found that LI was an independently significant predictive factor of a poor outcome of salvage RT, while VI was not significantly associated with an inferior outcome of treatment. This finding might resolve the discrepancies among previous reports regarding the association between LVI and the outcome of salvage RT. The reason why negative VI increases the risk of biochemical failure is abstruse. Although the statistical problem of significance caused by small group setting was undeniable, the velocity of vascular metastasis might be more gradual than LI especially in case of vein invasion.
The radiation dose of salvage RT for local recurrence or biochemical failure following RP has been recommended to be greater than 64 Gy [5, 6]; however, an optimal dose has not yet been defined. In our study, the multivariate analysis suggested that dose escalation may slightly decrease the risk of biological failure. However, no significant difference was observed between bDFS curves constructed according to the radiation dose using a Kaplan–Meier analysis; thus, other confounding factors were thought to be present. The radiation dose also did not cause a significant difference in the bDFS among the patients with LI. Nevertheless, the efficacy of dose escalation among patients with LI may become evident if the number of cases is increased and if a multivariate method is used.
Regarding the widening of the radiation field, the results of the univariate analysis differed from those of the multivariate analysis, and the P values of the analyses were too large to prove a relation between the size of the radiation field and the treatment outcome. The reason was assumed to be that because the radiation field was directed at the prostate bed, the size of the radiation field largely depended on the patient's preoperative prostate volume. Therefore, the efficacy of extended irradiation to the pelvic region was not investigated in this study. Since cancer cells are thought to migrate from lymphatic vessels in the prostate to regional lymph nodes, the early initiation of RT before the cancer cells migrate to a distant area or additional radiation beyond the prostate bed might offer some benefit to patients with LI and no lymph node metastasis. A prospective study is needed to resolve this problem.
Though the diagnosis of biochemical failure is important for the initiation of secondary therapy, such as salvage RT, a standardized definition of biochemical failure after RP does not exist. Most investigators have suggested a PSA level of 0.2 ng/ml [3] or 0.4 ng/ml [19] as a PSA cut-off point for biochemical failure. While the PSA level prior to salvage RT is considered to be a predictor of the outcome of salvage RT and overall survival, the reported preradiotherapy PSA cut-off points for successful treatment range widely from 0.2 ng/ml to 2.5 ng/ml [6, 8, 10]. In our study, the difference between the overall bDFS rates of patients with a preradiotherapy PSA level > 0.2 ng/ml and those with a level < 0.2 ng/ml was significant. Among patients with LI, 3 of the 17 patients who received salvage RT before the preradiotherapy PSA level reached 0.21 ng/ml did not experience biochemical failure following salvage RT. Meanwhile, 12 of the other 14 patients with LI who received salvage RT after their PSA level had risen above 0.2 ng/ml experienced biochemical failure. These findings suggest that for high-risk patients, especially those with LI, a PSA level of >0.2 ng/ml may be too late for the initiation of salvage RT, and earlier RT before the PSA level reaches 0.2 ng/ml seems to be advisable.
Geinitz et al. reported that distant metastases were more common in patients with a postradiotherapy PSA nadir >0.05 ng/ml [20]. We investigated the association between the prognostic factors and the postradiotherapy PSA nadir level and examined the outcome of patients with a postradiotherapy PSA nadir >0.05 ng/ml. We identified several factors such as LI, a negative surgical margin and the preradiotherapy PSA level as being significantly associated with a high postradiotherapy PSA nadir of >0.05 ng/mL using a logistic regression analysis. These factors were the same as the predictors of biochemical failure following salvage RT. The overall 5-year bDFS rate of patients with a postradiotherapy PSA nadir >0.05 ng/mL was calculated to be 10% using the Kaplan–Meier method. These facts indicate that patients with LI, a negative surgical margin and a high preradiotherapy PSA are highly likely to experience a postradiotherapy PSA nadir of >0.05 ng/ml, subsequent biochemical failure and distant metastasis. Thus, a postradiotherapy PSA nadir of >0.05 ng/ml may predict a poor outcome of salvage RT and a poor prognosis.
A limitation of our study is its retrospective design and the small group setting. A randomized clinical trial is needed to define the most appropriate technique for performing salvage RT. Furthermore, since the methods and sensitivities of PSA measurements have changed, studies with a unified protocol are needed.
In conclusion, the pathological features affected the outcome of salvage RT following RP. LI was strongly associated with a risk of biochemical failure despite salvage RT. Meanwhile, VI was not a significantly hazardous factor for biochemical failure.
ACKNOWLEDGEMENTS
We thank Dr Jiro Kumagai (Department of Pathology, Yokohama City Minato Red Cross Hospital) for his pointed advice on the pathological findings.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, et al. Cancer Statistics. A Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Lu-Yao GL, Friedman M, Yao SL. Use of radical prostatectomy among Medicare beneficiaries before and after the introduction of prostate specific antigen testing. J Urol. 1997;157:2219–22. [PubMed] [Google Scholar]
- 3.Pound CR, Partin AW, Eisenberger MA, et al. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 4.Choueiri TK, Chen MH, D'Amico AV, et al. Impact of postoperative prostate-specific antigen disease recurrence and the use of salvage therapy on the risk of death. Cancer. 2010;116:1887–92. doi: 10.1002/cncr.25013. [DOI] [PubMed] [Google Scholar]
- 5.Anscher MS, Clough R, Dodge R. Radiotherapy for a rising prostate-specific antigen after radical prostatectomy: the first 10 years. Int J Radiat Oncol Biol Phys. 2000;48:369–75. doi: 10.1016/s0360-3016(00)00645-3. [DOI] [PubMed] [Google Scholar]
- 6.Cox JD, Gallagher MJ, Hammond EH, et al. Consensus statements on radiation therapy of prostate cancer: guidelines for prostate re-biopsy after radiation and for radiation therapy with rising prostate-specific antigen levels after radical prostatectomy. American Society for Therapeutic Radiology and Oncology Consensus Panel. J Clin Oncol. 1999;17:1155–63. doi: 10.1200/JCO.1999.17.4.1155. [DOI] [PubMed] [Google Scholar]
- 7.Pisansky TM, Kozelsky TF, Myers RP, et al. Radiotherapy for isolated serum prostate specific antigen elevation after prostatectomy for prostate cancer. J Urol. 2000;163:845–50. [PubMed] [Google Scholar]
- 8.Stephenson AJ, Shariat SF, Zelefsky MJ, et al. Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA. 2004;291:1325–32. doi: 10.1001/jama.291.11.1325. [DOI] [PubMed] [Google Scholar]
- 9.Liauw SL, Webster WS, Pistenmaa DA, et al. Salvage radiotherapy for biochemical failure of radical prostatectomy: a single-institution experience. Urology. 2003;61:1204–10. doi: 10.1016/s0090-4295(03)00044-x. [DOI] [PubMed] [Google Scholar]
- 10.Leventis AK, Shariat SF, Kattan MW, et al. Prediction of response to salvage radiation therapy in patients with prostate cancer recurrence after radical prostatectomy. J Clin Oncol. 2001;19:1030–9. doi: 10.1200/JCO.2001.19.4.1030. [DOI] [PubMed] [Google Scholar]
- 11.Partin AW, Pearson JD, Landis PK, et al. Evaluation of serum prostate-specific antigen velocity after radical prostatectomy to distinguish local recurrence from distant metastases. Urology. 1994;43:649–59. doi: 10.1016/0090-4295(94)90180-5. [DOI] [PubMed] [Google Scholar]
- 12.Pound CR, Partin AW, Epstein JI, et al. Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol Clin North Am. 1997;24:395–406. doi: 10.1016/s0094-0143(05)70386-4. [DOI] [PubMed] [Google Scholar]
- 13.Shariat SF, Khoddami SM, Saboorian H, et al. Lymphovascular invasion is a pathological feature of biologically aggressive disease in patients treated with radical prostatectomy. J Urol. 2004;171:1122–7. doi: 10.1097/01.ju.0000113249.82533.28. [DOI] [PubMed] [Google Scholar]
- 14.Kihara K, Kobayashi T, Kawakami S, et al. Minimum incision endoscopic surgery (MIES) in Japanese urology: results of adrenalectomy, radical nephrectomy and radical prostatectomy. Aktuelle Urol. 2010;41(Suppl 1):S15–9. doi: 10.1055/s-0029-1224662. [DOI] [PubMed] [Google Scholar]
- 15.Schmid HP, McNeal JE, Stamey TA. Observations on the doubling time of prostate cancer. The use of serial prostate-specific antigen in patients with untreated disease as a measure of increasing cancer volume. Cancer. 1993;71:2031–40. doi: 10.1002/1097-0142(19930315)71:6<2031::aid-cncr2820710618>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 16.Brooks JP, Albert PS, O'Connell J, et al. Lymphovascular invasion in prostate cancer: prognostic significance in patients treated with radiotherapy after radical prostatectomy. Cancer. 2006;106:1521–6. doi: 10.1002/cncr.21774. [DOI] [PubMed] [Google Scholar]
- 17.Salomao DR, Graham SD, Bostwick DG. Microvascular invasion in prostate cancer correlates with pathologic stage. Arch Pathol Lab Med. 1995;119:1050–4. [PubMed] [Google Scholar]
- 18.Bahnson RR, Dresner SM, Gooding W, et al. Incidence and prognostic significance of lymphatic and vascular invasion in radical prostatectomy specimens. Prostate. 1989;15:149–55. doi: 10.1002/pros.2990150208. [DOI] [PubMed] [Google Scholar]
- 19.Stephenson AJ, Kattan MW, Eastham JA, et al. Defining biochemical recurrence of prostate cancer after radical prostatectomy: a proposal for a standardized definition. J Clin Oncol. 2006;24:3973–8. doi: 10.1200/JCO.2005.04.0756. [DOI] [PubMed] [Google Scholar]
- 20.Geinitz H, Riegel MG, Thamm R, et al. Outcome after conformal salvage radiotherapy in patients with rising prostate-specific antigen levels after radical prostatectomy. Int J Radiat Oncol Biol Phys. 2012;82:1930–7. doi: 10.1016/j.ijrobp.2011.03.003. [DOI] [PubMed] [Google Scholar]