Abstract
The key pathogenetic event of many retinopathies is apoptosis of retinal cells. Our previous studies have demonstrated that Coenzyme Q10 (CoQ10) prevents apoptosis of corneal keratocytes both in vitro and in vivo, by virtue of its ability to inhibit mitochondrial depolarization, independently of its free radical scavenger role. The aim of this study was to evaluate whether CoQ10 can protect cultured retinal cells and the retinas of rats from radiation-induced apoptosis, if instilled as eye drops in the cornea. In vitro experiments were carried out on cultured ARPE-19 or RGC-5 cells pretreated with CoQ10 before eliciting apoptosis by UV- and γ-radiation, chemical hypoxia (Antimycin A) and serum starvation. Cell viability was evaluated by light microscopy and fluorescence activated cell sorting analysis. Apoptotic events were scored by time-lapse videomicroscopy. Mitochondrial permeability transition was evaluated by JC-1. The anti-apoptotic effectiveness of CoQ10 in retina was also evaluated by an in situ end-labeling assay in Wistar albino rats treated with CoQ10 eye drops prior to UV irradiation of the eye. CoQ10 substantially increased cell viability and lowered retinal cell apoptosis in response both to UV- and γ-radiation and to chemical hypoxia or serum starvation by inhibiting mitochondrion depolarization. In the rat, CoQ10, even when applied as eye drops on the cornea, protected all retina layers from UVR-induced apoptosis. The ability of CoQ10 to protect retinal cells from radiation-induced apoptosis following its instillation on the cornea suggests the possibility for CoQ10 eye drops to become a future therapeutic countermeasure for radiation-induced retinal lesions.
Keywords: retina, apoptosis, coenzyme Q10, radiation
INTRODUCTION
The pivotal role of apoptosis in the pathogenesis of retinopathies or retinal damage has been largely recognized. Paradoxically, although light is essential for vision, its ultraviolet component is the main exogenous stimulus of retinal damage [1]. Nevertheless, little is known about the impact of phototoxicity on the retina [2]. Light-induced damage is strictly dependent on radiation wavelength, with ultraviolet radiation (UVR) being the most hazardous. Nevertheless, while UVR themselves with wavelengths as short as 295 nm has been recorded in children [3], UVB (315–280 nm) is strongly absorbed by the lens and most UVC (280–100 nm) is cut off by the cornea in adults [4]. In particular, young people absorb about 25% total UVR into the anterior eye, whereas octogenarians absorb up to 80%. In rats, both UVB and UVC can reach the retina, altering its structure and functions [5]. Although UVR toxicity in rats may not be directly applicable to humans [6], UVR has been proven to induce damage that is not well understood in the human eye [7], apart from its responsibility in the onset of ciliary body and choroid melanoma [8]. Besides solar light, two main types of natural radiation have an impact on the human eye: they are radioactive nuclides originating from the Earth's crust and high-energy cosmic rays entering the Earth's atmosphere (UNSCEAR Report 2000 and 2008). Since their energy is higher than 10 eV, they induce direct ionization of molecules, including DNA fragmentation leading to apoptosis.
Excessive exposure to sunlight and particularly to UVR and natural radiation may lead to oxidative stress of eye tissues. Being highly metabolically active, the retina is particularly susceptible to such stress [9–13], with retinal pigmented epithelium (RPE) and even more so its degenerated counterpart in retinopathies being the most susceptible [10, 13, 14]. The importance of oxidative stress as a mediator of apoptosis [15–17], as well as its role in the onset and progression of retinal degeneration [18–22] suggest application of antioxidants and anti-apoptotic molecules as possible therapies.
A variety of genetic retinopathies are characterized by retinal cell death. In particular, mutations/deletions of the rpe65 gene in RPE and cone photoreceptor cells characterize severe forms of early-onset retinal dystrophy, including Leber congenital amaurosis [23], which are driven by RPE cell apoptosis. Since rpe65 is a well-studied gene and associated with known phenotypes, cells carrying rpe65-inactivating mutations constitute a meaningful model of retinal dystrophy.
The opening of the mitochondrial permeability transition pore (mPTP) followed by extrusion of apoptogenic molecules to the cytoplasm [24] is recognized as the main trigger of apoptosis. We previously showed both in vitro [25] and in vivo [26] that CoQ10 (or ubiquinone Q10) inhibited corneal keratocyte apoptosis with a much higher effectiveness with respect to other antioxidants. We then demonstrated that this was due to the ability of CoQ10 to inhibit the mitochondrial permeability transition [27], independently from CoQ10 antioxidant properties. However, Devun et al. [28] have shown that synthetic ubiquinones were able not only to inhibit but also to induce the opening of mPTP and that this depended on the cell type. On the other hands, Fato et al. [29] have reported that corneal administration of CoQ10 eye drops markedly increased CoQ10 vitreous levels, which raised the possibility that CoQ10 instilled as eye drops on the cornea could reach the retina. All the above observations prompted us to explore the possibility that, following its corneal instillation, CoQ10 could extend its anti-apoptotic properties to retinal cells, using UV- and γ-radiation as the main apoptotic stimuli.
MATERIALS AND METHODS
Cell lines
In vitro experiments were performed on the human retina pigmented epithelial (RPE) cell line ARPE-19 (ATCC; Manassas, VA, USA) and the rat ganglion cell line RGC-5 (obtained from Professor Neeraj Agarval, University of North Texas Health Science Center, Fort Worth, TX, USA). ARPE-19 cells were cultured in 50% Dulbecco's Modified Eagles Medium (DMEM) and 50% F12 medium supplemented with 10% fetal calf serum (FCS), 100 U/ml penicillin G and 100 μg/ml streptomycin, in a humidified incubator at 37°C in 5% CO2. RGC-5 cells were cultured in DMEM medium, supplemented with 10% FCS, 100 U/ml penicillin G and 100 μg/ml streptomycin, in a humidified incubator at 37°C in 5% CO2.
Cell treatments
Cells were irradiated with UVR at a dose of 15 mJ/cm2 (254 nm; UV Stratalinker 1800, Stratagene) or with γ-rays, chosen as paradigmatic of ionizing radiation, emitted by 0.03 µM 3H-thymidine (specific activity 35 Ci/mmol) corresponding to 20 µCi/ml, added to the culture medium. The respiratory chain blocker Antimycin A at 200 µM concentration and fetal bovine serum restriction to 0.5%, were also used as ischemia-mimetic apoptotic stimuli non-inducers of free radicals. Each damaging agent was applied at doses experimentally established to induce apoptosis rather than necrosis. Cell pre-treatment with 10-µM CoQ10 dissolved in 0.04% Lutrol F127, used as vehicle to ensure cellular uptake, began 2 hs before application of the apoptotic stimuli. Vehicle alone-treated cells were used as controls. The treatments proceeded for 24–72 h as indicated.
Silencing of rpe65 by siRNA
ARPE-19 cells were transfected using Lipofectamine2000 reagent (Life Technology, Carlsbad, CA, USA) with a combination of two siRNAs (Sigma-Aldrich, Munich, Germany) specific for the rpe65 and one siRNA (Sigma-Aldrich) specific for the GFP at 100 nM final concentration. The sequences of rpe-65 mRNA targeted by the siRNAs were: 5′-TCAGAATCAGGAGATAAGC-3′ and 5′-ATCAACCTGCTTAATTGTC-3′. The GFP mRNA sequence targeted by the siRNA was: 5′-CGGCAAGCTGACCCTGAAGTTCAT-3′. For evaluation of silencing efficacy, 48 h post-transfection cells were pelleted at 100 × g for 5 min at 4°C, washed with ice-cold phosphate-buffered saline (PBS) and lysed in radioimmunoprecipitation buffer (50 mM Tris-HCl, pH 7.5, 1 mM EDTA, 1% NP-40, 150 mM NaCl, 0.25 mM Pefabloc, 2 µM leupeptin, 0.3 µM aprotinin and 0.1 mM sodium orthovanadate). Following incubation on ice for 10 min, cells were vortexed for 10 s, then centrifuged for 20 min at 16 000 × g. Supernatants were saved and protein concentrations were determined using the Qubit™ fluorimeter (Life Technologies). For immunoblot analysis, protein extracts (50 µg) were separated with NuPAGE 12% Bis-Tris Gel (Life Technologies) and electroblotted (Trans-Blot® Semi-Dry apparatus; Bio-Rad, Hercules, CA, USA) onto Protran nitrocellulose transfer membranes (Perkin Elmer, Boston, MA, USA). Primary antibodies used were mouse monoclonal RPE65 (Chemicon, Millipore, Billerica, MA, USA) and β-Tubulin (Sigma-Aldrich, Munich, Germany). The secondary antibody was goat anti-mouse IRDye 800CW (Li-Cor, Lincoln, NE, USA). The protein bands were analyzed using the Odyssey Infrared Imaging System (Li-Cor, USA).
Quantification of living and apoptotic cells
The viability of retinal cells was evaluated microscopically and confirmed by fluorescence-activated cell sorting (FACS) analysis on Guava PCA, with Guava ViaCount reagent and CytoSoft software (Guava Technologies, 4000-0040, Hayward, CA, USA). The cumulative apoptotic events were scored by time-lapse videomicroscopy using a Zeiss inverted phase contrast microscope equipped with a 10× objective and Panasonic CCD cameras. After cell detachment from the substrate, an apoptotic event was counted the moment the cell had shrunk completely and blebbing started, as previously reported [27]. The time when most treated cells were dead and the number of remaining living cells was too low for comparison was taken to be the experiment endpoint.
JC-1 assay
The shift in mitochondrial permeability transition (ΔΨ) occurring during apoptosis was detected in ARPE-19 cells with a fluorescence-based assay. Cells were cultured on cover slips in six-well tissue culture plates. Following application of indicated apoptotic stimulus, cells were maintained in appropriate culture medium containing 2.5 mg/ml of the lipophilic cationic probe 5,5′,6,6′-tetrachloro-1,1′3,3′-tetraethylbenzimidazol-carbocyanine iodide (JC-1; Molecular Probes, Eugene, OR, USA) for 20 min at 37°C. This dye has a unique feature: at hyperpolarized membrane potentials (to –140 mV) it forms a red fluorescent J-aggregate, whereas at depolarized membrane potentials (to –100 mV) it remains in the green fluorescent monomeric form. Prior to detection, cells were washed in BPS and placed in an open slide-flow loading chamber that was mounted on the stage of a confocal scanning microscope (Bio-Rad) equipped with a krypton/argon laser source. The emitted fluorescence was monitored at 488- and 568-nm wavelengths with a Nikon plan Apo 60X oil-immersion objective.
The rat model
Experiments with rats were performed in compliance with the Italian law on animal care (no. 116/1992), which is in accordance with the guidelines established by the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals. All efforts were made to reduce both animal suffering and the number of animals used. Adult Wistar albino rats weighing 250–300 g were anesthetized by intramuscular injection of ketamine hydrochloride (30 mg/kg) and xylazine hydrochloride (5 mg/kg) and UV-irradiated for 1 min at a distance of 3 cm, with lids clamped open at an intensity of 14 mW/cm2, using a high pressure mercury arc lamp (Osram HBO 100W/2) equipped with two concave quartz lenses and a UV filter, as reported by Wichert et al. [30]. During the three preoperative days each right eye was treated four times daily by instillation of an eye drop solution containing 0.2% CoQ10 in 10% Lutrol 127, 5% Cremophor EL, 0.45% NaCl and 0.001% benzalconium chloride in H2O, in a preparation suitable for topical application on the eye surface. Each left eye was treated with the same eye drop solution without CoQ10. At the 36th hour after UV irradiation, rats were sacrificed, eyes were rapidly removed and sectioned for in situ end labeling analysis.
In Situ End Labeling (ISEL)
Paraffin-fixed blocks were prepared and the microtome-sliced retinas were affixed to glass slides and analyzed microscopically and by ISEL (Boehringer Mannheim, Mannnheim, Germany) analysis of nicked DNA as a marker of apoptosis, as described previously [31]. The counting of ISEL-positive nuclei was carried out by two different observers.
Statistical analysis
Data are presented as means ± SEM of at least three to four independent experiments. Statistical comparisons were made by using Student's t-test. A value of P < 0.05 was considered statistically significant.
RESULTS
CoQ10 protects retinal cells from apoptosis caused by UV- or γ-radiation, serum starvation and chemical hypoxia
Cultured retinal pigment epithelial cells (ARPE-19) were subjected to the free radical inducer stimuli UV (15 mJ/cm2) or γ (0.03 µM 3H-thymidine, 1 µCi/ml) irradiation, or to serum starvation (FCS 0.5%) and chemical hypoxia (Antimycin A, 200 µM) used as ischemia mimetic stimuli, non-inducers of free radicals in order to investigate the apoptotic effects of CoQ10 on retinal damage. The cultured retinal ganglion cells (RGC-5) were also challenged with UV irradiation. Two hours before application of damaging stimuli, cells were pretreated with CoQ10 (10 µM) (+CoQ10) or with vehicle alone (–CoQ10) used as a control.
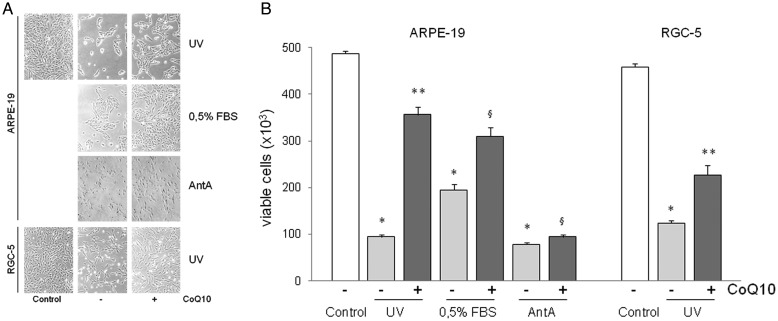
Figure 1A shows that ARPE-19 and RGC5 viable cells examined by light microscopy were markedly reduced following apoptotic stimuli, but to a much lower extent when CoQ10 was added to the culture medium. FACS analysis of viable cells with ViaCount reagent on Guava PCA is shown in Figure 1B. At 24 h following UV irradiation the number of ARPE-19 living cells dropped by 81% compared with untreated controls (from 487 ± 6 × 103 to 94 ± 4 × 103), but pretreatment with CoQ10 before UV irradiation markedly increased the number of living cells (356 ± 17 × 103), with a decrease of only 27%. Living cells following 72 h of serum starvation (0.5% FBS) diminished by 60% (195 ± 12 × 103) compared with the controls, but only by 36% when pretreated with CoQ10 (310 ± 18 × 103). Blocking the respiratory chain electron flow with Antimycin A (200 µM) for 48 h caused a very dramatic decrease (–84%) in the number of ARPE-19 living cells (78 ± 4 × 103) compared with the controls, but also in this case CoQ10 induced a relatively lower but significant reduction (–80%) in the number of living cells (94 ± 5 × 103) compared with the controls.
Fig. 1.
Evaluation of the number of living cultured ARPE-19 and RGC-5 cells pretreated (+CoQ10) or not (–CoQ10) with 10 µM CoQ10 for 2 h before being irradiated with UV (15 mJ/cm2) or maintained in a condition of serum starvation (0.5% FBS) or treated with 200 µM Antimycin A (AntA).
Untreated ARPE-19 cells were used as controls. (A) Light microscopy examination of ARPE19 cells, (B) FACS analysis of viable and non-viable nucleated cells with ViaCount reagent on Guava PCA. Data are means ±SEM of at least four to five experiments (*P < 0.001 vs. control; **P < 0.001 vs. to UV stimulated; § P < 0.005 vs. 0.5% FBS and Antimycin A stimulated).
Similar results were obtained with the RGC-5 cells subjected to UV irradiation. Treatment reduced RGC-5 cell viability by more than 72% compared with non-irradiated controls (from 459 ± 6 × 103 to 124 ± 5 × 103), but by 50% if cells were pretreated with CoQ10 (227 ± 21 × 103).
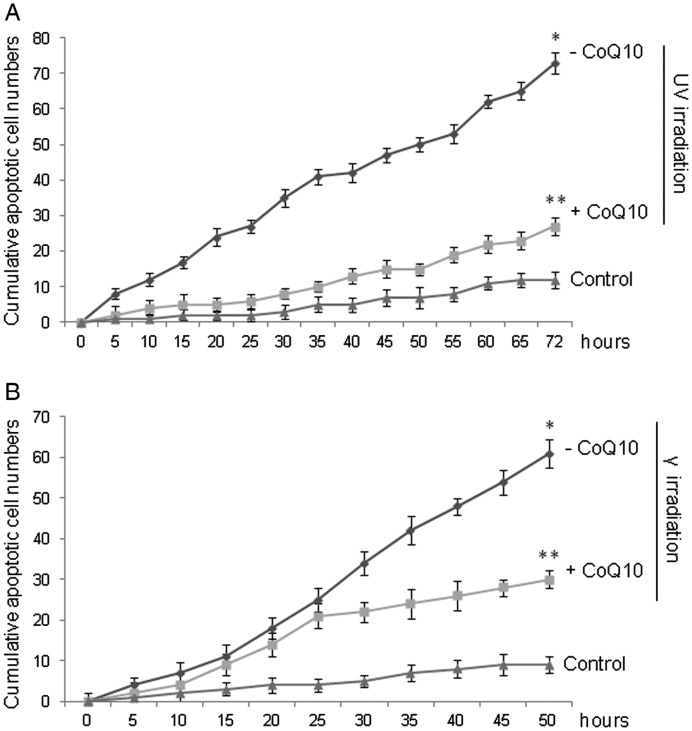
Scoring by time-lapse video-microscopy the cumulative apoptotic events in ARPE-19 cells subjected to UV or γ irradiation shown in Fig. 2 confirmed the anti-apoptotic effect of CoQ10. Indeed, UV irradiation of ARPE19 cells resulted in a progressive increase in the cumulative apoptotic events (Fig. 2A), whose number was six-fold higher (73 ± 3) than in the untreated controls (12 ± 2) at the 72nd hour. This number was only twice as high (27 ± 2) if cells were pretreated with CoQ10 2 h before UV irradiation. Similarly, γ radiation raised the number of cumulative apoptotic events 6.5-fold compared with the controls (from 9 ± 2 to 61 ± 3) at the 50th hour, which was considered the endpoint of these experiments as explained in the Materials and Methods (Fig. 2B). Also in this case, pre-treatment with CoQ10 halved the number of apoptotic events (30 ± 2) at the 50th hour.
Fig. 2.
Evaluation of anti-apoptotic effects of CoQ10 on ARPE-19 cells subjected to (A) UV irradiation (15 mJ/cm2) and (B) γ irradiation (20 µCi/ml). Where specified, the cells were pretreated (+CoQ10) or not (–CoQ10) with CoQ10. Untreated cells served as controls. The cumulative apoptotic events were detected and registered progressively by time-lapse videomicroscopy up to 72 h after stimulation. An apoptotic event was scored the moment the cell, after detachment from the substrate and shrinking, began apoptotic blebbing. Each value is the mean ± SEM of three experiments (*P < 0.005 –CoQ10 vs. control; **P < 0.01 +CoQ10 vs. –CoQ10).
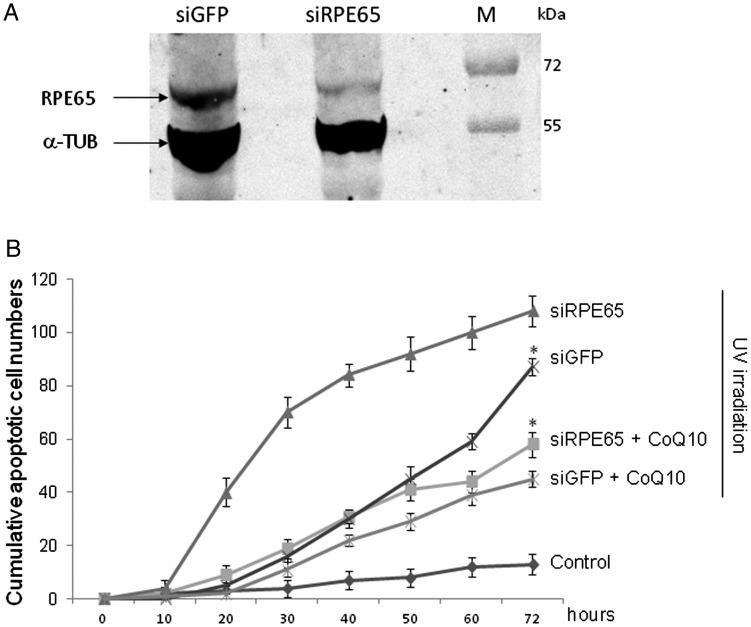
We then evaluated the effectiveness of CoQ10 in preventing the progression of retinopathies, extending our study to a cellular model of retinitis pigmentosa, a genetic retinopathy characterized by retinal cell hypersensitivity to the oxidative stress induced by sunlight and radiation, assumed as a retinopathy paradigm [32]. For this purpose we have used specific siRNAs to silence the rpe65 gene in the ARPE19 cell line, since RPE cell deletion is known to underlie retinitis pigmentosa, and then we challenged rpe65-silenced cells with UVR as described above. Figure 3A shows that the silencing of the rpe65 gene results in a significant reduction of relevant RPE65 protein, as compared with the control (siGFP). Figure 3B shows that ARPE19 cells knocked down for rpe65 are hypersensitive to UVR in terms of cumulative apoptotic events, which were significantly higher (siRPE65, 108 ± 6) at 72 h not only in comparison with the siGFP control (87 ± 4) but also with the UV-treated wt ARPE cells (73 ± 3, see Fig. 2A). Nevertheless, CoQ10 maintained its high efficacy, succeeding in reducing two-fold the number of apoptotic events in ARPE19 cells knocked-down for rpe65 compared with the CoQ10 untreated sample (from 108 ± 6 to 58 ± 5) at 72 h after UV irradiation.
Fig. 3.
(A) Immunoblot analysis of rpe65 or GFP silencing in ARPE-19 cells.
α-Tubulin served as an internal control (M is protein molecular weight marker). (B) Evaluation of anti-apoptotic effects by time-lapse videomicroscopy of CoQ10 on rpe65 or GFP knock-down ARPE-19 cells subjected to UV irradiation (15 mJ/cm2). Where specified, the cells were pretreated (+CoQ10) or not (–CoQ10) with CoQ10. Untreated cells served as controls. Each value is the mean ± SEM of three experiments (*P < 0.005 vs. siRPE65).
CoQ10 counteracts mitochondrial depolarization induced by apoptotic stimuli
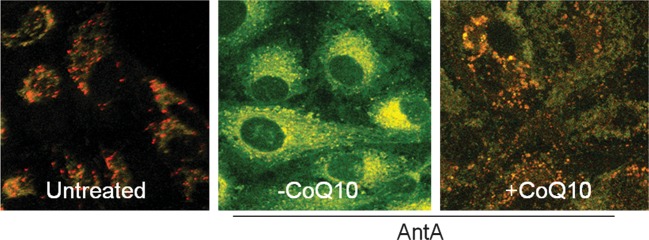
To evaluate if CoQ10 could prevent mitochondrion depolarization independently of its antioxidant property, chemical hypoxia, a non-inducer of free radical apoptotic stimulus, was used. Antimycin A treatment of ARPE-19 cells caused a shift of mitochondrial membrane charge (Δψ) evaluated by the uptake of JC-1, which evidences highly negative Δψ as fluorescent red-orange-stained mitochondria and low Δψ as an increase in fluorescent green-stained mitochondria (Fig. 4). CoQ10 counteracted this phenomenon, significantly preventing mitochondrial membrane depolarization in more than 50% of ARPE-19 cells examined.
Fig. 4.
Analysis of Δψ in JC-1 labeled mitochondria of ARPE-19 cells.
Representative dual emission confocal micrographs (525 and 590 nm) showing signals from monomeric (green) and J-aggregate (red) JC-1 fluorescence. The untreated (control) ARPE-19 showed the most cells with red-stained mitochondria (large negative Δψ). Most cells treated with 200 µM Antimycin A (–CoQ10) showed uniformly green-stained (loss of Δψ) mitochondria. Pretreatment with CoQ10 significantly protected cells against loss of Δψ as demonstrated by the maintenance of the red-stained mitochondria. Each image is representative of at least three independently performed experiments with similar results.
CoQ10 instilled in the cornea prevented UV-induced retinal damage in rats
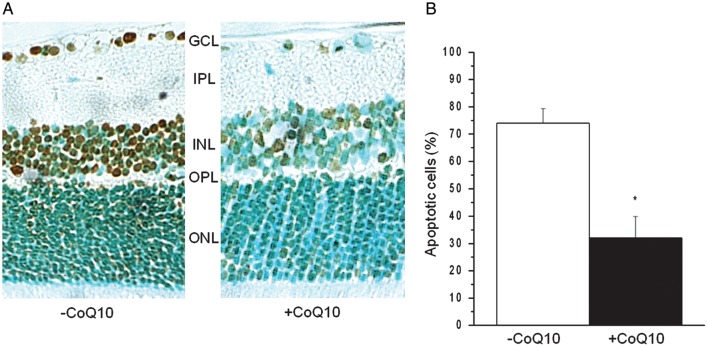
Wistar rats were sedated and treated with one drop of CoQ10 (right eye) solution or with the vehicle alone (left eye), administered 60 and 15 min before UV irradiation and 12, 24 and 36 h after UV irradiation (1 min, 14 mW/cm2, wavelength from 280 to 380 nm). The number of ISEL-positive apoptotic cells scored in all retinal layers at the 36th hour after UV irradiation (Fig. 5A) was dramatically reduced in the eyes treated with CoQ10 as compared with those treated with the vehicle alone. Quantification of ISEL-positive rat retinal cells counted in samples of ganglion (GCL), inner (INL) and outer (ONL) nuclear layer is reported as a percentage of ISEL-positive cells compared with the total number of counted cells (Fig. 5B). The high number (74 ± 5%) of retinal apoptotic cells in UV-irradiated eyes treated with the vehicle alone underwent a dramatic drop (more than 50%) in retinas explanted from eyes treated with CoQ10 instilled in the cornea (32 ± 8%).
Fig. 5.
Topical application of CoQ10 to rat cornea prevents retinal apoptosis in response to UV irradiation. (A) Representative microscopy image of the retina from UV-irradiated mice pretreated with CoQ10 (+CoQ10) or with the vehicle alone (–CoQ10) analyzed using the In Situ End Labeling (ISEL) technique of nicked DNA to detect DNA fragmentation. GCL (ganglion cell layer), IPL (inner plexiform layer), INL (inner nuclear layer), OPL (outer plexiform layer) and ONL (outer nuclear layer) are indicated. (B) The numbers of ISEL-positive (brown) and -negative (light blue) nuclei within at least three fields of retina specimens were scored by two different observers. The number of apoptotic cells was expressed as a percentage of total number of counted cells. Data are means ± SEM of three experiments; *P < 0.01.
DISCUSSION
Previous studies on anti-apoptotic molecules suitable for treatment of ophthalmologic diseases have shown that CoQ10 is significantly more effective than other antioxidants (Vitamin A, C, E) in preventing apoptosis of corneal keratocytes elicited by therapeutic excimer laser irradiation both in vitro [25] and in vivo [26]. This suggests that the anti-apoptotic property of CoQ10 could involve mechanisms independent from its antioxidant activity. The implication of complexes I and III of the respiratory chain with the mPTP and the association of ubiquinone Q10 with both complexes were in favor of this possibility, suggesting that CoQ10 could be part of the mPTP complex functioning as an inhibitor of its opening. Thereby, CoQ10 could prevent apoptosis independently from its role as a potent free radical scavenger. We confirmed this possibility by demonstrating that CoQ10 was able to prevent apoptosis in corneal keratocytes in response to a variety of apoptotic stimuli, both inducers and non-inducers of free radicals (ceramide, hypoxia, deprivation of growth factors), by maintaining the mPTP in a closed conformation [27].
The general effects of CoQ10 on the vision and, in particular, on retinal health have not been extensively investigated so far. A few studies have indicated that oral or intravitreal administration of CoQ10 significantly improved eyesight [33–35] and protected retinal cells against oxidative stress [36, 37] or high intraocular pressure-induced ischemia [38]. However, the molecular mechanisms have not been unraveled so far and the possibility that CoQ10 corneal eye drops could reach the retina to exert its antiapoptotic activity has not yet been explored.
In this paper we have shifted our studies on the anti-apoptotic property of CoQ10 from the cornea to the retina using UV- and γ-irradiation as the main apoptotic stimuli. The observation of Devun et al. [28] that various ubiquinone analogs can prevent or induce the opening of mPTP and, therefore, apoptosis, depending on the cell type, meant our investigation was not obvious. Our results clearly indicate that CoQ10 inhibits apoptosis both in RPE cells and in retinal ganglion cells (RGC), not only in response to free radical inducer stimuli, which were exciting (UVR) or ionizing (γ) radiation, but also in response to non-inducer free radical stimuli, which were chemical hypoxia and growth factor deprivation. This strongly suggests the role of CoQ10 is as a mitochondrion permeability transition inhibitor, as we previously demonstrated in corneal keratocytes [27] could be extended to RPE and RGC cells. We confirmed this possibility by demonstrating that CoQ10 was able to prevent mitochondrial depolarization in ARPE-19 cells also in response to chemical hypoxia (Antimycin A), an apoptotic stimulus that did not induce free radical production.
It should be noted that we also demonstrate for the first time that instillation of CoQ10 in the cornea of a rat model of UVR-induced retinal damage results in apoptosis inhibition in all retinal layers. The report of Fato et al. [29] that corneal administration of CoQ10 eye drops markedly increased CoQ10 vitreous levels suggests that CoQ10 could protect retinal layers from apoptosis by reaching the retina. Nevertheless, the possibility also exists that the presence of CoQ10 in the cornea or in the vitreous body may inhibit UVR-induced apoptosis by interfering with UVR reaching retinal layers consequently to its absorbance. Furthermore, the significant decrease of endogenous CoQ10 in the choroid and retina during aging (by about 40% in humans older than 80 years of age compared with those under 30 years of age) reported by Qu et al. [39], suggests that the age dependence of dry age-related macular degeneration progression may be consequent to this reduction and raises the possibility that enhancing CoQ10 levels in the retina, especially in older patients, could have therapeutic value.
The results reported in this paper and discussed above confirm our working hypothesis that CoQ10 is able to prevent UV- and γ-radiation-induced apoptosis of retinal cells by inhibiting mitochondrial permeability transition. In addition, the ability of CoQ10 to prevent UVR-induced apoptosis also in rpe65-silenced ARPE-19 cells, chosen as a cellular model of retinal degeneration, suggests that CoQ10 could be effective also in inhibiting the progression of retinopathies, for which solar UVR is recognized as one of the main causes. Furthermore, the evidence that CoQ10 extends its anti-apoptotic property also to retinal cells committed to apoptosis by serum deprivation or chemical hypoxia allows us to hypothesize a general therapeutic application of CoQ10 eye drops for the treatment of apoptosis-sustained retinopathies. In conclusion, the ability of CoQ10 eye drops instilled on the cornea to prevent UVR-induced apoptosis in all retinal layers suggests that it would be a good idea to evaluate the possibility that CoQ10 eye drops could become a future easily administrable therapy for treatment of acute retinal lesions induced by UVR.
ACKNOWLEDGEMENTS
This work was supported by grants from the Agenzia Spaziale Italiana (ASI) and Ente Cassa di Risparmio di Firenze (ECRF).
REFERENCES
- 1.Boulton M, Rózanowska M, Rózanowski B. Retinal photodamage. J Photochem Photobiol B. 2001;64:144–61. doi: 10.1016/s1011-1344(01)00227-5. [DOI] [PubMed] [Google Scholar]
- 2.Roduit R, Schorderet DF. MAP kinase pathways in UV-induced apoptosis of retinal pigment epithelium ARPE19 cells. Apoptosis. 2008;13:343–53. doi: 10.1007/s10495-008-0179-8. [DOI] [PubMed] [Google Scholar]
- 3.Brainard GC, Beacham S, Sanford BE, et al. Near ultraviolet radiation elicits visual evoked potentials in children. Clin Neurophysiol. 1999;110:379–83. doi: 10.1016/s1388-2457(98)00022-4. [DOI] [PubMed] [Google Scholar]
- 4.Gillardon F, Zimmermann M, Uhlmann E. Expression of c-Fos and c-Jun in the cornea, lens, and retina after ultraviolet irradiation of the rat eye and effects of topical antisense oligodeoxynucleotides. Br J Ophthalmol. 1995;79:277–81. doi: 10.1136/bjo.79.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roduit R, Schorderet DF. MAP kinase pathways in UV-induced apoptosis of retinal pigment epithelium ARPE19 cells. Apoptosis. 2008;13(3):343–53. doi: 10.1007/s10495-008-0179-8. [DOI] [PubMed] [Google Scholar]
- 6.Dillon J, Zheng L, Merriam JC, et al. The optical properties of the anterior segment of the eye: implications for cortical cataract. Exp Eye Res. 1999;68:785–95. doi: 10.1006/exer.1999.0687. [DOI] [PubMed] [Google Scholar]
- 7.de Oliveira Miguel NC, Meyer-Rochow VB, Allodi S. A structural study of the retinal photoreceptor, plexiform and ganglion cell layers following exposure to UV-B and UV-C radiation in the albino rat. Micron. 2003;34(8):395–404. doi: 10.1016/S0968-4328(03)00081-7. [DOI] [PubMed] [Google Scholar]
- 8.Vajdic CM, Kricker A, Giblin M, et al. Sun exposure predicts risk of ocular melanoma in Australia. Int J Cancer. 2002;101:175–82. doi: 10.1002/ijc.10579. [DOI] [PubMed] [Google Scholar]
- 9.Cai JY, Nelson KC, Wu M, et al. Oxidative damage and protection of the RPE. Prog Retin Eye Res. 2000;19:205–21. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- 10.Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp Eye Res. 2003;76:397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- 11.Zuclich JA. Ultraviolet-induced photochemical damage in ocular tissues. Health Phys. 1989;56:671–82. doi: 10.1097/00004032-198905000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Kerr JB, McElroy CT. Evidence for large upward trends of UVB radiation linked to ozone depletion. Science. 1993;262:1032–4. doi: 10.1126/science.262.5136.1032. [DOI] [PubMed] [Google Scholar]
- 13.Biesemeier A, Kokkinou D, Julien S, et al. UV-A induced oxidative stress is more prominent in naturally pigmented aged human RPE cells compared to non-pigmented human RPE cells independent of zinc treatment. J Photochem Photobiol B. 2008;90:113–20. doi: 10.1016/j.jphotobiol.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 14.Beatty S, Koh H, Phil M, et al. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2000;45:115–34. doi: 10.1016/s0039-6257(00)00140-5. [DOI] [PubMed] [Google Scholar]
- 15.Clutton S. The importance of oxidative stress in apoptosis. Br Med Bull. 1997;53:662–8. doi: 10.1093/oxfordjournals.bmb.a011637. [DOI] [PubMed] [Google Scholar]
- 16.Pourzand C, Tyrrell RM. Apoptosis, the role of oxidative stress and the example of solar UV radiation. Photochem Photobiol. 1999;70:380–90. [PubMed] [Google Scholar]
- 17.Buttke TM, Sandstrom PA. Oxidative stress as a mediator of apoptosis. Immunol Today. 1994;15:7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 18.Bailey TA, Kanuga N, Romero IA, et al. Oxidative stress affects the junctional integrity of retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2004;45(2):675–84. doi: 10.1167/iovs.03-0351. [DOI] [PubMed] [Google Scholar]
- 19.Sacca SC, Bolognesi C, Battistella A, et al. Gene-environment interactions in ocular diseases. Mutat Res. 2009;667(1–2):98–117. doi: 10.1016/j.mrfmmm.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Lu L, Oveson BC, Jo YJ, et al. Increased expression of glutathione peroxidase 4 strongly protects retina from oxidative damage. Antioxid Redox Signal. 2009;11(4):715–24. doi: 10.1089/ars.2008.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Usui S, Oveson BC, Lee SY, et al. NADPH oxidase plays a central role in cone cell death in retinitis pigmentosa. J Neurochem. 2009;110(3):1028–37. doi: 10.1111/j.1471-4159.2009.06195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sari A, Adiguzel U, Canacankatan N, et al. Effects of intravitreal bevacizumab in repeated doses: an experimental study. Retina. 2009;29(9):1346–55. doi: 10.1097/IAE.0b013e3181b26343. [DOI] [PubMed] [Google Scholar]
- 23.Cideciyan AV. Leber Congenital Amaurosis due to RPE65 mutations and its treatment with gene therapy. Prog Retin Eye Res. 2010;29:398–427. doi: 10.1016/j.preteyeres.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasola A, Bernardi P. The mitochondrial permeability transition pore and its involvement in cell death and in disease pathogenesis. Apoptosis. 2007;5:815–33. doi: 10.1007/s10495-007-0723-y. [DOI] [PubMed] [Google Scholar]
- 25.Brancato R, Schiavone N, Siano S, et al. Prevention of corneal keratocyte apoptosis after argon fluoride excimer laser irradiation with the free radical scavenger ubiquinone Q10. Eur J Ophthalmol. 2000;10:32–8. doi: 10.1177/112067210001000106. [DOI] [PubMed] [Google Scholar]
- 26.Brancato R, Fiore T, Papucci L, et al. Concomitant effect of topical ubiquinone Q10 and vitamin E to prevent keratocyte apoptosis after excimer laser photoablation in rabbits. J Refract Surg. 2002;18:135–39. doi: 10.3928/1081-597X-20020301-06. [DOI] [PubMed] [Google Scholar]
- 27.Papucci L, Schiavone N, Witort E, et al. Coenzyme Q10 prevents apoptosis by inhibiting mitochondrial depolarization independently of its free radical scavenging property. JBC. 2003;278:28220–8. doi: 10.1074/jbc.M302297200. [DOI] [PubMed] [Google Scholar]
- 28.Devun F, Walter L, Belliere J, et al. Ubiquinone analogs: a mitochondrial permeability transition pore-dependent pathway to selective cell death. PLoS One. 2012;5:e11792. doi: 10.1371/journal.pone.0011792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fato R, Bergamini C, Leoni S, et al. Coenzyme Q10 vitreous levels after administration of coenzyme Q10 eyedrops in patients undergoing vitrectomy. Acta Ophthalmologica. 2009;3:1–2. doi: 10.1111/j.1755-3768.2009.01632.x. [DOI] [PubMed] [Google Scholar]
- 30.Wickert H, Zaar K, Grauer A, et al. Differential induction of proto-oncogene expression and cell death in ocular tissues following ultraviolet irradiation of the rat eye. Br J Ophthalmol. 1999;83:225–30. doi: 10.1136/bjo.83.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nucci C, Schiavone N, Carella E, et al. Ganglionic cell apoptogenesis in glaucoma. Acta Ophthalmologica Scandinavica. 2000;7:24–7. [Google Scholar]
- 32.Bok D. The role of RPE65 in inherited retinal diseases. Retina. 2005;25:S61–2. doi: 10.1097/00006982-200512001-00028. [DOI] [PubMed] [Google Scholar]
- 33.Nucci C, Tartaglione R, Cerulli A, et al. Retinal damage caused by high intraocular pressure-induced transient ischemia is prevented by coenzyme Q10 in rat. Int Rev Neurobiol. 2007;82:397–406. doi: 10.1016/S0074-7742(07)82022-8. [DOI] [PubMed] [Google Scholar]
- 34.Feher J, Papale A, Mannino G, et al. Mitotropic compounds for the treatment of age-related macular degeneration. The metabolic approach and a pilot study. Ophthalmologica. 2003;217:351–7. doi: 10.1159/000071351. [DOI] [PubMed] [Google Scholar]
- 35.Feher J, Kovacs I, Artico M. Mitochondrial alterations of retinal pigment epithelium in age-related macular degeneration. Neurobiol Aging. 2006;27:983–93. doi: 10.1016/j.neurobiolaging.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 36.Nakajima Y, Inokuchi Y, Nishi M, et al. Coenzyme Q10 protects retinal cells against oxidative stress in vitro and in vivo. Brain Res. 2008;1226:226–33. doi: 10.1016/j.brainres.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 37.Feher J, Papale A, Mannino G, et al. Mitotropic compounds for the treatment of age-related macular degeneration. The metabolic approach and a pilot study. Ophthalmologica. 2003;217:351–7. doi: 10.1159/000071351. [DOI] [PubMed] [Google Scholar]
- 38.Nucci C, Tartaglione R, Rombolà L, et al. Neurochemical evidence to implicate elevated glutamate in the mechanisms of high intraocular pressure (IOP)-induced retinal ganglion cell death in rat. NeuroToxicology. 2005;26:935–41. doi: 10.1016/j.neuro.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Qu J, Kaufman Y, Washington I. Coenzyme Q10 in the human retina. IOVS. 2009;50:1814–18. doi: 10.1167/iovs.08-2656. [DOI] [PubMed] [Google Scholar]