Abstract
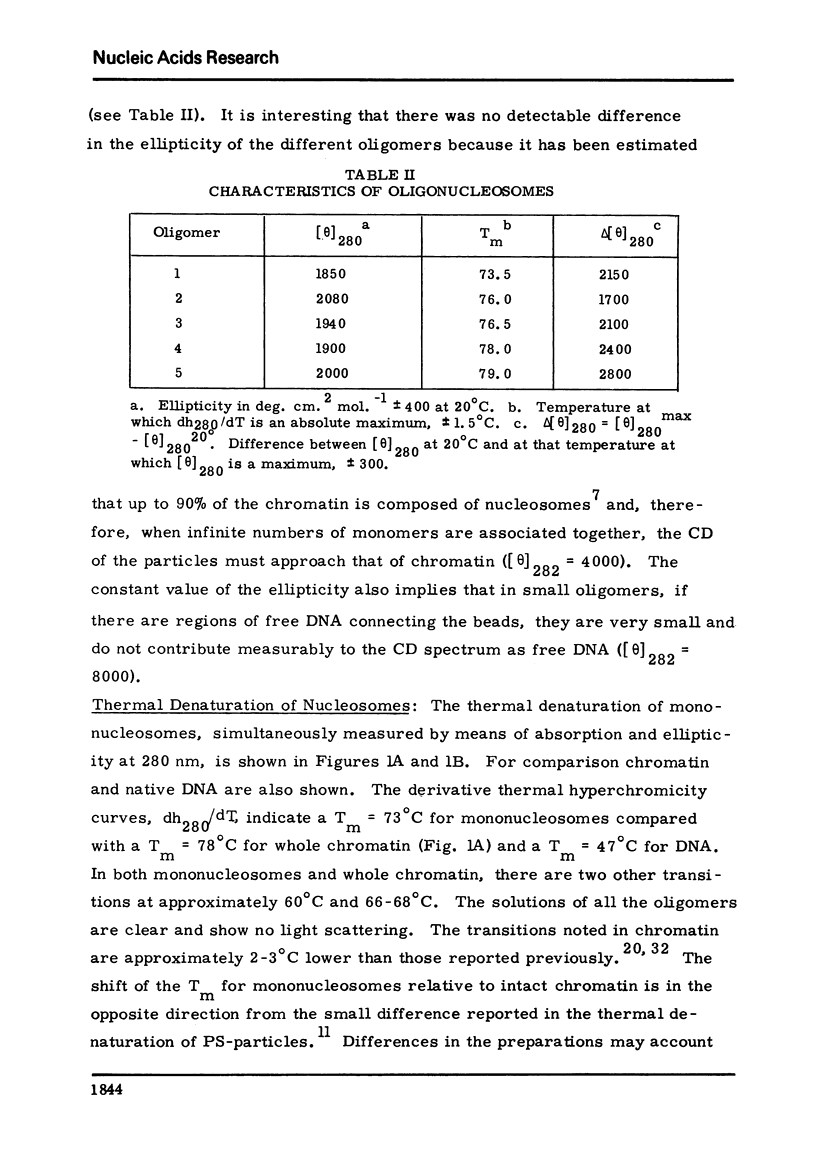
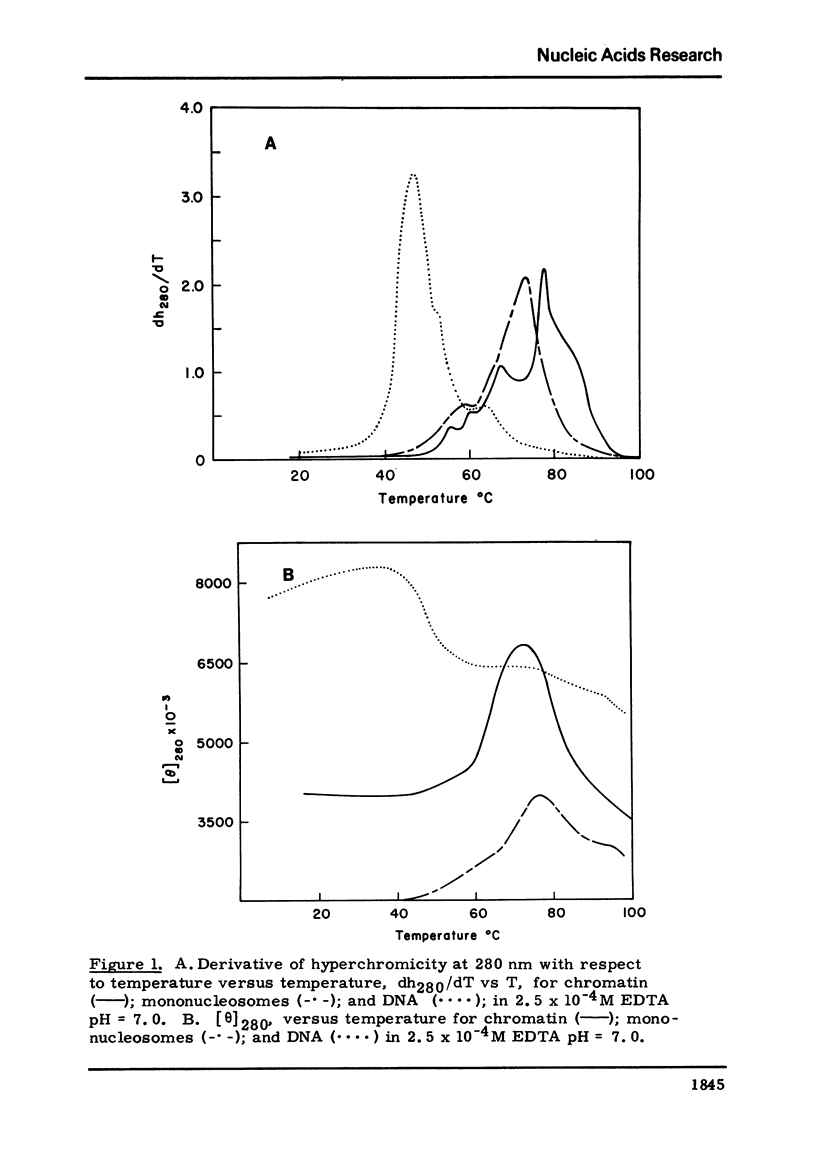
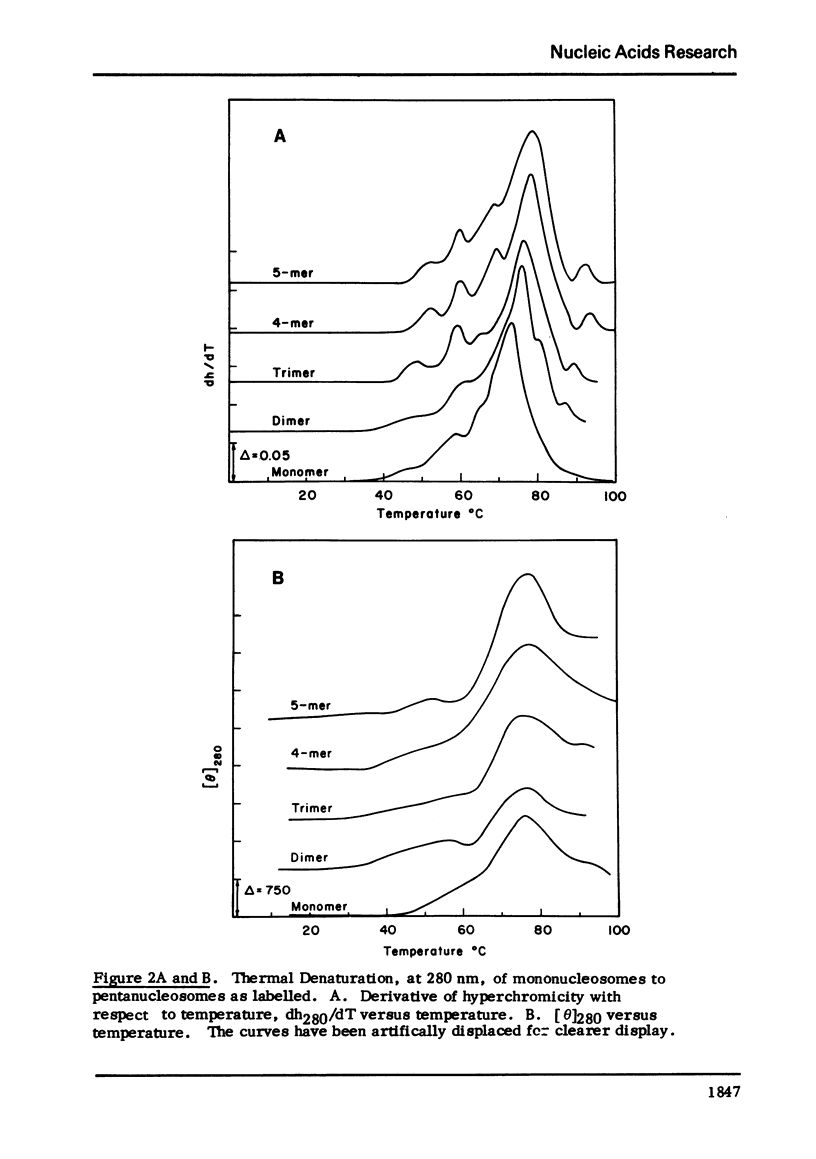
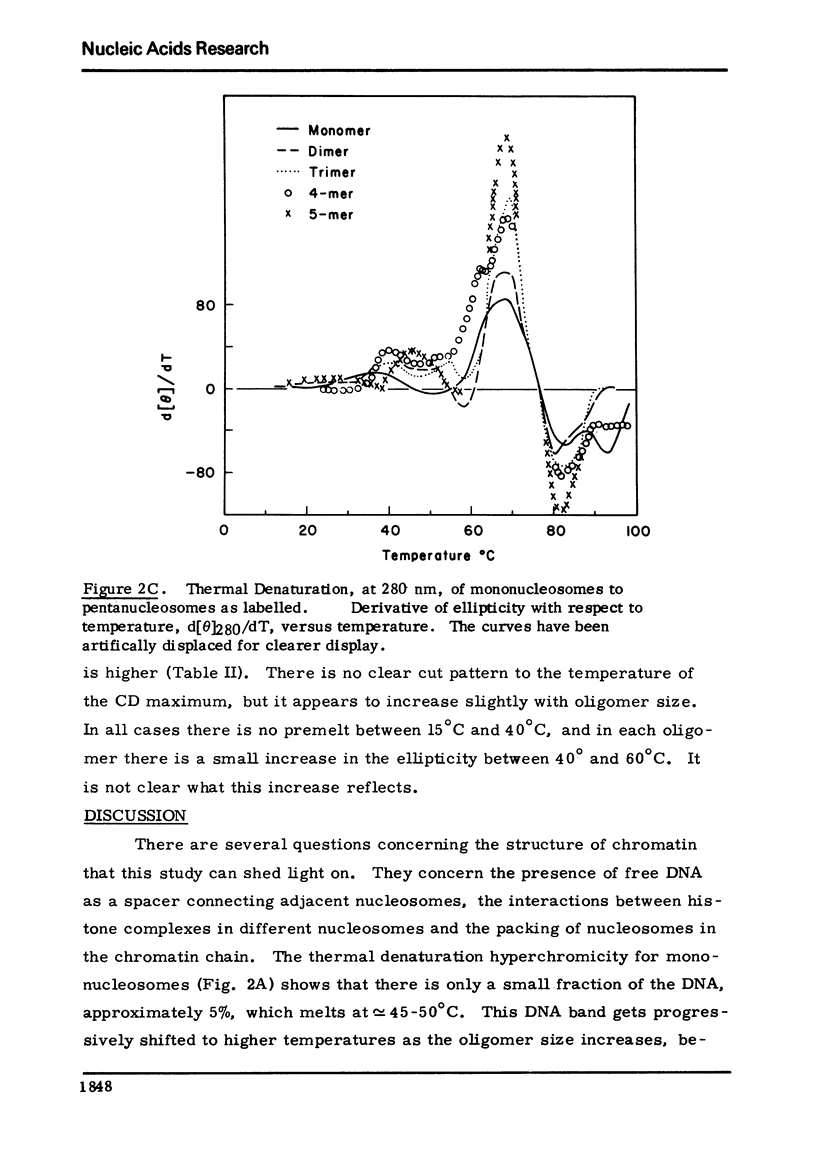
Chromatin nucleosomes (mononucleosomes through pentanucleosomes) have been isolated by staphylococcal nuclease digestion of calf thymus nuclei. The peak value ellipticity is the same for all oligomers, 1900 deg cm2, mol-1 at 280-nm, 23 degrees C. The dh280/dT vs T show a progressive increase in Tm of the main thermal band (73.5 degrees C, monomer; 79 degrees C, pentamer). Very small amounts of free DNA can be observed in the melting profiles, and shoulders at 60 degrees C and 93 degrees C appear and increase in magnitude as the particle size increases. The magnitude of the change, delta[theta]280, increases with oligomer size. This pattern could result from an initial unfolding of an asymmetric assembly of nucleosomes (polynucleosome superhelix) in addition to the denaturation of the internal nucleosome structure, and a subsequent or simultaneous denaturation of the double strand DNA. The extent of this unfolding appears to depend upon the size of the oligomer and therefore implies interactions between asymmetrically assembled neighboring nucleosomes.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler A. J., Ross D. G., Chen K., Stafford P. A., Woiszwillo M. J., Fasman G. D. Interaction of deoxyribonucleic acid with histone f2b and its half-molecules. Circular dichroism studies. Biochemistry. 1974 Jan 29;13(3):616–623. doi: 10.1021/bi00700a033. [DOI] [PubMed] [Google Scholar]
- Axel R., Melchior W., Jr, Sollner-Webb B., Felsenfeld G. Specific sites of interaction between histones and DNA in chromatin. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4101–4105. doi: 10.1073/pnas.71.10.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakayev V. V., Melnickov A. A., Osicka V. D., Varshausky A. J. Studies on chromatin. II. Isolation and characterization of chromatin subunits. Nucleic Acids Res. 1975 Aug;2(8):1401–1419. doi: 10.1093/nar/2.8.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin J. P., Boseley P. G., Bradbury E. M., Ibel K. The subunit structure of the eukaryotic chromosome. Nature. 1975 Jan 24;253(5489):245–249. doi: 10.1038/253245a0. [DOI] [PubMed] [Google Scholar]
- Bram S., Butler-Browne G., Baudy P., Ibel K. Quaternary structure of chromatin. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1043–1045. doi: 10.1073/pnas.72.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bustin M., Goldblatt D., Sperling R. Chromatin structure visualization by immunoelectron microscopy. Cell. 1976 Feb;7(2):297–304. doi: 10.1016/0092-8674(76)90029-5. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Noll M., Kornberg R. D. Electron microscopy of defined lengths of chromatin. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3320–3322. doi: 10.1073/pnas.72.9.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon S., Johnson R. S., Chan A. Relationship between protein and DNA structure in calf thymus chromatin. II. Conformational aspects. Biochemistry. 1974 Sep 10;13(19):3972–3981. doi: 10.1021/bi00716a024. [DOI] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Huang E. S., Newbold J. E., Pagano J. S. Analysis of simian virus 40 DNA with the restriction enzyme of Haemophilus aegyptius, endonuclease Z. J Virol. 1973 Apr;11(4):508–514. doi: 10.1128/jvi.11.4.508-514.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan C. F., Lerman L. S., Venable J. H. Structure and circular dichroism of DNA in concentrated polymer solutions. Nat New Biol. 1972 Mar 22;236(64):67–70. doi: 10.1038/newbio236067a0. [DOI] [PubMed] [Google Scholar]
- Langmore J. P., Wooley J. C. Chromatin architecture: investigation of a subunit of chromatin by dark field electron microscopy. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2691–2695. doi: 10.1073/pnas.72.7.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lis J. T., Schleif R. The regulatory region of the L-arabinose operon: its isolation on a 1000 base-pair fragment from DNA heteroduplexes. J Mol Biol. 1975 Jul 5;95(3):409–416. doi: 10.1016/0022-2836(75)90199-0. [DOI] [PubMed] [Google Scholar]
- Mandel R., Fasman G. D. Thermal denaturation of DNA and DNA:polypeptide complexes. Simultaneous absorption and circular dichroism measurements. Biochem Biophys Res Commun. 1974 Jul 24;59(2):672–679. doi: 10.1016/s0006-291x(74)80032-x. [DOI] [PubMed] [Google Scholar]
- Maurer H. R., Chalkley G. R. Some properties of a nuclear binding site of estradiol. J Mol Biol. 1967 Aug 14;27(3):431–441. doi: 10.1016/0022-2836(67)90049-6. [DOI] [PubMed] [Google Scholar]
- Mirsky A. E., Burdick C. J., Davidson E. H., Littau V. C. The role of lysine-rich histone in the maintenance of chromatin structure in metaphase chromosomes. Proc Natl Acad Sci U S A. 1968 Oct;61(2):592–597. doi: 10.1073/pnas.61.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll M. Subunit structure of chromatin. Nature. 1974 Sep 20;251(5472):249–251. doi: 10.1038/251249a0. [DOI] [PubMed] [Google Scholar]
- Noll M., Thomas J. O., Kornberg R. D. Preparation of native chromatin and damage caused by shearing. Science. 1975 Mar 28;187(4182):1203–1206. doi: 10.1126/science.187.4182.1203. [DOI] [PubMed] [Google Scholar]
- Olins D. E., Olins A. L., Von Hippel P. H. Model nucleoprotein complexes: studies on the interaction of cationic homopolypeptides with DNA. J Mol Biol. 1967 Mar 14;24(2):157–176. doi: 10.1016/0022-2836(67)90324-5. [DOI] [PubMed] [Google Scholar]
- Oosterhof D. K., Hozier J. C., Rill R. L. Nucleas action on chromatin: evidence for discrete, repeated nucleoprotein units along chromatin fibrils. Proc Natl Acad Sci U S A. 1975 Feb;72(2):633–637. doi: 10.1073/pnas.72.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudet P., Gross-Bellard M., Chambon P. Electron microscopic and biochemical evidence that chromatin structure is a repeating unit. Cell. 1975 Apr;4(4):281–300. doi: 10.1016/0092-8674(75)90149-x. [DOI] [PubMed] [Google Scholar]
- Permogorov V. I., Debabov V. G., Sladkova I. A., Rebentish B. A. Structure of DNA and histones in the nucleohistone. Biochim Biophys Acta. 1970 Feb 18;199(2):556–558. doi: 10.1016/0005-2787(70)90107-3. [DOI] [PubMed] [Google Scholar]
- Rill R., Van Holde K. E. Properties of nuclease-resistant fragments of calf thymus chromatin. J Biol Chem. 1973 Feb 10;248(3):1080–1083. [PubMed] [Google Scholar]
- Sahasrabuddhe C. G., Van Holde K. E. The effect of trypsin on nuclease-resistant chromatin fragments. J Biol Chem. 1974 Jan 10;249(1):152–156. [PubMed] [Google Scholar]
- Shaw B. R., Corden J. L., Sahasrabuddhe C. G., Van Holde K. E. Chromatographic separation of chromatin subunits. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1193–1198. doi: 10.1016/s0006-291x(74)80410-9. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Fasman G. D. Conformation of deoxyribonucleic acid in chromatin: a circular dichroism study. J Mol Biol. 1970 Aug 28;52(1):125–129. doi: 10.1016/0022-2836(70)90182-8. [DOI] [PubMed] [Google Scholar]
- Simpson R. T., Sober H. A. Circular dichroism of calf liver nucleohistone. Biochemistry. 1970 Aug 4;9(16):3103–3109. doi: 10.1021/bi00818a001. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Felsenfeld G. A comparison of the digestion of nuclei and chromatin by staphylococcal nuclease. Biochemistry. 1975 Jul;14(13):2915–2920. doi: 10.1021/bi00684a019. [DOI] [PubMed] [Google Scholar]
- Subirana J. A., Puigjaner L. C. X-ray diffraction studies of nucleohistone: a polyhelical model of chromosome organization. Proc Natl Acad Sci U S A. 1974 May;71(5):1672–1676. doi: 10.1073/pnas.71.5.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Holde K. E., Sahasrabuddhe C. G., Shaw B. R. A model for particulate structure in chromatin. Nucleic Acids Res. 1974 Nov;1(11):1579–1586. doi: 10.1093/nar/1.11.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm F. X., de Murcia G. M., Champagne M. H., Daune M. P. Conformational changes of histones and DNA during the thermal denaturation of nucleoprotein. Eur J Biochem. 1974 Jun 15;45(2):431–443. doi: 10.1111/j.1432-1033.1974.tb03567.x. [DOI] [PubMed] [Google Scholar]
- Wilhelm F. X., de Murcia G. M., Daune M. P. The premelting of nucleoprotein: role of non-histone proteins. Nucleic Acids Res. 1974 Aug;1(8):1043–1057. doi: 10.1093/nar/1.8.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock C. L., Frado L. L. Thermal denaturation of subchromosomal particles. Biochem Biophys Res Commun. 1975 Sep 2;66(1):403–410. doi: 10.1016/s0006-291x(75)80342-1. [DOI] [PubMed] [Google Scholar]



