Abstract
Introduction:
Secondhand smoke exposure (SHSe) threatens fragile infants discharged from a neonatal intensive care unit (NICU). Smoking practices were examined in families with a high respiratory risk infant (born at very low birth weight; ventilated > 12 hr) in a Houston, Texas, NICU. Socioeconomic status, race, and mental health status were hypothesized to be related to SHSe and household smoking bans.
Methods:
Data were collected as part of The Baby's Breath Project, a hospital-based SHSe intervention trial targeting parents with a high-risk infant in the NICU who reported a smoker in the household (N = 99). Measures of sociodemographics, smoking, home and car smoking bans, and depression were collected.
Results:
Overall, 26% of all families with a high-risk infant in the NICU reported a household smoker. Almost half of the families with a smoker reported an annual income of less than $25,000. 46.2% of families reported having a total smoking ban in place in both their homes and cars. Only 27.8% families earning less than $25,000 reported having a total smoking ban in place relative to almost 60% of families earning more (p < .01). African American and Caucasian families were less likely to have a smoking ban compared with Hispanics (p < .05). Mothers who reported no smoking ban were more depressed than those who had a household smoking ban (p < .02).
Conclusions:
The most disadvantaged families were least likely to have protective health behaviors in place to reduce SHSe and, consequently, are most at-risk for tobacco exposure and subsequent tobacco-related health disparities. Innovative SHSe interventions for this vulnerable population are sorely needed.
Over one-third (34.4%) of all children live with at least one parent who smokes cigarettes (King et al., 2009). Even more striking, almost half (49.4%) of children living at or below the poverty level live with a smoker, and often with multiple smokers, compared with those of higher SES (King et al., 2009). Children exposed to secondhand smoke have diminished pulmonary function and are more likely to suffer from respiratory infections, asthma, middle ear disease, poor growth, neurocognitive deficits, and sudden infant death syndrome (Cook & Strachan, 1999). Indeed, 50,000–300,000 cases per year of bronchitis and pneumonia are attributed to secondhand smoke exposure (SHSe) among generally healthy children ≤18 months of age (Emmons et al., 2001). SHSe is also associated with increased cardiovascular stress hyperreactivity (G. Cohen, Vella, Jeffery, Lagercrantz, & Katz-Salamon, 2008), respiratory-related emergency department visits, and hospitalizations for children (Kwok et al., 2008).
SHSe may be especially dangerous for low birth weight infants (LBW: <2,500 g; 5 lbs, 8 oz), very low birth weight infants (VLBW: <1,500 g; 3 lbs, 5 oz), and mechanically ventilated infants discharged from a neonatal intensive care unit (NICU). U.S. trends indicate a steady increase in preterm delivery and in LBW rates, with lower birth weights and higher mortality rates among African-American infants (Martin et al., 2005). NICU infants are particularly vulnerable to SHSe for both social and medical reasons. These infants are often born to members of impoverished populations. Smoking is more common among these lower SES groups and is known to decrease birth weight and increase the risk of neonatal respiratory problems, often requiring treatment with oxygen and mechanical ventilation (DiFranza, Aligne, & Weitzman, 2004). These life-saving treatments unfortunately can cause lung injury, which has been associated with decreased lung volumes, lower airway obstruction, hyperinflation, and residual abnormalities of varying severity that may persist until later childhood or even adulthood (Koumbourlis et al., 1996). More than 50% of VLBW infants require mechanical ventilation and 22% will develop bronchopulmonary dysplasia (BPD). Infants with BPD are at an especially increased risk for pneumonia, asthma, repeated hospitalizations, neurodevelopmental deficits, and death (Martin et al., 2005). There is a dearth of research in this area, and consequently, the specific effects of SHSe on infants discharged from NICUs are relatively unknown (Kitchen et al., 1992), although substantially increased odds of asthma, wheezing, and longer hospitalizations have been documented among VLBW populations (Chan, Noble-Jamieson, Elliman, Bryan, & Silverman, 1989; Doyle, Ford, Olinsky, Knoches, & Callanan, 1996; Kitchen et al., 1992). More recently, early life SHSe, particularly in the first 6 months, has been associated with increased chances of hospitalization for infectious illness up to 8 years of age (Kwok et al., 2008).
Racial disparities are noted between both NICU and smoking populations. Survey data indicate that African-American and Caucasian children are more likely to live with one or more smokers compared with children of Hispanic or Asian/Pacific Island origin (King et al., 2009). Despite limited race-related data on home smoking bans, one study found significantly more Hispanic families reported a home smoking ban relative to other racial/ethnic groups (Yousey, 2006), and another found that U.S.-born, Hispanic mothers were less likely to have a smoking ban in place than Mexico-born mothers (Gonzales, Malcoe, Kegler, & Espinoza, 2006). A review by Vidrine, Reitzel & Wetter (2009) documents the substantial disparities in rates of smoking as well as health consequences among adults of lower SES and minority status. For example, while the smoking rate of African-American men is now similar to Caucasian (non-Hispanic) men, African American men who smoke have significantly higher incidence and mortality rates for tobacco-related cancers (e.g., lung, pancreas, esophagus, larynx). Hispanic populations, who have lower smoking rates relative to African American and non-Hispanic Whites, also suffer adverse health consequences at disproportionately higher rates (e.g., lung cancer). Thus, it is likely that lower SES and minority children suffer disparate SHSe-related health consequences. Research is needed to examine the potential for tobacco-related health disparities among infants and children living with a smoker and in particular infants already vulnerable from birth.
The purpose of this article is to report on family smoking habits and prevalence of SHSe protective practices among families of high-risk NICU infants enrolled in an intervention study aimed at reducing infant SHSe. Differential protective health behaviors based on income or race may inform future interventions specific to high-risk groups for which exposure rates are highest and/or health consequences of SHSe are most severe or frequent. We contend that within a generally low-income, smoker group there may be differences in smoking safety practices related to SES, racial minority status, and mental health status. Specifically, we hypothesized that fewer NICU families of lower income and minority status would have a total smoking ban (i.e., both home and car) in place. In addition, we postulated that higher depression and perceived stress levels, and lower social support, would be associated with no smoking ban in the home or car.
Methods
Study Design and Procedures
This is a secondary analysis of baseline data from The Baby's Breath Project, a three-group randomized controlled trial. Parents with a high-risk infant in the NICU who have at least one smoker living in their household were randomized to receive a brief hospital-based SHSe prevention intervention or were assigned to one of two control groups. Primary caregivers were recruited from a large children's hospital with a 128-bed NICU and approximately 1,100 admissions per year. Research assistants approached parents of infants at high respiratory risk (HRR) in the NICU to determine the potential for household SHSe. Eligibility criteria included: (a) having an infant at HRR in the NICU, with HRR defined as VLBW or having received mechanical ventilation for >12 hr; (b) report of at least one smoker living in the household; (c) being able to read English or Spanish; and (d) living within a 50 mile radius of the hospital (due to follow-up home assessments).
Eligible families were consented and randomized to one of three groups: (a) Motivational Interviewing intervention (MI); (b) Usual Care (UC); and (c) Usual Care–Reduced Measurement (UC-RM). While their babies were in the NICU, MI participants received two individual, hospital-based, MI sessions targeting SHSe provided by experienced MI counselors. The UC and UC-RM groups received usual hospital care, including written materials and a discussion of many health issues with follow-up care coordinators. Follow-up home assessments were conducted at 1-, 3-, and 6-months postdischarge for the MI and UC groups; UC-RM participants only received the 6-month assessment, our primary evaluation endpoint. Inclusion of the UC-RM group was based on data from other SHSe studies indicating that measuring SHSe at multiple timepoints alone may be reactive and decrease exposure (e.g., Hovell et al., 1994). This study reports on baseline data from the first 94 participants randomized to the study plus 5 pilot participants on whom the intervention was piloted (N = 99).
Measures
A home smoking ban was assessed by asking, “How is cigarette smoking handled in your home?” Response options were (a) no one is allowed to smoke in my home, (b) only special guests are allowed to smoke in my home, (c) people are allowed to smoke only in certain areas of my home where my child rarely goes, and (d) people are allowed to smoke in any common room in my house. Only answer “a” indicated a home smoking ban was in place. Multiple choice questions (vs. dichotomous yes or no questions) have been shown to improve disclosure of smoking (Mullen, Carbonari, Tabak, & Glenday, 1991). A car smoking ban was assessed with a similar multiple choice question: (a) no one is allowed to smoke in my car, (b) only special guests are allowed to smoke in my car, (c) people are sometimes allowed to smoke in my car, but only when the windows are open, (d) smoking is sometimes allowed in my car, but only when my child is not present; and (e) people are allowed to smoke anytime in my car. A Total Smoking Ban (TSB) was considered to be in place when a participant answered (a) to both questions, that is, reported that no one is allowed to smoke in the home or in the car.
Measures of depression, perceived stress, and social support were administered to assess the degree to which psychological distress is related to having a smoking ban. The Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff, 1977) measured depressive symptoms over the past week with 20 Likert-scale items, ranging from “Rarely/None (<1 day/week)” (score = 0) to “Most/All of the time (5–7 days/week)” (score = 3). In community populations, it has been found to be reliable and valid. A score of 16 or higher was found in initial studies to identify subjects with depressive illness. The Mental Health Inventory-5 (Veit & Ware, 1983) measures overall mental health functioning, including depression and anxiety. Only one item, found to be equivalent to the full scale in its validity for predicting depression (Berwick et al., 1991), was used in this study in order to reduce assessment burden: “How much of the time, during the past month, have you felt downhearted and blue?” The question is answered on a 6-point Likert scale anchored by 1 = All of the time and 5 = None of the time. The 14-item Perceived Stress Scale (PSS) (S. Cohen, Kamarck, & Mermelstein, 1983) measured the degree to which individuals appraise situations in their lives as stressful (e.g., “dealt successfully with irritating life hassles”). Item responses range from “Never” (score = 0) to “Very often” (score = 4). The PSS has been found to discriminate quitters and nonquitters in studies of smoking cessation (Glasgow, Whitlock, Eakin, & Lichtenstein, 2000). The 12-item Interpersonal Support Evaluation List (ISEL) (S. Cohen, Mermelstein, Kamarck, & Hoberman, 1985) measured perceptions of the availability of potential social resources (i.e., social support), with responses spanning Definitely False (score = 1) to Definitely True (score = 4). The ISEL has been used to assess social support in a number of smoking cessation studies (e.g., Pollack & Mullen, 1997).
Analysis
Demographic and smoking history variables were analyzed descriptively by deriving means (SDs) or frequencies. Chi-square analyses were used to assess for differences among income levels, racial groups, and education levels on total smoking ban, smoking ban in the home, and smoking ban in the car. For analyses of income, the two highest of the five income categories were combined due to small subgroup sample sizes. Analysis of variance procedures were used to assess for differences in levels of depression and social support between those with and without a total smoking ban. Sample size varies across analyses due to missing data and because the UC-RM control group completed fewer measures at baseline.
Results
Recruitment to Date
From July 2008 through December 2009, 1,859 NICU infants were screened via medical records for birthweight and/or mechanical ventilation criteria. Based on these and the distance criteria (i.e., living within 50 miles of the hospital), 672 (out of 841 eligible families) were approached to determine household smoking status. Those who were not approached included 103 families who were never seen by the research assistant in the NICU prior to discharge, as well as 66 families grieving the loss of an infant. The 103 families not contacted primarily reflected those being discharged from the NICU within the first month of study start. Since this initial period, the “failure to locate” rate (i.e., families who could not be found in the NICU nor contacted via telephone prior to discharge) has been about 5%. Of those families contacted, 26% reported a household smoker, 77% of those with household smokers consented to participate, and 57% of those consented completed the baseline and were randomized. Figure 1 depicts the flow of recruitment. Families were typically contacted 1–3 weeks after the birth of their child (M = 17.6 (19.5) days; Median = 10 days). Losses between consent and randomization were due to: (a) discharged/failure to locate the family after consent; (b) no longer eligible, for example, lost custody, moved; (c) infant mortality; and (d) withdrew. The following results are from data collected at baseline only.
Figure 1.
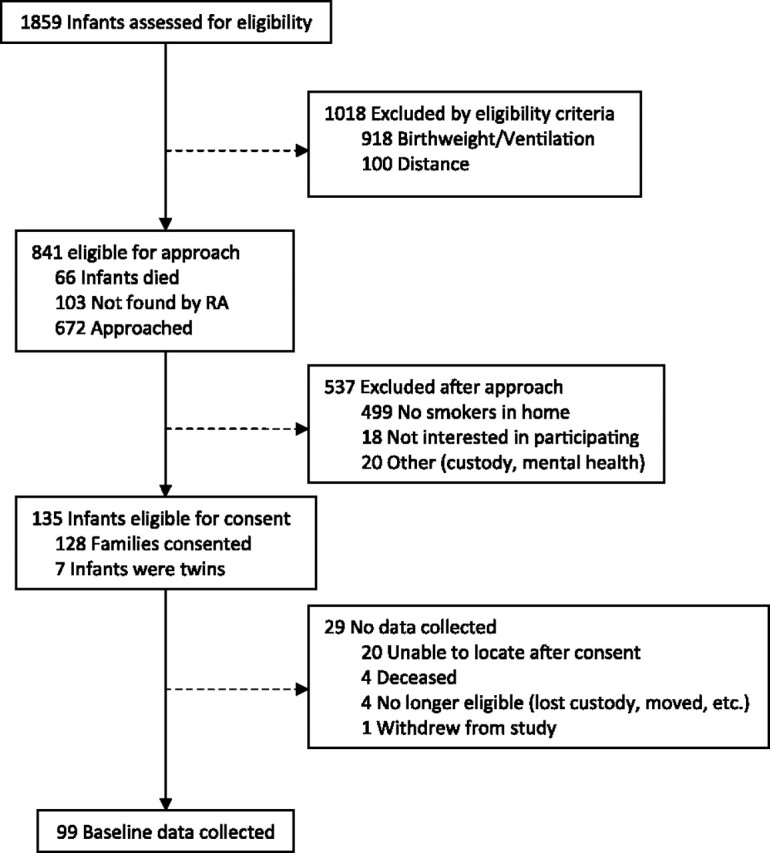
Screening and recruitment flow of participants to the intervention study conducted at the Children’s Memorial Hermann Hospital NICU in Houston, TX from July 2008 through May 2010.
Sociodemographic Characteristics
Table 1 describes the sample. The majority of participants were of racial minority status and primarily African American. All primary caregivers consented were mothers. Only about a fourth of the mothers were married, and most had a high school education, were unemployed, and were enrolled in Medicaid. The majority of families were overwhelmingly poor, with almost half of the sample reporting an annual income of less than $25,000 and a large minority of caregivers unable to cover basic living expenses (see Table 1). Almost 75% of the sample reported this pregnancy to be unplanned.
Table 1.
Sociodemographic and Smoking History Characteristics of the Primary Caregivers and Their Families
| Characteristic | n (%) |
| Gender (female) | 99 (100) |
| Race | |
| Asian | 2 (2.0) |
| Black | 51 (51.5) |
| White | 21 (21.2) |
| Hispanic | 25 (25.3) |
| Marital status | |
| Single | 33 (33.3) |
| Married | 28 (28.3) |
| Separated | 6 (6.1) |
| Divorced | 2 (2.0) |
| Living together | 30 (30.3) |
| Employment | |
| Full time | 17 (21.5) |
| Part time | 5 (6.3) |
| Not employed | 56 (71.0) |
| Income ($) | |
| <15,000 | 19 (24.1) |
| 15,000–24,999 | 18 (22.8) |
| 25,000–34,999 | 14 (17.7) |
| 35,000–44,999 | 7 (8.9) |
| >50,000 | 6 (7.6) |
| In past year, not enough money for | |
| Food | 27 (35.5) |
| Housing | 28 (36.8) |
| Utilities | 31 (40.8) |
| Medical care | 20 (26.3) |
| Medicine | 22 (29.3) |
| Medicaid recipient | 62 (80.5) |
| Living situation | |
| Living independently | 32 (40.5) |
| Relying on others for a place to live | 47 (59.4) |
| Pregnancy | |
| Planned | 21 (27.6) |
| Unplanned | 55 (72.4) |
| Delivery method | |
| Vaginal | 33 (42.3) |
| Caesarean section | 45 (57.7) |
| Premature births | |
| 0 | 12 (15.2) |
| 1 | 56 (70.9) |
| 2 | 10 (12.7) |
| 5 or more | 1 (1.3) |
| Breastfeeding or pumping | |
| Plan to breastfeed | 2 (2.5) |
| Do not plan to breastfeed | 21 (26.6) |
| Currently breastfeeding | 44 (55.7) |
| No longer breastfeeding | 12 (15.2) |
| Smoking status | |
| Dad only smokes | 36 (51.4) |
| Mom only smokes | 5 (7.1) |
| Both smoke | 18 (25.7) |
| Neither smoke | 11 (15.7) |
| M (SD) | |
| Maternal age in years | 25.7 (6.53) |
| Maternal education in years | 12.3 (2.2) |
| Pregnancies | 2.5 (1.6) |
| Live births | 1.9 (1.3) |
| Prenatal visits | 11.9 (10.9) |
| Cigarettes smoked per daya | 11.2 (17.1) |
| Cigarettes smoked in house per daya | 3.5 (9.8) |
Note.aRepresents all smokers in the household (not just the primary caregiver).
Smoking History and Current Smoking Practices
The majority of household smokers were fathers, with few families reporting that only the mother smoked (see Table 1). In almost 16% of families, neither parent smoked; in these cases, a grandparent or other relative with whom they were living was the household smoker. According to primary caregivers, 64.3% of enrolled families have a smoking ban in place in their home, while 56.6% reported having a smoking ban in their cars; 46.2% of families reported having a TSB (both home and car) in place. When asked whether they had ever received information or materials about SHSe, 50% reported their doctors had provided information; 44.9% received information from other health care providers; 48.7% heard about SHSE from friends and family, and 64.6% from TV, magazine, newspaper, or Internet sources.
Comparisons of TSB by Income, Race, and Education
Income Comparisons
Total ban
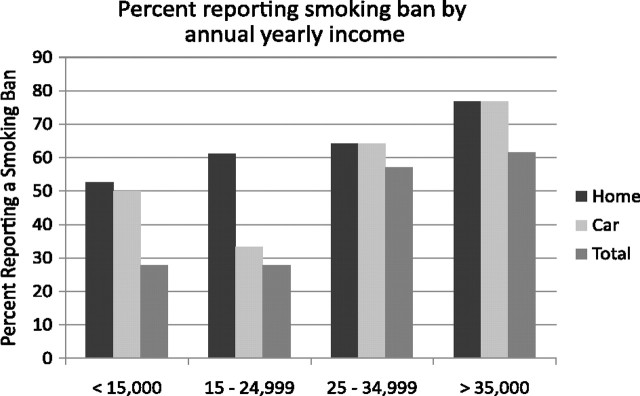
Differences in the frequency of having a TSB by income did not reach statistical significance across the four income groups likely due to insufficient power (see Figure 2), X2(N = 63) = 6.36, p = .095, yet a distinct pattern emerged, suggesting that lower income was associated with higher proportions of families without a TSB. Income and proportion of families without a TSB were correlated at −.31, p < .01. When yearly income was collapsed into two groups, < or ≥ $25,000, differences were revealed, X2 (N = 63) = 6.31, p = .01. Fewer families earning less than $25,000 annually had a total smoking ban in place (27.8%) relative to families earning $25,000 or more (59.3%).
Figure 2.
Percent of sample reporting a smoking ban in home, car and both (total) by reported annual household income.
Home ban.
Although a similar pattern emerged, no differences were found on frequency of reporting a smoking ban in the home across the four income levels, X2(N = 63) = 1.55, p = .67, nor the levels dichotomized at $25,000, X2(N = 63) = .96, p = .33. See Figure 2.
Car ban.
Fewer families in the lower income ranges were likely to have a smoking ban in place in their cars. Although differences failed to reach significance using the four income ranges, X2(N = 63) = 6.56, p = .09 (see Figure 2), differences were found with the dichotomous income variable, X2(N = 63) = 5.12, p = .02. Again, fewer families with a yearly income of less than $25,000 reported having a smoking ban in place in their cars (41.7%) relative to families earning $25,000 or more (70.4%).
Race Comparisons
Total ban.
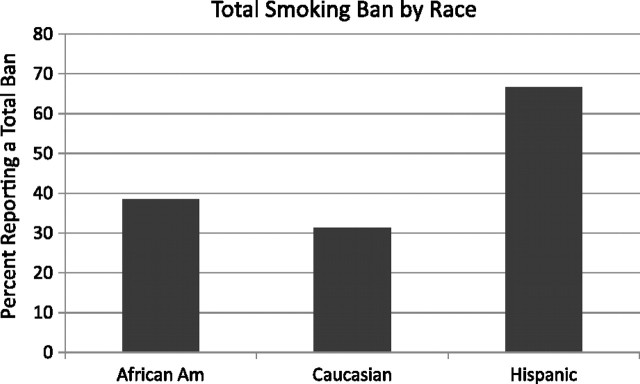
Differences across the three primary race groups on Total Smoking Ban were found, X2(N = 76) = 5.12, p = .02. Overall, more Hispanic families reported having a smoking ban in both their homes and cars relative to either African American or Caucasian families (see Figure 3).
Figure 3.
Percent of sample reporting a total smoking ban by race.
Home ban.
Differences among racial groups reporting a home smoking ban approached significance, X2(N = 76) = 4.54, p = .10. 81%, of Hispanic families reported having a home ban compared with 68.8% of Caucasians and 53.8% of African Americans.
Car ban.
Differences were found across racial groups with regard to having a smoking ban in the car, X2 (N = 76) = 7.47, p = .02. Caucasians were least likely to report a car smoking ban (31.3%), followed by African Americans (56.4%) and Hispanics (76.2%).
Education Comparisons
No differences were found on level of education for the presence of a Total, Home or Car smoking ban, X2 (N = 78) = .41, p = .52, X2 (N = 63) = .12, p = .73, X2 (N = 78) = 1.85, p = .17.
Comparisons of Total Smoking Ban by Social and Psychological Variables
Depression
Mothers of infants currently in the NICU who reported already having a TSB tended to be less depressed on the CES-D than those who reported no ban. Although this difference did not reach statistical significance, F(1, 74) = 3.43, p = .07, it is likely a meaningful difference. Mothers who reported having a TSB scored an average of 13.2 (SD = 11.5) on the CES-D, while those who did not have a Total Ban had a mean of 17.6 (SD = 8.9), which is above the typical threshold suggestive of depressive illness for this measure. Significant differences were found, X2(N = 78) = 7.38, p = .02, on a separate one-item depression question: “How much of the time, during the past month, have you felt downhearted and blue?” Of those who had a TSB in place 73.3% answered, “None of the time” compared with 26.7% who did not have a TSB. Conversely, of the mothers who reported not having a TSB, 75% selected “A lot or all of the time,” compared with 25% who did have a TSB. Differences were also found between those with and without a TSB on number of times the primary caregiver had been seen by a doctor or professional for psychological problems, such as depression or anxiety, F(1, 76) = 6.57, p = .01. Mothers with a TSB were seen for mental health problems an average .33 times, while those without a TSB were seen an average of 3.19 times.
Perceived Stress and Social Support
No differences were found between those reporting versus not reporting a TSB on level of perceived stress nor on level of social support, F(1, 72) = .76, p = .39 and F(1, 75) = .10, p = .75. This may be due to the measures’ lack of specificity to the NICU population.
Discussion
SHSe is an environmental condition that threatens fragile infants discharged from an NICU. To our knowledge, this is the first study to examine in depth the smoking and protective practices of parents of high-risk infants who have a smoker in the household and to assess how these practices are related to characteristics commonly associated with health disparities. Collectively, the data depict a population of families at extreme risk for social, psychological, and physical health problems. The majority of the sample was of racial minority status, only about a quarter of mothers were married, most were not employed and were living at or below poverty level, with the majority receiving Medicaid to cover their child's healthcare expenses. With regard to smoking, over a quarter of families approached reported a smoker living in the home, comparable to rates found by Bock, Becker, & Borelli (2008). The majority of smokers were the fathers, although for a quarter of the babies both parents smoked. A startlingly large majority (over 72%) reported that their preterm, LBW infant was the result of an unplanned pregnancy and 85% reported one or more previous premature births.
Our data suggest a significant potential for tobacco-related health disparities. Important differences in preventative smoke exposure practices were found among various sociodemographic categories. Income and race/ethnicity differences were found with regard to the existence of a TSB. Families were much less likely to have a TSB if they reported making less than $25,000 per year, which is just slightly over the U.S. annual poverty level for a family of four ($22,050). Thus, high-risk NICU children born into the poorest of families with a household smoker are less likely to be protected from SHSe and, thus, are more prone to developing associated acute and chronic health conditions (e.g., SIDS, asthma, respiratory infections).
With regard to race, according to our data and that of others (e.g., Yousey, 2006), being of minority status does not uniformly indicate poorer preventative SHSe practices, in fact, although not significantly different from African American families, Caucasian families were the least likely to have a total smoking ban in place. Further, it appears that Hispanic families may be more likely to protect their children from SHSe. There is also evidence that increased acculturation is associated with decreased SHSe protection in Hispanic families who migrated to the United States (Gonzales et al., 2006). African American families were least likely to have a smoking ban in their homes, whereas Caucasian families were least likely to have a ban in their cars. The lower rates of SHSe protection practices among African American families are especially important given their higher incidence of tobacco-related cancer mortality (Vidrine et al., 2009) and higher asthma prevalence and hospitalization rates compared with Caucasians (Carr, Zeitel, & Weiss 1992; Evans 1992; Miller 2000). Moreover, African-American children have been found to have higher rates of respiratory illness (e.g., asthma), independent of income (Miller, 2000). SHSe may be a contributing factor to this disparity in health, particularly among children.
Also compelling were the mental health differences found between those with and without a TSB. Across multiple measures, including a community-normed depression scale and assessment of previous treatment episodes, a significantly larger proportion of mothers who reported they did not have a TSB struggled with depression. A recent study using Behavioral Risk Factor Surveillance System data similarly found that the risk of major depression was significantly higher for those living in households where smoking was allowed anywhere versus those living in homes with complete smoking bans (Bandiera et al. 2010). It is well established that smokers are at greater risk for depression, although the exact direction of this relationship is unclear. Failure to have a smoking ban could be a direct result of maternal depression or alternatively could be a marker of a more generally chaotic or unstable lifestyle or living situation.
Data also indicated that healthcare professionals in the NICU setting are not routinely addressing SHSe with their families. Only half of the families with a baby at HRR and a smoker in the home reported that a physician or other healthcare provider provided them information on SHSe, and the extent of information or assistance provided is unknown. Moreover, to our knowledge, there have been almost no efforts to develop programs to reduce NICU infants’ exposure to their parents’ cigarette smoke. Previous research has found socioeconomic disparity in the delivery of tobacco dependence treatment (Browning, Ferketich, Salsberry, & Wewers, 2008), perhaps due to the highly complex health and social situations common among people from disadvantaged backgrounds.
Programs to assist NICU parents in protecting their fragile infants from SHSe will need to be targeted to several levels, consistent with the Behavioral Ecological Model (Hovell & Hughes, 2009). At the local hospital level, healthcare professionals in the NICU could be trained and systems could be developed to deliver SHSe information and education on a more routine basis. Previous research suggests that 5 minutes of physician-delivered smoking cessation counseling can significantly increase quit rates (Folsom & Grimm, 1987; Janz et al., 1987). Further, raising awareness throughout the NICU of the dangers of SHSe will perpetuate an anti-tobacco culture (e.g., Hovell & Hughes, 2009), to which parents will be exposed throughout their infant's hospital stay. On an individual level, SHSe counseling as well as evidence-based smoking treatments, such as cessation counseling and pharmacotherapy (e.g., nicotine replacement therapies, bupropion, varenicline) should be offered. To our knowledge, only one smoking cessation study has been conducted in an NICU setting (Ling, Wooderson, Rees, Neild, & Wright, 2008). Randomized, controlled trials are needed to verify outcomes as well as improve upon interventions for this vulnerable population of infants and their families.
Our data as well as our experiences with the parents of NICU infants in our study, however, revealed significant and difficult social situations, such as poverty, divorce, substance abuse, and domestic violence, in addition to caring for a medically fragile infant. As such, tangible and emotional resources for affecting behavior change are limited. As seen with other impoverished populations, offering external reinforcers (e.g., cash vouchers, gift cards) of sufficient magnitude for desired behavior change in this context is likely one of the few ways to raise the saliency of a problem that lacks immediacy in its consequences (e.g., infant illness). Novel incentive-based interventions based on behavioral principles of contingency management may be especially effective, as demonstrated in similar populations (Glenn & Dallery, 2007; Higgins, Silverman, & Heil, 2008; Lussier, Heil, Mongeon, Badger, & Higgins, 2006; Petry et al., 2006; Prendergast, Podus, Finney, Greenwell, & Roll, 2006).
Limitations of this study include a reliance on self-report to determine smoking ban status, sample size, and appropriateness of some measures. Although self-report has been found to be a valid means of assessing smoking status (Patrick et al., 1994), misrepresentation of smoking safety practices may have occurred, particularly given the NICU healthcare setting. Misrepresentation may be due to social desirability as the dangers of secondhand smoke and smoking indoors have become well publicized, with many major cities now having smoking bans in restaurants and bars. Also possible as a result of the NICU experience is a new resolve on the part of parents to protect their fragile infant, thus biasing report of current practices. Objective measurement of home nicotine levels and/or biomarkers of nicotine exposure (e.g., cotinine) is needed to reduce this possible bias. Our sample size for the study recruited over 1.5 years was not large; therefore, small yet important differences among groups may not have been detected due to a lack of power. We believe the sample is representative of NICU families with an HRR baby living near an urban area that have a smoker in the household, and thus, our findings are relevant to other NICU settings serving similar populations.
Conclusions
NICU hospitalization offers a rare opportunity to reach a young segment of the low-income, smoker population that rarely presents in health care settings, thereby precluding exposure to messages regarding the hazards of smoking for families and their children. Those who are poor, depressed, African American, or Caucasian are less likely to have protective health behaviors in place to reduce SHSe and are therefore at higher risk for tobacco-related health disparities. Innovative interventions to address smoking behaviors in this vulnerable population are sorely needed.
Funding
U.S. Department of Health and Human Services, Maternal and Child Health Research Program (grant R40MC08962).
Declaration of Interests
None declared.
Acknowledgments
Special thanks to Shireen Hayatghaibi Fung and Tiffany Dean for their superior data collection efforts, as well as the staff and physicians at the Children's Memorial Hermann Hospital, Houston, TX.
References
- Bandiera FC, Caban-Martinez AJ, Arheart KL, Davila EP, Fleming LE, Dietz NA, et al. Secondhand smoke policy and the risk of depression. Annals of Behavioral Medicine. 2010;39:198–203. doi: 10.1007/s12160-010-9174-8. doi:10.1007/s12160-010-9174-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwick DM, Murphy JM, Goldman PA, Ware JE, Jr., Barsky AJ, Weinstein MC. Performance of a five-item mental health screening test. Medical Care. 1991;29:169–176. doi: 10.1097/00005650-199102000-00008. doi:10.1097/00005650-199102000-00008. [DOI] [PubMed] [Google Scholar]
- Bock BC, Becker BM, Borrelli B. Smoking behavior and risk perception among the parents of infants in the neonatal intensive care unit. Nicotine & Tobacco Research. 2008;10:47–54. doi: 10.1080/14622200701767795. doi:10.1080/14622200701767795. [DOI] [PubMed] [Google Scholar]
- Browning KK, Ferketich AK, Salsberry PJ, Wewers ME .Socioeconomic disparity in provider-delivered assistance to quit smoking. Nicotine & Tobacco Research. 2008;10:55–61. doi: 10.1080/14622200701704905. doi:10.1080/14622200701704905. [DOI] [PubMed] [Google Scholar]
- Carr W, Zeitel L, Weiss K. Variations in asthma hospitalizations and deaths in New York City. American Journal of Public Health. 1992;82:59–65. doi: 10.2105/ajph.82.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KN, Noble-Jamieson CM, Elliman A, Bryan EM, Silverman M. Lung function in children of low birth weight. Archives of Disease in Childhood. 1989;64:1284–1293. doi: 10.1136/adc.64.9.1284. doi:10.1136/adc.64.9.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen G, Vella S, Jefferery H, Lagercrantz H, Katz-Salamon M. Cardiovascular stress hyperreactivity in babies of smokers and in babies born preterm. Circulation. 2008;118:1848–1853. doi: 10.1161/CIRCULATIONAHA.108.783902. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. Journal of Health and Social Behavior. 1983;24:385–396. doi:10.2307/2136404. [PubMed] [Google Scholar]
- Cohen S, Mermelstein R, Kamarck T, Hoberman HM. Measuring the functional components of social support. In: Sarason IGSBR, editor. Social support: Theory, research, and applications. 1985. pp. 73–94. Retrieved from http://www.psy.cmu.edu/∼scohen/publications.html. [Google Scholar]
- Cook DG, Strachan DP. Health effects of passive smoking-10: Summary of effects of parental smoking on the respiratory health of children and implications for research. Thorax. 1999;54:357–366. doi: 10.1136/thx.54.4.357. doi:10.1136/thx.54.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Aligne CA, Weitzman M. Prenatal and postnatal environmental tobacco smoke exposure and children's health. Pediatrics. 2004;113(Suppl):1007–1015. Retrieved from http://pediatrics.aappublications.org/ [PubMed] [Google Scholar]
- Doyle LW, Ford GW, Olinsky A, Knoches AM, Callanan C. Passive smoking and respiratory function in very low birthweight children. The Medical Journal of Australia. 1996;164:266–269. doi: 10.5694/j.1326-5377.1996.tb94185.x. Retrieved from http://www.mja.com.au/ [DOI] [PubMed] [Google Scholar]
- Emmons KM, Wong M, Hammond SK, Velicer WF, Fava JL, Monroe AD, et al. Intervention and policy issues related to children's exposure to environmental tobacco smoke. Preventive Medicine. 2001;32:321–331. doi: 10.1006/pmed.2000.0822. doi:10.1006/pmed.2000.0822. [DOI] [PubMed] [Google Scholar]
- Folsom AR, Grimm RH., Jr. Stop smoking advice by physicians: A feasible approach? American Journal of Public Health. 1987;77:849–850. doi: 10.2105/ajph.77.7.849. doi:10.2105/AJPH.77.7.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow RE, Whitlock EP, Eakin EG, Lichtenstein E. A brief smoking cessation intervention for women in low-income planned parenthood clinics. American Journal of Public Health, 2000;90:786–789. doi: 10.2105/ajph.90.5.786. doi:10.2105/AJPH.90.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn IM, Dallery J. Effects of internet-based voucher reinforcement and a transdermal nicotine patch on cigarette smoking. Journal of Applied Behavior Analysis. 2007;40:1–13. doi: 10.1901/jaba.2007.40-1. doi:10.1901/jaba.2007.40-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales M, Malcoe LH, Kegler MC, Espinoza J. Prevalence and predictors of home and automobile smoking bans and child environmental tobacco smoke exposure: A cross-sectional study of U.S.- and Mexico-born Hispanic women with young children. BMC Public Health. 2006;6:265. doi: 10.1186/1471-2458-6-265. doi:10.1186/1471-2458-6-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins ST, Silverman K, Heil SH. Contingency management in substance abuse treatment. New York: Guilford Press; 2008. Retrieved from http://www.guilford.com/cgi-bin/cartscript.cgi?page=home.html&cart_id=525254.30581; [Google Scholar]
- Hovell MF, Hughes SC. The behavioral ecology of secondhand smoke exposure: A pathway to complete tobacco control. Nicotine & Tobacco Research. 2009;11:1254–1264. doi: 10.1093/ntr/ntp133. doi:10.1093/ntr/ntp133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovell MF, Meltzer SB, Zakarian JM, Wahlgren DR, Emerson JA, Hofstetter CR, et al. Reduction of environmental tobacco smoke exposure among asthmatic children: A controlled trial. Chest. 1994;106:440–446. doi: 10.1378/chest.106.2.440. doi:10.1378/chest.106.2.440. [DOI] [PubMed] [Google Scholar]
- Janz NK, Becker MH, Kirscht JP, Eraker SA, Billi JE, Woolliscroft JO. Evaluation of a minimal-contact smoking cessation intervention in an outpatient setting. American Journal of Public Health. 1987;77:805–809. doi: 10.2105/ajph.77.7.805. doi:10.2105/AJPH.77.7.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King K, Martynenko M, Bergman MH, Liu YH, Winickoff JP, Weitzman M. Family composition and children's exposure to adult smokers in their homes. Pediatrics. 2009;123:e559–e564. doi: 10.1542/peds.2008-2317. doi:10.1542/peds.2008-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchen WH, Olinsky A, Doyle LW, Ford GW, Murton LJ, Slonim L, et al. Respiratory health and lung function in 8-year-old children of very low birth weight: A cohort study. Pediatrics. 1992;89(Pt 2):1151–1158. Retrieved from http://pediatrics.aappublications.org/ [PubMed] [Google Scholar]
- Koumbourlis AC, Motoyama EK, Mutich RL, Mallory GB, Walczak SA, Fertal K. Longitudinal follow-up of lung function from childhood to adolescence in prematurely born patients with neonatal chronic lung disease. Pediatric Pulmonology. 1996;21:28–34. doi: 10.1002/(SICI)1099-0496(199601)21:1<28::AID-PPUL5>3.0.CO;2-M. doi:10.1002/(SICI) 1099-0496(199601)21:1<28::AID-PPUL5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Evans R., 3rd. Asthma among minority children. A growing problem. Chest. 1992;101(6 Suppl):368S–371S. [PubMed] [Google Scholar]
- Kwok MK, Schooling CM, Ho LM, Leung SS, Mak KH, McGhee SM, et al. Early life second-hand smoke exposure and serious infectious morbidity during the first 8 years: Evidence from Hong Kong's “Children of 1997” birth cohort. Tobacco Control. 2008;17:263–270. doi: 10.1136/tc.2007.023887. doi: tc.2007.023887. [DOI] [PubMed] [Google Scholar]
- Ling SK, Wooderson S, Rees K, Neild R, Wright I. A smoking cessation program in the neonatal intensive care unit. Journal of Smoking Cessation. 2008;3:73–76. Retrieved from http://search.informit.com.au/browseJournalTitle;res=IELHEA;issn=1834-2612. [Google Scholar]
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101:192–203. doi: 10.1111/j.1360-0443.2006.01311.x. doi:10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson MD, et al. Births: Final data from 2003. 2005 National Vital Statistics Reports, 54, 1–116. Retrieved from http://www.cdc.gov/nchs/products/nvsr.htm. [PubMed] [Google Scholar]
- Miller JE. The effects of race/ethnicity and income on early childhood asthma prevalence and health care use. American Journal of Public Health. 2000;90:428–430. doi: 10.2105/ajph.90.3.428. doi:10.2105/AJPH.90.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen PD, Carbonari JP, Tabak ER, Glenday MC. Improving disclosure of smoking by pregnant women. American Journal of Obstetrics & Gynecology. 1991;165:409–413. doi: 10.1016/0002-9378(91)90105-z. Retrieved from http://www.ajog.org/ [DOI] [PubMed] [Google Scholar]
- Patrick DL, Cheadle A, Thompson DC, Diehr P, Koepsell T, Kinne S. The validity of self-reported smoking: A review and meta-analysis. American Journal of Public Health. 1994;84:1086–1093. doi: 10.2105/ajph.84.7.1086. doi:10.2105/AJPH.84.7.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Alessi SM, Carroll KM, Hanson T, MacKinnon S, Rounsaville B, Sierra S. Contingency management treatments: Reinforcing abstinence versus adherence with goal-related activities. Journal of Consulting and Clinical Psychology. 2006;74:592–601. doi: 10.1037/0022-006X.74.3.592. doi:10.1037/0022-006X.74.3.592. [DOI] [PubMed] [Google Scholar]
- Pollack H, Mullen PD. An exploration of the effects of partner smoking, type of social support, and stress on ‘partum smoking in married women who stopped smoking during pregnancy. Psychology of Addictive Behaviors. 1997;11:182–189. Retrieved from http://www.apa.org/pubs/journals/adb/ [Google Scholar]
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: A meta-analysis. Addiction. 2006;101:1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi:10.1177/014662167700100306. [Google Scholar]
- Veit CT, Ware JE. The structure of psychological distress and well-being in general populations. Journal of Consulting and Clinical Psychology. 1983;51:730–742. doi: 10.1037//0022-006x.51.5.730. doi:10.1037//0022-006X.51.5.730. [DOI] [PubMed] [Google Scholar]
- Vidrine J, Reitzel L, Wetter DW. The role of tobacco in cancer health disparities. Current Oncology Reports. 2009;11:475–481. doi: 10.1007/s11912-009-0064-9. doi:10.1007/s11912-009-0064-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousey YK. Household characteristics, smoking bans, and passive smoke exposure in young children. Journal of Pediatric Health Care. 2006;20:98–105. doi: 10.1016/j.pedhc.2005.08.006. doi:10.1016/j.pedhc.2005.08.006. [DOI] [PubMed] [Google Scholar]