Abstract
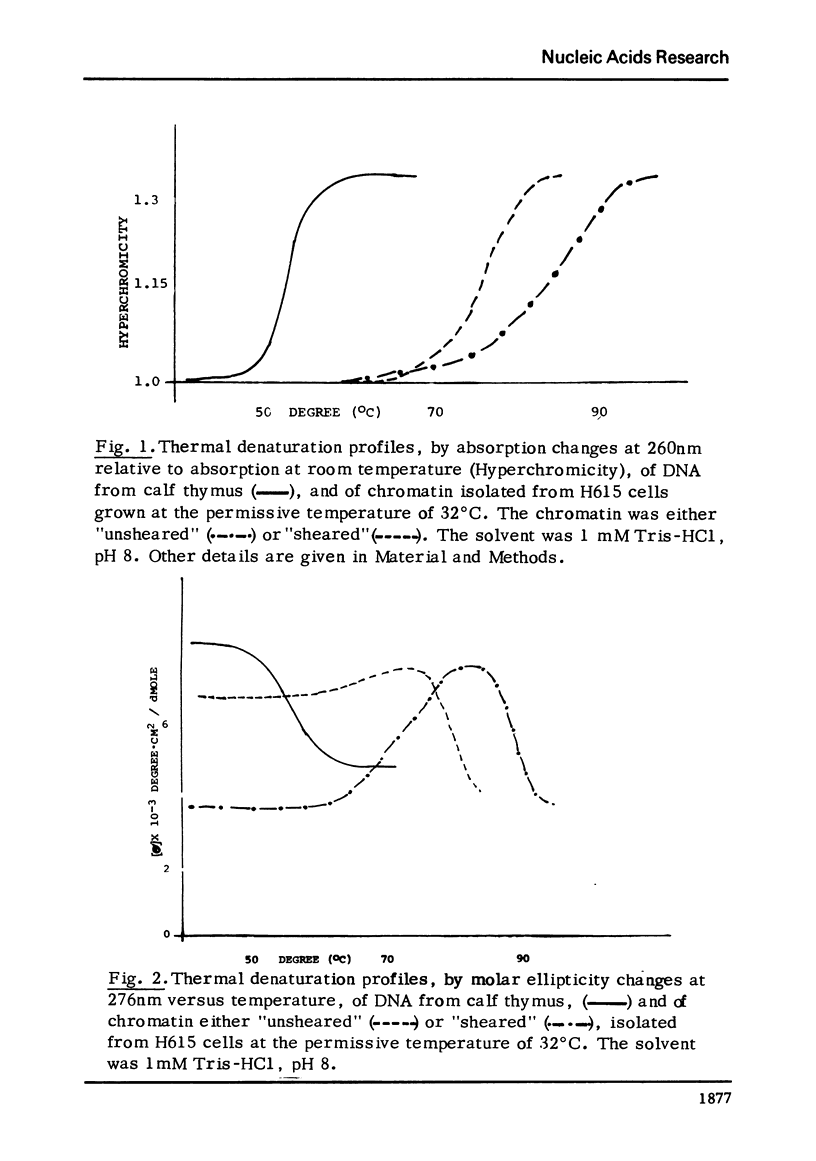
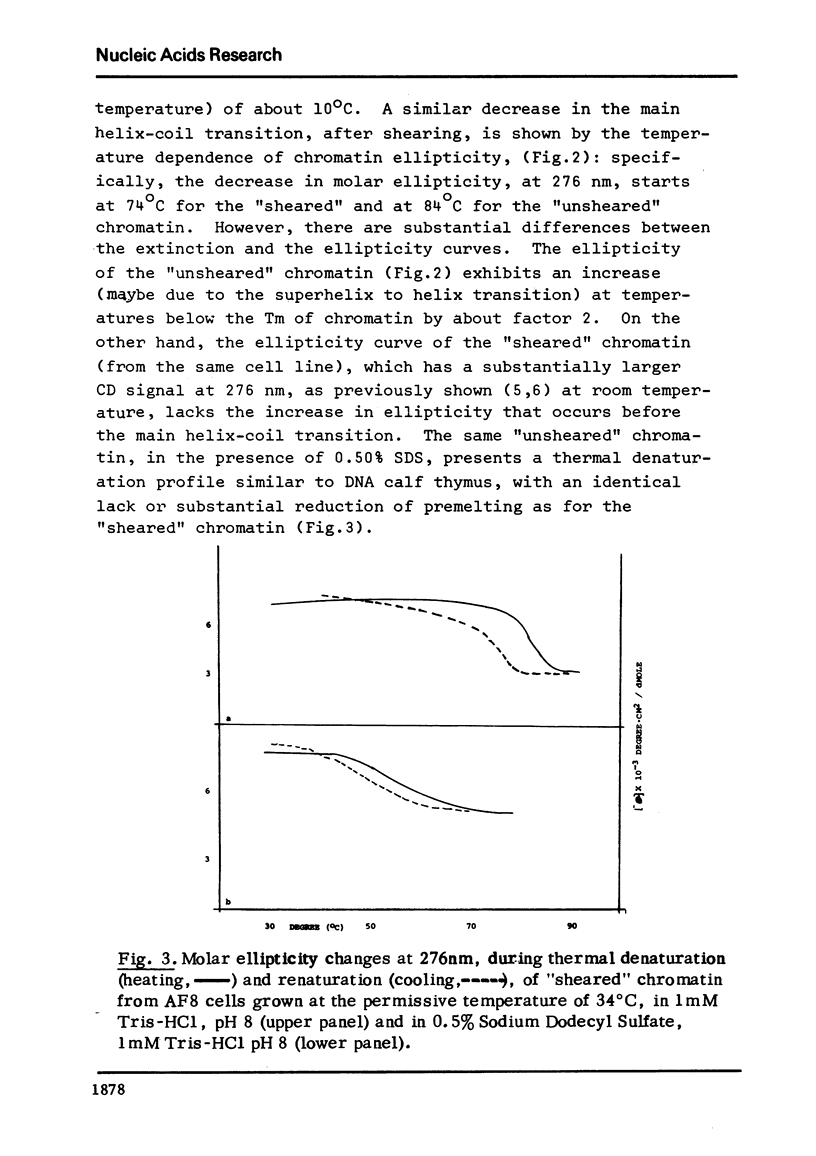
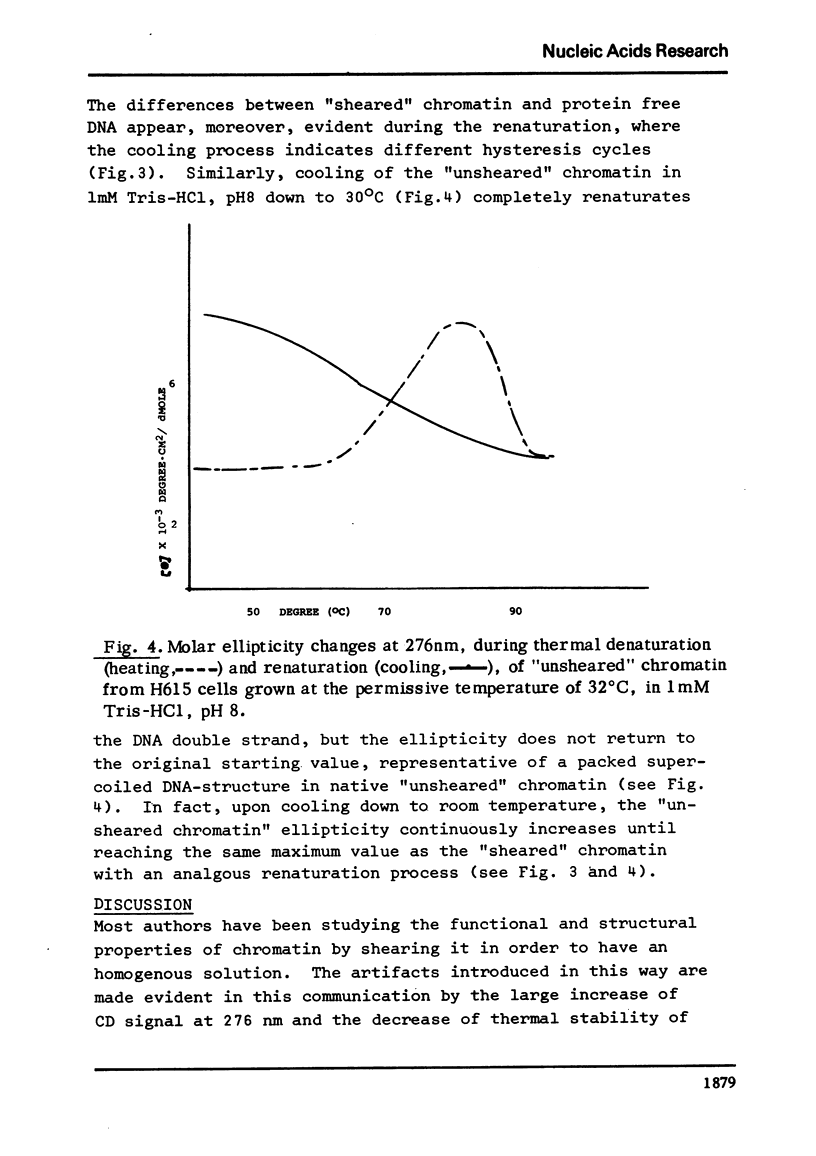
Thermal denaturation of chromatin is observed by simultaneously monitoring absorption and circular dichroism at 276 nm as functions of temperature. Either observation indicates that sheared chromatins shows less thermal stability than native chromatin. The temperature-dependent ellipticities at 276 nm of these chromatins show features not seen in the absorption curves: the ellipticity of unsheared chromatin increases with temperature, while this increase is abolished or greatly reduced in the same chromatin after shearing. After its first thermal transition (prior to the helix-coli transition) the unsheared chromatin achieves the same ellipticity as sheared chromatin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Augenlicht L., Nicolini C., Baserga R. Circular dichroism and thermal denaturation studies of chromatin and DNA from BrdU-treated mouse fibroblasts. Biochem Biophys Res Commun. 1974 Aug 5;59(3):920–926. doi: 10.1016/s0006-291x(74)80067-7. [DOI] [PubMed] [Google Scholar]
- Burstin S. J., Meiss H. K., Basilico C. A temperature-sensitive cell cycle mutant of the BHK cell line. J Cell Physiol. 1974 Dec;84(3):397–408. doi: 10.1002/jcp.1040840308. [DOI] [PubMed] [Google Scholar]
- Chesterton C. J., Coupar B. E., Butterworth P. H. Transcription of fractionated mammalian chromatin by mammalian ribonucleic acid polymerase. Demonstration of temperature-dependent rifampicin-resistant initiation sites in euchromatin deoxyribonucleic acid. Biochem J. 1974 Oct;143(1):73–81. doi: 10.1042/bj1430073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. J., Felsenfeld G. Structure of chromatin. Nat New Biol. 1971 Jan 27;229(4):101–106. doi: 10.1038/newbio229101a0. [DOI] [PubMed] [Google Scholar]
- Gennis R. B., Cantor C. R. Optical studies of a conformational change in DNA before melting. J Mol Biol. 1972 Apr 14;65(3):381–399. doi: 10.1016/0022-2836(72)90196-9. [DOI] [PubMed] [Google Scholar]
- Henson P., Walker I. O. The partial dissociation of nucleohistone by salts. Hydrodynamic and denaturation studies. Eur J Biochem. 1970 Jun;14(2):345–350. doi: 10.1111/j.1432-1033.1970.tb00295.x. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Nicolini C., Baserga R., Kendall F. DNA structure in sheared and unsheared chromatin. Science. 1976 May 21;192(4241):796–798. doi: 10.1126/science.1265482. [DOI] [PubMed] [Google Scholar]
- Nicolini C., Ng S., Baserga R. Effect of chromosomal proteins extractable with low concentrations of NaCl on chromatin structure of resting and proliferating cells. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2361–2365. doi: 10.1073/pnas.72.6.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll M., Thomas J. O., Kornberg R. D. Preparation of native chromatin and damage caused by shearing. Science. 1975 Mar 28;187(4182):1203–1206. doi: 10.1126/science.187.4182.1203. [DOI] [PubMed] [Google Scholar]
- Parodi S., Mulivor R. A., Martin J. T., Nicolini C., Sarma D. S., Farber E. Alkaline lysis of mammalian cells for sedimentation analysis of nuclear DNA. Conformation of released DNA as monitored by physical, electron microscopic and enzymological techniques. Biochim Biophys Acta. 1975 Oct 1;407(2):174–190. doi: 10.1016/0005-2787(75)90283-x. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Bonner J. Template properties of DNA-polypeptide complexes. J Mol Biol. 1970 Jun 14;50(2):333–344. doi: 10.1016/0022-2836(70)90196-8. [DOI] [PubMed] [Google Scholar]
- Subirana J. A. Studies on the thermal denaturation of nucleohistones. J Mol Biol. 1973 Mar 5;74(3):363–386. doi: 10.1016/0022-2836(73)90378-1. [DOI] [PubMed] [Google Scholar]
- Wilhelm F. X., de Murcia G. M., Champagne M. H., Daune M. P. Conformational changes of histones and DNA during the thermal denaturation of nucleoprotein. Eur J Biochem. 1974 Jun 15;45(2):431–443. doi: 10.1111/j.1432-1033.1974.tb03567.x. [DOI] [PubMed] [Google Scholar]
- de Pomerai D. I., Chesterton C. J., Butterworth P. H. Preparation of chromatin. Variation in the template properties of chromatin dependent on the method of perparation. Eur J Biochem. 1974 Aug 1;46(3):461–471. doi: 10.1111/j.1432-1033.1974.tb03639.x. [DOI] [PubMed] [Google Scholar]



