Abstract
Traumatic axonal injury (TAI) accounts for at least 35% of the morbidity and mortality in traumatic brain injury (TBI) patients without space-occupying lesions. It is also believed to be a key determinant of adverse outcomes such as cognitive dysfunction across the spectrum of TBI severity. Previous studies have shown that COG1410, a synthetic peptide derived from the apolipoprotein E (apoE) receptor binding region, has anti-inflammatory effects after experimental TBI, with improvements in cognitive recovery. However, the effects of COG1410 on axonal injury following TBI are not known. The current study evaluated the effects of 1 mg/kg daily COG1410 versus saline administered intravenously starting 30 min after controlled cortical impact (CCI) injury on pericontusional TAI in young, wild-type C57BL6/J male mice. We found that COG1410 did not affect the number of amyloid precursor protein (APP)-immunoreactive axonal varicosities in the pericontusional corpus callosum and external capsule at 24 h, but reduced APP-immunoreactive varicosities by 31% at 3 days (p=0.0023), and 36% at 7 days (p=0.0009). COG1410 significantly reduced the number of Iba1-positive cells with activated microglial morphology at all three time points by 21–30%. There was no effect of COG1410 on pericontusional white matter volume or silver staining at any time point. This indicates a possible effect of COG1410 on delayed but not immediate TAI. Future studies are needed to investigate the underlying mechanisms, therapeutic time window, and physiological implications of this effect.
Key words: COG1410, controlled cortical impact injury, microglia, neuroprotection, traumatic axonal injury
Introduction
Neuronal cell death has received much attention in the field of traumatic brain injury (TBI). However, traumatic axonal injury (TAI), sometimes referred to as diffuse axonal injury (DAI), is well known to be associated with TBI of all types, ranging from mild to severe (Adams et al., 1989; Blumbergs et al., 1995, 1989; Smith et al., 2003; Strich, 1956, 1961). TAI may be of crucial importance in determining adverse outcomes such as cognitive dysfunction across the spectrum of TBI severity (Buki and Povlishock, 2006; Gennarelli et al., 1982; Kraus et al., 2007; Lipton et al., 2009; Niogi and Mukherjee, 2010; Niogi et al., 2008a, 2008b; Perlbarg et al., 2009; Smith et al., 2000; Wang et al., 2008).
The pathophysiology of TBI is extremely complex. In the past decades, several biochemical mechanisms involved in TBI have been demonstrated, including cellular calcium homeostasis, inflammation, apoptosis, free radical generation, and lipid peroxidation (Werner and Engelhard, 2007). Multiple pharmacological agents based on these mechanisms have reached Phase III clinical trials after promising results of animal testing. However, to date, all of them have failed to show a therapeutic benefit, and some have produced worse outcomes for acute or chronic treatment of TBI (Margulies and Hicks, 2009; Narayan et al., 2002). Of note, relatively few of the candidate therapeutics have demonstrated preclinical efficacy in TAI (Buki et al., 1999; Fujita et al., 2011; Koizumi and Povlishock, 1998; Oda et al., 2011; Mbye et al., 2008; Okonkwo et al., 2003; Smith et al., 2003).
Genetic polymorphisms may play a role in the susceptibility of a given individual to adverse outcomes following TBI (Dardiotis et al., 2010). Apolipoprotein E polymorphisms (APOE=gene, apoE=protein) are the most extensively studied genetic factor in neurotrauma research (Jellinger et al., 2001, 2004; Sun and Jiang, 2008; Teasdale et al., 2005). In humans, apoE is the major apolipoprotein expressed in the brain and exists as three isoforms, designated E2, E3, and E4. Many studies, including our previous clinical research, have demonstrated that the presence of the APOEɛ4 allele predisposes individuals to clinical deterioration in the acute phase and poor long-term outcome (Jiang et al., 2006; Sorbi et al., 1995; Teasdale et al., 1997,2005; Zhou et al., 2008). Under normal physiologic conditions, apoE serves as an important mediator of cholesterol and lipid transport in the brain. In response to injury, apoE may exert neuroprotective effects via multiple mechanisms including antioxidant, anti-inflammatory, anti-excitotoxic, and neurotrophic mechanisms (Hatters et al., 2006; Verghese et al., 2011). Studies of genetically modified mice further suggested that apoE modulates the response to brain injury. For example, apoE-deficient mice have exacerbated cerebral edema and worse functional deficits than their wild-type counterparts after acute closed head injury (Chen et al., 1997; Lynch et al., 2002). The above observations suggest a role for apoE in remodeling and modulating recovery in response to injury, and indicate the potential of neuroprotective therapy based on apoE. In fact, several studies have demonstrated that exogenous administration of intrathecal apoE improved outcomes of ischemic injury (Horsburgh et al., 2000; McAdoo et al., 2005). However, apoE does not cross the blood–brain barrier (BBB) in appreciable quantities (Linton et al., 1991). Since this makes it unsuitable for systemic administration, apoE itself would be of limited practical use in clinical trials.
To attempt to harness the therapeutic potential of endogenous apoE, apoE-mimetic drugs have been developed that retain the receptor-binding and effective functional characteristics of the intact apoE holoprotein, while being modified to cross the BBB. COG133, a peptide based on apoE amino acid residues 133–149, has been reported to demonstrate neuroprotective, antioxidant, anti-excitotoxic, and anti-inflammatory properties in vitro and in vivo (Aono et al., 2003; Laskowitz et al., 2001; Lynch et al., 2003, 2005; McAdoo et al., 2005; Misra et al., 2001). Subsequently, COG1410 was developed, composed of apoE residues 138–149, with aminoisobutyric acid (Aib) substitutions at positions 140 and 145 (Laskowitz et al., 2007). COG1410 reportedly retains most of the properties of COG133, and expands the therapeutic window to 120 min post-experimental TBI. The effects of COG1410 have been assessed based on vestibulomotor function, microglial activation, neuronal cell death in the hippocampus, and neurodegenerative pathology (Hoane et al., 2009; James et al., 2009; Laskowitz et al., 2010; Tukhovskaya et al., 2009).
However, to our knowledge, the effects of COG1410 on axonal injury have not been reported. Although TAI is considered a major contributor to cognitive dysfunction in TBI patients, and is a process potentially amenable to therapeutic intervention, the precise effects of COG1410 on the axonal damage seen following TBI are still unclear. In the current study, we explored the effects of COG1410 when administered following controlled cortical impact (CCI) injury on pericontusional TAI. We found that COG1410 attenuated amyloid precursor protein (APP) immunohistochemistry at subacute (3 and 7 days), but not acute (1 day) time points after injury. Pericontusional white matter microglial activation as indicated by Iba1 immunohistochemistry was reduced by COG1410 at all three time points.
Methods
In this study, all the following procedures were approved by the Washington University Animal Studies Committee, and are consistent with the National Institutes of Health (NIH) guidelines for the care and use of animals.
Experimental TBI: Controlled cortical impact
For these experiments, a total of 45 8- to 10-week-old C57BL/6J male mice (Jackson Laboratory, Bar Harbor, ME) weighing 18–23 g were used. Three separate groups of mice were sacrificed at 24 h, 3 days, and 7 days after experimental injury. For each time point, 5 mice received TBI + COG1410, 5 mice received TBI + saline, and 5 mice underwent sham injury. This injury model was adapted from a previously described experimental TBI method for mice (Brody et al., 2007). Briefly, the mice were anesthetized using 5% isoflurane, shaved, and then placed in a stereotaxic frame (MyNeuroLab, St. Louis, MO). Throughout the surgery, the mice were provided with constant 2% isoflurane in room air. Using sterile technique, a midline incision was made, and the scalp was reflected to expose the skull. A 5-mm left lateral craniotomy was performed using a motorized drill mounted to the stereotactic arm, and centered at 2.7 mm lateral from the midline and 3 mm anterior to the lambda. The skull was removed without disrupting the underlying dura. The impact depth of the stereotaxic device was set at 2.0 mm. The impactor, employing a 3-mm-diameter rod tip, was then driven at a velocity of 5 m/sec with a dwell time of 100 msec. This produces a moderately severe contusion in the left sensorimotor cortex and underlying hippocampus, with pronounced behavioral deficits but virtually no mortality. The sham group consisted of mice that received craniotomy but not cortical impact. Following the injury, a plastic skull cap was secured over the craniotomy and adhered to the skull using cyanoacrylate. The skin incision was sutured. The mice were kept at 37°C via a rectal temperature probe and feedback temperature controller throughout the duration of the surgery. The anesthetized mice were placed in an incubator (37°C) until they recovered the ability to ambulate.
Synthesis and administration of peptides
COG1410 was synthesized and kindly provided by Cognosci (Research Triangle Park, NC) at a purity of 95%. COG1410 is Ac-AS(Aib)LRKL(Aib)KRLLamide, which is derived from apoE residues 138–149 with Aib substitutions at positions 140 and 145. The peptide was reconstituted in a sterile 0.9% saline solution at a concentration of 1 mg/kg prior to administration. The mice were randomly assigned to either the COG1410 (1 mg/kg IV) or vehicle (0.9% saline IV) groups. Injections were given 30 min following injury, and then every 24 h (3-day group and 7-day group) via tail vein infusion. Infusions were performed under brief (<5 min), light isoflurane anesthesia (2%).
Histopathology
The mice were deeply anesthetized with an overdose of isoflurane and were perfused intracardially with ice-cold phosphate-buffered saline (PBS, pH 7.4) plus 0.3% heparin. After sacrifice, the brains were carefully removed, fixed in 4% paraformaldehyde for 24 h, and then equilibrated in 30% sucrose. Serial coronal slices 50 μm thick were cut on a freezing microtome. Two sets of sections spaced every 300 μm were mounted on glass slides and used for immunohistochemical studies as described previously (Mac Donald et al., 2007a). The controls underwent the same procedures. Briefly, all sections were washed with Tris-buffered saline (TBS) between applications of antibody solutions. Endogenous peroxidase activity was blocked with 0.3% hydrogen peroxide in TBS (10 min). After blocking of nonspecific binding sites with 3% normal goat serum (Sigma-Aldrich Chemie, Steinheim, Germany) in TBS containing 0.25% Triton X (TBS-X) for 30 min, the slides were incubated with primary antibodies overnight at 4°C. The following antibodies were used for immunohistochemistry: polyclonal rabbit anti-β-APP (Invitrogen, Carlsbad, CA) in a 1:500 dilution, and polyclonal rabbit anti-Iba1 antibody (Wako Chemicals USA, Richmond, VA) in a 1:1000 dilution. The slides were developed with biotinylated secondary antibodies. Bound antibodies were visualized with the horseradish peroxidase method (ABC Elite kit, PK6100; Vector Laboratories, Burlingame, CA) and diaminobenzidine (DAB). The sections were mounted on glass slides and allowed to dry. Once dry, the slides were dipped for 1 min each in 50%-70%-95%-95%-100% ethanol solutions, followed by 4 min each in xylene. Finally the slides were cover-slipped with Cytoseal 60 (Richard-Allan Scientific, Kalamazoo, MI) for stereological quantification. As a control for background staining, control sections were treated in the same manner, except TBS solution was substituted for the primary antibodies. Negative controls included sections from uninjured mice, and sections from injured mice with omission of the primary antibodies. The positive controls for APP staining were brain slices from PDAPP mice.
Silver staining was performed on a third set of sections to visualize degenerating neuronal elements in the brain. Sections were processed with the FD NeuroSilver kit II (FD NeuroTechnologies, Elicott City, MD), according to the manufacturer's instructions with modifications as in our previous study (Shitaka et al., 2011). The modified protocol was made more sensitive and resulted in slightly increased background. For the quantification of degeneration in the corpus callosum and external capsule, entire transverse sections were digitized with an image analysis system. NIH ImageJ software was used to measure the optical density of silver staining, as previously described (Shitaka et al., 2011).
Stereological quantification
Unbiased stereological methods were used to quantify the numbers of APP-stained axonal varicosities and Iba1-stained microglia cells per cubic millimeter in the region of the corpus callosum and external capsule via StereoInvestigator version 8.2 software (MicroBrightField, Williston, VT) and a Nikon (Tokyo, Japan) Eclipse E800 microscope. All assessments were made by a single investigator blinded to the injury status and treatment regimens of the animals. The optical fractionator technique was used to count a systematic random sample of positively-stained axonal varicosities over the entire rostral to caudal extent of the region of interest. Details of these stereological methods have been previously described (Mac Donald et al., 2007a, 2007b; Shitaka et al., 2011; Tran et al., 2011). In brief, for quantification of APP-stained axonal varicosities and Iba1-stained microglia, the region of the corpus callosum and external capsule from each coronal slice was outlined at low power (4×), followed by systematic counts of objects of interest at high power (60×, oil immersion), of sites within the counting region randomly chosen by the StereoInvestigator software. For quantification of APP, a 200×200-μm (24 h and 3 d), or 100×100 μm (7 days) sampling grid, and a 40×40-μm counting frame was used. Non-axonal DAB-stained structures (e.g., DAB-positive red blood cells) were not counted. For quantification of Iba1, a 180×180-μm sampling grid and an 80×80-μm counting frame was used. A dissector height of 15 μm and guard zones of 2.5 μm were used for all measurements. Seven sections per mouse for each stain were used for the stereological estimation.
Statistical analysis
All data were analyzed using Prism 5.0 software (GraphPad Software, San Diego, CA). The results are presented as the mean±standard error of the mean (SEM) of 5 animals. There was no evidence indicating significant deviations from the normal distribution in any of the data sets (p>0.05 by Shapiro-Wilk tests). Therefore, for each type of quantitative data (APP and Iba1 counts, silver staining density, and white matter volume), a two-way analysis of variance (ANOVA) was used. The factors were treatment group (COG1410 versus saline), and time (1, 3, or 7 days after injury). Significant main effects or interactions were subjected to post-hoc analysis for independent samples. Pre-specified post-hoc comparisons included COG1410 versus saline at each time point. A significance level of p<0.05 after Bonferroni correction for multiple comparisons was used for all statistical analyses. All p values have been reported before correction for multiple comparisons.
Results
Effect of COG1410 on APP immunohistochemistry in pericontusional TAI
The pericontusional corpus callosum and external capsule are among the white matter structures most substantially affected in CCI (Hall et al., 2005; Mac Donald et al., 2007a, 2007b). To explore the effects of COG1410 on pericontusional TAI, we examined APP accumulation in the corpus callosum and external capsule axonal fibers after COG1410 treatment.
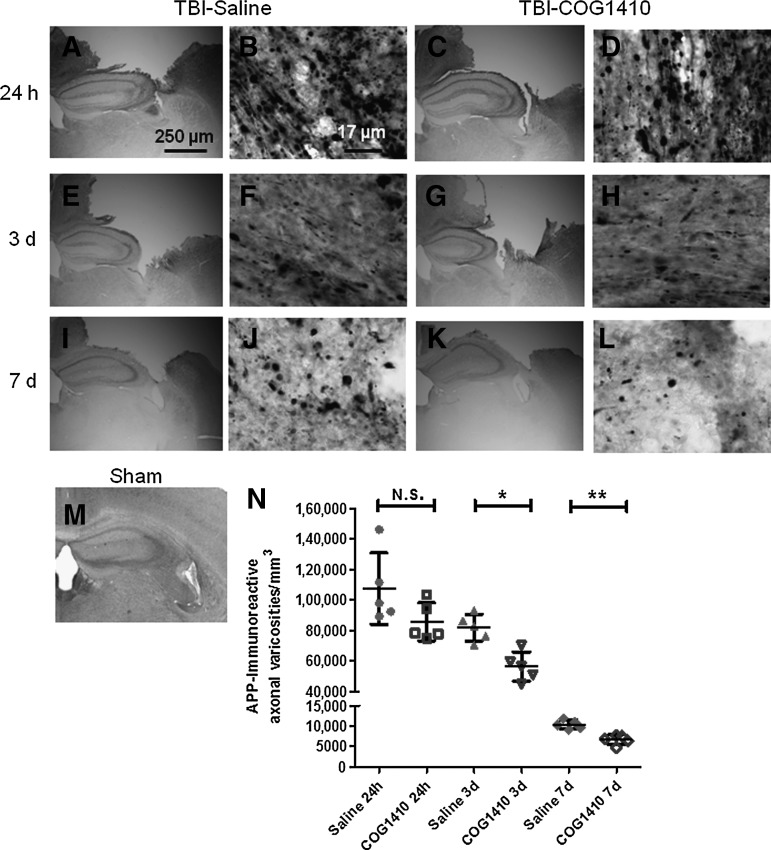
As reported previously, we found spheroidal APP accumulations in pericontusional white matter of injured mice. The ipsilateral corpus callosum and external capsule appeared to have the most prominent APP accumulations (Fig. 1). No such axonal APP staining was observed in uninjured (sham) controls (Fig. 1M). Although there were still numerous APP-stained varicosities (Fig. 1E–L) in injured mice at 3 days and 7 days, the extent of APP staining was reduced compared with 24 h after TBI (Fig. 1N).
FIG. 1.
Reduction in delayed but not acute pericontusional axonal injury after controlled cortical impact traumatic brain injury (CCI-TBI) after COG1410 treatment. (A–M) Micrographs of amyloid precursor protein (APP) immunoreactivity in pericontusional white matter of injured (A–L) and sham (M) wild-type C57BL/6J mice. Separate groups of mice were assessed at 24 h (A–D), 3 days (E–H), and 7 days (I–L) after injury. Scale bar in A applies also to panels C, E, G, I, and K. Scale bar in B applies also to panels D, F, H, J, and L. (N) Stereological quantification of numbers of APP-immunoreactive varicosities in the ipsilateral corpus callosum and external capsule of injured mice receiving either COG1410 or vehicle treatment (*p=0.0023; **p=0.0009 by Student's t-test).
We examined the effects of COG1410 or vehicle treatment on injured mice up to 7 days after TBI. There appeared to be no difference in the number of APP-stained varicosities in the ipsilateral corpus callosum and external capsule between groups at 24 h (Fig. 1B and D). However, there appeared to be fewer APP-stained varicosities in the COG1410 group (Fig. 1H and L), compared with the saline group (Fig. 1F and J), at 3 days and 7 days.
Quantitative analyses of APP-immunoreactive varicosities in these regions using stereology confirmed the qualitative histological findings (Fig. 1N). In a two-way ANOVA, there were significant main effects of treatment (F=14.92, p=0.0007), and of time since injury (F=140.8, p<0.0001), without a significant treatment×time interaction (F=2.3, n.s.). In prespecified post-hoc testing, COG1410 treatment was found not to affect the numbers of APP-stained varicosities in the ipsilateral corpus callosum and external capsule at 24 h (Fig. 1N; p=0.10 by two-sided Student's t-test). However, there was an approximately 31% reduction in APP-immunoreactive varicosities in the COG1410-treated group at 3 days (p=0.0023), and a 36% reduction at 7 days (p=0.0009).
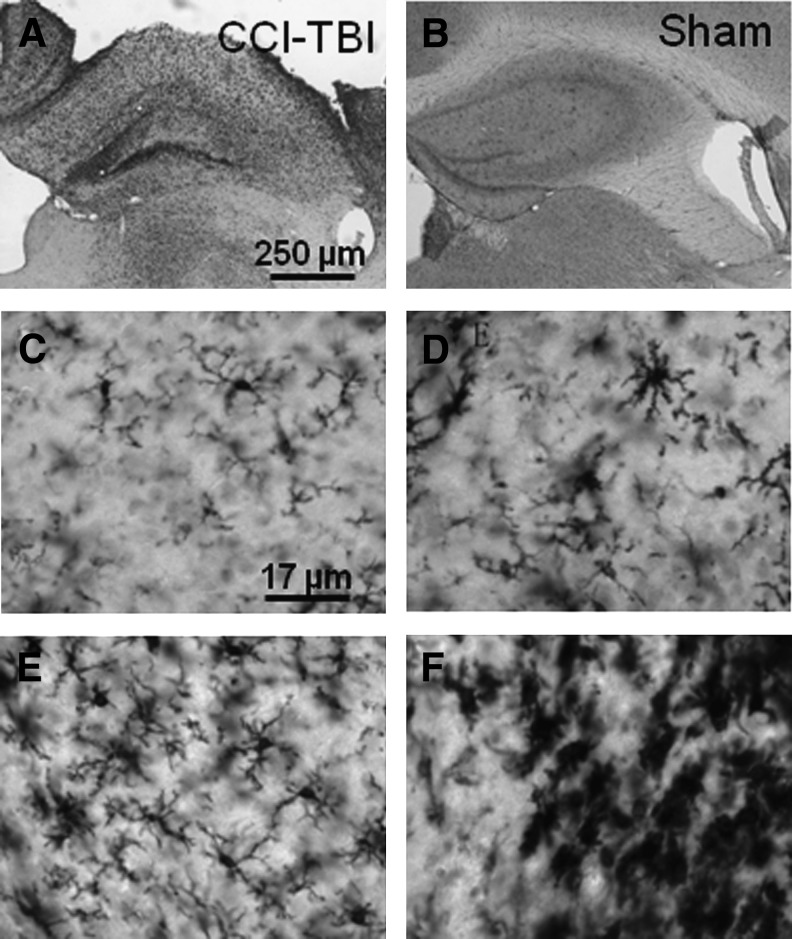
Effect of COG1410 on microglial activation in regions of pericontusional TAI
The ipsilateral corpus callosum and external capsule appeared to have prominent Iba1 immunoreactivity in cells with morphological characteristics of activated microglia following CCI (Fig. 2A). Minimal Iba1 staining was observed in sham-injured mice (Fig. 2B). Morphological criteria have been used to distinguish among four sub-populations of Iba1-immunoreactive cells in mice. All four were observed following CCI, including resting ramified microglia with spindle-shaped cell bodies and numerous long, thin, branched processes (Fig. 2C), hypertrophic microglia with extended thicker processes (Fig. 2D), bushy microglia with densely-branched processes (Fig. 2E), and amoeboid microglia with densely-labeled cell bodies and few processes (Fig. 2F). However, there were numerous cells for which an objective determination of morphological sub-population could not be made. Therefore, quantitative analyses were based on total numbers of Iba1-immunoreactive microglial cells (Shitaka et al., 2011).
FIG. 2.
Iba1 immunoreactivity in the ipsilateral corpus callosum and external capsule after controlled cortical impact traumatic brain injury (CCI-TBI). (A–B) Low-power micrographs of Iba1 immunoreactivity in pericontusional white matter of injured (A), sham (B), and wild-type C57BL/6J mice. (C–F) High-power micrographs of Iba1-immunoreactive microglia of various morphologies, including resting ramified (C), hypertrophic (D), bushy (E), and amoeboid (F).
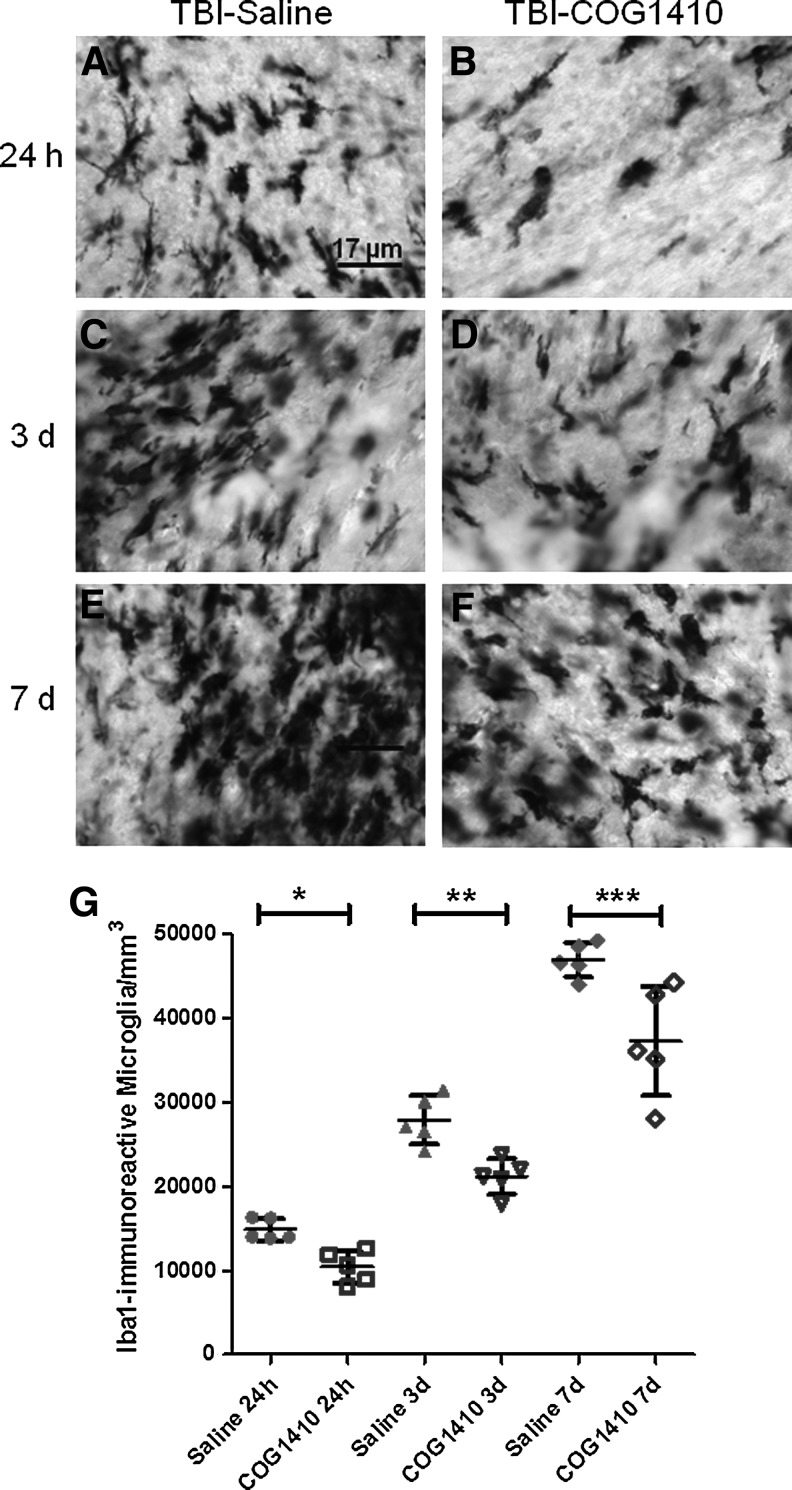
We examined the effects of COG1410 versus vehicle treatment on Iba1-immunoreactive cells in injured mice at 24 h, 3 days, and 7 days after TBI (Fig. 3). Overall, the numbers of Iba1-immunoreactive cells in pericontusional white matter were observed to increase with time after TBI. At all three time points, there appeared to be fewer Iba1-immunoreactive microglia in the ipsilateral corpus callosum and external capsule of COG1410-treated mice (Fig. 3A–F).
FIG. 3.
Reduction in pericontusional Iba1-immunoreactive microglia following controlled cortical impact traumatic brain injury (CCI-TBI) after COG1410 treatment. (A–F) High power micrographs of Iba1-immunoreactive microglia. Mice were assessed at 24 h (A–B), 3 days (C–D), and 7 days (E–F) after injury. (G) Stereological quantification of numbers of Iba1-immunoreactive activated microglia in the ipsilateral corpus callosum and external capsule of injured mice receiving either COG1410 or vehicle treatment (*p=0.0026, **p=0.0030, ***p=0.0132 by Student's t-test).
Quantitative analyses of Iba1-immunoreactive microglial cell counts in these regions using stereology confirmed the qualitative histological findings. In a two-way ANOVA, there were significant main effects of treatment (F=33.47, p<0.0001), and of time since injury (F=203.8, p<0.0001), without a significant treatment×time interaction (F=1.6, n.s.). In prespecified post-hoc testing, COG1410 treatment was found to reduce the numbers of APP-stained varicosities in the ipsilateral corpus callosum and external capsule at all three time points compared to vehicle-treated mice (Fig. 3G). The reduction was 30% at 24 h (p=0.0026), 24% at 3 days (p=0.003), and 21% at 7 days (p=0.013).
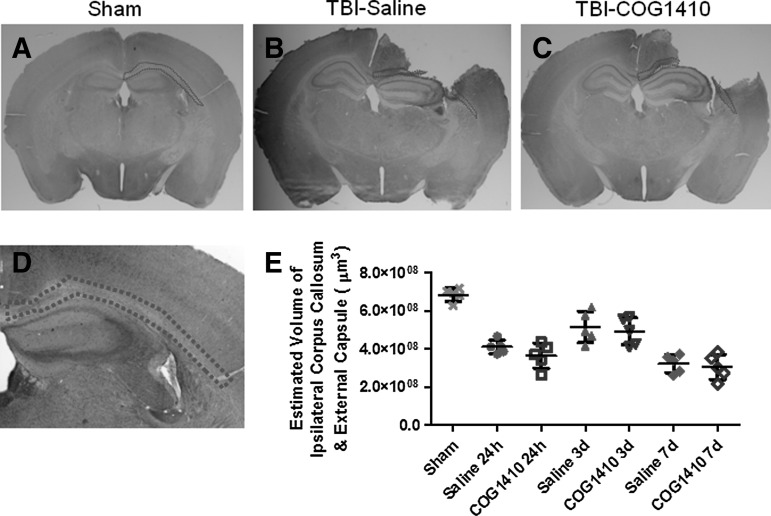
Effect of COG1410 on estimated volume of the ipsilateral corpus callosum and external capsule
To explore the effect of COG1410 on the white matter atrophy, the volume of remaining ipsilateral corpus callosum and external capsule (Fig. 4A–D) was estimated by the Cavalieri method. In a two-way ANOVA, there was no significant main effect of treatment (F=1.5, n.s.), a significant main effect of time since injury (F=22.7, p<0.0001), and no significant treatment×time interaction (F=0.1, n.s.). No differences between COG1410 treatment and saline groups were found at any time point in post-hoc testing. (Fig. 4E).
FIG. 4.
The volume of the ipsilateral corpus callosum and external capsule were not affected by COG1410. (A–C) Whole-slice images illustrating the region of interest tracing (dotted outlines) on a single slice in sham-injured mice (Sham, A), mice treated with saline (TBI-saline, B), and injured mice treated with COG1410 (TBI-COG1410, C). Full three-dimensional volumes were estimated using the Cavalieri method. (D) Low-power micrograph illustrating the region of interest. (E) Quantitative analysis of full three-dimensional volumes, estimated using the Cavalieri method across multiple slices. No significant differences were found between the COG1410- and saline vehicle-treated groups (p>0.05).
Effect of COG1410 on silver staining
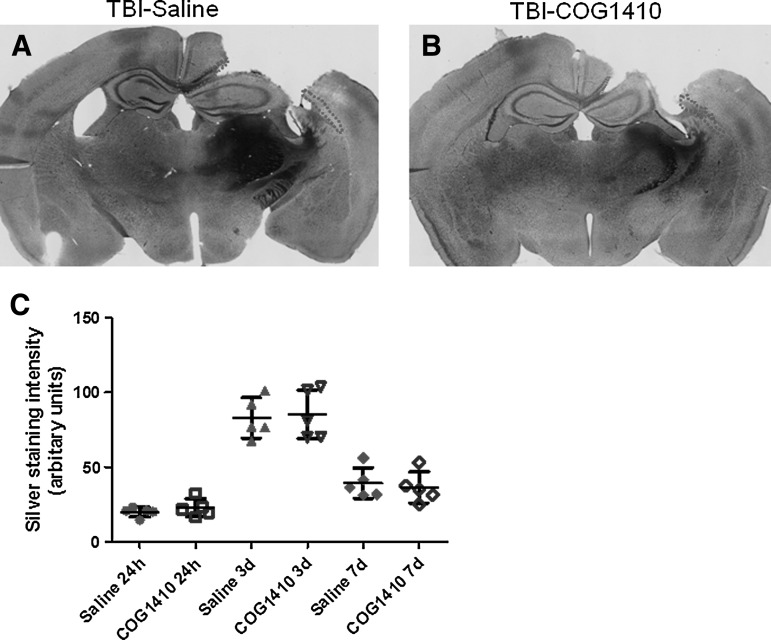
Controlled cortical impact has been reported to cause widespread silver-staining abnormalities (Hall et al., 2005). While the exact nature of these silver-staining abnormalities has not been determined, they have been suggested to reflect protein aggregation and neurodegenerative processes (Betarbet et al., 2000; Ross and Poirier, 2004). Here we confirmed that CCI in mice results in widespread silver staining in the injured brain. Densitometric methods were used to quantify the extent of silver staining in the ipsilateral corpus callosum and external capsule. We found that the peak in silver-staining density occurred at 3 days. However, there was no significant difference between the extent of silver staining in the ipsilateral corpus callosum and external capsule at any time point post-injury in the COG1410- and saline-treated groups (Fig. 5, p>0.05). In a two-way ANOVA, there was no significant main effect of treatment (F=0.4, n.s.), a significant main effect of time since injury (F=89.3, p<0.0001), and no significant treatment×time interaction (F=0.2, n.s.). No differences between COG1410 treatment the and saline groups were found at any time point in post-hoc testing. Thus, post-traumatic silver staining was unaffected by COG1410 at a dosing regimen that reduced APP and Iba1 immunoreactivity.
FIG. 5.
No effects of COG1410 on silver staining intensity were seen in the ipsilateral corpus callosum and external capsule following controlled cortical impact (CCI). (A–B) Low-power micrographs of silver staining in injured mice treated with saline (A), and COG1410 (B), at 7 days. (C) Densitometric methods were used to quantify the extent of silver staining in the full three-dimensional white matter region of interest (the areas in the single-slice images outlined in dotted lines in panels A and B). Silver staining intensity peaked at 3 days, but was no different between COG1410- and saline-treated mice at any time point (p>0.05).
Discussion
In summary, COG1410 reduced the number of APP-immunoreactive axons at 3 days and 7 days after controlled cortical impact in mice, but did not affect APP immunoreactivity at 24 h. COG1410 reduced the number of pericontusional white matter Iba1-positive cells with activated microglial morphology at all three time points. COG1410 did not have a detectable effect on silver staining or white matter atrophy in pericontusional regions at any time point. This provides some initial experimental evidence that COG1410 may have a beneficial effect on some aspects of pericontusional traumatic axonal injury.
There are several possible interpretations of these results. One is that COG1410 has no effect on initial pericontusional TAI, but inhibits one or more forms of delayed secondary pericontusional TAI. The reduction in Iba1-positive microglia suggests that an inflammatory component may play a role in this delayed secondary injury. Another interpretation is that COG1410 accelerates the clearance of APP-immunoreactive axonal varicosities or reduces delayed transport of APP to the site of injury. A third is that COG1410 impairs the immunohistochemical identification of APP-immunoreactive injured axons through other mechanisms (e.g., downregulating APP production, enhancing clearance of APP, or otherwise reducing APP immunoreactivity). The lack of effect on silver staining raises the question of which markers of white matter injury are most appropriate. Silver staining has been reported to be sensitive to the potential benefits of preclinical candidate therapeutics such as the free radical scavenger Tempol (Deng-Bryant et al., 2008). Further experiments using other markers of axonal injury such as neurofilament immunohistochemistry, diffusion tensor imaging, electron microscopy, and electrophysiology, will be required to fully address the effects of COG1410 on axonal injury. Thus it is not entirely clear whether COG1410 truly preserves pericontusional axonal health following CCI.
The effects of COG1410 on Iba1-immunoreactive activated microglia at all time points suggest an effect on the inflammatory response to injury, as has been reported previously (Laskowitz et al., 2007). Inflammatory responses may contribute to secondary injury cascades that promote continued cellular destruction, but it also could play a key role in repair and regeneration of the injury (Kelley et al., 2007; Namas et al., 2009; Shein et al., 2008). It is not known whether persistent microglial activation is beneficial, neutral, or harmful on the whole. One hypothesis is that microglia have a harmful effect on pericontusional axonal injury, and that reduction in the microglial response is the proximal mechanism by which COG1410 affects TAI. Additional interventions targeting microglia will be necessary to directly address this hypothesis (Elliott et al., 2011; Grathwohl et al., 2009; Namas et al., 2009). By analogy, minocycline, which may also inhibit microglial activation, has been shown to improve outcomes in other experimental injury models (Kim and Suh 2009). Future investigations specifically focused on microglial subclasses (Kigerl et al., 2009) are required to address the various roles of microglia.
We favor the hypothesis that the Iba1-immunoreactive cells with activated microglial morphology in the pericontusional white matter represent a response to axonal injury. Previous studies have indicated that apoE can suppress glial activation following stimulation with a variety of structurally-diverse agents (Barger and Harmon, 1997; Laskowitz et al., 1997). It has been demonstrated that apoE modulates the CNS inflammatory response by downregulating glial secretion of inflammatory cytokines and neurotoxic mediators such as nitric oxide (NO) and tumor necrosis factor-α (TNF-α) in vitro (Laskowitz et al., 2001).
There are several limitations of the current study. First, the effects of COG1410 have only been tested on one type of TAI, pericontusional. In contrast, human TAI can be diffuse or multifocal, associated with rapid angular (rotational) acceleration and deceleration of the brain. Therefore, an additional axonal injury paradigm such as fluid percussion injury or impact acceleration injury, which produces more diffuse axonal injury, is needed to verify our findings.
Second, these studies have been carried out in wild-type mice. Strain-matched mice with human APOE alleles “knocked in” to the mouse loci (Laskowitz et al., 2010; Sullivan et al., 1997) should be tested to explore the pharmacogenetic effects of COG1410 on axonal injury.
Third, the dose-response and therapeutic window following injury for COG1410 have not been determined. In the current study, COG1410 was first injected intravenously at 30 min following injury. In clinical trials, first doses starting 6–12 h after injury are considered the earliest feasible times, unless waiver of consent can be obtained. Furthermore, COG1410 was dosed every 24 h. A pharmacokinetic study of COG1410 has shown that the half-life of COG1410 is 13±5 min in blood plasma following intravenous administration (M. Vitek, unpublished data). However, the pharmacokinetics of COG1410 in the brain have not been described.
In conclusion, these findings indicate a possible protective effect of COG1410 on delayed traumatic axonal injury in the setting of controlled cortical impact TBI. However, several questions remain, and further studies are needed to determine whether COG1410 can be considered a well-validated preclinical therapeutic agent.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grants 81000528 and 30973087), the Foundation for Sci & Tech Research Project of Sichuan Province (grant 2009JY0126), the Project Foundation of Health Bureau of Sichuan Province (grant 090217), the Project Foundation of Sichuan Province Education Committee (grant 08zb049), and the NIH (grant NS065069). We thank Thomas Esparza, Rachel Bennett, and Hien Tran for technical assistance, and Dr. David Holtzman for the use of his stereology system. We thank Cognosci Inc. for providing COG1410. None of the authors has a financial interest in Cognosci Inc.
Author Disclosure Statement
No conflicting financial interests exist.
References
- Adams J.H. Doyle D. Ford I. Gennarelli T.A. Graham D.I. McLellan D.R. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989;15:49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- Aono M. Bennett E.R. Kim K.S. Lynch J.R. Myers J. Pearlstein R.D. Warner D.S. Laskowitz D.T. Protective effect of apolipoprotein E-mimetic peptides on N-methyl-D-aspartate excitotoxicity in primary rat neuronal-glial cell cultures. Neuroscience. 2003;116:437–445. doi: 10.1016/s0306-4522(02)00709-1. [DOI] [PubMed] [Google Scholar]
- Barger S.W. Harmon A.D. Microglial activation by Alzheimer amyloid precursor protein and modulation by apolipoprotein E. Nature. 1997;388:878–881. doi: 10.1038/42257. [DOI] [PubMed] [Google Scholar]
- Betarbet R. Sherer T.B. MacKenzie G. Garcia-Osuna M. Panov A.V. Greenamyre J.T. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nature Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Blumbergs P.C. Jones N.R. North J.B. Diffuse axonal injury in head trauma. J. Neurol. Neurosurg. Psychiatry. 1989;52:838–841. doi: 10.1136/jnnp.52.7.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumbergs P.C. Scott G. Manavis J. Wainwright H. Simpson D.A. McLean A.J. Topography of axonal injury as defined by amyloid precursor protein and the sector scoring method in mild and severe closed head injury. J. Neurotrauma. 1995;12:565–572. doi: 10.1089/neu.1995.12.565. [DOI] [PubMed] [Google Scholar]
- Brody D.L. Mac Donald C. Kessens C.C. Yuede C. Parsadanian M. Spinner M. Kim E. Schwetye K.E. Holtzman D.M. Bayly P.V. Electromagnetic controlled cortical impact device for precise, graded experimental traumatic brain injury. J. Neurotrauma. 2007;24:657–673. doi: 10.1089/neu.2006.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buki A. Povlishock J.T. All roads lead to disconnection?—Traumatic axonal injury revisited. Acta Neurochirurgica. 2006;148:181–193. doi: 10.1007/s00701-005-0674-4. [DOI] [PubMed] [Google Scholar]
- Buki A. Okonkwo D.O. Povlishock J.T. Postinjury cyclosporin A administration limits axonal damage and disconnection in traumatic brain injury. J. Neurotrauma. 1999;16:511–521. doi: 10.1089/neu.1999.16.511. [DOI] [PubMed] [Google Scholar]
- Chen Y. Lomnitski L. Michaelson D.M. Shohami E. Motor and cognitive deficits in apolipoprotein E-deficient mice after closed head injury. Neuroscience. 1997;80:1255–1262. doi: 10.1016/s0306-4522(97)00007-9. [DOI] [PubMed] [Google Scholar]
- Dardiotis E. Fountas K.N. Dardioti M. Xiromerisiou G. Kapsalaki E. Tasiou A. Hadjigeorgiou G.M. Genetic association studies in patients with traumatic brain injury. Neurosurgical Focus. 2010;28:E9. doi: 10.3171/2009.10.FOCUS09215. [DOI] [PubMed] [Google Scholar]
- Deng-Bryant Y. Singh I.N. Carrico K.M. Hall E.D. Neuroprotective effects of tempol, a catalytic scavenger of peroxynitrite-derived free radicals, in a mouse traumatic brain injury model. J. Cereb. Blood Flow Metab. 2008;28:1114–1126. doi: 10.1038/jcbfm.2008.10. [DOI] [PubMed] [Google Scholar]
- Elliott M.B. Tuma R.F. Amenta P.S. Barbe M.F. Jallo J.I. Acute effects of a selective cannabinoid-2 receptor agonist on neuroinflammation in a model of traumatic brain injury. J. Neurotrauma. 2011;28:973–981. doi: 10.1089/neu.2010.1672. [DOI] [PubMed] [Google Scholar]
- Fujita M. Oda Y. Wei E.P. Povlishock J.T. The combination of either tempol or FK506 with delayed hypothermia: implications for traumatically induced microvascular and axonal protection. J. Neurotrauma. 2011;28:1209–1218. doi: 10.1089/neu.2011.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennarelli T.A. Thibault L.E. Adams J.H. Graham D.I. Thompson C.J. Marcincin R.P. Diffuse axonal injury and traumatic coma in the primate. Ann. Neurol. 1982;12:564–574. doi: 10.1002/ana.410120611. [DOI] [PubMed] [Google Scholar]
- Grathwohl S.A. Kalin R.E. Bolmont T. Prokop S. Winkelmann G. Kaeser S.A. Odenthal J. Radde R. Eldh T. Gandy S. Aguzzi A. Staufenbiel M. Mathews P.M. Wolburg H. Heppner F.L. Jucker M. Formation and maintenance of Alzheimer's disease beta-amyloid plaques in the absence of microglia. Nat. Neurosci. 2009;12:1361–1363. doi: 10.1038/nn.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall E.D. Sullivan P.G. Gibson T.R. Pavel K.M. Thompson B.M. Scheff S.W. Spatial and temporal characteristics of neurodegeneration after controlled cortical impact in mice: more than a focal brain injury. J. Neurotrauma. 2005;22:252–265. doi: 10.1089/neu.2005.22.252. [DOI] [PubMed] [Google Scholar]
- Hatters D.M. Peters-Libeu C.A. Weisgraber K.H. Apolipoprotein E structure: insights into function. Trends Biochem. Sci. 2006;31:445–454. doi: 10.1016/j.tibs.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Hoane M.R. Kaufman N. Vitek M.P. McKenna S.E. COG1410 improves cognitive performance and reduces cortical neuronal loss in the traumatically injured brain. J. Neurotrauma. 2009;26:121–129. doi: 10.1089/neu.2008.0565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsburgh K. McCulloch J. Nilsen M. McCracken E. Large C. Roses A.D. Nicoll J.A. Intraventricular infusion of apolipoprotein E ameliorates acute neuronal damage after global cerebral ischemia in mice. J. Cereb. Blood Flow Metab. 2000;20:458–462. doi: 10.1097/00004647-200003000-00003. [DOI] [PubMed] [Google Scholar]
- James M.L. Sullivan P.M. Lascola C.D. Vitek M.P. Laskowitz D.T. Pharmacogenomic effects of apolipoprotein e on intracerebral hemorrhage. Stroke. 2009;40:632–639. doi: 10.1161/STROKEAHA.108.530402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger K.A. Head injury and dementia. Curr. Opin. Neurol. 2004;17:719–723. doi: 10.1097/00019052-200412000-00012. [DOI] [PubMed] [Google Scholar]
- Jellinger K.A. Paulus W. Wrocklage C. Litvan I. Effects of closed traumatic brain injury and genetic factors on the development of Alzheimer's disease. Eur. J. Neurol. 2001;8:707–710. doi: 10.1046/j.1468-1331.2001.00322.x. [DOI] [PubMed] [Google Scholar]
- Jiang Y. Sun X. Xia Y. Tang W. Cao Y. Gu Y. Effect of APOE polymorphisms on early responses to traumatic brain injury. Neurosci. Lett. 2006;408:155–158. doi: 10.1016/j.neulet.2006.08.082. [DOI] [PubMed] [Google Scholar]
- Kelley B.J. Lifshitz J. Povlishock J.T. Neuroinflammatory responses after experimental diffuse traumatic brain injury. J. Neuropathol. Exper. Neurol. 2007;66:989–1001. doi: 10.1097/NEN.0b013e3181588245. [DOI] [PubMed] [Google Scholar]
- Kigerl K.A. Gensel J.C. Ankeny D.P. Alexander J.K. Donnelly D.J. Popovich P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H.S. Suh Y.H. Minocycline and neurodegenerative diseases. Behavioural Brain Res. 2009;196:168–179. doi: 10.1016/j.bbr.2008.09.040. [DOI] [PubMed] [Google Scholar]
- Koizumi H. Povlishock J.T. Posttraumatic hypothermia in the treatment of axonal damage in an animal model of traumatic axonal injury. J. Neurosurg. 1998;89:303–309. doi: 10.3171/jns.1998.89.2.0303. [DOI] [PubMed] [Google Scholar]
- Kraus M.F. Susmaras T. Caughlin B.P. Walker C.J. Sweeney J.A. Little D.M. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130:2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- Laskowitz D.T. Goel S. Bennett E.R. Matthew W.D. Apolipoprotein E suppresses glial cell secretion of TNF alpha. J. Neuroimmunol. 1997;76:70–74. doi: 10.1016/s0165-5728(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Laskowitz D.T. McKenna S.E. Song P. Wang H. Durham L. Yeung N. Christensen D. Vitek M.P. COG1410, a novel apolipoprotein E-based peptide, improves functional recovery in a murine model of traumatic brain injury. J. Neurotrauma. 2007;24:1093–1107. doi: 10.1089/neu.2006.0192. [DOI] [PubMed] [Google Scholar]
- Laskowitz D.T. Song P. Wang H. Mace B. Sullivan P.M. Vitek M.P. Dawson H.N. Traumatic brain injury exacerbates neurodegenerative pathology: improvement with an apolipoprotein E-based therapeutic. J. Neurotrauma. 2010;27:1983–1995. doi: 10.1089/neu.2010.1396. [DOI] [PubMed] [Google Scholar]
- Laskowitz D.T. Thekdi A.D. Thekdi S.D. Han S.K. Myers J.K. Pizzo S.V. Bennett E.R. Downregulation of microglial activation by apolipoprotein E and apoE-mimetic peptides. Exper. Neurol. 2001;167:74–85. doi: 10.1006/exnr.2001.7541. [DOI] [PubMed] [Google Scholar]
- Linton M.F. Gish R. Hubl S.T. Butler E. Esquivel C. Bry W.I. Boyles J.K. Wardell M.R. Young S.G. Phenotypes of apolipoprotein B and apolipoprotein E after liver transplantation. J. Clin. Invest. 1991;88:270–281. doi: 10.1172/JCI115288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton M.L. Gulko E. Zimmerman M.E. Friedman B.W. Kim M. Gellella E. Gold T. Shifteh K. Ardekani B.A. Branch C.A. Diffusion-tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology. 2009;252:816–824. doi: 10.1148/radiol.2523081584. [DOI] [PubMed] [Google Scholar]
- Lynch J.R. Pineda J.A. Morgan D. Zhang L. Warner D.S. Benveniste H. Laskowitz D.T. Apolipoprotein E affects the central nervous system response to injury and the development of cerebral edema. Ann. Neurol. 2002;51:113–117. doi: 10.1002/ana.10098. [DOI] [PubMed] [Google Scholar]
- Lynch J.R. Tang W. Wang H. Vitek M.P. Bennett E.R. Sullivan P.M. Warner D.S. Laskowitz D.T. APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. J. Biological Chem. 2003;278:48529–48533. doi: 10.1074/jbc.M306923200. [DOI] [PubMed] [Google Scholar]
- Lynch J.R. Wang H. Mace B. Leinenweber S. Warner D.S. Bennett E.R. Vitek M.P. McKenna S. Laskowitz D.T. A novel therapeutic derived from apolipoprotein E reduces brain inflammation and improves outcome after closed head injury. Exper. Neurol. 2005;192:109–116. doi: 10.1016/j.expneurol.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Mac Donald C.L. Dikranian K. Bayly P. Holtzman D. Brody D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J. Neurosci. 2007a;27:11869–11876. doi: 10.1523/JNEUROSCI.3647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald C.L. Dikranian K. Song S.K. Bayly P.V. Holtzman D.M. Brody D.L. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exper. Neurol. 2007b;205:116–131. doi: 10.1016/j.expneurol.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulies S. Hicks R. Combination therapies for traumatic brain injury: prospective considerations. J. Neurotrauma. 2009;26:925–939. doi: 10.1089/neu.2008.0794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbye L.H. Singh I.N. Sullivan P.G. Springer J.E. Hall E.D. Attenuation of acute mitochondrial dysfunction after traumatic brain injury in mice by NIM811, a non-immunosuppressive cyclosporin A analog. Exper. Neurol. 2008;209:243–253. doi: 10.1016/j.expneurol.2007.09.025. [DOI] [PubMed] [Google Scholar]
- McAdoo J.D. Warner D.S. Goldberg R.N. Vitek M.P. Pearlstein R. Laskowitz D.T. Intrathecal administration of a novel apoE-derived therapeutic peptide improves outcome following perinatal hypoxic-ischemic injury. Neurosci. Lett. 2005;381:305–308. doi: 10.1016/j.neulet.2005.02.036. [DOI] [PubMed] [Google Scholar]
- Misra U.K. Adlakha C.L. Gawdi G. McMillian M.K. Pizzo S.V. Laskowitz D.T. Apolipoprotein E and mimetic peptide initiate a calcium-dependent signaling response in macrophages. J. Leukoc. Biol. 2001;70:677–683. [PubMed] [Google Scholar]
- Namas R. Ghuma A. Hermus L. Zamora R. Okonkwo D.O. Billiar T.R. Vodovotz Y. The acute inflammatory response in trauma/hemorrhage and traumatic brain injury: current state and emerging prospects. Libyan J. Med. 2009;4:97–103. doi: 10.4176/090325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan R.K. Michel M.E. Ansell B. Baethmann A. Biegon A. Bracken M.B. Bullock M.R. Choi S.C. Clifton G.L. Contant C.F. Coplin W.M. Dietrich W.D. Ghajar J. Grady S.M. Grossman R.G. Hall E.D. Heetderks W. Hovda D.A. Jallo J. Katz R.L. Knoller N. Kochanek P.M. Maas A.I. Majde J. Marion D.W. Marmarou A. Marshall L.F. McIntosh T.K. Miller E. Mohberg N. Muizelaar J.P. Pitts L.H. Quinn P. Riesenfeld G. Robertson C.S. Strauss K.I. Teasdale G. Temkin N. Tuma R. Wade C. Walker M.D. Weinrich M. Whyte J. Wilberger J. Young A.B. Yurkewicz L. Clinical trials in head injury. J. Neurotrauma. 2002;19:503–557. doi: 10.1089/089771502753754037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niogi S.N. Mukherjee P. Diffusion tensor imaging of mild traumatic brain injury. J. Head Trauma Rehabil. 2010;25:241–255. doi: 10.1097/HTR.0b013e3181e52c2a. [DOI] [PubMed] [Google Scholar]
- Niogi S.N. Mukherjee P. Ghajar J. Johnson C.E. Kolster R. Lee H. Suh M. Zimmerman R.D. Manley G.T. McCandliss B.D. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain. 2008b;131:3209–3221. doi: 10.1093/brain/awn247. [DOI] [PubMed] [Google Scholar]
- Niogi S.N. Mukherjee P. Ghajar J. Johnson C. Kolster R.A. Sarkar R. Lee H. Meeker M. Zimmerman R.D. Manley G.T. McCandliss B.D. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. Am. J. Neuroradiol. 2008a;29:967–973. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y. Gao G. Wei E.P. Povlishock J.T. Combinational therapy using hypothermia and the immunophilin ligand FK506 to target altered pial arteriolar reactivity, axonal damage, and blood-brain barrier dysfunction after traumatic brain injury in rat. J. Cereb. Blood Flow Metab. 2011;31:1143–1154. doi: 10.1038/jcbfm.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okonkwo D.O. Melon D.E. Pellicane A.J. Mutlu L.K. Rubin D.G. Stone J.R. Helm G.A. Dose-response of cyclosporin A in attenuating traumatic axonal injury in rat. Neuroreport. 2003;14:463–466. doi: 10.1097/00001756-200303030-00033. [DOI] [PubMed] [Google Scholar]
- Perlbarg V. Puybasset L. Tollard E. Lehericy S. Benali H. Galanaud D. Relation between brain lesion location and clinical outcome in patients with severe traumatic brain injury: a diffusion tensor imaging study using voxel-based approaches. Hum. Brain Mapp. 2009;30:3924–3933. doi: 10.1002/hbm.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C.A. Poirier M.A. Protein aggregation and neurodegenerative disease. Nat. Med. 2004;10(Suppl.):S10–S17. doi: 10.1038/nm1066. [DOI] [PubMed] [Google Scholar]
- Shein N.A. Grigoriadis N. Horowitz M. Umschwief G. Alexandrovich A.G. Simeonidou C. Grigoriadis S. Touloumi O. Shohami E. Microglial involvement in neuroprotection following experimental traumatic brain injury in heat-acclimated mice. Brain Res. 2008;1244:132–141. doi: 10.1016/j.brainres.2008.09.032. [DOI] [PubMed] [Google Scholar]
- Shitaka Y. Tran H.T. Bennett R.E. Sanchez L. Levy M.A. Dikranian K. Brody D.L. Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J. Neuropathol. Exper. Neurol. 2011;70:551–567. doi: 10.1097/NEN.0b013e31821f891f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.H. Meaney D.F. Shull W.H. Diffuse axonal injury in head trauma. J. Head Trauma Rehabil. 2003;18:307–316. doi: 10.1097/00001199-200307000-00003. [DOI] [PubMed] [Google Scholar]
- Smith D.H. Nonaka M. Miller R. Leoni M. Chen X.H. Alsop D. Meaney D.F. Immediate coma following inertial brain injury dependent on axonal damage in the brainstem. J. Neurosurg. 2000;93:315–322. doi: 10.3171/jns.2000.93.2.0315. [DOI] [PubMed] [Google Scholar]
- Sorbi S. Nacmias B. Piacentini S. Repice A. Latorraca S. Forleo P. Amaducci L. ApoE as a prognostic factor for post-traumatic coma. Nat. Med. 1, 1995:852. doi: 10.1038/nm0995-852. [DOI] [PubMed] [Google Scholar]
- Strich S.J. Diffuse degeneration of the cerebral white matter in severe dementia following head injury. J. Neurol. Neurosurg. Psychiatry. 1956;19:163–185. doi: 10.1136/jnnp.19.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strich S.J. Shearing of nerve fibres as cause of brain damage due to head injury. Lancet. 1961;2:443–448. [Google Scholar]
- Sullivan P.M. Mezdour H. Aratani Y. Knouff C. Najib J. Reddick R.L. Quarfordt S.H. Maeda N. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J. Biological Chem. 1997;272:17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- Sun X.C. Jiang Y. Genetic susceptibility to traumatic brain injury and apolipoprotein E gene. Chin. J. Traumatol. 2008;11:247–252. doi: 10.1016/s1008-1275(08)60051-6. [DOI] [PubMed] [Google Scholar]
- Teasdale G.M. Murray G.D. Nicoll J.A. The association between APOE epsilon4, age and outcome after head injury: a prospective cohort study. Brain. 2005;128:2556–2561. doi: 10.1093/brain/awh595. [DOI] [PubMed] [Google Scholar]
- Teasdale G.M. Nicoll J.A. Murray G. Fiddes M. Association of apolipoprotein E polymorphism with outcome after head injury. Lancet. 1997;350:1069–1071. doi: 10.1016/S0140-6736(97)04318-3. [DOI] [PubMed] [Google Scholar]
- Tran H.T. Laferla F.M. Holtzman D.M. Brody D.L. Controlled cortical impact traumatic brain injury in 3xTg-AD mice causes acute intra-axonal amyloid-beta accumulation and independently accelerates the development of tau abnormalities. J. Neurosci. 2011;31:9513–9525. doi: 10.1523/JNEUROSCI.0858-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukhovskaya E.A. Yukin A.Y. Khokhlova O.N. Murashev A.N. Vitek M.P. COG1410, a novel apolipoprotein-E mimetic, improves functional and morphological recovery in a rat model of focal brain ischemia. J. Neurosci. Res. 2009;87:677–682. doi: 10.1002/jnr.21874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese P.B. Castellano J.M. Holtzman D.M. Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.Y. Bakhadirov K. Devous M.D., Sr. Abdi H. McColl R. Moore C. Marquez de la Plata C.D. Ding K. Whittemore A. Babcock E. Rickbeil T. Dobervich J. Kroll D. Dao B. Mohindra N. Madden C.J. Diaz-Arrastia R. Diffusion tensor tractography of traumatic diffuse axonal injury. Arch. Neurol. 2008;65:619–626. doi: 10.1001/archneur.65.5.619. [DOI] [PubMed] [Google Scholar]
- Werner C. Engelhard K. Pathophysiology of traumatic brain injury. Br. J. Anaesthesia. 2007;99:4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- Zhou W. Xu D. Peng X. Zhang Q. Jia J. Crutcher K.A. Meta-analysis of APOE4 allele and outcome after traumatic brain injury. J. Neurotrauma. 2008;25:279–290. doi: 10.1089/neu.2007.0489. [DOI] [PubMed] [Google Scholar]