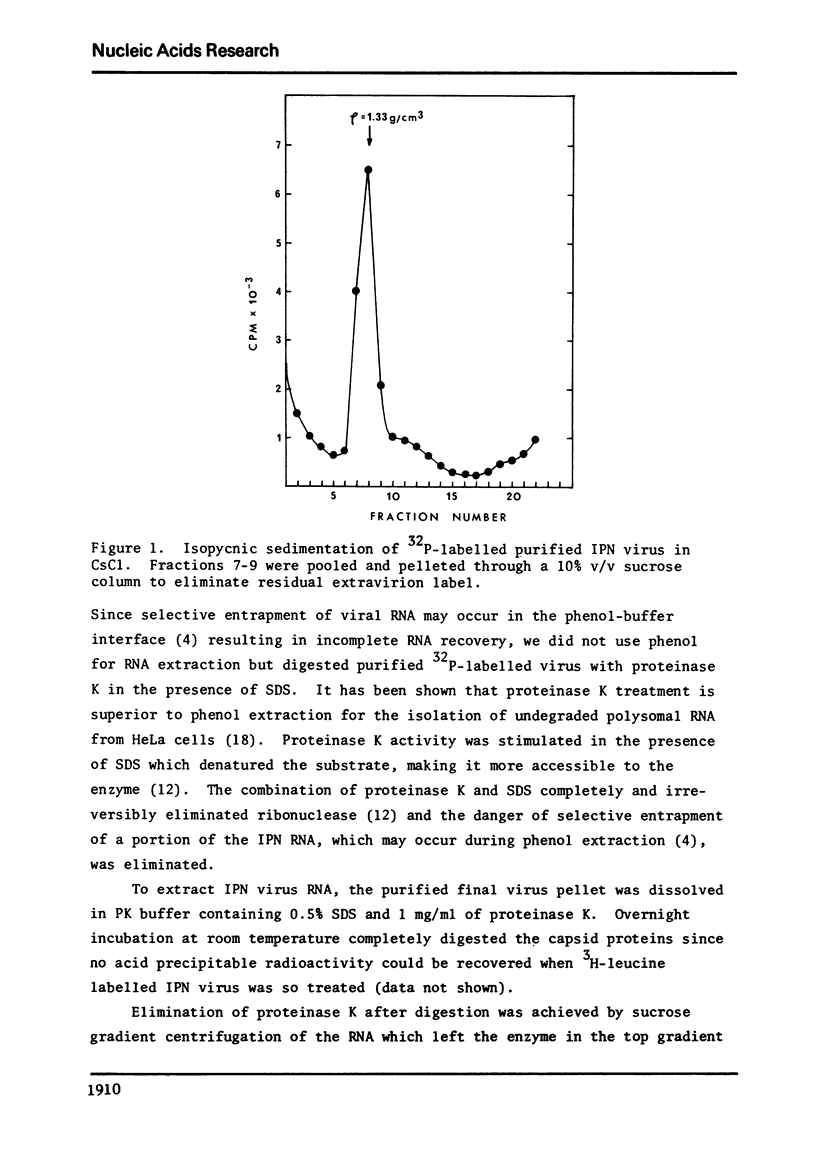
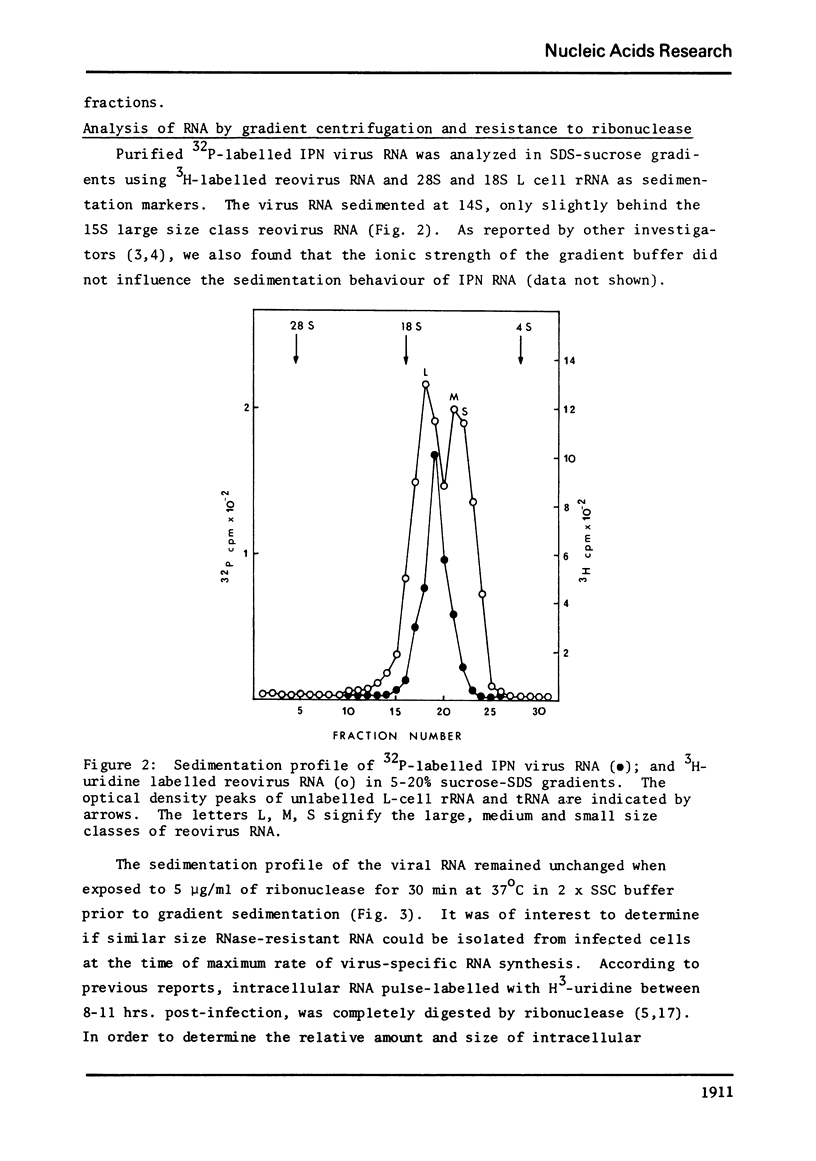
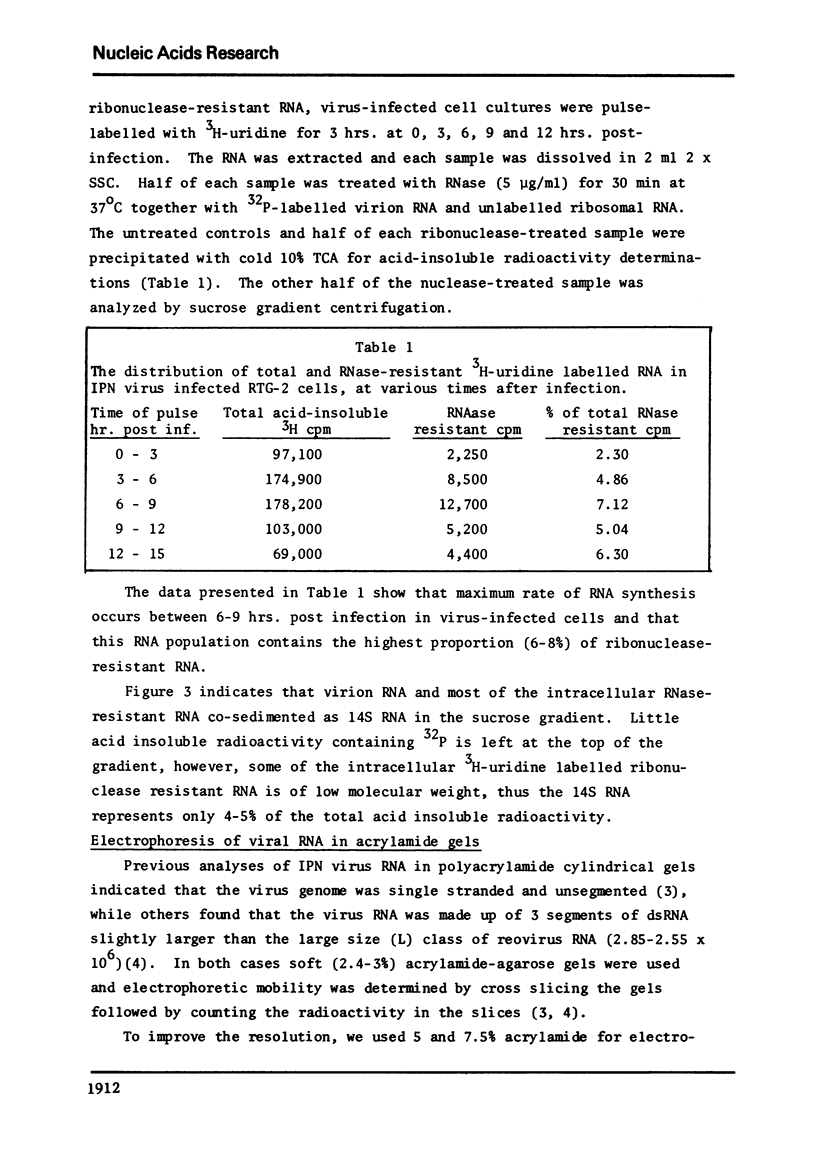
Abstract
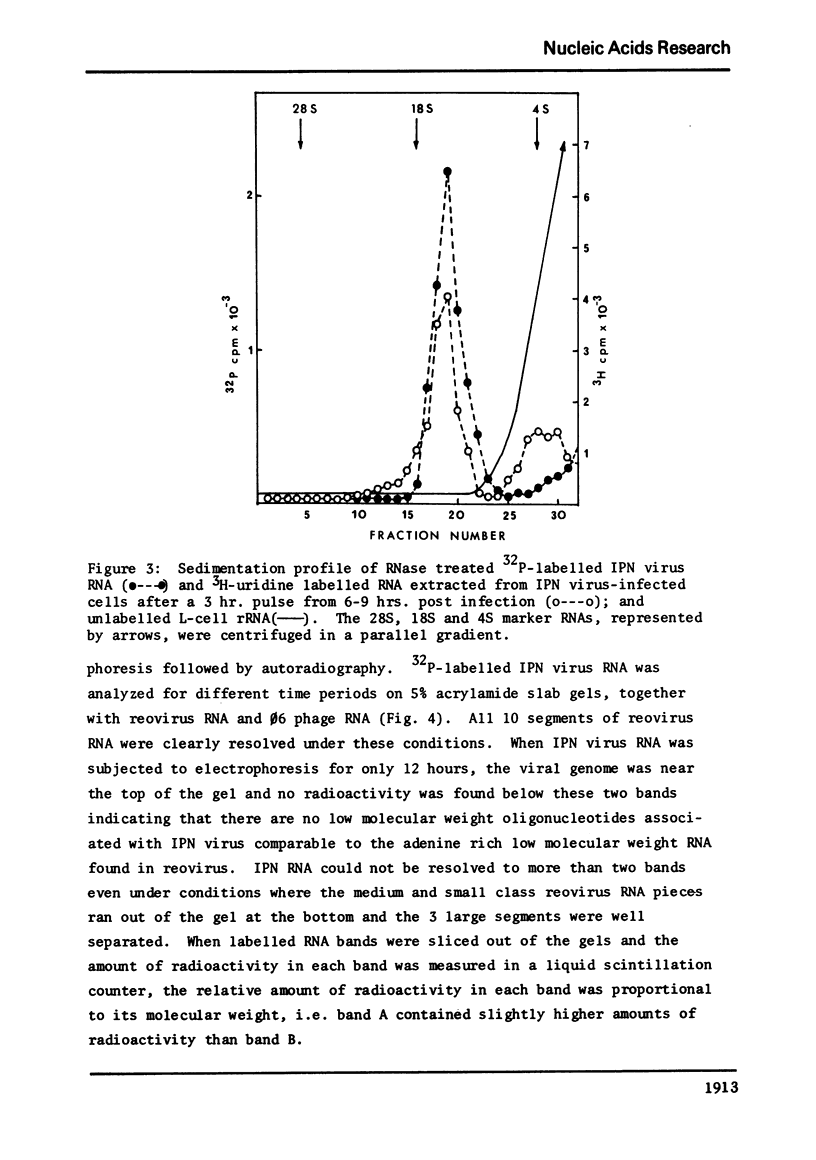
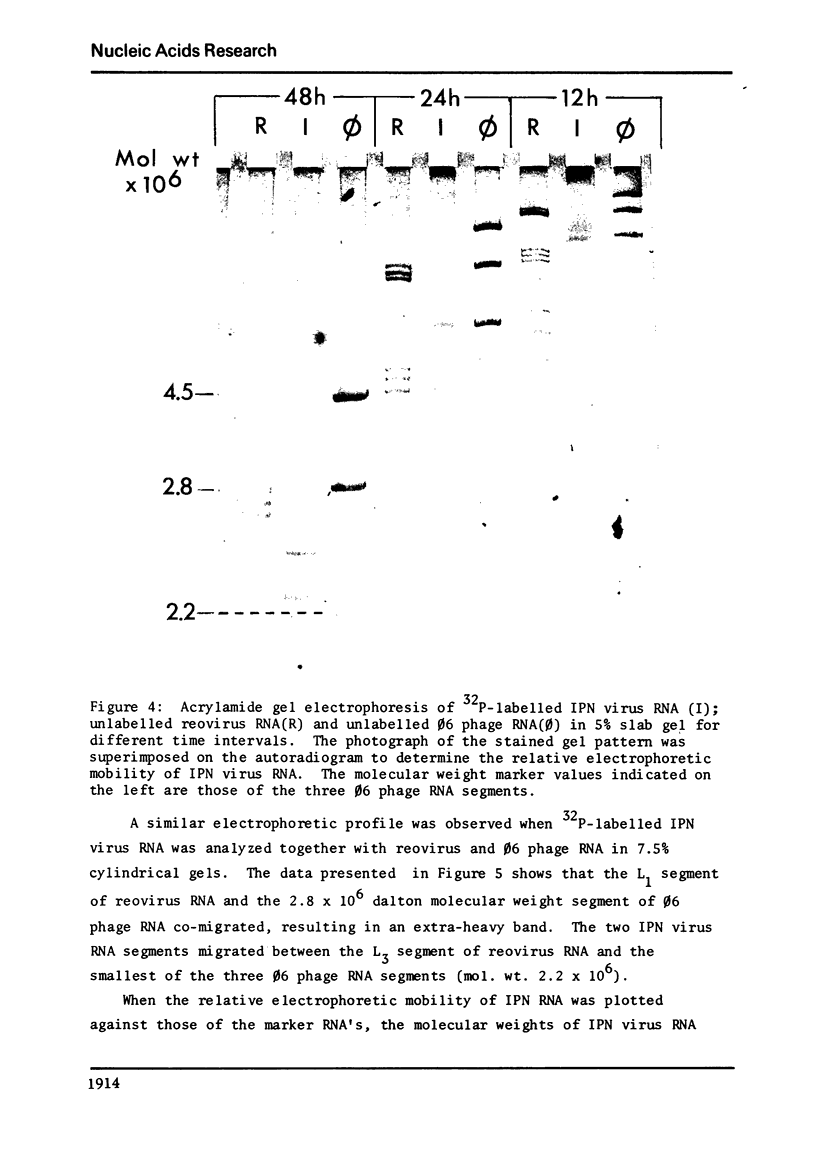
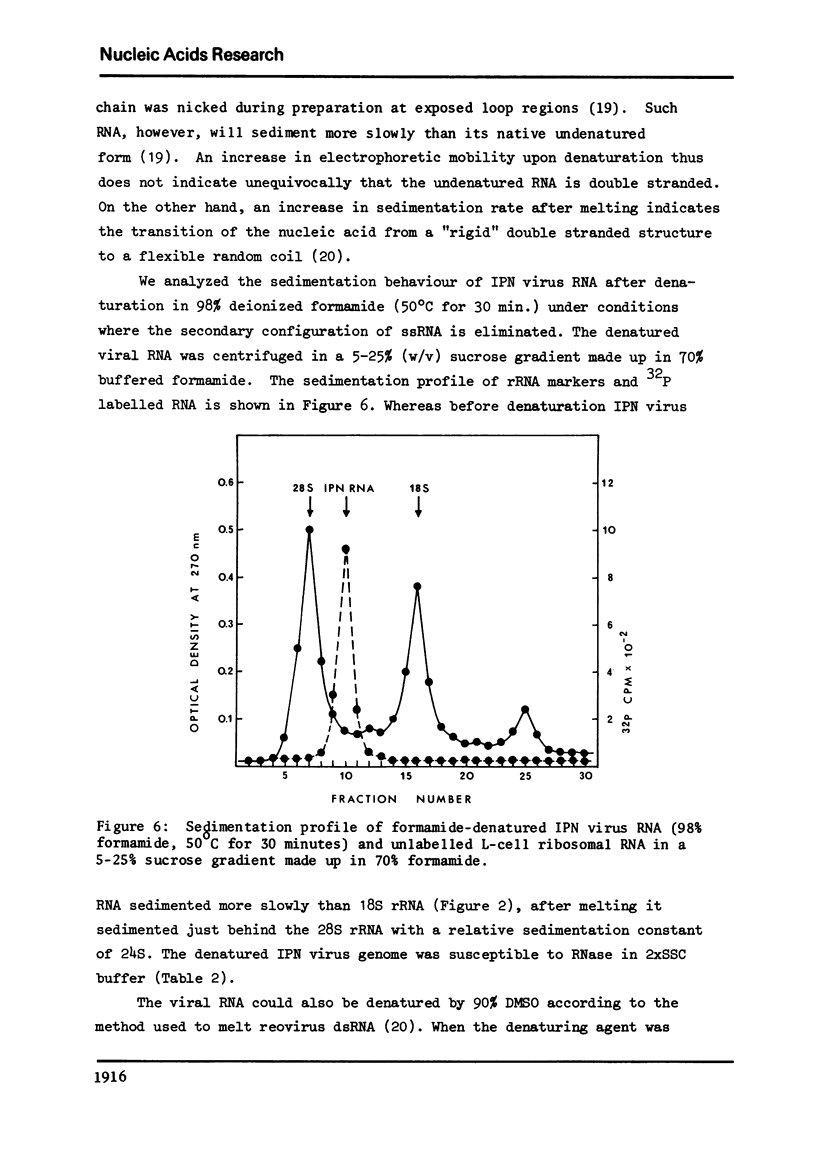
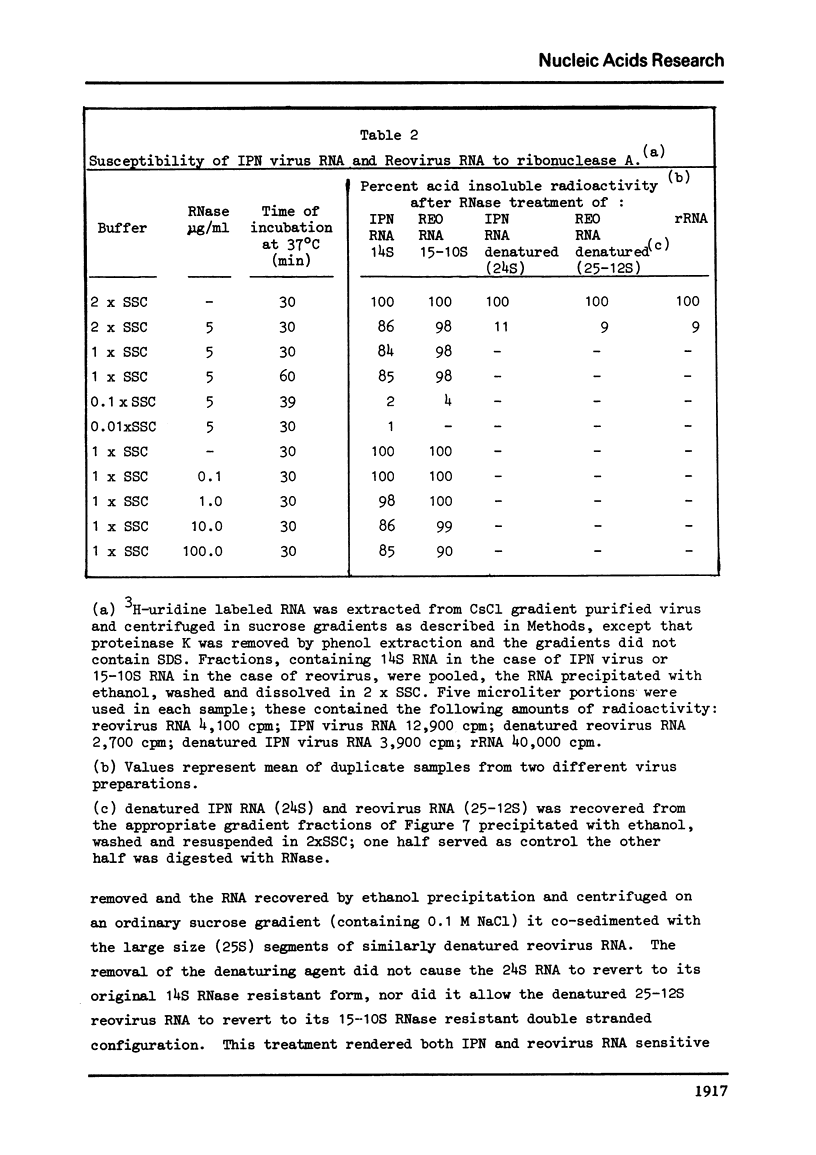
The genome of infectious pancreatic necrosis virus consists of two segments of dsRNA, in equimolar amounts, with molecular weights of 2.5 X 10(6) and 2.3 X 10(6) daltons, as determined by polyacrylamide gel electrophoresis and autoradiography. The viral RNA was resistant to ribonuclease, and in sucrose gradient it co-sedimented at 14S with RNase resistant RNA from virus infected cells. Upon denaturation in 98% formamide, the viral genome sedi-mented at 24S in formamide sucrose gradient and became sensitive to RNase. Denatured 24S viral RNA did revert to its undenatured 14S form upon recentrifugation in aquaeous sucrose gradient (0.1 M NaCL), but co-sedimented with the denatured large size class of reovirus 25S RNA. The same results were obtained if the native viral RNA was pre-treated with ribonuclease before denaturation, indicating the absence of exposed single strainded regions in the viral genome. Since infectious pancreatic necrosis virus contains only two dsRNA segments it does not belong to the family Reoviridae and may represent a new group of viruses.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arif B. M., Faulkner P. Genome of Sindbis virus. J Virol. 1972 Jan;9(1):102–109. doi: 10.1128/jvi.9.1.102-109.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy A. R., Shapiro L., August J. T., Joklik W. K. Studies on reovirus RNA. I. Characterization of reovirus genome RNA. J Mol Biol. 1967 Oct 14;29(1):1–17. doi: 10.1016/0022-2836(67)90177-5. [DOI] [PubMed] [Google Scholar]
- Boedtker H. Dependence of the sedimentation coefficient on molecular weight of RNA after reaction with formaldehyde. J Mol Biol. 1968 Jul 14;35(1):61–70. doi: 10.1016/s0022-2836(68)80036-1. [DOI] [PubMed] [Google Scholar]
- Cohen J., Poinsard A., Scherrer R. Physico-chemical and morphological features of infectious pancreatic necrosis virus. J Gen Virol. 1973 Dec;21(3):485–498. doi: 10.1099/0022-1317-21-3-485. [DOI] [PubMed] [Google Scholar]
- Gravell M., Malsberger R. G. A permanent cell line from the fathead minnow (Pimephales promelas). Ann N Y Acad Sci. 1965 Aug 10;126(1):555–565. doi: 10.1111/j.1749-6632.1965.tb14302.x. [DOI] [PubMed] [Google Scholar]
- Hilz H., Wiegers U., Adamietz P. Stimulation of proteinase K action by denaturing agents: application to the isolation of nucleic acids and the degradation of 'masked' proteins. Eur J Biochem. 1975 Aug 1;56(1):103–108. doi: 10.1111/j.1432-1033.1975.tb02211.x. [DOI] [PubMed] [Google Scholar]
- Huang A. S., Baltimore D., Stampfer M. Ribonucleic acid synthesis of vesicular stomatitis virus. 3. Multiple complementary messenger RNA molecules. Virology. 1970 Dec;42(4):946–957. doi: 10.1016/0042-6822(70)90343-0. [DOI] [PubMed] [Google Scholar]
- Kelly R. K., Loh P. C. Electron microscopical and biochemical characterization of infectious pancreatic necrosis virus. J Virol. 1972 Oct;10(4):824–834. doi: 10.1128/jvi.10.4.824-834.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. K., Loh P. C. Replication of IPN virus: a cytochemical and biochemical study in SWT cells. Proc Soc Exp Biol Med. 1975 Mar;148(3):688–693. doi: 10.3181/00379727-148-38611. [DOI] [PubMed] [Google Scholar]
- Kudo H., Graham A. F. Synthesis of reovirus ribonucleic acid in L cells. J Bacteriol. 1965 Oct;90(4):936–945. doi: 10.1128/jb.90.4.936-945.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo P. C., Lee M. H., Kelly R. K. The polypeptides of infectious pancreatic necrosis virus. J Gen Virol. 1974 Mar;22(3):421–423. doi: 10.1099/0022-1317-22-3-421. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Independent translation of the genes of bacteriophage f2 RNA. J Mol Biol. 1968 Mar 28;32(3):681–685. doi: 10.1016/0022-2836(68)90351-3. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malsberger R. G., Cerini C. P. Multiplication of infectious pancreatic necrosis virus. Ann N Y Acad Sci. 1965 Aug 10;126(1):320–327. doi: 10.1111/j.1749-6632.1965.tb14283.x. [DOI] [PubMed] [Google Scholar]
- McGuire P. M., Swart C., Hodge L. D. Adenovirus messenger RNA in mammalian cells: failure of polyribosome association in the absence of nuclear cleavage. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1578–1582. doi: 10.1073/pnas.69.6.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson B. L. Macromolecule synthesis in RTG-2 cells following infection with infectious pancreatic necrosis (IPN) virus. J Gen Virol. 1971 Nov;13(2):369–372. doi: 10.1099/0022-1317-13-2-369. [DOI] [PubMed] [Google Scholar]
- Oie H., Loh P. C., Soergel M. Growth characteristics and immunocytochemical studies of reovirus type 2 in a line of human amnion cells. Arch Gesamte Virusforsch. 1966;18(1):16–24. doi: 10.1007/BF01241697. [DOI] [PubMed] [Google Scholar]
- Pinder J. C., Staynov D. Z., Gratzer W. B. Properties of RNA in formamide. Biochemistry. 1974 Dec 17;13(26):5367–5373. doi: 10.1021/bi00723a018. [DOI] [PubMed] [Google Scholar]
- Reddy D. V., Black L. M. Electrophoretic separation of all components of the double-stranded RNA of wound tumor virus. Virology. 1973 Aug;54(2):557–562. doi: 10.1016/0042-6822(73)90168-2. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Vidaver A. K., Van Etten J. L. Characterization of segmented double-helical RNA from bacteriophage phi6. J Mol Biol. 1973 Aug 25;78(4):617–625. doi: 10.1016/0022-2836(73)90283-0. [DOI] [PubMed] [Google Scholar]
- Toneguzzo F., Ghosh H. P. Characterization and translation of methylated and unmethylated vesicular stomatitis virus mRNA synthesized in vitro by ribonucleoprotein particles from vesicular stomatitis virus-infected L cells. J Virol. 1976 Feb;17(2):477–491. doi: 10.1128/jvi.17.2.477-491.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLF K., QUIMBY M. C. Established eurythermic line of fish cells in vitro. Science. 1962 Mar 23;135(3508):1065–1066. doi: 10.1126/science.135.3508.1065. [DOI] [PubMed] [Google Scholar]
- Wiegers U., Hilz H. Rapid isolation of undegraded polysomal RNA without phenol. FEBS Lett. 1972 Jun 1;23(1):77–82. doi: 10.1016/0014-5793(72)80289-8. [DOI] [PubMed] [Google Scholar]
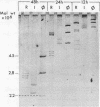
- Wood H. A. Viruses with double-stranded RNA genomes. J Gen Virol. 1973 Jun;20(Suppl):61–85. doi: 10.1099/0022-1317-20-Supplement-61. [DOI] [PubMed] [Google Scholar]