Abstract
Conventional imaging is unable to detect damage that accounts for permanent cognitive impairment in patients with mild traumatic brain injury (mTBI). While diffusion tensor imaging (DTI) can help to detect diffuse axonal injury (DAI), it is a limited indicator of tissue complexity. It has also been suggested that the thalamus may play an important role in the development of clinical sequelae in mTBI. The purpose of this study was to determine if diffusional kurtosis imaging (DKI), a novel quantitative magnetic resonance imaging (MRI) technique, can provide early detection of damage in the thalamus and white matter (WM) of mTBI patients, and can help ascertain if thalamic injury is associated with cognitive impairment. Twenty-two mTBI patients and 14 controls underwent MRI and neuropsychological testing. Mean kurtosis (MK), fractional anisotropy (FA), and mean diffusivity (MD) were measured in the thalamus and several WM regions classically identified with DAI. Compared to controls, patients examined within 1 year after injury exhibited variously altered DTI- and DKI-derived measures in the thalamus and the internal capsule, while in addition to these regions, patients examined more than 1 year after injury also showed similar differences in the splenium of the corpus callosum and the centrum semiovale. Cognitive impairment was correlated with MK in the thalamus and the internal capsule. These findings suggest that combined use of DTI and DKI provides a more sensitive tool for identifying brain injury. In addition, MK in the thalamus might be useful for early prediction of permanent brain damage and cognitive outcome.
Key words: cognitive impairment, diffusion tensor imaging, mild traumatic brain injury, thalamus, white matter
Introduction
Mild traumatic brain injury (mTBI) is a major public health problem. While about 85% of patients eventually recover from trauma-related cognitive deficits, approximately 15% have protracted symptoms with serious social and economic consequences (National Center for Injury Prevention and Control, 2003). Clinical and neuropsychological predictors are suboptimal to forecast cognitive outcome, and more objective measures such as conventional magnetic resonance imaging (MRI) usually fail to detect any macroscopic and microscopic evidence of brain injury (Mittl et al., 1994). It has been suggested that in addition to the presence of diffuse axonal injury (DAI) in white matter (WM), injury to the thalamus may play an important role in the development of clinical sequelae in mTBI (Abdel-Dayem et al.,1998; Anderson et al., 1996; Ge et al., 2009; Henninger et al., 2007; Little et al., 2010; Wood and Bigler, 1995). Its possible function in mTBI has, however, remained largely uninvestigated.
Diffusion tensor imaging (DTI) can detect microscopic tissue damage, grade its severity, and provide prognostic markers for clinical outcome in mTBI (Arfanakis et al., 2002; Inglese et al., 2005; Little et al., 2010; Miles et al., 2008). The theory on which DTI is based assumes that water molecules move through a homogeneous environment in which diffusion can be estimated using a gaussian displacement probability distribution. Therefore, it is most useful in the evaluation of highly organized systems such as WM tracts (Basser et al., 2002). Most biological tissues, however, have an intricate microstructure that impedes the movement of water, and causes diffusion to deviate substantially from a gaussian form. This means that DTI is a limited indicator of complexity, especially in brain regions that exhibit a substantial amount of tissue heterogeneity.
This limitation can be partially overcome with diffusional kurtosis imaging (DKI), a recently developed noninvasive MRI technique that measures non-gaussian properties of water diffusion (Jensen and Helpern, 2010; Jensen et al., 2005; Lu et al., 2006b; Wu and Cheung, 2010). A scalar index derived from DKI called the mean kurtosis (MK) provides an average measure over all directions of the amount by which the diffusion displacement probability distribution departs from a gaussian form (Jensen and Helpern, 2010; Jensen et al., 2005; Wu and Cheung, 2010). Recent studies have demonstrated that DKI may provide different and complementary information to DTI in a variety of brain disorders (Helpern et al., 2011; Jensen et al., 2010; Lu et al., 2006a; Raab et al., 2010; Ramani et al., 2007). This suggests that employing both DTI and DKI indices in the investigation of mTBI might produce more sensitive and specific markers for brain injury. Since a DKI dataset generally includes a DTI dataset as part of its total measurements, it can be used to calculate both types of indices, implying that they can be regarded as belonging to a larger unified set of diffusion metrics.
The aims of this study were: (1) to investigate whether DKI can detect the presence, if any, and extent of brain tissue changes in the thalamus and WM of mTBI patients; (2) to assess whether DKI provides complementary information about brain tissue injury; and (3) to identify associations between the cognitive performance of mTBI patients with DTI- and DKI-derived measures.
Methods
Subjects
Twenty-two consecutive patients with mTBI (14 male, 8 female; mean age 38.2±11.7 years; mean formal education 16.0±2.5 years) were prospectively recruited and classified with mTBI using diagnostic criteria developed by the Mild Traumatic Brain Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine, which has been reviewed by Esselman and Uomoto (1995). Enrollment was permitted only in cases in whom there was no other history of brain damage or disorders of the central nervous system, no history of systemic illness, and no history of alcoholism or drug dependency. Patients were divided into two groups based on whether they were studied within or more than 1 year after injury, since this period is close to the end of the stage in which sustained spontaneous recovery of cognitive function is expected (Szymanski and Linn, 1992). The first group, referred to as Group 1, consisted of seven patients to whom MRI and a neuropsychological battery were administered within 1 year after injury (mean interval 0.18 years; range 0.04–0.59 years), and the second group, referred to as Group 2, consisted of 15 patients to whom MRI and a neuropsychological battery were administered more than 1 year after injury (mean interval 3.9 years; range 1.33–9.58 years). Fourteen healthy controls matched to the patients according to gender, age, and formal education (9 male, 5 female; mean age 36.5±12.3 years; mean formal education 17.0±2.4 years) underwent the same MRI and neuropsychological protocols. Approval for the study was obtained from the Institutional Review Board of the New York University School of Medicine, and all participants provided informed written consent.
Neuropsychological assessment
Neuropsychological testing was conducted on the same day that subjects underwent MRI, and was administered by a psychologist blinded to imaging results. All neuropsychological test results were converted to z scores using published norms for easier comparison between subject groups with higher indices indicative of better performance. Patients that scored at or below the fifth percentile (−1.6 standard deviations below the normative mean) on two or more of the neuropsychological tests administered compared to controls were categorized as cognitively impaired.
The neuropsychological battery was composed of tests that were chosen to yield measures of attention, concentration, executive functioning, memory, learning, and psychomotor ability. Meta-analysis studies comparing the cognitive performance of mTBI patients to controls have found significant differences in attention and processing speed, as well as aspects of executive functioning such as cognitive flexibility, abstraction, and verbal fluency (Binder, 1997; Frencham et al., 2005; Zakzanis et al., 1999). Memory impairments (Tay et al., 2010; Tsirka et al., 2010), as well as psychomotor and reaction time slowing (Heitger et al., 2006; Miles et al., 2008; Paré et al., 2009) have also been identified in individuals with mTBI.
Attention and concentration were assessed with the Weinberg Visual Cancellation Test (WVCT; Rath et al., 2004), which measures sustained visual attention and concentration as well as accuracy of visual scanning. Executive functioning was assessed using the Stroop Test (ST; Batchelor et al., 1995; Bohnen, 1992; Cicerone and Azulay, 2002; Rath et al., 2004; Trenerry et al., 1989), and Prioritization Form A (PriA) and Form B (PriB) tests (Miles et al., 2008; Rath et al., 2004), which are measures of cognitive flexibility and abstraction, and the Controlled Oral Word Association Test (FAS; Lezak et al., 2004; Mathias et al., 2004; McHugh et al., 2006; Ruff et al., 1997), which is a measure of verbal fluency. The HeadMinder Cognitive Stability Index (Erlanger et al., 2002), an internet-based computerized screening tool designed to measure the cognitive functioning of patients with neurological impairments, was used to assess performance in four areas: Attention and Concentration (A/C), Memory and Learning (M/L), Processing Speed (PS), and Response Speed (RS). A/C is comprised of the Number Recall subtest, which assesses focused attention, and the Number Sequencing subtest, which assesses focused attention while placing a greater demand on working memory. M/L is comprised of the Memory Cabinet 1 subtest, which assesses nonverbal incremental learning of visual material presented within a selective reminding format, and the Memory Cabinet 2 subtest, which assesses delayed recall of the visual material presented in Memory Cabinet 1. PS is comprised of the Animal Decoding and Symbol Scanning subtests. RS is comprised of the Response Direction 1 subtest, which provides an assessment of reaction time with speeded discrimination response requirements, and the Response Direction 2 subtest, which provides an assessment of reaction time with more complex discrimination response requirements.
Image acquisition
The theory underlying DKI has been previously described elsewhere (Jensen and Helpern, 2010; Jensen et al., 2005; Wu and Cheung, 2010). In brief, it employs a diffusion sensitizing gradient similar to what is used in DTI, but acquires three or more diffusion weighting b values instead of two. These additional b values are needed to determine the nonlinearity of the logarithm for the signal decay caused by numerous distinct biophysical environments with different diffusion properties. The b values are fitted to the following equation from which the apparent diffusional kurtosis (ADK) can be estimated:
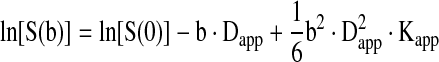 |
(1) |
Here S(b) is the signal intensity as a function of b, S(0) is the signal intensity at b=0, Dapp is the apparent diffusion coefficient (ADC) for a given direction, and Kapp is the ADK. By fitting experimental data to Eq. (1), obtained while applying multiple gradient encoding directions, it is possible to calculate tensor information about the ADC and the ADK. From the apparent diffusion tensor, several standard measures can be derived, including fractional anisotropy (FA) and mean diffusivity (MD), and from the diffusional kurtosis tensor a number of other parameters can be similarly elicited, including MK.
MRI was performed on all subjects using a 3-Tesla (T) Magnetom Trio whole-body scanner (Siemens Medical Solutions, Erlangen, Germany) with a 12-channel head matrix coil. The protocol included the following sequences: (1) two-dimensional T2-weighted (T2W) turbo spin echo (TSE), which was applied to 50 axial slices that were 3.0 mm thick and contiguous with repetition time (TR)=9090 msec, echo time (TE)=93 msec, flip angle (FA)=120°, field of view (FOV)=220×220 mm2, and matrix=256×256; (2) three-dimensional T1-weighted (T1W) turbo fast low angle shot (turboFLASH) magnetization prepared rapid acquisition gradient echo (MPRAGE), which was applied to 160 axial slices that were 1.0 mm thick and contiguous with TR=2100 msec, TE=3.9 msec, inversion time (TI)=1100 msec, FA=12°, FOV=165×220 mm2, and matrix=192×256; (3) susceptibility-weighted imaging (SWI) which was acquired using a 2D gradient echo (GRE), and applied to 32 axial slices that were 2.0 mm thick and contiguous, with TR=50 msec, TE=25 msec, FA=20°, FOV=165×220 mm2, and matrix=192×256; and (4) DKI, which was applied to 13 axial slices that were 3.0 mm thick and had an interslice gap of 3.0 mm with TR=2100 msec, TE=115 msec, FOV=220×220 mm2, matrix=128×128, and voxel size=1.7×1.7×3.0 mm3. The DKI sequence was carried out by means of a twice-refocused spin echo diffusion technique (Reese et al., 2003), using 30 different gradient encoding directions and an optimized sampling strategy (Jones et al., 1999; Skare et al., 2000), in which there were six b values (0, 500, 1000, 1500, 2000, and 2500 s/mm2). Images from all sequences were evaluated by a neuroradiologist and analyzed for structural abnormalities, including microinfarcts and gliotic spots in the region of the small penetrating arteries and arterioles.
Data processing and analysis
The DKI data were processed with in-house MATLAB (The Mathworks, Natick, MA) scripts. Three-dimensional motion correction and spatial smoothing (gaussian filter full-width-half-maximum ;FWHM]=2.5 mm) were performed with the Statistical Parametric Mapping package (SPM; Wellcome Trust Centre for Neuroimaging at University College London, London, U.K.). The signal intensities of the diffusion-weighted data acquired from each gradient direction were then fitted to Eq. (1) on a voxel-by-voxel basis to obtain ADC and ADK maps using a Levenberg-Marquardt nonlinear fitting algorithm (Bates and Watts, 1988). The ADC values from all 30 gradient directions were used to calculate diffusion tensor indices, which were then employed to obtain FA and MD maps. The ADK values from all 30 gradient directions were averaged to generate MK maps (Lu et al., 2006b).
Analyses of the MK, FA, and MD maps were performed with ImageJ (National Institutes of Health, Bethesda, MD) software. Anatomical regions of interest (ROIs) were selected on T2-weighted scans by an experienced observer, and then transferred onto co-registered MK, FA, and MD maps. Rectangular ROIs that varied in size (range 4–96 pixels2), depending on the anatomical region studied, were placed bilaterally on three consecutive slices in the thalamus, and the anterior limb, genu, and posterior limb of the internal capsule along the anterior commissure-posterior commissure (AC-PC) line, the splenium of the corpus callosum at the most ventral-caudal aspect, and the centrum semiovale at the most ventral aspect. The average MK, FA, and MD were calculated for each ROI, and these values were then averaged over all slices and both sides to derive one mean for each brain region on every map. To assess variations in measurement, the same observer conducted two sets of ROI analyses for the above-mentioned brain regions in five controls on two occasions separated by 1 week.
Statistical analysis
Statistical computations were performed with SAS (SAS Institute, Cary, NC) software. A Wilcoxon-Mann-Whitney test was used to assess differences between control and patient group neuropsychological z-score results. Restricted maximum likelihood (REML) estimation of variance components in a random effects model was used to assess variations in the replicate assessment of regional imaging measures acquired by a single observer, and reproducibility was summarized in terms of the coefficient of variation (CV). Analysis of covariance (ANCOVA) was used to compare subject groups (controls, all patients, Group 1, and Group 2), in terms of regional imaging measures. A separate assessment of every brain region was conducted in which the observed value for each imaging measure converted to ranks was employed as the dependent variable to better satisfy underlying distributional assumptions, and subject group was included as a fixed classification factor with age and gender as numerical covariates. Spearman correlation coefficients were used to test associations between imaging measures for patients compared to controls in the thalamus and WM. Partial Spearman correlation coefficients adjusted for age and gender were used to test associations between imaging measures in every brain region of controls and patients with their z-scores from each neuropsychological test. Logistic regression analysis adjusted for age and gender was used to assess whether regional imaging measures for patients were predictive of cognitive impairment, or had diagnostic utility for the detection of mTBI. All p values were two-sided, adjusted for age and gender, and declared statistically significant only when <0.05. While p values have been reported without multiple comparison adjustments, explicit mention has been made with regard to results that would remain statistically significant after Bonferroni correction.
Results
Patient demographics and clinical data are summarized in Table 1. Conventional MRI scans showed the presence of right frontal lobe encephalomalacia in two patients, while no abnormalities were observed in any of the controls. Eighteen patients had an emergency department Glasgow Coma Scale (GCS) score of 15, two had a score of 14, and two had a score of 13. All patients complained of post-concussive symptoms, such as headache, photophobia, nausea, dizziness, fatigue, memory deficits, and sleep disturbances, except two Group 2 patients, who reported a complete recovery at the time they were studied. These two patients were not classified as cognitively impaired.
Table 1.
mTBI Patient Demographics and Clinical Data Sorted by Elapsed Time Since Injury
| Patient | Age/gender | Cause of injury | GCS score | Years since injury | T2W MRI findings |
|---|---|---|---|---|---|
| 1 | 21/M | MVA | 13 | 0.04 | Unremarkable |
| 2 | 36/F | Fall | 14 | 0.05 | Unremarkable |
| 3 | 43/F | Fall | 15 | 0.08 | Unremarkable |
| 4 | 47/M | Falling object | 15 | 0.09 | Unremarkable |
| 5 | 32/M | Fall | 15 | 0.15 | Unremarkable |
| 6 | 26/M | Assault | 15 | 0.28 | Unremarkable |
| 7 | 24/M | Fall | 15 | 0.59 | Unremarkable |
| 8 | 34/F | MVA | 15 | 1.33 | Unremarkable |
| 9 | 36/M | Falling object | 14 | 1.75 | Unremarkable |
| 10 | 50/F | Assault | 14 | 2.38 | Unremarkable |
| 11 | 23/M | Assault | 15 | 2.42 | Unremarkable |
| 12 | 28/M | Ped/auto | 15 | 2.54 | Unremarkable |
| 13 | 42/F | MVA | 15 | 2.58 | Unremarkable |
| 14 | 30/M | Assault | 15 | 2.58 | Unremarkable |
| 15 | 60/M | Falling object | 15 | 2.58 | Right frontal lobe encephalomalacia |
| 16 | 33/F | Assault | 15 | 3.00 | Unremarkable |
| 17 | 25/M | Fall | 15 | 3.00 | Right frontal lobe encephalomalacia |
| 18 | 50/F | MVA | 15 | 4.50 | Unremarkable |
| 19 | 49/M | MVA | 15 | 6.75 | Unremarkable |
| 20 | 45/M | MVA | 15 | 6.75 | Unremarkable |
| 21 | 59/F | Fall | 15 | 7.33 | Unremarkable |
| 22 | 48/M | Fall | 15 | 9.58 | Unremarkable |
mTBI, mild traumatic brain injury; GCS, Glasgow Coma Scale score; T2W MRI, T2-weighted magnetic resonance imaging; MVA, motor vehicle accident; Ped/auto, pedestrian struck by automobile.
The mean and standard deviations of neuropsychological test z scores obtained from the control and patient groups, as well as cases where they demonstrated significant differences are shown in Table 2. Thirteen (59%) patients, four from Group 1 and nine from Group 2, were classified as cognitively impaired, with most impairment found in the domains of executive functioning (50%) and memory (33%). The bias towards long-term cognitive impairment over what is usually reported in mTBI reflects the fact that patients used in this study do not represent a random sample group, since the majority of them were recruited from hospital centers while receiving treatment for post-concussive symptoms.
Table 2.
Neuropsychological Test Z-Score Results for Controls and mTBI Patients
| |
Mean z score±SD |
|||
|---|---|---|---|---|
| Neuropsychological test | Controls | All patients | Group 1 | Group 2 |
| WVCTT | −0.05±0.71 | 0.15±0.75 | 0.37±0.91 | 0.05±0.67 |
| WVCTE | −0.30±0.76a | 0.77±1.54 | 1.08±1.92 | 0.62±1.38 |
| ST | 0.36±0.31a | −1.85±2.11 | −1.41±1.30 | −2.06±2.42 |
| PriA | −0.11±0.82 | 0.14±0.98 | 0.27±0.99 | 0.07±0.99 |
| PriB | 0.13±0.61 | 0.36±0.88 | 0.39±0.97 | 0.35±0.88 |
| FAS | 0.05±0.65 | −0.32±1.02 | −0.74±1.42 | −0.13±0.75 |
| A/C | 0.14±0.86a | −0.59±1.11 | −0.58±1.03 | −0.60±1.19 |
| M/L | 0.13±1.21a | −0.99±1.55 | −0.75±1.63 | −1.11±1.56 |
| PS | 0.33±0.55a | −0.62±1.19 | −0.37±1.09 | −0.71±1.26 |
| RS | 0.16±1.06a | −0.85±1.38 | −0.52±0.90 | −1.02±1.57 |
Demonstrated differences of p<0.01 when compared to all patients.
mTBI, mild traumatic brain injury; SD, standard deviation; All patients, group 1+group 2; Group 1, patients studied within 1 year after injury; Group 2, patients studied more than 1 year after injury; WVCTT, Weinberg Visual Cancellation Test Time; WVCTE, Weinberg Visual Cancellation Test Error; ST, Stroop Test; PriA, Prioritization Form A; PriB, Prioritization Form B; FAS, Controlled Oral Word Association Test; A/C, HeadMinder Attention and Concentration; M/L, HeadMinder Memory and Learning; PS, HeadMinder Processing Speed; RS, HeadMinder Response Speed.
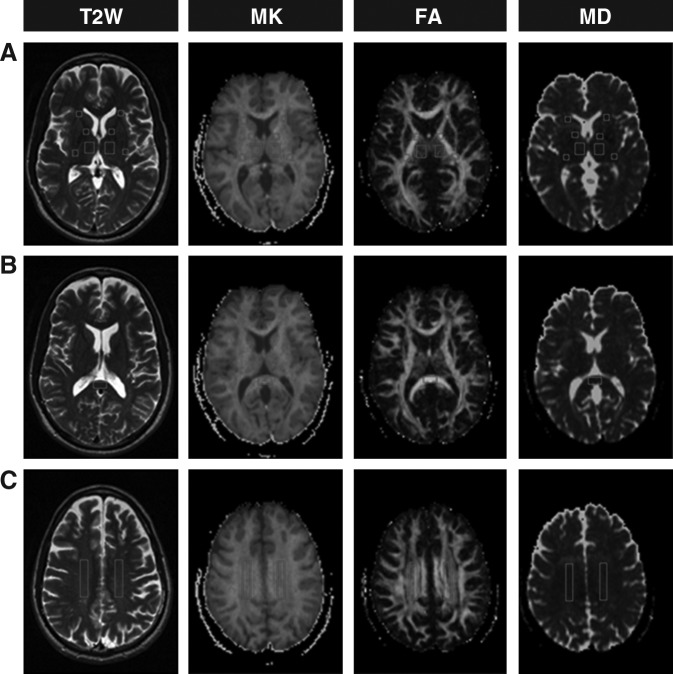
Examples of MK, FA, and MD maps from one mTBI patient are shown in Figure 1. Variations in measurement differed across ROIs for each DTI and DKI measure, with CV ranging from 1.9–5.9% for MK, 3.9–12.5% for FA, and 2.2–7.1% for MD.
FIG. 1.
Representative T2-weighted (T2W) images and corresponding mean kurtosis (MK), fractional anisotropy (FA), and mean diffusivity (MD) maps (A, B, and C), from one 49 year old male patient with mild traumatic brain injury. Locations for regions of interest that were investigated in this study are indicated bilaterally as follows: (A) the thalamus and the anterior limb, genu, and posterior limb of the internal capsule; (B) the splenium of the corpus callosum; and (C) the centrum semiovale.
Comparisons between controls and patients in terms of regional DTI and DKI measures are reported in Table 3. Compared to controls, the group of patients as a whole showed significantly lower MK and FA and higher MD in the thalamus (MK: p<0.01), and the internal capsule (MK: p=0.04; FA: p<0.01; MD=0.01). All differences remained statistically significant after Bonferroni correction, except for MK in the internal capsule. Regression analysis showed that DTI and DKI measures in the thalamus were significantly correlated with those in WM (MK thalamus and MD total WM: r=−0.58, p<0.01; MD thalamus and MD total WM: r=0.56, p=0.01; MD thalamus and FA total WM: r=−0.59, p<0.01).
Table 3.
DTI and DKI Comparative Regional Brain Measures for Controls and mTBI Patients
| |
|
Controls |
All patients |
Controls versus All Patients |
Group 1 |
Controls versus Group 1 |
Group 2 |
Controls versus Group 2 |
Group 1 versus Group 2 |
|---|---|---|---|---|---|---|---|---|---|
| Brain region | Measure | Mean±SE | Mean±SE | p Valuesa | Mean±SE | p Valuesa | Mean±SE | p Valuesa | p Valuesa |
| Thalamus | MK | 1.26±0.02 | 1.23±0.01 | <0.01b,c | 1.24±0.02 | 0.02b | 1.23±0.02 | <0.01b,c | 0.59 |
| FA | 0.32±0.01 | 0.30±0.01 | 0.06 | 0.30±0.01 | 0.13 | 0.30±0.01 | 0.10 | 0.99 | |
| MD | 0.83±0.01 | 0.84±0.01 | 0.20 | 0.83±0.01 | 0.81 | 0.84±0.01 | 0.02b | 0.02b | |
| Internal capsule | MK | 1.39±0.02 | 1.38±0.02 | 0.04b | 1.38±0.03 | 0.39 | 1.37±0.02 | 0.02b | 0.35 |
| FA | 0.64±0.01 | 0.62±0.01 | <0.01b,c | 0.62±0.02 | <0.01b,c | 0.62±0.02 | <0.01b,c | 0.74 | |
| MD | 0.84±0.01 | 0.85±0.01 | 0.01b,c | 0.85±0.02 | 0.97 | 0.85±0.02 | 0.91 | 0.61 | |
| Splenium of corpus callosum | MK | 1.42±0.02 | 1.40±0.03 | 0.62 | 1.46±0.01 | 0.48 | 1.37±0.03 | 0.26 | 0.11 |
| FA | 0.74±0.01 | 0.71±0.02 | 0.13 | 0.75±0.02 | 0.76 | 0.70±0.02 | 0.06 | 0.22 | |
| MD | 1.00±0.02 | 1.06±0.03 | 0.14 | 0.96±0.03 | 0.7 | 1.10±0.04 | 0.02b | 0.02b | |
| Centrum semiovale | MK | 1.37±0.01 | 1.33±0.01 | 0.13 | 1.36±0.01 | 0.25 | 1.33±0.02 | <0.01b,c | <0.01b,c |
| FA | 0.50±0.02 | 0.48±0.01 | 0.06 | 0.52±0.03 | 0.44 | 0.47±0.02 | 0.80 | 0.02b | |
| MD | 0.82±0.01 | 0.83±0.01 | 0.14 | 0.82±0.01 | 0.95 | 0.84±0.01 | <0.01b,c | <0.01b,c |
Significant after adjustments for differences in age and gender.
Demonstrated significant differences without Bonferroni correction.
Demonstrated significant differences with Bonferroni correction.
DTI, diffusion tensor imaging; DKI, diffusional tensor imaging; mTBI, mild traumatic brain injury; All patients, Group 1+Group 2; Group 1, patients studied within 1 year after injury; Group 2, patients studied more than 1 year after injury; SE, standard error; MK, mean kurtosis; FA, fractional anisotropy; MD, mean diffusivity.
When patient groups were analyzed separately and compared to controls, Group 1 patients showed significantly lower MK in the thalamus (p=0.02), and FA in the internal capsule (p<0.01), and Group 2 patients had significantly lower MK and FA and higher MD in the thalamus (MK: p<0.01; MD=0.02), the internal capsule (MK: p=0.02; FA: p<0.01), the splenium of the corpus callosum (MD: p=0.02), and the centrum semiovale (MK and MD: p<0.01). All differences remained statistically significant after Bonferroni correction, except for MK in the thalamus of Group 1 patients and MD in the thalamus and the splenium of the corpus callosum and FA in the centrum semiovale of Group 2 patients.
When Group 2 patients were compared to Group 1 patients they showed significantly lower MK and FA and higher MD in the thalamus (MD: p=0.02), the splenium of the corpus callosum (MD: p=0.02), and the centrum semiovale (MK and MD: p<0.01; FA: p=0.02). All differences remained statistically significant after Bonferroni correction, except for MD in the thalamus and the splenium of the corpus callosum, and FA in the centrum semiovale.
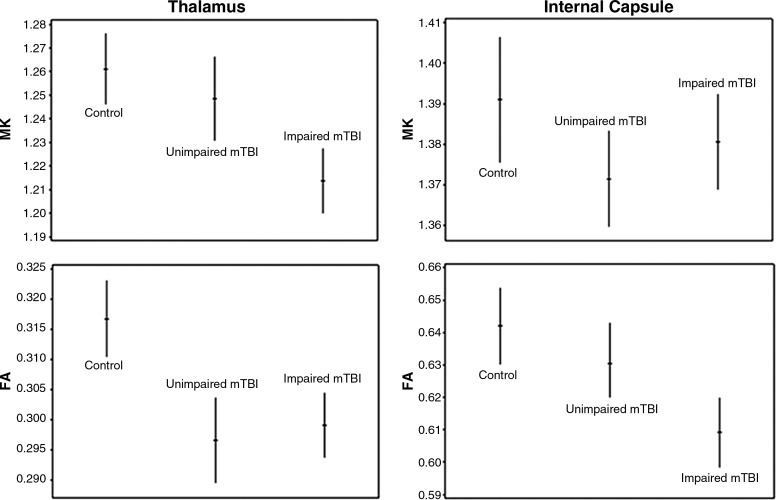
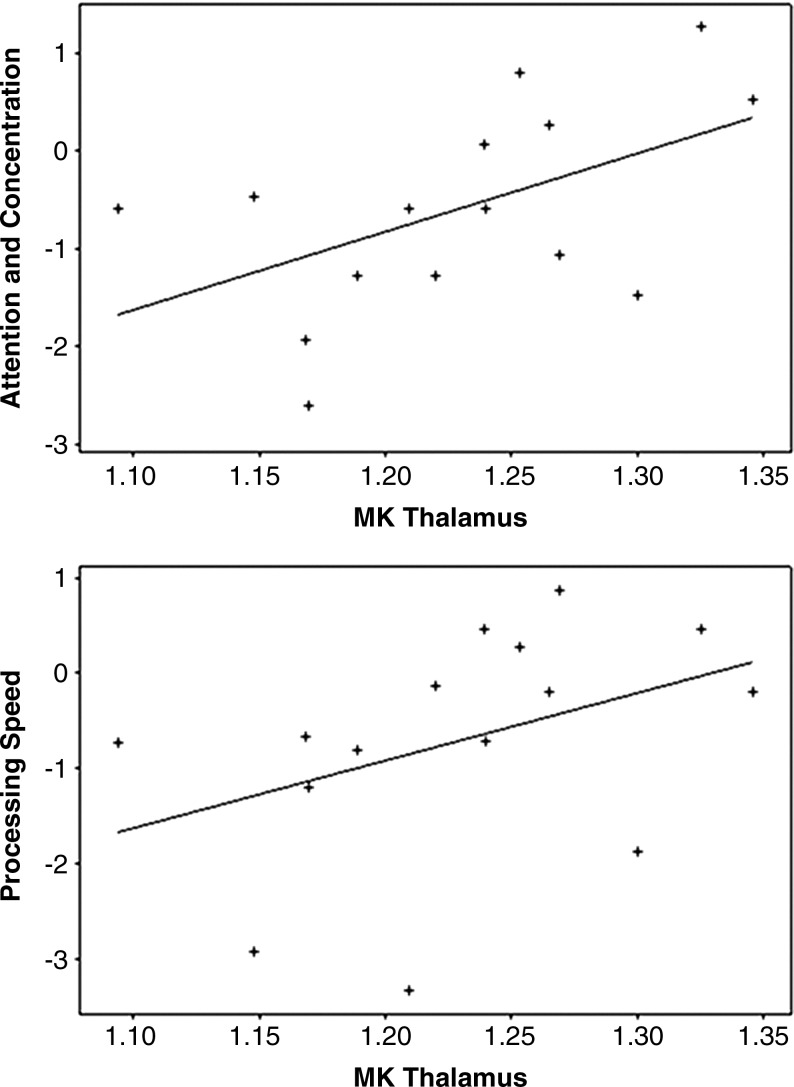
When cognitively-impaired patients were compared with cognitively-unimpaired patients they showed significantly lower MK in the thalamus (p<0.01) and FA in the internal capsule (p=0.02), which is illustrated in Figure 2. Only the difference for MK in the thalamus remained significant after Bonferroni correction. In addition, regression analysis showed that for Group 2 patients, MK in the thalamus was significantly correlated with neuropsychological z score results for A/C (r=0.67, p=0.03) and PS (r=0.65, p=0.02), which is illustrated in Figure 3.
FIG. 2.
Plots displaying age- and gender-adjusted means (hash marks), and 95% confidence intervals (lines), for mean kurtosis (MK) and fractional anisotropy (FA) in the thalamus and the internal capsule of controls, cognitively-unimpaired mild traumatic brain injury (mTBI) patients, and cognitively-impaired mTBI patients. When cognitively-impaired patients were evaluated with respect to cognitively-unimpaired patients they exhibited significantly lower MK in the thalamus (p<0.01), and FA in the internal capsule (p=0.02).
FIG. 3.
Scatterplots with best-fit lines showing significant correlations between the baseline average values for mean kurtosis (MK) in the thalamus of Group 2 mild traumatic brain injury (mTBI) patients and their z scores on neuropsychological tests evaluating attention and concentration (r=0.67, p=0.03), and processing speed (r=0.65, p=0.02).
DTI and DKI measures were not found to be predictive of cognitive status, since the number of subjects in each of the cognitively-impaired and cognitively-unimpaired patient groups was relatively small. They were, however, found to predictive when considered with respect to the larger patient group represented by mTBI status in the thalamus (FA: p=0.04), the internal capsule (FA: p=0.03), and the centrum semiovale (MK: p=0.04).
Discussion
This study shows that the combined use of DTI and DKI indices provides higher detection and better characterization of regional brain injury in mTBI patients. These findings also suggest that DTI and DKI have the potential to advance our understanding of pathophysiology in mTBI, and may possibly yield valuable predictors for the severity of brain damage and cognitive outcome.
While this study is in line with previous DTI investigations of mTBI in identifying that, compared to controls, the group of patients as a whole demonstrated lower FA and higher MD in the internal capsule, and reflected the presence of DAI (Arfanakis et al., 2002; Inglese et al., 2005; Miles et al., 2008) a more interesting finding is that they also exhibited lower MK in the thalamus. Although there is some evidence to suggest that the thalamus is involved in mTBI (Abdel-Dayem et al., 1998; Anderson et al., 1996; Basser and Jones, 2002; Ge et al., 2009; Henninger et al., 2007; Wood and Bigler, 1995), very few MRI investigations have addressed the structural and functional vulnerability of this region during such injury (Anderson et al., 1996; Ge et al., 2009; Henninger et al., 2007; Little et al., 2010). This raises an important question about whether thalamic damage is directly caused by traumatic insult or results from secondary phenomena involving longer-term cellular processes related to WM injury. Experimental models suggest that these mechanisms are not mutually exclusive, and it is likely that they both contribute to thalamic injury in mTBI. For example, Zhang and associates (2004) investigated the susceptibility of different cerebral tissues to direct injury during traumatic insult in mTBI using a simulated human head, and demonstrated that the highest stress shear concentration in the brain was localized to the thalamus and midbrain. In contrast, Anderson and colleagues (1996) proposed a model of secondary injury in mTBI based on transneuronal degeneration, showing that if a lesion interrupts WM fibers connecting the cerebral cortex and the thalamus, then target cells in both regions might undergo apoptosis. Furthermore, although speculative at this time, it would appear that damage to axons controlling the blood flow and permeability of vessels supplying the thalamus might also contribute to secondary injury (Feig and Guillery, 2000). In the present study the correlation in patients between DTI and DKI measures in the thalamus with those in WM does indeed support the secondary nature of thalamic injury. Since, however, this was only a moderate correlation, it suggests that damage caused directly by traumatic insult also had the potential to contribute, at least in part, to thalamic injury.
The precise meaning of DTI and DKI measures and their relationship to the underlying cellular environment is not yet completely understood. According to experimental animal models of DTI a decrease in FA and an increase in MD reflects alterations in microstructural integrity that may be associated with the disorganization and loss of axonal membranes and myelin sheaths (Basser, 1995). A decrease in MK can be interpreted as a reduction in overall diffusional heterogeneity, and while it is only possible to speculate about the underlying pathological substrate, this is probably associated with degenerative changes and neuronal shrinkage (Jensen and Helpern, 2010; Jensen et al., 2005; Lu et al., 2006b; Wu and Cheung, 2010).
Perhaps the most important finding in this study is that cognitively-impaired patients demonstrated significantly lower MK and FA in the thalamus and internal capsule compared to cognitively-unimpaired patients. In addition, there were correlations in Group 2 patients between MK in the thalamus and neuropsychological performance on tests of attention, concentration, and processing speed. Unlike the thalamus the internal capsule has been widely accepted as a probable anatomical correlate for cognitive dysfunction in mTBI (Mittl et al., 1994). Pathological investigations have shown that the anterior limb and genu of the internal capsule are related to disorders of attention and executive functioning, delayed visuospatial memory, and perceptuomotor slowness (Rao, 1995; Tatemichi et al., 1992), while the posterior limb of the internal capsule is related to pure motor deficits (Arboix et al., 2000).
Despite the paucity of research on the possible function of the thalamus in mTBI there is evidence to suggest that if this region is injured it might play a role in cognitive impairment. The thalamus has been described as the central relay station of the brain because it has reciprocal projections to the entire cerebral cortex and plays a principal role in the processing and transmission of information between the sensory, motor, and associative regions (Sherman and Guillery, 2009). These multiple functional pathways influence many global activities and therefore could potentially account for a great deal of the morbidity associated with mTBI. The relationship between the thalamus and cognitive function has been described with respect to several neurological diseases, as well as preliminarily mTBI, and includes the domains of attention, concentration, and processing speed, which are relevant to the results of this study (Ge et al., 2009; Little et al., 2010; Salmond et al., 2005; Van Der Werf et al., 2000, 2001).
Finally, since changes detected in the thalamus and the internal capsule of patients were present during both early and late time intervals following injury compared to controls, it is possible to infer that damage occurring in these regions is sustained, and DTI and DKI measures might therefore represent early indicators and prognostic measures for neuropsychological outcome. This is important because predicting which patients are at risk of experiencing protracted adverse cognitive changes enables early intervention that has been shown to reduce the possibility of long-term deficits (Cicerone et al., 1996; Mittenberg et al. 1996; Warden et al., 2006).
There are several potential limitations to this study. One concern is the lack of histological confirmation for the imaging findings that have been reported. Nevertheless, DTI and DKI WM abnormalities appear to be in locations that are characteristic for DAI and consistent with what has been identified in previous DTI investigations of mTBI. Another concern is the relatively small number of patients examined. Recruiting a larger cohort would certainly improve statistical power and will be addressed in subsequent studies. Finally, to determine their true predictive utility, longitudinal studies will be required to better assess changes in imaging measures over time, and more precisely ascertain their relationship to cognitive outcome.
In conclusion, it has been demonstrated that the combined use of DTI and DKI facilitates the detection of subtle brain tissue injury in both the thalamus and WM of mTBI patients. Since the acquisition protocol used to obtain a DKI dataset includes all the information necessary to derive a standard DTI dataset, it can be used to calculate both types of indices and provide more sensitive and specific markers for tissue injury. Finally, since the thalamus and the internal capsule exhibited changes in DTI and DKI measures at both early and late time intervals following injury, and were associated with neuropsychological dysfunction, it is possible they might be useful in the early prediction of permanent brain damage and cognitive outcome.
Acknowledgments
The authors would like to thank Drs. Stewart Bloomfield, Qun Chen, Henry Rusinek, and Edward Ziff of the New York University School of Medicine for advice on this study, and Dr. Hanzhang Lu of the University of Texas Southwestern Medical Center for developing the MATLAB scripts used to process the DKI data. This work was supported by the National Institutes of Health grant numbers R01NS039135 and R01NS051623.
Author Disclosure Statement
No competing financial interests exist.
References
- Abdel-Dayem H.M. Abu-Judeh H. Kumar M. Atay S. Naddaf S. El-Zeftawy H. Luo J.Q. SPECT brain perfusion abnormalities in mild or moderate traumatic brain injury. Clin. Nucl. Med. 1998;23:309–317. doi: 10.1097/00003072-199805000-00009. [DOI] [PubMed] [Google Scholar]
- Anderson C.V. Wood D.M. Bigler E.D. Blatter D.D. Lesion volume, injury severity, and thalamic integrity following head injury. J. Neurotrauma. 1996;13:59–65. doi: 10.1089/neu.1996.13.59. [DOI] [PubMed] [Google Scholar]
- Arboix A. Garcia-Eroles L. Massons J. Oliveres M. Targa C. Hemorrhagic lacunar stroke. Cerebrovasc. Dis. 2000;10:229–234. doi: 10.1159/000016061. [DOI] [PubMed] [Google Scholar]
- Arfanakis K. Haughton V.M. Carew J.D. Rogers B.P. Dempsey R.J. Meyerand M.E. Diffusion tensor MR imaging in diffuse axonal injury. Am. J. Neuroradiol. 2002;23:794–802. [PMC free article] [PubMed] [Google Scholar]
- Basser P.J. Jones D.K. Diffusion-tensor MRI: theory, experimental design and data analysis—a technical review. NMR Biomed. 2002;15:456–467. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- Basser P.J. Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 1995;8:333–344. doi: 10.1002/nbm.1940080707. [DOI] [PubMed] [Google Scholar]
- Batchelor M. Harvey A.G. Bryant R.A. Stroop Colour Word Test as a measure of attentional deficit following mild head injury. Clin. Neuropsychol. 1995;9:180–186. [Google Scholar]
- Bates D.M. Watts D.G. Nonlinear Regression Analysis and its Applications. Wiley Press: New York; 1988. [Google Scholar]
- Binder L.M. A review of mild head trauma. Part II: Clinical implications. J. Clin. Exp. Neuropsychol. 1997;19:421–431. doi: 10.1080/01688639708403870. [DOI] [PubMed] [Google Scholar]
- Bohnen N. Twijnstra A. Jolles J. Performance in the Stroop color word test in relationship to the persistence of symptoms following mild head injury. Acta Neurol. Scand. 1992;85:116–121. doi: 10.1111/j.1600-0404.1992.tb04009.x. [DOI] [PubMed] [Google Scholar]
- Cicerone K.D. Azulay J. Diagnostic utility of attention measures in postconcussion syndrome. Clin. Neuropsychol. 2002;16:280–289. doi: 10.1076/clin.16.3.280.13849. [DOI] [PubMed] [Google Scholar]
- Cicerone K.D. Smith L.C. Ellmo W. Mangel H.R. Nelson P. Chase R.F. Kalmar K. Neuropsychological rehabilitation of mild traumatic brain injury. Brain Inj. 1996;10:277–286. doi: 10.1080/026990596124458. [DOI] [PubMed] [Google Scholar]
- Erlanger D.M. Kaushik T. Broshek D. Freeman J. Feldman D. Festa J. Development and validation of a web-based screening tool for monitoring cognitive status. J. Head Trauma Rehabil. 2002;5:458–476. doi: 10.1097/00001199-200210000-00007. [DOI] [PubMed] [Google Scholar]
- Esselman P.C. Uomoto J.M. Classification of the spectrum of mild traumatic brain injury. Brain Inj. 1995;9:417–424. doi: 10.3109/02699059509005782. [DOI] [PubMed] [Google Scholar]
- Feig S.L. Guillery R.W. Corticothalamic axons contact blood vessels as well as nerve cells in the thalamus. Eur. J. Neurosci. 2000;12:2195–2198. doi: 10.1046/j.1460-9568.2000.00093.x. [DOI] [PubMed] [Google Scholar]
- Frencham K.A.R. Fox A.M. Maybery M.T. Neuropsychological studies of mild traumatic brain injury: A meta-analytic review of research since 1995. J. Clin. Exp. Neuropsychol. 2005;27:334–351. doi: 10.1080/13803390490520328. [DOI] [PubMed] [Google Scholar]
- Ge Y. Patel M.B. Chen Q. Grossman E.J. Zhang K. Miles L. Babb J.S. Reaume J. Grossman R.I. Assessment of thalamic perfusion in patients with mild traumatic brain injury by true FISP arterial spin labelling MR imaging at 3T. Brain Inj. 2009;23:666–674. doi: 10.1080/02699050903014899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitger M.H. Jones R.D. Dalrymple-Alford J.C. Fampton C.M. Ardagh M.W. Anderson T.J. Motor deficits and recovery during the first year following mild closed head injury. Brain Inj. 2006;20:807–824. doi: 10.1080/02699050600676354. [DOI] [PubMed] [Google Scholar]
- Helpern J.A. Adisetiyo V. Falangola M.F. Hu C. Di Martino A. Williams K. Castellanos F.X. Jensen J.H. Preliminary evidence of altered gray and white matter microstructural development in the frontal lobe of adolescents with ADHD: a diffusional kurtosis imaging study. J. Magn. Reson. Imaging. 2011;33:17–23. doi: 10.1002/jmri.22397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henninger N. Sicard K.M. Li Z. Kulkarni P. Dützmann S. Urbanek C. Schwab S. Fisher M. Differential recovery of behavioral status and brain function assessed with functional magnetic resonance imaging after mild traumatic brain injury in the rat. Crit. Care Med. 2007;35:2607–2614. doi: 10.1097/01.CCM.0000286395.79654.8D. [DOI] [PubMed] [Google Scholar]
- Inglese M. Makani S. Johnson G. Cohen B.A. Silver J.A. Gonen O. Grossman R.I. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J. Neurosurg. 2005;103:298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- Jensen J.H. Helpern J.A. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010;23:698–710. doi: 10.1002/nbm.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J.H. Falangola M.F. Hu C. Tabesh A. Rapalino O. Lo C. Helpern J.A. Preliminary observations of increased diffusional kurtosis in human brain following recent cerebral infarction. NMR Biomed. 2010;24:452–457. doi: 10.1002/nbm.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen J.H. Helpern J.A. Ramani A. Lu H. Kaczynski K. Diffusional kurtosis imaging: the quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn. Reson. Med. 2005;53:1432–1440. doi: 10.1002/mrm.20508. [DOI] [PubMed] [Google Scholar]
- Jones D.K. Horsfield M.A. Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn. Reson. Med. 1999;42:515–525. [PubMed] [Google Scholar]
- Lezak M.D. Howieson D.B. Loring D.W. Neuropsychological Assessment. 4th. Oxford University Press; New York: 2004. [Google Scholar]
- Little D.M. Kraus M.F. Joseph J. Geary E.K. Susmaras T. Zhou X.J. Pliskin N. Gorelick P.B. Thalamic integrity underlies executive dysfunction in traumatic brain injury. Neurology. 2010;74:558–564. doi: 10.1212/WNL.0b013e3181cff5d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. Jensen J.H. Hu C. Falangola M.F. Ramani A. Ferris S. Helpern J.A. Alterations in cerebral microstructual integrity in normal aging and in Alzheimer's disease: a multi-contrast diffusion MRI study. Proc. Int. Soc. Magn. Reson. Med. 2006a;14:723. [Google Scholar]
- Lu H. Jensen J.H. Ramani A. Helpern J.A. Three-dimensional characterization of non-gaussian water diffusion in humans using diffusion kurtosis imaging. NMR Biomed. 2006b;19:236–247. doi: 10.1002/nbm.1020. [DOI] [PubMed] [Google Scholar]
- Mathias J.L. Beall J. Bigler E.D. Neuropsychological and information processing deficits following mild traumatic brain injury. J. Int. Neuropsychol. Soc. 2004;10:286–297. doi: 10.1017/S1355617704102117. [DOI] [PubMed] [Google Scholar]
- McHugh T. Laforce R., Jr. Gallagher P. Quinn S. Diggle P. Buchanan L. Natural history of the long-term cognitive, affective, and physical sequelae of mild traumatic brain injury. Brain Cog. 2006;60:209–211. [PubMed] [Google Scholar]
- Miles L. Grossman R.I. Johnson G. Babb J.S. Diller L. Inglese M. Short-term DTI predictors of cognitive dysfunction in mild traumatic brain injury. Brain Inj. 2008;22:115–122. doi: 10.1080/02699050801888816. [DOI] [PubMed] [Google Scholar]
- Mittenberg W. Tremont G. Zielinski R.E. Fichera S. Rayls K.R. Cognitive-behavioral prevention of postconcussion syndrome. Arch. Clin. Neuropsychol. 1996;11:139–145. [PubMed] [Google Scholar]
- Mittl R.L. Grossman R.I. Hiehle J.F. Hurst R.W. Kauder D.R. Gennarelli T.A. Alburger G.W. Prevalence of MR evidence of diffuse axonal injury in patients with mild head injury and normal head CT findings. Am. J. Neuroradiol. 1994;15:1583–1589. [PMC free article] [PubMed] [Google Scholar]
- National Center for Injury Prevention and Control. Report to Congress on Mild Traumatic Brain Injury in the United States: Steps to Prevent a Serious Public Health Problem. Centers for Disease Control and Injury Prevention; Atlanta: 2003. [Google Scholar]
- Paré N. Rabin L.A. Fogel J. Pépin M. Mild traumatic brain injury and its sequelae: characterization of divided attention deficits. Neuropsychol. Rehabil. 2009;19:110–137. doi: 10.1080/09602010802106486. [DOI] [PubMed] [Google Scholar]
- Raab P. Hattingen E. Franz K. Zanella F.E. Lanfermann H. Cerebral gliomas: diffusional kurtosis imaging analysis of microstructural differences. Radiology. 2010;254:876–881. doi: 10.1148/radiol.09090819. [DOI] [PubMed] [Google Scholar]
- Ramani A. Jensen J.H. Szulc K.U. Ali O. Hu C. Lu H. Brodle J.D. Helpern J.A. Assessment of abnormalities in the cerebral microstructure of schizophrenia patients: a diffusional kurtosis imaging study. Proc. Int. Soc. Magn. Reson. Med. 2007;15:648. [Google Scholar]
- Rao S.M. Neuropsychology of multiple sclerosis. Curr. Opin. Neurol. 1995;8:216–220. doi: 10.1097/00019052-199506000-00010. [DOI] [PubMed] [Google Scholar]
- Rath J.F. Langenbahn D.M. Simon D. Sherr R.L. Fletcher J. Diller L. The construct of problem solving in higher level neuropsychological assessment and rehabilitation. Arch. Clin. Neuropsychol. 2004;19:613–635. doi: 10.1016/j.acn.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Reese T.G. Heid O. Weisskoff R.M. Wedeen V.J. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn. Reson. Med. 2003;49:177–182. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Ruff R.M. Light R.H. Parker S.B. Levin H.S. The psychological construct of word fluency. Brain Lang. 1997;57:394–405. doi: 10.1006/brln.1997.1755. [DOI] [PubMed] [Google Scholar]
- Salmond C.H. Chatfield D.A. Menon D.K. Pickard J.D. Sahakian B.J. Cognitive sequelae of head injury: involvement of basal forebrain and associated structures. Brain. 2005;128:189–200. doi: 10.1093/brain/awh352. [DOI] [PubMed] [Google Scholar]
- Sherman S.M. Guillery R.W. Exploring the Thalamus and Its Role in Cortical Function. 2nd. The MIT Press; Cambridge: 2009. [Google Scholar]
- Skare S. Hedehus M. Moseley M.E. Li T.Q. Condition number as a measure of noise performance of diffusion tensor data acquisition schemes with MRI. J. Magn. Reson. 2000;147:340–352. doi: 10.1006/jmre.2000.2209. [DOI] [PubMed] [Google Scholar]
- Szymanski H.V. Linn R. A review of the postconcussion syndrome. Int. J. Psychiatry Med. 1992;22:357–375. doi: 10.2190/XARA-B1EF-J2HC-VAE0. [DOI] [PubMed] [Google Scholar]
- Tatemichi T.K. Desmond D.W. Prohovnik I. Cross D.T. Gropen T.I. Mohr J.P. Stern Y. Confusion and memory loss from capsular genu infarction: a thalamocortical disconnection syndrome? Neurology. 1992;42:1966–1979. doi: 10.1212/wnl.42.10.1966. [DOI] [PubMed] [Google Scholar]
- Tay S.Y. Ang B.T. Lau X.Y. Meyyappan A. Collinson S.L. Chronic impairment of prospective memory after mild traumatic brain injury. J. Neurotrauma. 2010;27:77–83. doi: 10.1089/neu.2009.1074. [DOI] [PubMed] [Google Scholar]
- Trenerry M.R. Crosson B. DeBoe J. Leber W.R. Stroop Neuropsychological Screening Test Manual. Psychological Assessment Resources; Odessa: 1989. [Google Scholar]
- Tsirka V. Simos P. Vakis A. Vourkas M. Arzoglou B. Symros N. Stavropoulos S. Material-specific difficulties in episodic memory tasks in mild traumatic brain injury. Int. J. Neurosci. 2010;120:184–191. doi: 10.3109/00207450903585308. [DOI] [PubMed] [Google Scholar]
- Van Der Werf Y.D. Tisserand D.J. Visser P.J. Hofman P.A. Vuurman E. Uylings H.B. Jolles J. Thalamic volume predicts performance on tests of cognitive speed and decreases in healthy aging. A magnetic resonance imaging-based volumetric analysis. Brain Res. Cogn. Brain Res. 2001;11:377–385. doi: 10.1016/s0926-6410(01)00010-6. [DOI] [PubMed] [Google Scholar]
- Van der Werf Y.D. Witter M.P. Uylings H.B. Jolles J. Neuropsychology of infarctions in the thalamus: a review. Neuropsychologia. 2000;38:613–627. doi: 10.1016/s0028-3932(99)00104-9. [DOI] [PubMed] [Google Scholar]
- Warden D.L. Gordon B. McAllister T.W. Silver J.M. Barth J.T. Bruns J. Drake A. Gentry T. Jagoda A. Katz D.I. Kraus J. Labbate L.A. Ryan L.M. Sparling M.B. Walters B. Whyte J. Zapata A. Zitnay G. Guidelines for the pharmacologic treatment of neurobehavioral sequelae of traumatic brain injury. J. Neurotrauma. 2006;23:1468–1501. doi: 10.1089/neu.2006.23.1468. [DOI] [PubMed] [Google Scholar]
- Wood D.M. Bigler E.D. Diencephalic changes in traumatic brain injury: relationship to sensory perceptual function. Brain Res. Bull. 1995;38:545–549. doi: 10.1016/0361-9230(95)02026-0. [DOI] [PubMed] [Google Scholar]
- Wu E.X. Cheung M.M. MR diffusion kurtosis imaging for neural tissue characterization. NMR Biomed. 2010;23:836–848. doi: 10.1002/nbm.1506. [DOI] [PubMed] [Google Scholar]
- Zakzanis K.K. Leach L. Kaplan E. Neuropsychological Differential Diagnosis. Swets & Zeitlinger Publishers; Exton: 1999. [Google Scholar]
- Zhang L. Yang K.H. King A.I. A proposed injury threshold for mild traumatic brain injury. J. Biomech. Eng. 2004;126:226–236. doi: 10.1115/1.1691446. [DOI] [PubMed] [Google Scholar]