Abstract
A prolonged QT interval is a marker for an increased risk of ventricular tachyarrhythmias. Both endogenous and exogenous sex hormones have been shown to affect the QT interval. Endogenous testosterone and progesterone shorten the action potential, and estrogen lengthens the QT interval. During a single menstrual cycle, progesterone levels, but not estrogen levels, have the dominant effect on ventricular repolarization in women. Studies of menopausal hormone therapy (MHT) in the form of estrogen-alone therapy (ET) and estrogen plus progesterone therapy (EPT) have suggested a counterbalancing effect of exogenous estrogen and progesterone on the QT. Specifically, ET lengthens the QT, whereas EPT has no effect. To date, there are no studies on oral contraception (OC) and the QT interval, and future research is needed. This review outlines the current literature on sex hormones and QT interval, including the endogenous effects of estrogen, progesterone, and testosterone and the exogenous effects of estrogen and progesterone therapy in the forms of MHT and hormone contraception. Further, we review the potential mechanisms and pathophysiology of sex hormones on the QT interval.
Introduction
The electrocardiogram (ECG) is a useful noninvasive tool for the diagnosis and prognosis of a wide range of cardiovascular conditions. A typical ECG tracing of the cardiac cycle consists of a P wave, a QRS complex, and a T wave. The QT interval on the ECG, measured from the beginning of the QRS complex to the end of the T wave, represents the duration of activation and recovery of the ventricular myocardium. Corrected QT (QTc) takes into account the physiologic shortening of the QT with increases in heart rate and has commonly been calculated using Bazett's formula (QTc=QT/√RR) Other heart rate correction formulas are also available, including Fridericia's formula, which uses the cube root of RR. These methods are limited by overcorrection at faster heart rates and undercorrection at slower heart rates. As a result, linear regression formulas for QT correction have been developed and are preferred to Bazett's and Fridericia's formulas.1
A prolonged QTc interval is a marker for an increased risk of ventricular tachyarrhythmias, specifically torsades de pointes (TdP), and of sudden cardiac death (SCD).2 Female gender is an independent risk factor for developing drug-related TdP.3 Further, although SCD is more common in men than women,4 women who experience SCD are less likely to have coronary heart disease (CHD) as the culprit etiology and less likely to have a prior history of heart disease than men.5 Thus, the ongoing study into arrhythmic mechanisms of SCD in women and such risk markers as the QT interval has been an important area of research over the past decade.
Both endogenous and exogenous sex hormones have been shown to affect the QT interval.6–11 Endogenous testosterone and progesterone shorten the action potential8,9,11 and estrogen lengthens the QT interval in animals.10 During a single menstrual cycle, progesterone levels but not estrogen levels have the dominant effect on ventricular repolarization in women.8,9 Studies of menopause hormone therapy (MHT) in the form of estrogen-alone therapy (ET) and estrogen plus progesterone therapy (EPT) have suggested a counterbalancing effect of exogenous estrogen and progesterone on the QT. Specifically, ET lengthens the QT while EPT has no effect.6,7 To date, there are no studies on oral contraception (OC) and the QT interval. The new fourth generation OC, which are nontestosterone derived and antiandrogenic, might contribute to overall lengthening of the QT interval, and studies are needed.
This review outlines the current literature on sex hormones and QT interval, including the endogenous effects of estrogen, progesterone, and testosterone and the exogenous effects of estrogen and progesterone therapy in the forms of MHT and hormone contraception. Further, we review the potential mechanisms and pathophysiology of sex hormones on the QT interval.
Endogenous Sex Hormones and QT interval
Adult men have shorter QTc intervals than women.12 This gender difference is absent at birth and in young children.13 Throughout puberty, the QTc interval in males shortens by 20 msec, whereas the QTc of females remains unchanged, resulting in a 6% shorter QTc in males compared to females.14 With aging, the QTc in men gradually lengthens and approximates that of women by the age of 50.14 This suggests that gender differences exist in the QTc interval.
Observational and small prospective interventional studies have supported an association between testosterone and shorter ventricular repolarization. Van Noord et al.15 studied random testosterone samples and QTc intervals in 445 males from the Rotterdam study cohort and 1,428 males from the Study of Health In Pomerania (SHIP). They found that QTc intervals decreased with increasing tertiles of endogenous testosterone (p value for trend=0.024). Similarly, Zhang et al.11 evaluated 727 men enrolled in the Third National Health and Nutrition Examination Survey (NHANES III). They demonstrated that middle-aged men with the highest quartile of endogenous total testosterone (>6.0 ng/mL) had significantly shorter uncorrected QT intervals (by 8.5 msec) than those with the lowest quartile (<3.3 ng/mL).11 Charbit et al.16 recorded digital ECGs at low (median level=52.6 nmol/L), medium (median level=35.8 nmol/L), and high (median level=6 nmol/L) levels of testosterone in 11 hypogonadic men after a single injection of testosterone. The QTc interval was significantly shorter at high testosterone levels than at low levels (mean difference of 13.6 msec, p=0.0007), and a negative linear relationship was demonstrated between QTc and testosterone levels (p=0.004). Percori Geraldi et al.17 compared the QTc in 26 men with hypogonadism to the QTc in 26 age-matched controls. They reported a higher prevalence of a prolonged QTc in hypogonadal men (15%) than in controls (0%) (p<0.05) and demonstrated normalization of the QTc after testosterone therapy. Finally, a case-control study with 27 orchiectomized men with no exogenous testosterone therapy and 53 nonorchiectomized controls demonstrated that orchiectomized men had significantly longer JTc intervals (from the start of the J wave to the end of the T wave) than nonorchiectomized men (p<0.01).18 These studies support the role of testosterone in QT shortening.
Conflicting results of endogenous estrogen on the QT interval have been described. Saito et al.10 compared the QTc of mice with high endogenous estrogen to the QTc of ovariectomized mice with no detectable endogenous estrogen. They found a significantly shorter QTc in the ovariectomized group (p<0.05). Further, when estradiol was added back to the ovariectomized group, the QTc lengthened to presurgical values. In humans, these results have not been replicated. De Leo et al.19 studied the QTc of 26 premenopausal women before and after bilateral oophorectomy. Estradiol levels dropped significantly from 67±20 pg/mL to 15±5 pg/mL postoophorectomy, but the authors were unable to detect a significant change in the QTc 20–25 days after surgery. Finally, Saba et al.20 compared 36 premenopausal women (mean age 36 years) to 65 postmenopausal women (mean age 72 years). They found no significant difference between the average QTc of premenopausal (405±21 msec) and postmenopausal (419±30 msec) women (p=NS) despite significantly lower estradiol levels in the postmenopausal group. All the studies cited used Bazett's formula to correct for QTc; thus, the differences in results cannot be explained by the method of QTc correction.
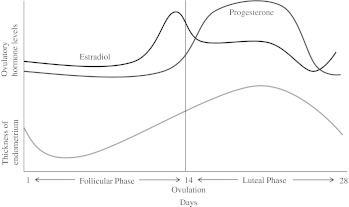
Progesterone shortens the action potential duration and QT interval in women.8,9 The best evidence for this finding has been derived from studies on QT alterations during the menstrual cycle. During a single menstrual cycle, estrogen and progesterone levels fluctuate; estrogen levels are lowest at the onset of menses and peak at ovulation approximately 11–13 days later. Estrogen levels gradually increase during the follicular phase and fall in the luteal phase (Fig. 1). Progesterone levels, in contrast, are low during the follicular phase and increase through the luteal phase.
FIG. 1.
Estrogen and progesterone levels over a single menstrual cycle in women.
Studies have reported conflicting results of the hormonal fluctuations observed during a single menstrual cycle on the QT interval. Hulot et al.21 studied the QTc (corrected for heart rate [HR] using a regression formula) of 21 healthy women with regular menstrual cycles and did not find a difference in QTc duration between menses (taken 24 hours after onset) and preovulation (taken 14 days before the next menstrual cycle) (p=0.98). Similarly, Burke et al.22 did not demonstrate a difference in QTc (corrected for HR using Bazett's formula) duration between the menstrual (taken within 4 days of the onset of menses), follicular (taken days 12–14 of the cycle), and luteal (taken 6–8 days after ovulation) phases of 23 healthy premenopausal women during resting conditions. With double autonomic blockade consisting of atropine and propranolol administration, however, the QTc was shorter in the luteal phase (438 msec) compared to the menstrual (446 msec) and follicular (444 msec) phases (p=0.05), suggesting an important role for autonomic tone in alterations in the QTc during the menstrual cycle. Rodriguez et al.9 examined QTc responses (corrected for HR using Bazett's formula) to ibutilide, an antiarrhythmic, in 58 healthy premenopausal women with normal menstrual cycles. They reported the greatest increase in QTc during the menstrual (taken 24 hours after the onset of menses) and ovulatory (taken 24 hours after a positive urine ovulation test) phases compared to the luteal phase (taken 7–9 days after ovulation) (p=0.02). Further, they reported that measured progesterone levels, but not estrogen, were inversely correlated with ibutilide-induced QTc prolongation (r=-.40, p=0.01 for progesterone; r=0.14, p=0.28 for estrogen). Nakagawa et al.8 reported longer QTc intervals during the follicular phase (taken days 7-12 after the onset of menses) than the luteal phase (taken days 18–26 after the onset of menses) in healthy women aged 18–32 years on Holter ECG recordings (p<0.05). They too found no correlation with estrogen levels but an inverse relationship between high progesterone levels and QT length.
In summary, endogenously, testosterone and progesterone shorten the action potential. Estrogen lengthens the QT interval in animals; however, this has not been supported by human studies. Further, studies on the menstrual cycle indicate that fluctuations in progesterone levels and not estrogen levels has the dominant effect on ventricular repolarization in women.8,9 A summary of the effects of endogenous and exogenous hormones on the QT interval in addition to the responsible mechanisms is displayed in Table 1. Further, a summary of the endogenous hormone studied, method of QTc correction, study population and design, and major findings is shown in Table 2.
Table 1.
Summary of Effects and Mechanisms of Endogenous and Exogenous Estrogen, Progesterone, and Testosterone on QT Interval
| Estrogen effect on QT | Progesterone effect on QT | Testosterone effect on QT | |
|---|---|---|---|
| Endogenous hormone studies | Animal studies support overall QT lengthening; human studies have found no change | Menstrual cycle studies support overall QT shortening | Observational human studies support overall QT shortening |
| Hormone replacement therapy studies | Studies of ET support QT lengthening | Studies of EPT have found no change in the QT, thus supporting a counteractive role for progesterone against estrogen | Studies of normalization of the QT with testosterone therapy in hypogonadal men support a QT shortening effect of testosterone |
| Hormonal contraception studies | No studies performed to date | No significant change in QTc with progestin only subdermal implant device | N/A |
| Proposed mechanisms | Suppression of IKr, IKs, and IK1 channel currents | Upregulation of IKs and suppression of ICa,L channel currents | Upregulation of IKr, IKs, and IK1 and suppression of ICa,L channel currents |
EPT, estrogen plus progesterone therapy; ET, estrogen-alone therapy; ICa,L, L-type calcium channel; IKr, rapidly activating delayed rectifier potassium channel; IKs, slowly activating delayed rectifier potassium channel; IK1 inward rectifier channel; QTc, corrected QT interval.
Table 2.
Summary of Studies Examining Effects of Endogenous Hormones on the QTc
| Hormone tested | Citation | Method of QT correction | Study population and design | Main findings |
|---|---|---|---|---|
| Testosterone | Van Noord et al.15 | Bazett's formula | Studied testosterone levels and QTc in 445 males from Rotterdam study cohort and 1428 males from Study of Health In Pomerania (SHIP) | QTc intervals decreased with increasing tertiles of endogenous testosterone |
| Testosterone | Zhang et al.11 | Uncorrected QT | Studied QT and endogenous testosterone levels in 727 men enrolled in Third National Health and Nutrition Examination Survey (NHANES III) | Middle-aged men with highest quartile of endogenous total testosterone had significantly shorter uncorrected QT intervals (by 8.5 msec) than those with the lowest quartile |
| Testosterone | Charbit et al.16 | QT interval was taken at a predetermined RR interval of 1000 msec | Studied QTc and endogenous testosterone levels in 11 hypogonadal men after single injection of exogenous testosterone | QTc was significantly shorter at high endogenous testosterone levels than low levels after single injection of testosterone |
| Testosterone | Percori Geraldi et al.17 | Bazett's formula | Measured QTc in 26 men with hypogonadism before and after testosterone therapy compared to 26 age-matched controls | Higher prevalence of prolonged QTc in hypogonadal men than in controls and normalization of prolonged QTc after testosterone therapy |
| Testosterone | Bidoggia et al.18 | JTc intervals (from start of J wave to end of T wave, corrected for by Bazett's formula) | Studied JTc in 27 orchiectomized men with no exogenous testosterone therapy and 53 nonorchiectomized controls | Orchiectomized men had significantly longer JTc intervals than nonorchiectomized men |
| Estrogen | Saito et al.10 | QTc=QT/√RR/100 (variation on Bazett's formula) | Measured QTc in mice with high endogenous estrogen compared to ovariectomized mice with no detectable endogenous estrogen | Significantly shorter QTc in ovariectomized group; when estradiol was added back to ovariectomized group, QTc lengthened to presurgical values |
| Estrogen | De Leo et al.19 | Bazett's formula | Measured estradiol levels and QTc in 26 premenopausal women before and after bilateral oophorectomy | Estradiol levels dropped significantly postoophorectomy with no significant change in QTc |
| Estrogen | Saba et al.20 | Bazett's formula | Compared QTc and estradiol levels in 36 premenopausal women to 65 postmenopausal women | No significant difference between QTc of premenopausal and postmenopausal women despite significantly lower estradiol levels in postmenopausal group |
| Estrogen | Hulot et al.21 | Regression formula | Measured QTc and estradiol levels during menses and preovulation of 21 healthy women with regular menstrual cycles | No difference in QTc duration between menses and preovulation despite large differences in estradiol at different phases |
| Hormones not measured | Burke et al.22 | Bazett's formula | Measured QTc in 23 healthy women with normal menstrual cycles during menstrual, follicular, and luteal phases before and after double autonomic blockade | No difference in QTc between menstrual, follicular, and luteal phases during resting conditions; QTc was shorter in luteal phase compared to menstrual and follicular phases with double autonomic blockade |
| Progesterone | Rodriguez et al.9 | Bazett's formula | Examined QTc responses to ibutilide, an antiarrhythmic, in 58 healthy premenopausal women with normal menstrual cycles | Greatest increase in QTc during menstrual and ovulatory phases compared to luteal phase; progesterone levels, but not estrogen, were inversely correlated with ibutilide-induced QTc prolongation |
| Progesterone | Nakagawa et al.12 | Bining method (pairs of RR and QT intervals are collected and distributed into “bins” according to immediately preceding RR value) | Examined QTc during different phases of menstrual cycle in healthy women aged 18–32 years on Holter ECG recordings | Longer QTc intervals during follicular than luteal phase; no correlation with estrogen levels but an inverse relationship between high progesterone levels and QT length |
Exogenous Sex Hormones and QT Interval
Menopause hormone therapy and QT interval
MHT in terms of ET and EPT and the effects on the QT interval have been reported in numerous studies.6,7,23 These studies define ET as 0.625 mg/day of conjugated equine estrogen (CEE) and EPT as 0.625 mg/day of CEE plus 2.5 mg/day of medroxyprogesterone (MPA). A retrospective study by Larsen et al.23 on 277 postmenopausal women in the Women's Cardiology Screening database at the Northwestern Medical Faculty Foundation found no difference in the QTc of women on MHT (426 msec) compared to women not on MHT (423 msec) (p=NS). This study, however, did not separate MHT into ET or EPT. Further, they corrected for the QTc using both Bazett's and linear regression methods and found no significant difference in the results based on method of correction. Kadish et al.6 reported on 34,378 postmenopausal women from the Women's Health Initiative (WHI) and did stratify by ET and EPT. They found that women on ET had significantly longer QTc intervals (corrected for HR by both Bazett's and linear regression methods) compared with women who were never treated with MHT (p<0.05). Women on EPT, in contrast, had no difference in QTc intervals compared with controls. They too corrected for HR using both Bazett's and linear regression methods and found no significant influence of correction method on the results. These results were further supported by Carnethon et al.7 who studied 3,101 women from the Atherosclerosis Risk in Communities cohort. They reported that the likelihood of QTc prolongation in women on ET was nearly twice that compared to women never treated with MHT (odds ratio [OR] 1.9, 95% confidence interval [CI] 1.2-2.0). EPT, in contrast, was not significantly associated with QTc length (OR 1.1, 95% CI 0.6-1.9). These studies suggest a counterbalancing effect of exogenous oral estrogen and progesterone on the QTc.
One study to date has examined the effects of transdermal MHT on the QTc. Nowinski et al.24 randomized 60 postmenopausal women into three groups: oral CEE 0.625 mg/day for 18 days followed by 10 days combined with oral MPA 5 mg/day, transdermal 17β-estradiol 50 μg/24 hours for 18 days followed by 10 days combined with oral MPA 5 mg/day, and transdermal placebo for 18 days followed by 10 days combined with oral placebo tablets. They measured QTc intervals at baseline and at 6 and 12 months and found no significant difference in QTc among the three different groups at any of these time intervals.
In summary, exogenous ET lengthens the QT, whereas EPT has no effect. This suggests a counterbalancing effect of exogenous estrogen and progesterone on the QTc. A summary of the exogenous hormones studied, method of QTc correction, study population and design, and major findings is displayed in Table 3. At this time, there is no evidence to suggest that the route of delivery has any effect on QT alterations with MHT, although further studies need to be done.
Table 3.
Summary of Studies Examining Effects of Exogenous Hormones on QTc
| Hormone therapy tested | Citation | Method of QT correction | Study population and design | Main findings |
|---|---|---|---|---|
| MHT | Larsen et al.23 | Bazett's and linear regression | Compared QTc of women on MHT and not on MHT among 277 postmenopausal women in the Women's Cardiology Screening database | No difference in QTc (by either correction method) of women on MHT compared to women not on MHT |
| MHT | Kadish et al.6 | Bazett's and linear regression | Compared QTc in women on ET or EPT compared to controls in 34,378 postmenopausal women from the WHI | Women on ET had significantly longer QTc intervals compared with women who were never treated with MHT (by either correction method); women on EPT had no difference in QTc compared with controls |
| MHT | Carnethon et al.7 | Bazett's formula | Compared QTc of women on ET or EPT compared to controls in 3,101 women from Atherosclerosis Risk in Communities cohort | Likelihood of QTc prolongation in women on ET was nearly twice that compared to women never treated with MHT; EPT was not significantly associated with QTc length |
| MHT | Nowinski et al.24 | Bazett's formula | Randomized 60 postmenopausal women into three groups: oral CEE/oral MPA, transdermal 17β-estradiol/oral MPA, and transdermal placebo/oral placebo and measured QTc | No significant difference in QTc among the three different groups at baseline, 6 months, and 12 months |
| Hormonal contraception | Okeahialam et al.25 | Bazett's formula | Measured QTc in 21 patients on a progestin-only subdermal inplant and at 3, 6, and 12 months of use | QTc increased at 1 year compared to baseline; however, this did not reach statistical significance |
CEE, conjugated equine estrogen; MHT, menopause hormone therapy; WHI, Women's Health Initiative.
Hormonal contraception and QT interval
In general, hormonal contraception, such as that used OC pills, use doses of estrogen and progesterone that are 5–10-fold higher than that of MHT. To date, four generations of OC by progestin type have been developed: first and second generation OCs have progestins that are androgenic with relatively high levels of estrogen. The third generation OCs have progestins that are less androgenic, and fourth generation OCs are nontestosterone derived and antiandrogenic. As estrogen lengthens the QT and testosterone and progesterone shorten ventricular repolarization, it can be hypothesized that the different generations of hormonal contraception might contribute to the QT interval according to Table 4.
Table 4.
Proposed Effects of Different Generations of Hormonal Contraceptives on QT Interval
| Estrogen effect on QT | Progestin effect on QT | Overall effect on QT | |
|---|---|---|---|
| First generation OC | ↑↑ | ↓↓↓ | ↓ |
| Second generation OC | ↑ | ↓ | ↔/↓ |
| Third generation OC | ↑ | ↓ | ↔ |
| Fourth generation OC | ↑ | ↑ | ↑↑ |
OC, oral contraception.
To date, no studies have been performed on OC and the QT interval, although research is currently underway. Specifically, the fourth generation OCs currently approved for noncontraceptive indications, such as acne and premenstrual dysmorphic disorder, are in widespread use and have a relatively unknown cardiovascular safety record. Because of the difference in progestin preparation, the fourth generation OCs might contribute to overall lengthening of the QT and associated cardiovascular risk.
A single study of a progestin-only subdermal implant device measured the QTc preinsertion and at 3, 6, and 12 months of use.25 They found that the QTc increased over the 1-year study period, but this did not reach statistical significance compared to baseline (p=0.48). Thus, further studies are needed to clarify the interaction between hormonal contraceptive therapy and QT.
Mechanisms and Pathophysiology of Sex Hormonal Effects on QT Interval
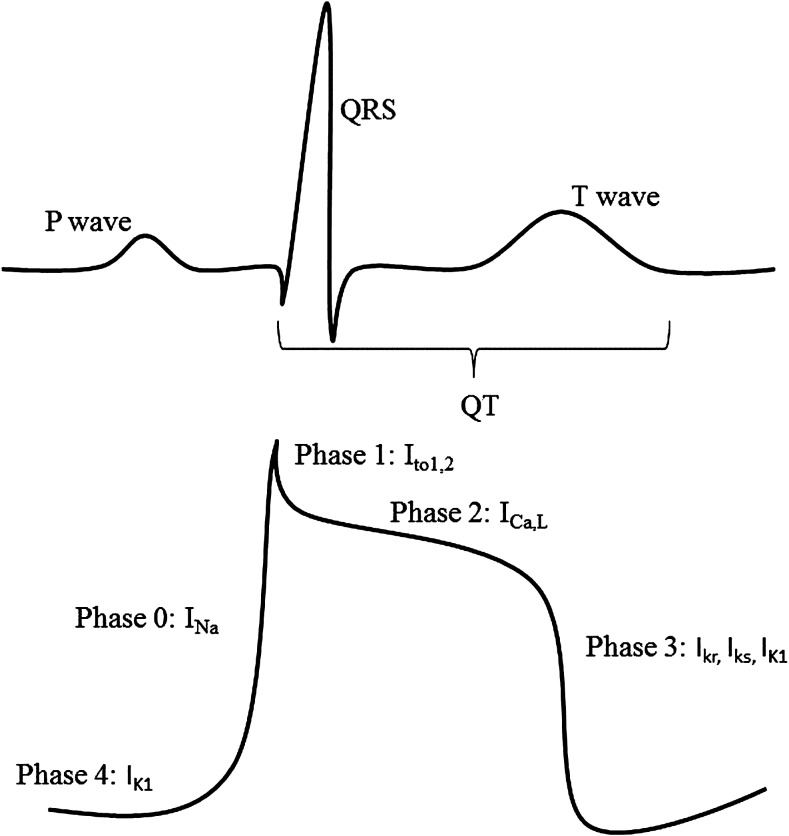
The electrical events underlying the QT interval correspond with the phases of action potential (AP) generation at the cellular level (Fig. 2). Specifically, the interval between the onset of the Q wave and the beginning of the S wave corresponds to the initial rapid upstroke of the AP (phase 0) and the early phase of repolarization (phase 1). The interval between the S wave and the peak of the T wave corresponds to the plateau phase of the AP (phase 2), and the peak of the T wave to the end of the T wave corresponds to the final repolarization phase (phase 3). The duration of the AP (and, therefore, the duration of QT) is most affected by alterations in phases 2 and 3.26 During phase 2, the L-type calcium channel current (ICa,L) plays a dominant role.27 Upregulation of ICa,L channel currents lengthens the QT, whereas downregulation shortens the QT. During phase 3, the delayed rectifier potassium channel currents (consisting of the rapidly activating (IKr) and slowly activating (IKs) channel types) and the inward rectifier current (IK1) play a dominant role.28 Upregulation of these channel currents shortens the QT, whereas downregulation lengthens the QT.
FIG. 2.
Correlation of the electrocardiogram (ECG) intervals with the phases of the ventricular muscle action potential and the ion channels responsible for each phase. Ica,L, L-type calcium channel; Itol,2, transient outward potassium current; Ikr, rapidly activating delayed rectifier potassium channel; Iks, slowly activating delayed rectifier calcium channel; Ik1, inward rectifier channel; Ina, sodium current.
Mechanistic studies suggest that testosterone, estrogen, and progesterone have varying effects on the ICa,L, IKr, IKs, and IK1 channel currents, providing a plausible mechanism for the QT alterations described in humans.29–34 Testosterone decreases calcium channel current and increases potassium channel current and may shorten the QT interval through these mechanisms. For example, in the guinea pig model, administration of 100 nM of testosterone caused dose-dependent shortening of the action potential duration through suppression of ICa,L channels and enhancement of IKs channels in ventricular myocytes. 29 Chronic testosterone treatment in male rabbits led to faster AP repolarization through upregulation of IKr and IK1 currents in the ventricle.32 Further, testosterone treatment suppressed ICa,L expression in human embryonic kidney 293 (HEK293) cell lines.35
Estrogen decreases potassium channel current and may lengthen the QT interval through this mechanism. For example, in the guinea pig model, Tanabe et al.34 reported lengthening of ventricular repolarization with 30 μM of 17ß-estradiol through suppression of IKr and IKs currents. In the rat model, 30 μM of 17ß-estradiol lengthened the AP through suppression of IK1 channels.30 Other estrogens, including estrone 3-sulfate, suppressed IKr channel expression in HEK293 cell lines in a dose-dependent manner.31
Finally, progesterone decreases calcium channel current and increases potassium channel current and may shorten the QT interval through these mechanisms. This is supported by the observation that in the guinea pig model, progesterone administration shortened the AP duration through upregulation of IKs currents and downregulation of ICa,L currents.33 A summary of the effects of estrogen, progesterone, and testosterone on ion channel expression in the myocyte is provided in Table 5.
Table 5.
Effects of Estrogen, Progesterone, and Testosterone on Ion Channel Expression in Myocytes and Their Overall Effect on QT Interval
| L-type calcium channel current (ICa,L) Phase 2 | Rapidly activating delayed rectifier current (IKr) Phase 3 | Slowly activating delayed rectifier current (IKs) Phase 3 | Inward rectifier current (IK1) Phase 3 | Overall Effect on QT interval | |
|---|---|---|---|---|---|
| Estrogen | ↓ | ↓ | ↓ | ↓ | ↑ |
| Progesterone | ↓ | N/A | ↑ | N/A | ↓ |
| Testosterone | ↓ | ↑ | ↑ | ↑ | ↓ |
NA, not applicable.
Conclusions
Both endogenous and exogenous preparations of sex hormones have been shown to affect the QTc interval. Endogenous testosterone and progesterone shorten the action potential,8,9,11 and estrogen lengthens the QTc interval in animals.10 These effects occur through alterations in myocardial ICa,L, IKr, IKs and IK1 channels, which control phases 2 and 3 of the cardiac action potential. Exogenous ET lengthens the QTc interval, and EPT has no effect.6,7 This supports a counterbalancing effect of estrogen and progesterone on action potential duration. To date, no studies have examined the effects of OC on the QTc. The newer fourth generation hormonal contraception preparations have progestins that are antiandrogenic and may adversely impact the QT interval by not counteracting the lengthening effects of estrogen. Future research needs to be directed toward answering this important research question.
Acknowledgments
This work was supported by contracts from the National Heart, Lung and Blood Institutes, Nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, and N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, T32HL69751, and 1R03AG032631 from the National Institute on Aging, GCRC grant MO1-RR00425 from the National Center for Research Resources, and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women's Guild of Cedars-Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, QMED, Inc., Laurence Harbor, NJ, the Edythe L. Broad Women's Heart Research Fellowship, Cedars-Sinai Medical Center, Los Angeles, CA, the Barbra Streisand Women's Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, CA, and The Society for Women's Health Research (SWHR), Washington, DC.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.Rautaharju PM. Surawicz B. Gettes LS, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: Part iv: The ST segment, T and U waves, and the QT interval: A scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: Endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119:e241–250. doi: 10.1161/CIRCULATIONAHA.108.191096. [DOI] [PubMed] [Google Scholar]
- 2.Algra A. Tijssen JG. Roelandt JR. Pool J. Lubsen J. QTc prolongation measured by standard 12-lead electrocardiography is an independent risk factor for sudden death due to cardiac arrest. Circulation. 1991;83:1888–1894. doi: 10.1161/01.cir.83.6.1888. [DOI] [PubMed] [Google Scholar]
- 3.Makkar RR. Fromm BS. Steinman RT. Meissner MD. Lehmann MH. Female gender as a risk factor for torsades de pointes associated with cardiovascular drugs. JAMA. 1993;270:2590–2597. doi: 10.1001/jama.270.21.2590. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB. Wilson PW. D'Agostino RB. Cobb J. Sudden coronary death in women. Am Heart J. 1998;136:205–212. doi: 10.1053/hj.1998.v136.90226. [DOI] [PubMed] [Google Scholar]
- 5.Albert CM. Chae CU. Grodstein F, et al. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107:2096–2101. doi: 10.1161/01.CIR.0000065223.21530.11. [DOI] [PubMed] [Google Scholar]
- 6.Kadish AH. Greenland P. Limacher MC. Frishman WH. Daugherty SA. Schwartz JB. Estrogen and progestin use and the QT interval in postmenopausal women. Ann Noninvas Electrocardiol. 2004;9:366–374. doi: 10.1111/j.1542-474X.2004.94580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carnethon MR. Anthony MS. Cascio WE, et al. A prospective evaluation of the risk of QT prolongation with hormone replacement therapy: The atherosclerosis risk in communities study. Ann Epidemiol. 2003;13:530–536. doi: 10.1016/s1047-2797(03)00050-4. [DOI] [PubMed] [Google Scholar]
- 8.Nakagawa M. Ooie T. Takahashi N, et al. Influence of menstrual cycle on QT interval dynamics. PACE. 2006;29:607–613. doi: 10.1111/j.1540-8159.2006.00407.x. [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez I. Kilborn MJ. Liu XK. Pezzullo JC. Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle. JAMA. 2001;285:1322–1326. doi: 10.1001/jama.285.10.1322. [DOI] [PubMed] [Google Scholar]
- 10.Saito T. Ciobotaru A. Bopassa JC. Toro L. Stefani E. Eghbali M. Estrogen contributes to gender differences in mouse ventricular repolarization. Circulation Res. 2009;105:343–352. doi: 10.1161/CIRCRESAHA.108.190041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y. Ouyang P. Post WS, et al. Sex-steroid hormones and electrocardiographic QT-interval duration: Findings from the Third National Health and Nutrition Examination Survey and the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2011;174:403–411. doi: 10.1093/aje/kwr172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakagawa M. Ooie T. Ou B, et al. Gender differences in autonomic modulation of ventricular repolarization in humans. J Cardiovasc Electrophysiol. 2005;16:278–284. doi: 10.1046/j.1540-8167.2005.40455.x. [DOI] [PubMed] [Google Scholar]
- 13.Stramba-Badiale M. Spagnolo D. Bosi G. Schwartz PJ. Are gender differences in QTc present at birth? MISNES investigators. Multicenter Italian Study on Neonatal Electrocardiography and Sudden Infant Death Syndrome. Am J Cardiol. 1995;75:1277–1278. [PubMed] [Google Scholar]
- 14.Rautaharju PM. Zhou SH. Wong S, et al. Sex differences in the evolution of the electrocardiographic QT interval with age. Can J Cardiol. 1992;8:690–695. [PubMed] [Google Scholar]
- 15.van Noord C. Dorr M. Sturkenboom MC, et al. The association of serum testosterone levels and ventricular repolarization. Eur J Epidemiol. 2010;25:21–28. doi: 10.1007/s10654-009-9406-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charbit B. Christin-Maitre S. Demolis JL. Soustre E. Young J. Funck-Brentano C. Effects of testosterone on ventricular repolarization in hypogonadic men. Am J Cardiol. 2009;103:887–890. doi: 10.1016/j.amjcard.2008.11.041. [DOI] [PubMed] [Google Scholar]
- 17.Pecori Giraldi F. Toja PM. Filippini B, et al. Increased prevalence of prolonged QT interval in males with primary or secondary hypogonadism: A pilot study. Int J Androl. 2010;33:e132–138. doi: 10.1111/j.1365-2605.2009.00985.x. [DOI] [PubMed] [Google Scholar]
- 18.Bidoggia H. Maciel JP. Capalozza N, et al. Sex differences on the electrocardiographic pattern of cardiac repolarization: Possible role of testosterone. Am Heart J. 2000;140:678–683. doi: 10.1067/mhj.2000.109918. [DOI] [PubMed] [Google Scholar]
- 19.De Leo V. la Marca A. Agricola E. Morgante G. Mondillo S. Setacci C. Resting ECG is modified after oophorectomy and regresses with estrogen replacement therapy in premenopausal women. Maturitas. 2000;36:43–47. doi: 10.1016/s0378-5122(00)00123-7. [DOI] [PubMed] [Google Scholar]
- 20.Saba S. Link MS. Homoud MK. Wang PJ. Estes NA., 3rd Effect of low estrogen states in healthy women on dispersion of ventricular repolarization. Am J Cardiol. 2001;87:354–356. doi: 10.1016/s0002-9149(00)01377-1. [DOI] [PubMed] [Google Scholar]
- 21.Hulot JS. Demolis JL. Riviere R. Strabach S. Christin-Maitre S. Funck-Brentano C. Influence of endogenous oestrogens on QT interval duration. Euro Heart J. 2003;24:1663–1667. doi: 10.1016/s0195-668x(03)00436-6. [DOI] [PubMed] [Google Scholar]
- 22.Burke JH. Ehlert FA. Kruse JT. Parker MA. Goldberger JJ. Kadish AH. Gender-specific differences in the QT interval and the effect of autonomic tone and menstrual cycle in healthy adults. Am J Cardiol. 1997;79:178–181. doi: 10.1016/s0002-9149(96)00707-2. [DOI] [PubMed] [Google Scholar]
- 23.Larsen JA. Tung RH. Sadananda R, et al. Effects of hormone replacement therapy on QT interval. Am J Cardiol. 1998;82:993–995. doi: 10.1016/s0002-9149(98)00523-2. [DOI] [PubMed] [Google Scholar]
- 24.Nowinski K. Pripp U. Carlstrom K. Landgren BM. Schenck-Gustafsson K. Bergfeldt L. Repolarization measures and their relation to sex hormones in postmenopausal women with cardiovascular disease receiving hormone replacement therapy. Am J Cardiol. 2002;90:1050–1055. doi: 10.1016/s0002-9149(02)02768-6. [DOI] [PubMed] [Google Scholar]
- 25.Okeahialam BN. Sagay AS. Imade GE. Prolongation of electrocardiographic intervals in women on norplant contraceptive: What dangers? Afr J Med Med Sci. 2004;33:11–13. [PubMed] [Google Scholar]
- 26.James AF. Choisy SC. Hancox JC. Recent advances in understanding sex differences in cardiac repolarization. Prog Biophys Mol Biol. 2007;94:265–319. doi: 10.1016/j.pbiomolbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Linz KW. Meyer R. Control of L-type calcium current during the action potential of guinea-pig ventricular myocytes. Physiol. 1998;513:425–442. doi: 10.1111/j.1469-7793.1998.425bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snyders DJ. Structure and function of cardiac potassium channels. Cardiovasc Res. 1999;42:377–390. doi: 10.1016/s0008-6363(99)00071-1. [DOI] [PubMed] [Google Scholar]
- 29.Bai CX. Kurokawa J. Tamagawa M. Nakaya H. Furukawa T. Nontranscriptional regulation of cardiac repolarization currents by testosterone. Circulation. 2005;112:1701–1710. doi: 10.1161/CIRCULATIONAHA.104.523217. [DOI] [PubMed] [Google Scholar]
- 30.Berger F. Borchard U. Hafner D. Putz I. Weis TM. Effects of 17beta-estradiol on action potentials and ionic currents in male rat ventricular myocytes. Naunyn-Schmiedeberg Arch Pharmacol. 1997;356:788–796. doi: 10.1007/pl00005119. [DOI] [PubMed] [Google Scholar]
- 31.Kakusaka S. Asayama M. Kaihara A, et al. A receptor-independent effect of estrone sulfate on the herg channel. J Pharmacol Sci. 2009;109:152–156. doi: 10.1254/jphs.08257sc. [DOI] [PubMed] [Google Scholar]
- 32.Liu XK. Katchman A. Whitfield BH, et al. In vivo androgen treatment shortens the QT interval and increases the densities of inward and delayed rectifier potassium currents in orchiectomized male rabbits. Cardiovasc Res. 2003;57:28–36. doi: 10.1016/s0008-6363(02)00673-9. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura H. Kurokawa J. Bai CX, et al. Progesterone regulates cardiac repolarization through a nongenomic pathway: An in vitro patch-clamp and computational modeling study. Circulation. 2007;116:2913–2922. doi: 10.1161/CIRCULATIONAHA.107.702407. [DOI] [PubMed] [Google Scholar]
- 34.Tanabe S. Hata T. Hiraoka M. Effects of estrogen on action potential and membrane currents in guinea pig ventricular myocytes. Am J Physiol. 1999;277:H826–833. doi: 10.1152/ajpheart.1999.277.2.H826. [DOI] [PubMed] [Google Scholar]
- 35.Scragg JL. Jones RD. Channer KS. Jones TH. Peers C. Testosterone is a potent inhibitor of l-type ca(2+) channels. Biochem Biophys Res Commun. 2004;318:503–506. doi: 10.1016/j.bbrc.2004.04.054. [DOI] [PubMed] [Google Scholar]