Abstract
Advanced neuroimaging techniques have shown promise in highlighting the subtle changes and nuances in mild traumatic brain injury (MTBI) even though clinical assessment has shown a return to pre-injury levels. Here we use 1H-magnetic resonance spectroscopy (1H-MRS) to evaluate the brain metabolites N-acetyl aspartate (NAA), choline (Cho), and creatine (Cr) in the corpus callosum in MTBI. Specifically, we looked at the NAA/Cho, NAA/Cr, and Cho/Cr ratios in the genu and splenium. We recruited 20 normal volunteers (NV) and 28 student athletes recovering from the subacute phase of MTBI. The MTBI group was categorized based upon the number of MTBIs and time from injury to 1H-MRS evaluation. Significant reductions in NAA/Cho and NAA/Cr ratios were seen in the genu of the corpus callosum, but not in the splenium, for MTBI subjects, regardless of the number of MTBIs. MTBI subjects recovering from their first MTBI showed the greatest alteration in NAA/Cho and NAA/Cr ratios. Time since injury to 1H-MRS acquisition was based upon symptom resolution and did not turn out to be a significant factor. We observed that as the number of MTBIs increased, so did the length of time for symptom resolution. Unexpected findings from this study are that MTBI subjects showed a trend of increasing NAA/Cho and NAA/Cr ratios that coincided with increasing number of MTBIs.
Key words: concussion, 1H-MRS, MTBI, multiple MTBI
Introduction
Research into concussion has revealed the complexities of a commonly misdiagnosed condition. A single episode of mild traumatic brain injury (MTBI) sets off a complex chain of neurochemical and neurometabolic reactions caused by mechanical trauma produced by acceleration and deceleration forces on the brain (Barkhoudarian et al., 2011). Rotational forces from MTBI can also lead to unwanted axonal strain and stress causing diffuse axonal injury (DAI) (Maruta et al., 2010). After MTBI, the destructive biochemical sequelae include activation of inflammatory response, imbalances of ion concentrations, increase in the presence of excitatory amino acids, dysregulation of neurotransmitter synthesis and release, and production of free radicals (Wheaton et al., 2011). As a result of the complex response to MTBI, individuals present with many clinical symptoms including: headache, nausea, visual disturbances, light sensitivity, dizziness, fatigue, and irritability (Bryant and Harvey, 1999). Even more disturbing is the time frame for MTBI symptom resolution, with 15% of cases in which individuals report physical, cognitive, and emotional symptoms that persist for more than 1 year post-injury (Kiraly and Kiraly, 2007; Witt et al., 2010; Sedney et al., 2011).These persistent symptoms from MTBI can lead to post-concussive syndrome (PCS) and long-term disabilities (Hughes et al., 2004).
Despite this cascade of destructive pathophysiological events that occur after MTBI, conventional neuroimaging techniques and neuropsychological tests fail to be sensitive enough to detect these differences in the subacute phase of injury (Mayer et al., 2011), and lack specificity in being able to distinguish individuals who have had previous MTBIs (Iverson et al., 2006). This lack of sensitivity and specificity of current clinical measures for assessing MTBI is a major concern, as research is mounting that demonstrates the damaging effects of cumulative concussions (Echemendia and Cantu, 2003; Guskiewicz et al., 2003; De Beaumont et al., 2007). Premature return-to-play after MTBI may be a critical component in why there is a higher risk of recurrent concussions (Guskiewicz et al., 2003), in addition to neurological and cognitive deficiencies seen in chronic PCS (Cantu, 2006).
The use of advanced neuroimaging techniques has demonstrated cognitive, structural, and metabolic alterations after a single concussion (McAllister et al., 2001; Ptito et al., 2007; Lipton et al., 2008; Gasparovic et al., 2009; Zhang et al., 2010; Johnson et al., 2011; Slobounov et al., 2011). These imaging techniques have highlighted subtle changes and nuances in brain morphology, physiology, and function caused by MTBI (Gasparovic et al., 2009), even though clinical assessment has shown a return to pre-injury levels. Based upon the damaging biochemical sequelae that occur following MTBI, in vivo proton 1H-magnetic resonance spectroscopy (1H-MRS) studies used to evaluate brain metabolites (Gasparovic et al., 2009) offer promise in examining the metabolic vulnerability after MTBI (Hovda et al., 1995). MTBI studies utilizing 1H-MRS, commonly report on a various number of metabolites that include: N-acetyl aspartate (NAA), choline (Cho), and creatine-phosphocreatine (Cr) (Cecil et al., 1998; Belanger et al., 2007; Govind et al., 2010). However, there is still no consensus on whether or not metabolites are better reported as ratios or absolute concentrations, but several MTBI studies have indicated that Cr may not be as stable as once thought (Gasparovic et al., 2009, Yeo et al., 2011). However, disruptions in brain metabolite ratios after MTBI have been reported within all lobes of the brain as well as in both white and gray matter (Belanger et al., 2007; Govind et al., 2010; Henry et al., 2011). NAA is found in the brain in high concentrations, and it is synthesized in the neuronal mitochondria and cytoplasm (Patel and Clark, 1979, Arun et al., 2009); however, little is known about its function (Miller, 1991). Decreased cellular levels of NAA are associated with neuronal loss and metabolic dysfunction (Gasparovic et al., 2009). Reductions in NAA following head trauma are the most common and persistent 1H-MRS findings (Ross et al., 1998) and indicative of DAI (Babikian et al., 2006). Cho is a marker for membrane damage and/or repair (Gasparovic et al., 2009), and elevated levels are the second most common 1H-MRS finding associated with brain injury (Ross et al., 1998). The Cr peak is an accepted indicator of cell energy metabolism (Signoretti et al., 2009), and low levels are associated with anoxia, a major factor in TBI (Ross et al., 1998).
The aim of this study was to use 1H-MRS to evaluate the metabolite ratios NAA/Cho, NAA/Cr, and Cho/Cr in clinically “asymptomatic” athletes recovering from single and multiple MTBIs. We focused on the corpus callosum because it is a major predilection site in TBI (Smits et al., 2010, Sponheim et al., 2011), and is highly susceptible to the rotational acceleration and decelerations forces inducing MTBI (Rutgers et al., 2008, Smits et al., 2010, Sponheim et al., 2011). Specifically, we looked at two regions of interest (ROI) within the corpus callosum, the genu, and splenium. We hypothesized that following a single episode of MTBI, there would be a reduction in both the NAA/Cho and NAA/Cr ratios, whereas the Cho/Cr ratio would remain stable. Furthermore, we predicted that these decreases in NAA/Cho and NAA/Cr ratios would be further exacerbated as the number of MTBIs increased.
Methods
For this study, 28 student-athletes (13 male, 15 female) who had recently had a sports-related grade (Cantu Data Driven Revised Concussion Grading Guideline, 2006) and 20 neurologically normal volunteer (NV) student-athletes with no history of MTBI (10 male, 10 female) were recruited. The initial diagnosis of MTBI was made on the field by certified athletic trainers as a part of the routine protocol of the Sport Concussion Program at the Pennsylvania State University. MTBI subjects were sorted into three categories (1st, 2nd, 3+) based on the history of the number of clinically diagnosed MTBIs that they reported (see Table 1 for a summary of demographics). Strict adherence to a symptoms-based testing schedule was followed, with all MTBI subjects being scanned within 24 h of meeting three criteria: 1) clinical self-reported symptom resolution, 2) return to baseline on cognitive and clinical balance testing (Sport Concussion Assessment Tool 2 [SCAT-2] and Balance Error Scoring System [BESS]), and 3) clearance from a medical professional for the first stage of aerobic activity. None of the subjects under study were taking nonsteroidal anti-inflammatory drugs for concomitant musculoskeletal injury, symptom management, or sleep disturbances. It should be noted that as the number of MTBIs accumulated so did the length of time from injury to scanning. Therefore, an additional categorization based on time from injury to 1H-MRS acquisition was performed to investigate the effect that time from injury to scanning might have on the restoration of brain metabolites. Again MTBI subjects were placed into one of three categories (1 week, 2 weeks, 3+ weeks) (Table 1). Nonetheless, all MTBI subjects were clinically “asymptomatic” and scanned within 30 days of injury. The Institutional Review Board of the Pennsylvania State University approved this protocol, and all subjects gave informed consent.
Table 1.
Demographic Information for Subjects
| NV | MTBI | 1st | 2nd | 3+ | 1 Week | 2 Weeks | 3+ Weeks | |
|---|---|---|---|---|---|---|---|---|
| Age | 20.2 (0.83) | 20.3 (1.53) | 20 (1.5) | 20.5 (1.5) | 20.3 (1.7) | 19.75 (1.4) | 20.5 (1.5) | 20.5 (1.9) |
| n | 20 | 28 | 9 | 10 | 9 | 8 | 14 | 6 |
| Male n | 10 | 13 | 2 | 5 | 6 | 4 | 5 | 4 |
| Fem. n | 10 | 15 | 7 | 5 | 3 | 4 | 9 | 2 |
| No. MTBI | — | 2 (0.82) | 1 (0.0) | 2 (0.0) | 3 (0.0) | 1.75 (0.9) | 1.8 (0.7) | 2.83 (0.4) |
| No. days | — | 11.4 (6.1) | 8 (1.6) | 9.7 (3.3) | 16.6 (8.0) | 6.4 (1.1) | 10.1 (1.9) | 21 (5.8) |
Age, the number of mild traumatic brain injuries (MTBIs) and the number of days from injury to scanning (no. days) are averages with standard deviations in parentheses. NV, normal volunteers; MTBI, all MTBI subjects collectively; 1st, subjects recovering from their first MTBI; 2nd, subjects recovering from their second MTBI; 3+, subjects recovering from three or more MTBIs; 1 week, MTBI subjects that were scanned within 7 days of injury; 2 weeks, MTBI subjects scanned 8–14 days after injury; and 3+ weeks, MTBI subjects scanned >14 days after injury.
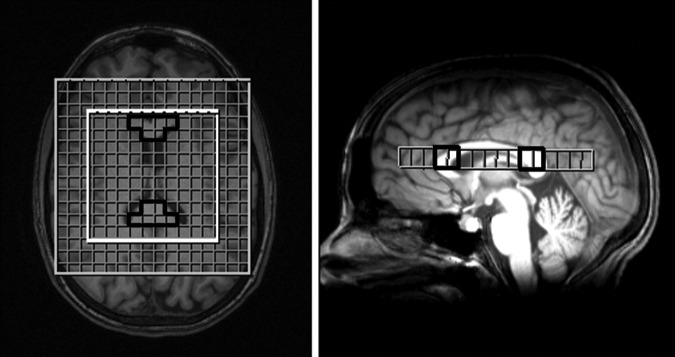
1H-MRS and anatomical images were acquired on a 3.0 Tesla Siemens Trio whole-body scanner (Siemens, Erlangen, Germany) using a 12 channel head coil. Three-dimensional isotropic T1-weighted magnetization prepared rapid gradient echo (MP-RAGE) anatomical images were acquired in the axial plane parallel with the anterior and posterior commissure axis covering the entire brain (0.9 mm×0.9 mm×0.9mm resolution, TE=3.46 ms, TR=2300 ms, TI=900 ms, flip angle=9 degrees, 160 slices, integrated parallel acquisition techniques iPAT=none, number of signal averages NSA=1). Three-dimensional multivoxel 1H-MRS chemical shift imaging (CSI) (120 mm×120mm×80 mm field of view, 10.0 mm×10.0mm×12.5 mm voxel size, TE=135 ms, TR=1510 ms, iPAT=none, NSA=1, acquisition time=7:56) was implemented to evaluate in vivo NAA, Cho, and Cr metabolites. Placement of the CSI volume of interest was centered anteriorly/posteriorly and inferiorly/superiorly over the corpus callosum, ensuring that both the genu and splenium were acquired within the same CSI slice (Fig. 1). Structural MRI scans did not reveal any radiological abnormalities.
FIG. 1.
Axial and sagittal views showing placement of chemical shift imaging (CSI) slice to acquire both the genu and splenium regions of interest (ROIs). Superimposed grid shows voxels acquired during CSI within the inner white box and genu and splenium ROIs outlined in black.
Data analysis
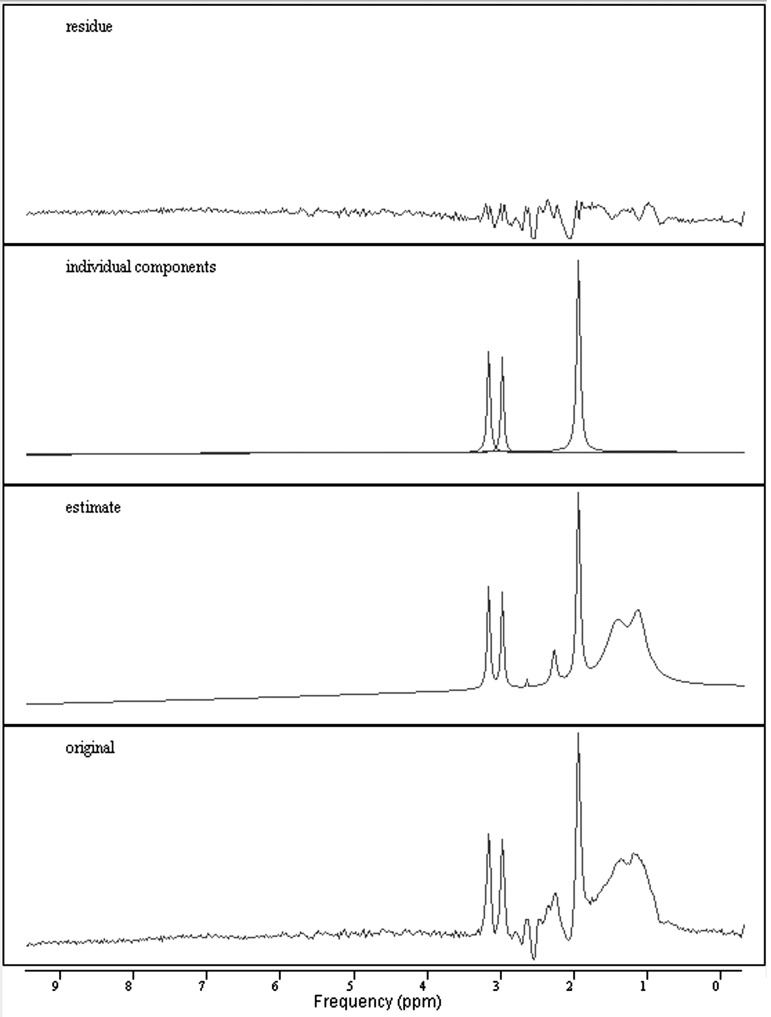
The 1H-MRS CSI spectra were processed offline in the time domain using the jMRUI v. 5.0 software (http://www.mrui.uab.es/mrui/) (Fig. 2) (Stefan et al., 2009). A Hankel Lanczos Singular Value Decomposition (HLSVD) filter was used to remove unwanted resonance frequencies and the residual water line from the free induction decay (FID) as described by Covaciu et al. (2010). Further analysis was performed on the FID using the advanced method for accurate, robust, and efficient spectral fitting (AMARES), a nonlinear least square fitting algorithm (Vanhamme et al., 1997) for the metabolite peaks of NAA (2 ppm), Cho (3.2 ppm), and Cr (3ppm). The two ROIs, genu and splenium of the corpus callosum, were made up of six voxels that were individually selected from the 1H-MRS acquisition grid superimposed on anatomical T1 images (see Fig. 1). Segmentation of the T1 anatomical images was done in order to compensate for differences in tissue compositions within the voxels. Similar to McLean et al. (2000), segmentation was performed using Statistical Parametric Mapping (SPM) version 8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) resulting in maps for gray matter, white matter, and cerebrospinal fluid (CSF). NAA/Cho, NAA/Cr, and Cho/Cr ratios of these selected voxels were then averaged across the ROI to come up with a mean value (Vagnozzi et al., 2010). Any voxel that was determined to be composed of <75% white matter based on segmentation was not included in the metabolite average for the ROI.
FIG. 2.
Example of 1H-magnetic resonance spectroscopy (1H-MRS) spectra processed with jMRUI showing original, estimate, individual components, and residue spectra. Individual components visualized are for N-acetyl aspartate (NAA) (2.0 ppm), creatine (Cr) (3.0 ppm), and choline (Cho) (3.2 ppm).
Minitab 16 Statistical Software (Minitab, Inc., State College, PA www.minitab.com) was used to perform statistical analysis. In order to test the multiple dependent variables and potential correlations, factorial multivariate analysis of variance (MANOVA) was performed on NAA/Cho, NAA/Cr, and Cho/Cr ratios between the NV and MTBI groups. Values were considered significant if p<0.05 (Wilks', Lawley-Hotelling's and Pillai's methods tested). The number of MTBIs and the time between injury and scan were imported as covariates into the MANOVA analysis. Further post-hoc univariate F-tests were done comparing the NV group to the MTBI group with the number of MTBIs and time from injury to scanning used as confounding effects. Once again, p<0.05 was used to determine significance.
Results
In the genu of the corpus callosum MANOVA revealed that there was a significant main effect of group (NV vs. MTBI) (F=10.276, p=0.000). Specifically the NAA/Cho (F=20.085, p=0.000) and NAA/Cr (F=23.173, p=0.000) ratios were reduced in the MTBI group compared with the NVs, although there was no significant alteration to the Cho/Cr ratio. Post-hoc analysis based on the number of previous MTBIs revealed that these reductions of NAA/Cho and NAA/Cr in the genu were seen in all three categories (1st, 2nd, 3+). The subjects (1st) recovering from their first MTBI showed the largest decrease in NAA/Cho (F=33.40, p=0.000) and NAA/Cr (F=56.12, p=0.000) ratios. Contrary to our initial hypothesis, we observed an unexpected result in the subjects (2nd and 3+) with a history of multiple MTBIs. Where we had predicted that as the number of MTBIs increased we would see a further reduction in NAA/Cho and NAA/Cr ratios, we saw the opposite response. As the number of MTBIs increased, we still saw a reduction in NAA/Cho and NAA/Cr ratios compared with NVs, but this depression was not exacerbated by accumulation of subsequent MTBIs (Fig. 3). For subjects (2nd) recovering from their second MTBI, reductions in NAA/Cho (F=22.56, p=0.000) and NAA/Cr (F=27.74, p=0.000) ratios were significant, as well as for NAA/Cho (F=11.72, p=0.002) and NAA/Cr (F=27.03, p=0.000) ratios in subjects (3+) with three or more MTBIs.
FIG. 3.
Bar graphs with average brain metabolite ratios for the genu and splenium of the corpus callosum. NV, normal volunteers; 1st, subjects recovering from their first mild traumatic brain injury (MTBI); 2nd, subjects recovering from their second MTBI; 3+, subjects recovering from three or more MTBIs. *Denotes a significant (p<0.05) difference between NVs and MTBI groups, and error bars indicate standard deviations.
In contrast to the genu, there was no significant (p>0.05) main effect of group (NV vs. MTBI) or alterations of brain metabolite ratios observed in the splenium of the corpus callosum based upon the number of MTBIs. However, it should be noted that the splenium displayed the same trend as the genu, as the number of MTBIs increased there was not a further reduction in NAA/Cho and NAA/Cr ratios, but a surprising elevation compared with subjects who had only received a single MTBI (Fig. 3). Similar to the splenium, post-hoc analysis performed as a function of time from injury to scanning revealed no significant (p>0.05) changes in NAA/Cho, NAA/Cr, and Cho/Cr in either the genu or the splenium of the corpus callosum.
Discussion
In this study, we used 1H-MRS to investigate the metabolic integrity of the genu and splenium of the corpus callosum in the subacute phase of MTBI. Additionally we examined the effects of multiple concussions and time since injury to scanning in 1H-MRS evaluation of MTBI. The major finding in this study was that a single concussive episode, whether it was an initial or subsequent injury, produced a significant decrease of NAA/Cho and NAA/Cr ratios in the genu of the corpus callosum. Furthermore, it appeared that there was no progressive reduction of NAA/Cho and NAA/Cr ratios in the genu or the splenium as a result of an increase in the number of MTBIs.
Our findings of decreased NAA/Cho and NAA/Cr ratios are consistent with previous 1H-MRS research indicating that concussion opens a window of brain metabolite imbalance that is not restored to premorbid levels in subjects recovering from concussion, even though they are clinically “asymptomatic” (Vagnozzi et al., 2008; Henry et al., 2011). Govindaraju et al. (2004) showed that there are widespread metabolic disruptions throughout the brain in the subacute phase of MTBI. Specifically, NAA/Cho and NAA/Cr ratios were reduced globally, consistent with decreases in NAA and increases in Cho. Similarly, Cohen et al. (2007), showed that whole brain NAA levels were significantly reduced in MTBI as well as in both white and gray matter (Belanger et al., 2007; Govind et al., 2010; Henry et al., 2011). Other studies assessing focal brain regions with 1H-MRS have found complimentary results (Vagnozzi et al., 2010). Although not all brain regions and tissue types are uniformly altered following MTBI, as there have been differences reported in the degree of metabolic alterations, tissue type, lobes, and hemispheres (Govind et al., 2010).
It is important to note that we only observed reductions in NAA/Cho and NAA/Cr ratios in the genu and not in the splenium of the corpus callosum. One reason for this may be credited to anatomical differences between these two regions of the corpus callosum. There are local histological differences that include: a higher density of thin fibers in the genu and an increase in larger diameter fibers in the posterior pole (Aboitiz et al., 1992). The genu and splenium also differ in their connectivity, with the genu interconnecting the prefrontal cortex, whereas fibers from the parietal, occipital, and temporal lobes pass through the splenium (Park et al., 2008). There have also been regional differences in brain metabolite levels seen by 1H-MRS between the genu and splenium within healthy NVs and MTBI cohorts. Most notably, the genu showed higher levels of Cho than did the splenium, and the splenium had increased NAA compared with the genu (Cecil et al., 1998; Degaonkar et al., 2005; Babikian et al., 2006).This would presumably have an effect on metabolite ratios that are similar to the ones we have reported here. Another reason the genu showed metabolic alterations may be the nature of injury in MTBI. Finite element models of MTBI have predicted that the brainstem and genu of the corpus callosum experience the highest shear stress during frontal and lateral impacts (Zhang et al., 2001), as well as the frontal lobe being the most common site of injury following a moving head impact (Cantu, 1997). Also, degradation of the genu of the corpus callosum as a result of frontal lobe injury has been indicated in other neuroimaging studies on TBI (Kraus et al., 2007; Kinnunen et al., 2011).
Our novel findings from this study are not as straightforward as was initially hypothesized, most likely because of the complex nature of MTBI (Cantu, 2006). Contradictory to our initial hypothesis that the history of previous successive concussions will be further compounded in the form of lower brain metabolite ratios, we observed a paradoxical increase in both the genu and splenium. The most simplistic explanation for the increases in the metabolic ratios may be solely attributable to the time since injury. As we reported, earlier subjects recovering from multiple MTBIs on average took longer to become “asymptomatic,” and as dictated by our research protocol, all scanning was dependent upon clinical symptom resolution. In a pilot study by Vagnozzi et al. (2008), they looked at three athletes who were recovering from MTBI who had received a second subsequent MTBI within 15 days of the initial injury. They saw that the slowest rate of recovery was within the first 15 days post-impact followed by a five times higher accelerated rate in days 15–30. In this study, the doubly concussed group's 30 day scan showed significant decreases in NAA/Cho compared with the singly concussed group's, but at 45 days (30 days following the second MTBI) there were no significant differences. To date, there is still sporadic research looking at multiple MTBIs with 1H-MRS. In their animal model, Vagnozzi et al. (2005) showed that concussions that were not within the “vulnerability window” acted as two independent events, and that NAA levels after the second concussion were not decreased further. It is also important to note that the repeated TBIs designed and implemented in this experiment were the same, which is hardly the case in human MTBI, and that these subsequent impacts happened while recovery from the initial MTBI was still in progress. This suggests that time since injury is a major factor that needs to be taken into consideration in MTBI research/recovery and when comparing one versus multiple concussions.
The exact role of NAA is not known, and there are several hypotheses as to why NAA decreases following MTBI, including; the mitochondrial permeability of NAA causes an increased neuronal efflux of NAA leading to reduced synthesis, and an increase in degradation by oligodendrocytes (Vagnozzi et al., 2007). It is also known that that the initial depression of NAA following MTBI is a reversible phenomenon (Brooks et al., 2000, Vagnozzi et al., 2005). Whether or not elevated NAA levels following MTBI are a recovery mechanism or an indication of a pathological process is yet to be determined. However, an explanation for the improvement in NAA is more likely associated with metabolic recovery than axonal death (Brooks et al., 2000), and may be related to physiological adaptation to the primary insult. NAA is produced within the mitochondria of the neuron for use in neuronal regulatory functions such as cell membrane myelination, regulation of neuronal osmolarity, and energy production (Baslow, 2003). Each of these processes has been previously documented as a physiologic consequence of MTBI (Giza and Hovda, 2001). We suggest that one reason we observed higher NAA/Cho and NAA/Cr ratios in the subjects recovering from multiple concussions than in those recovering from a single concussive episode may be the result of an adaptive processes in the neuron caused by previous injury or oxidative stress. It has been reported that mitochondrial biosynthesis can occur as a result of hypoxia in controlled stroke animal models (Gutsaeva et al., 2008, Yin et al., 2008). This subsequent increase in neuronal mitochondrial density could account for the increased concentrations of NAA seen in athletes with a history of previous concussions, indicating a physiological adaptation to mechanical injury in the brain.
Limitations
There are a few limitations of this study. First, in our analysis, we based the results on metabolite ratios not absolute measurements, which may weaken the magnitude of measured changes if both metabolites are similarly affected (Govindaraju et al., 2004). Moreover, recent 1H-MRS studies have drawn into question the reliability of Cr as a divisor in metabolite ratios, as its concentrations may vary and may contribute to alterations in metabolite ratios (Munoz Maniega et al., 2008; Gasparovic et al., 2009; Yeo et al., 2011). As is the nature of MTBI, all injuries are unique, and many areas of the brain can be affected to varying degrees. There are differences in the ROIs under study, inclusion criteria, and time since injury throughout the MTBI literature that may lead to discrepancies among studies. Second, 1H-MRS metabolic information is obtained from a small area of the brain, although voxel sizes are relatively large and the corpus callosum is well known to be a target area for TBI (Marino et al., 2010). All data collected were from the most recent episode of concussion and there was no 1H-MRS information relating to previous concussions available. Even though the subjects in this study were clinically “asymptomatic” and scanned within 24 h of being cleared by a medical professional, this cohort of subjects was restricted to collegiate athletes.
Conclusion
In conclusion, MTBI proves to be a complex pathology, and more intricate research strategies need to be implemented to gain even subtle insights. Our major findings partly support our initial hypothesis that in the subacute phase of MTBI, there will be alterations in brain metabolites; however, the effect of multiple MTBIs on brain metabolites requires further exploration. Future studies investigating the effects of multiple concussions are needed in order to shed light on the differences that subsequent concussions may have on the brain morphology, physiology, and function.
Acknowledgments
This work was supported by National Institutes of Health Grant RO1 NS056227-01A2 “Identification of Athletes at Risk for Traumatic Brain Injury.”
Author Disclosure Statement
No competing financial interests exist.
References
- Aboitiz F. Scheibel A.B. Fisher R.S. Zaidel E. Fiber composition of the human corpus-callosum. Brain Res. 1992;598:143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- Arun P. Moffett J.R. Namboodiri A.M.A. Evidence for mitochondrial and cytoplasmic N-acetylaspartate synthesis in SH-SY5Y neuroblastoma cells. Neurochem. Int. 2009;55:219–225. doi: 10.1016/j.neuint.2009.03.003. [DOI] [PubMed] [Google Scholar]
- Babikian T. Freier M.C. Ashwal S. Riggs M.L. Burley T. Holshouser B.A. MR spectroscopy: predicting long-term neuropsychological outcome following pediatric TBI. J. Magn. Reson. Imaging. 2006;24:801–811. doi: 10.1002/jmri.20696. [DOI] [PubMed] [Google Scholar]
- Barkhoudarian G. Hovda D.A. Giza C.C. The molecular pathophysiology of concussive brain injury. Clin. Sports Med. 2011;30:33–48. doi: 10.1016/j.csm.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Baslow M.H. N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem. Res. 2003;28:941–953. doi: 10.1023/a:1023250721185. [DOI] [PubMed] [Google Scholar]
- Belanger H.G. Vanderploeg R.D. Curtiss G. Warden D.L. Recent neuroimaging techniques in mild traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2007;19:5–20. doi: 10.1176/jnp.2007.19.1.5. [DOI] [PubMed] [Google Scholar]
- Brooks W.M. Stidley C.A. Petropoulos H. Jung R.E. Weers D.C. Friedman S.D. Barlow M.A. Sibbitt W.L. Yeo R.A. Metabolic and cognitive response to human traumatic brain injury: a quantitative proton magnetic resonance study. J. Neurotrauma. 2000;17:629–640. doi: 10.1089/089771500415382. [DOI] [PubMed] [Google Scholar]
- Bryant R.A. Harvey A.G. Postconcussive symptoms and posttraumatic stress disorder after mild traumatic brain injury. J. Nerv. Ment. Dis. 1999;187:302–305. doi: 10.1097/00005053-199905000-00006. [DOI] [PubMed] [Google Scholar]
- Cantu R. Concussion classification: ongoing controversy, in: Foundations of Sport-Related Brain Injuries. In: Slobounov S., editor; Sebastianelli W., editor. New York: Springer; 2006. pp. 87–110. [Google Scholar]
- Cantu R.C. Athletic head injuries. Clin. Sports Med. 1997;16:531. doi: 10.1016/s0278-5919(05)70038-7. [DOI] [PubMed] [Google Scholar]
- Cecil K.M. Hills E.C. Sandel E. Smith D.H. McIntosh T.K. Mannon L.J. Sinson G.P. Bagley L.J. Grossman R.I. Lenkinski R.E. Proton magnetic resonance spectroscopy for detection of axonal injury in the splenium of the corpus callosum of brain-injured patients. J. Neurosurgery. 1998;88:795–801. doi: 10.3171/jns.1998.88.5.0795. [DOI] [PubMed] [Google Scholar]
- Cohen B.A. Inglese M. Rusinek H. Babb J.S. Grossman R.I. Gonen O. Proton MR spectroscopy and MRI-volumetry in mild traumatic brain injury. Am. J. Neuroradiol. 2007;28:907–913. [PMC free article] [PubMed] [Google Scholar]
- Covaciu L. Rubertsson S. Ortiz–Nieto F. Ahlstrom H. Weis J. Human brain MR spectroscopy thermometry using metabolite aqueous-solution calibrations. J. Magn. Reson. Imaging. 2010;31:807–814. doi: 10.1002/jmri.22107. [DOI] [PubMed] [Google Scholar]
- De Beaumont L. Brisson B. Lassonde M. Jolicoeur P. Long-term electrophysiological changes in athletes with a history of multiple concussions. Brain Inj. 2007;21:631–644. doi: 10.1080/02699050701426931. [DOI] [PubMed] [Google Scholar]
- Degaonkar M.N. Pomper M.G. Barker P.B. Quantitative proton magnetic resonance spectroscopic imaging: regional variations in the corpus callosum and cortical gray matter. J. Magn. Reson. Imaging. 2005;22:175–179. doi: 10.1002/jmri.20353. [DOI] [PubMed] [Google Scholar]
- Echemendia R.J. Cantu R.C. Return to play following sports-related mild traumatic brain injury: the role for neuropsychology. Appl. Neuropsychol. 2003;10:48–55. doi: 10.1207/S15324826AN1001_7. [DOI] [PubMed] [Google Scholar]
- Gasparovic C. Yeo R. Mannell M. Ling J. Elgie R. Phillips J. Doezema D. Mayer A.R. Neurometabolite concentrations in gray and white matter in mild traumatic brain injury: An H-1-magnetic resonance spectroscopy study. J. Neurotrauma. 2009;26:1635–1643. doi: 10.1089/neu.2009.0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giza C.C. Hovda D.A. The neurometabolic cascade of concussion. J. Athl. Train. 2001;36:228–235. [PMC free article] [PubMed] [Google Scholar]
- Govind V. Gold S. Kaliannan K. Saigal G. Falcone S. Arheart K.L. Harris L. Jagid J. Maudsley A.A. Whole-brain proton MR spectroscopic imaging of mild-to-moderate traumatic brain injury and correlation with neuropsychological deficits. J. Neurotrauma. 2010;27:483–496. doi: 10.1089/neu.2009.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindaraju V. Gauger G.E. Manley G.T. Ebel A. Meeker M. Maudsley A.A. Volumetric proton spectroscopic imaging of mild traumatic brain injury. Am. J. Neuroradiol. 2004;25:730–737. [PMC free article] [PubMed] [Google Scholar]
- Guskiewicz K.M. McCrea M. Marshall S.W. Cantu R.C. Randolph C. Barr W. Onate J.A. Kelly J.P. Cumulative effects associated with recurrent concussion in collegiate football players – The NCAA Concussion Study. J.A.M.A. 2003;290:2549–2555. doi: 10.1001/jama.290.19.2549. [DOI] [PubMed] [Google Scholar]
- Gutsaeva D.R. Carraway M.S. Suliman H.B. Demchenko I.T. Shitara H. Yonekawa H. Piantadosi C.A. Transient hypoxia stimulates mitochondrial biogenesis in brain subcortex by a neuronal nitric oxide synthase dependent mechanism. J. Neurosci. 2008;28:2015–2024. doi: 10.1523/JNEUROSCI.5654-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry L.C. Tremblay J. Tremblay S. Lee A. Brun C. Lepore N. Theoret H. Ellemberg D. Lassonde M. Acute and chronic changes in diffusivity measures after sports concussion. J. Neurotrauma. 2011;28:2049–2059. doi: 10.1089/neu.2011.1836. [DOI] [PubMed] [Google Scholar]
- Hovda D.A. Lee S.M. Smith M.L. Vonstuck S. Bergsneider M. Kelly D. Shalmon E. Martin N. Caron M. Mazziotta J. Phelps M. Becker D.P. The neurochemical and metabolic cascade following brain injury – moving from animal-models to man. J. Neurotrauma. 1995;12:903–906. doi: 10.1089/neu.1995.12.903. [DOI] [PubMed] [Google Scholar]
- Hughes D. Jackson A. Mason D. Berry E. Hollis S. Yates D. Abnormalities on magnetic resonance imaging seen acutely following mild traumatic brain injury: correlation with neuropsychological tests and delayed recovery. Neuroradiology. 2004;46:550–558. doi: 10.1007/s00234-004-1227-x. [DOI] [PubMed] [Google Scholar]
- Iverson G.L. Brooks B.L. Lovell M.R. Collins M.W. No cumulative effects for one or two previous concussions. Br. J. Sports Med. 2006;40:72–75. doi: 10.1136/bjsm.2005.020651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson B. Zhang K. Gay M. Horovitz S. Hallett M. Sebastianelli W. Slobounov S. Alteration of brain default network in subacute phase of injury in concussed individuals: resting-state fMRI study. NeuroImage. 2012;59:511–518. doi: 10.1016/j.neuroimage.2011.07.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen K.M. Greenwood R. Powell J.H. Leech R. Hawkins P.C. Bonnelle V. Patel M.C. Counsell S.J. Sharp D.J. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134:449–463. doi: 10.1093/brain/awq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiraly M.A. Kiraly S.J. Traumatic brain injury and delayed sequelae: a review – traumatic brain injury and mild traumatic brain injury (concussion) are precursors to later-onset brain disorders, including early-onset dementia. Scientific World Journal. 2007;7:1768–1776. doi: 10.1100/tsw.2007.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus M.F. Susmaras T. Caughlin B.P. Walker C.J. Sweeney J.A. Little D.M. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130:2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- Lipton M.L. Gellella E. Lo C. Gold T. Ardekani B.A. Shifteh K. Bello J.A. Branch C.A. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: a voxel-wise analysis of diffusion tensor imaging. J. Neurotrauma. 2008;25:1335–1342. doi: 10.1089/neu.2008.0547. [DOI] [PubMed] [Google Scholar]
- Marino S. Ciurleo R. Bramanti P. Federico A. Stefano N. 1H-MR spectroscopy in traumatic brain injury. Neurocrit. Care. 2010;14:127–133. doi: 10.1007/s12028-010-9406-6. [DOI] [PubMed] [Google Scholar]
- Maruta J. Suh M. Niogi S.N. Mukherjee P. Ghajar J. Visual tracking synchronization as a metric for concussion screening. J. Head Trauma Rehabil. 2010;25:293–305. doi: 10.1097/HTR.0b013e3181e67936. [DOI] [PubMed] [Google Scholar]
- Mayer A.R. Mannell M.V. Ling J. Gasparovic C. Yeo R.A. Functional connectivity in mild traumatic brain injury. Hum. Brain Mapp. 2011;32:1825–1835. doi: 10.1002/hbm.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister T.W. Sparling M.B. Flashman L.A. Guerin S.J. Mamourian A.C. Saykin A.J. Differential working memory load effects after mild traumatic brain injury. NeuroImage. 2001;14:1004–1012. doi: 10.1006/nimg.2001.0899. [DOI] [PubMed] [Google Scholar]
- McLean M.A. Woermann F.G. Barker G.J. Duncan J.S. Quantitative analysis of short echo time H-1-MRSI of cerebral gray and white matter. Magn. Reson. Med. 2000;44:401–411. doi: 10.1002/1522-2594(200009)44:3<401::aid-mrm10>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Miller B.L. A review of chemical issues in H-1-NMR spectroscopy –N-acetyl-L-aspartate, creatine and choline. NMR Biomed. 1991;4:47–52. doi: 10.1002/nbm.1940040203. [DOI] [PubMed] [Google Scholar]
- Munoz Maniega S. Cvoro V. Armitage P. Marshall I. Bastin M. Wardlaw J. Choline and creatine are not reliable denominators for calculating metabolite ratios in acute ischemic stroke. Stroke. 2008;39:2467–2469. doi: 10.1161/STROKEAHA.107.507020. [DOI] [PubMed] [Google Scholar]
- Park H.J. Kim J.J. Lee S.K. Seok J.H. Chun J. Kim D.I. Lee J.D. Corpus callosal connection mapping using cortical gray matter parcellation and DT-MRI. Hum. Brain Mapp. 2008;29:503–516. doi: 10.1002/hbm.20314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel T.B. Clark J.B. Synthesis of N-acetyl-L-aspartate by rat-brain mitochondria and its involvement in mitochondrial-cytosolic carbon transport. Biochem J. 1979;184:539–546. doi: 10.1042/bj1840539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptito A. Chen J.K. Johnston K.M. Contributions of functional magnetic resonance imaging (fMRI) to sport concussion evaluation. Neurorehabilitation. 2007;22:217–227. [PubMed] [Google Scholar]
- Ross B.D. Ernst T. Kreis R. Haseler L.J. Bayer S. Danielsen E. Bluml S. Shonk T. Mandigo J.C. Caton W. Clark C. Jensen S.W. Lehman N.L. Arcinue E. Pudenz R. Shelden C.H. H-1 MRS in acute traumatic brain injury. J. Magn. Reson. Imaging. 1998;8:829–840. doi: 10.1002/jmri.1880080412. [DOI] [PubMed] [Google Scholar]
- Rutgers D.R. Fillard P. Paradot G. Tadie M. Lasjaunias P. Ducreux D. Diffusion tensor imaging characteristics of the corpus callosum in mild, moderate, and severe traumatic brain injury. Am. J. Neuroradiol. 2008;29:1730–1735. doi: 10.3174/ajnr.A1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedney C.L. Orphanos J. Bailes J.E. When to consider retiring an athlete after sports-related concussion. Clin. Sports Med. 2011;30:189–200. doi: 10.1016/j.csm.2010.08.005. [DOI] [PubMed] [Google Scholar]
- Signoretti S. Pietro V. Vagnozzi R. Lazzarino G. Amorini A.M. Belli A. D'Urso S. Tavazzi B. Transient alterations of creatine, creatine phosphate, N-acetylaspartate and high-energy phosphates after mild traumatic brain injury in the rat. Mol. Cell. Biochem. 2009;333:269–277. doi: 10.1007/s11010-009-0228-9. [DOI] [PubMed] [Google Scholar]
- Slobounov S.M. Gay M. Zhang K. Johnson B. Pennell D. Sebastianelli W. Horovitz S. Hallett M. Alteration of brain functional network at rest and in response to YMCA physical stress test in concussed athletes: RsFMRI study. NeuroImage. 2011;55:1716–1727. doi: 10.1016/j.neuroimage.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits M. Houston G.C. Dippel D.W.J. Wielopolski P.A. Vernooij M.W. Koudstaal P.J. Hunink M.G.M. Lugt A. Microstructural brain injury in post-concussion syndrome after minor head injury. Neuroradiology. 2010;53:553–563. doi: 10.1007/s00234-010-0774-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sponheim S.R. McGuire K.A. Kang S.S. Davenport N.D. Aviyente S. Bernat E.M. Lim K.O. Evidence of disrupted functional connectivity in the brain after combat-related blast injury. NeuroImage. 2011;54:S21–S29. doi: 10.1016/j.neuroimage.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Stefan D. Di Cesare F. Andrasescu A. Popa E. Lazariev A. Vescovo E. Strbak O. Williams S. Starcuk Z. Cabanas M. van Ormondt D. Graveron–Demilly D. Quantitation of magnetic resonance spectroscopy signals: the jMRUI software package. Meas. Sci. Technol. 2009;20:104035–104044. [Google Scholar]
- Vagnozzi R. Signoretti S. Cristofori L. Alessandrini F. Floris R. Isgro E. Ria A. Marziale S. Zoccatelli G. Tavazzi B. Del Bolgia F. Sorge R. Broglio S.P. McIntosh T.K. Lazzarino G. Assessment of metabolic brain damage and recovery following mild traumatic brain injury: a multicentre, proton magnetic resonance spectroscopic study in concussed patients. Brain. 2010;133:3232–3242. doi: 10.1093/brain/awq200. [DOI] [PubMed] [Google Scholar]
- Vagnozzi R. Signoretti S. Tavazzi B. Cimatti M. Amorini A.M. Donzelli S. Delfini R. Lazzarino G. Hypothesis of the postconcussive vulnerable brain: experimental evidence of its metabolic occurrence. Neurosurgery. 2005;57:164–171. doi: 10.1227/01.neu.0000163413.90259.85. [DOI] [PubMed] [Google Scholar]
- Vagnozzi R. Signoretti S. Tavazzi B. Floris R. Ludovici A. Marziali S. Tarascio G. Amorini A.M. Di Pietro V. Delfini R. Lazzarino G. Temporal window of metabolic brain vulnerability to concussion: a pilot (1)H magnetic resonance spectroscopic study in concussed athletes – Part III. Neurosurgery. 2008;62:1286–1295. doi: 10.1227/01.neu.0000333300.34189.74. [DOI] [PubMed] [Google Scholar]
- Vagnozzi R. Tavazzi B. Signoretti S. Amorini A.M. Belli A. Cimatti M. Delfini R. Di Pietro V. Finocchiaro A. Lazzarino G. Temporal window of metabolic brain vulnerability to concussions: mitochondrial-related impairment - Part I. Neurosurgery. 2007;61:379–388. doi: 10.1227/01.NEU.0000280002.41696.D8. [DOI] [PubMed] [Google Scholar]
- Vanhamme L. van den Boogaart A. Van Huffel S. Improved method for accurate and efficient quantification of MRS data with use of prior knowledge. J. Magn. Reson. 1997;129:35–43. doi: 10.1006/jmre.1997.1244. [DOI] [PubMed] [Google Scholar]
- Wheaton P. Mathias J.L. Vink R. Impact of pharmacological treatments on cognitive and behavioral outcome in the postacute stages of adult traumatic brain injury: a meta-analysis. J. Clin. Psychopharmacol. 2011;31:745–757. doi: 10.1097/JCP.0b013e318235f4ac. [DOI] [PubMed] [Google Scholar]
- Witt S.T. Lovejoy D.W. Pearlson G.D. Stevens M.C. Decreased prefrontal cortex activity in mild traumatic brain injury during performance of an auditory oddball task. Brain Imaging Behav. 2010;4:232–247. doi: 10.1007/s11682-010-9102-3. [DOI] [PubMed] [Google Scholar]
- Yeo R.A. Gasparovic C. Merideth F. Ruhl D. Doezema D. Mayer A.R. A longitudinal proton magnetic resonance spectroscopy study of mild traumatic brain injury. J. Neurotrauma. 2011;28:1–11. doi: 10.1089/neu.2010.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W. Signore A.P. Iwai M. Cao G.D. Gao Y.Q. Chen J. Rapidly increased neuronal mitochondrial biogenesis after hypoxic-ischemic brain injury. Stroke. 2008;39:3057–3063. doi: 10.1161/STROKEAHA.108.520114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K. Johnson B. Pennell D. Ray W. Sebastianelli W. Slobounov S. Are functional deficits in concussed individuals consistent with white matter structural alterations: combined FMRI & DTI study. Exp. Brain Res. 2010;204:57–70. doi: 10.1007/s00221-010-2294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.Y. Yang K.H. King A.I. Comparison of brain responses between frontal and lateral impacts by finite element modeling. J. Neurotrauma. 2001;18:21–30. doi: 10.1089/089771501750055749. [DOI] [PubMed] [Google Scholar]