Abstract
We have investigated the in vitro effects and regulatory mechanism of CGRP (calcitonin gene-related peptide) on the differentiation of OB (osteoblasts) in co-culture with HUVEC (human umbilical vein endothelial cells). Primary human MOB (mandibular OB) and OB-like cells (MG-63) were either cultured directly or indirectly co-cultured with HUVEC at a 1:1 ratio. Expression of OC (osteocalcin) was measured by ELISA, and expression of ALP (alkaline phosphatase) and collagen mRNA was measured by quantitative fluorescent PCR. For mineralization nodus, OB were stained with Alizarin Red-S. When co-cultured with HUVEC, expression of OC and ALP mRNA were increased in MG-63 (P<0.01), and the expression of OC, ALP and collagen mRNA were increased in MOB (P<0.01 or 0.05). When treated with CGRP, OC and ALP mRNA and mineralization nodus numbers were increased in the MG-63 co-culture system (P<0.01 or 0.05); OC, ALP and collagen mRNA, and mineralization nodus numbers were increased in the MOB co-culture system (P<0.01 or 0.05). The effect of CGRP regulation on the differentiation of OB is not only direct but also indirect, via its effect on HUVEC and stimulation of OB.
Keywords: calcitonin gene-related peptide, co-culture, endothelial cell, osteoblast
Abbreviations: ALP, alkaline phosphatase; BMP, bone morphogenetic protein; CGRP, calcitonin gene-related peptide; DiI, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate; DMEM, Dulbecco's modified Eagle's medium; EC, endothelial cells; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HBMSC, human bone marrow stromal cells; HUVEC, human umbilical vein endothelial cells; MOB, mandibular OB; OB, osteoblast; OC, osteocalcin; RQ%, relative quantity %; VEC, vascular endothelial cells; VEGF, vascular endothelial growth factor
1. Introduction
Bone remodelling is a process of renewal accomplished by two opposing activities of bone cells: resorption by osteoclasts and formation by OB (osteoblasts) (Irie et al., 2002). However, an intimate connection between blood vessels and bone cells has been found that are also jointly involved in the regulation of bone remodelling. Genetic, biochemical and pharmacological studies have identified and characterized factors involved in the conversion of EC (endothelial cells) to OB during both bone formation and repair (Ha et al., 2001; Carano and Filvaroff, 2003). Although the mechanism is not clearly understood, there is mounting evidence that the nervous system and certain neuropeptides may have effects on bone metabolism. Therefore the connection between bone cells, blood vessels and the nervous system during bone remodelling has been investigated.
In vivo identification of regulators of bone remodelling is complex and nearly impossible because of many other factors in callus, such as cytokines, growth factors and the bone matrix itself. The co-culture system using OB and EC provided a simpler and useful method of exploring these problems. There is a reciprocal regulatory and functional relationship between ECs and OB during osteogenesis (Villars et al., 2002; Guillotin et al., 2008; Santos et al., 2009). Numerous regulatory molecules [e.g. VEGF (vascular endothelial growth factor), BMP (bone morphogenetic protein), endothelins and prostaglandins] that exert major effects on the control of differentiation and activity of bone-forming cells are secreted by ECs (Yu et al., 2006). However, OB influence EC activity through the release of diverse angiogenic growth factors, such as VEGF and bFGF (basic fibroblast growth factor) (Deckers et al., 2000; Mayr-Wohlfart et al., 2002). This co-culture system is not only used in cell biology studies but also in studies of bone tissue engineering, for example, the creation of vasculature inside a piece of engineered bone tissue before implantation (Choong et al., 2006).
CGRP (calcitonin gene-related peptide), a 37-amino acid peptide generated by tissue-specific alternative splicing of the calcitonin gene, may play a key role in promoting OB recruitment and osteogenic activity (Irie et al., 2002). CGRP is expressed in nerve fibres during bone development and regeneration (Imai et al., 1997). The CGRP-immunoreactive fibres appear to be related to blood vessels, and the others are associated with bone marrow cells, bone cells and other cells in bone tissue (Elefteriou, 2005). These nerve fibres often have a beaded appearance, also called varicosities. CGRP may possess multiple functions in neurons, including acting as a neurotransmitter, vasodilator and neurotrophic effector. As CGRP is one of the most potent vasodilators and is generally thought to control local blood flow, this suggests that CGRP is involved in the regulation of regional blood flow (Carano and Filvaroff, 2003). However, there is still some disagreement on the effect of CGRP on OB differentiation from in vitro studies (Kawase et al., 2005; Villa et al., 2006; Kanazawa et al., 2008). Functional studies of CGRP-knockouts in mice have demonstrated that CGRP is an important factor during bone formation and repair (McDonald et al., 2004; Ma et al., 2009). It may be that there is indirect regulation of CGRP on OB differentiation via VEC (vascular endothelial cells), for example, by stimulating the VEC secreting VEGF and BMP.
We therefore propose that CGRP exerts a biological effect on the OB-VEC co-culture system, and have focused on its regulation of OB differentiation. The OB-VEC co-culture system was established using OB-like cells (MG-63) and human MOB (mandibular OB) by co-culturing with VEC. We identified OB differentiation via the detection of OC (osteocalcin) production, collagen and ALP (alkaline phosphatase) mRNA expression, ALP staining and the detection of mineralization nodus. An understanding of the regulation of CGRP on the cellular and molecular interactions of OB and VEC should enhance the overall understanding of bone remodelling and aid in the future development of bone tissue engineering.
2. Materials and methods
2.1. Materials
Human CGRP was purchased from Sigma–Aldrich. CGRP was dissolved in distilled water to a stock concentration of 100 μM and stored in 100 μl aliquots at −70°C. DiI (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate), the ALP staining kit and Alizarin red-S were purchased from Beyotime. The human OC ELISA kit was purchased from Nb Diagnostics. The RNAiso Plus, the PrimeScript RT reagent Kit and SYBRR Premix Ex Taq II were purchased from Takara.
2.2. Cell culture
MG-63, an osteogenic human osteosarcoma cell line, was obtained from the A.T.C.C. The cells were cultured in DMEM (Dulbecco's modified Eagle's medium) supplemented with 10% FCS (fetal calf serum), 100 units/ml penicillin and 0.1 mg/ml streptomycin. The cells were sub-cultured every 72 h in a humidified air plus 5% CO2 incubator. Cells from passages 4–6 were used in the experiments.
Primary cultured human MOB were isolated from the mandible of a 24-year-old patient undergoing reconstruction surgery who had no evidence of metabolic bone disease. Following guidelines from the local ethics committee, the patient was informed of the study prior to any surgical procedure and provided consent for use of the materials. Fragments (2 mm×2 mm) of mandibular bone were mechanically isolated and washed several times in PBS to remove blood cells. The fragments were placed in tissue culture flasks with the same medium and culture conditions as MG-63 cells. After 1–2 weeks in culture, the bone fragments were discarded and the cells were harvested by trypsinization. Cells from passages 4–6 were used in the experiments.
HUVEC (human umbilical vein endothelial cells) were obtained from ATCC and cultured with the same medium and culture conditions as MG-63 cells. Cells from passages 4–6 were used in the experiments.
2.3. Co-culture of OB and HUVEC
In direct contact co-culture conditions, MG-63 or MOB were mixed with HUVEC in a ratio of 2:1 in DMEC medium (10 μg/ml PBS) and plated in 12 multiwell dishes at 5×104 cells/well. During microscopic observation, HUVEC were incubated with 0.2 μmol/l DiI in culture medium for 4 h, and stained with red fluorescence to identify HUVEC from OB. They were mixed with the two types of OB in co-culture.
In indirect contact co-culture conditions, a 0.4 μm transwell membrane insert (Corning Company) was used to separate HUVEC from OB. After MG-63 or MOB had been plated in a 12 multiwell at 5×104 cells/well and cultured for 24 h, the transwell was added, followed by the HUVEC to the insert chamber at 5×104 cells/well.
2.4. ELISA detect OC production
MG-63, MOB and HUVEC were plated in 12 multiwell dishes at 5×104 cells/well, and direct and indirect contact co-culture cells were plated as mentioned above. To determine the time-dependent effect of HUVEC on differentiation of OB, 0, 12, 24, 36, 48 and 60 h cell culture supernatants of OB, direct and indirect contact co-culture cells were collected for measurement. The blank control group (0 h) was given DMEC medium (10 μg/ml PBS). To determine the effect of CGRP on co-culture, several types of cells were incubated with or without 10 nM CGRP for 48 h, and cell culture supernatants were collected for measurement. The control group was treated with DMEC medium (10 μg/ml PBS). Six supernatant samples in every experimental group were removed for analysis.
OC production in supernatants was measured with a human OC ELISA kit. In total 50 μl of the reconstituted standards and the sample supernatants were incubated at room temperature in a 96-well plate that had been pre-coated with an anti-OC monoclonal antibody, followed by the addition of 100 μl of secondary polyclonal antibody, labelled with the enzyme HRP (horseradish peroxidase) for 2 h. During incubation, the 96-well plates were vigorously shaking at 500 rpm on an orbital shaker. After incubation and washing, each well was incubated with 100 μl of the chromogenic substrate tetramethylbenzidine, and 100 μl of an acidic stopping solution (0.2 M sulfuric acid) was added to each well. The degree of enzymatic turnover of the substrate was determined by absorbance measurement at 450 nm.
2.5. Real-time PCR expression analysis of collagen I and ALP mRNAs
The time-dependent effect of HUVEC experiments was designed for time-points at 12, 24, 36 and 48 h; the effect of CGRP on co-culture experiments was designed as OC production. For each cell group, six mRNA samples in every group were extracted using RNAiso Plus (Takara) and reverse transcribed using a PrimeScript reagent Kit (Takara).
GAPDH (glyceraldehyde-3-phosphate dehydrogenase) and β-cytoskeletal actin (β-actin) were used as internal controls. Primers were designed using Primer Express software, based on the published human sequences in GenBank. ALP forward: 5′-CACCCACGTCGATTGCATCT-3′, reverse: 5′-TAGCCACGTTGGTGTTGAGC-3′(211 bp); collagen forward: 5′-CAAGAACTCGGACCTCCTCAC-3′, reverse: 5′-CTCCTGCTCATCTGTCACGTT-3′(160 bp); β-actin forward: 5′-CATGTACGTTGCTATCCAGGC-3′, reverse: 5′-CTCCTTAATGTCACGCACGAT-3′(250 bp); GAPDH forward, 5′-GCACCGTCAAGGCTGAGAAC-3′, reverse, 5′-ATGGTGGTGAAGACGCCAGT-3′(142 bp). Quantitative PCR detection involved SYBRR Premix Ex Taq II (Takara). The thermal cycling conditions consisted of an initial denaturation at 95°C for 5 min, followed by 40 cycles of 30 s denaturation at 95°C, 30 s annealing at 61°C, 45 s elongation at 72°C and a terminal elongation for 5 min at 72°C. All samples and standards were run in triplicate. The quantification of gene expression was performed using the comparative CT (threshold cycle) method and is reported as the fold difference relative to GAPDH and β-actin and the obtained RQ% (relative quantity %) of target mRNA relative to the control sample (set to 1).
2.6. ALP stain and mineralization nodus stain
Only the effect of CGRP on co-culture experiments was designed by ALP and mineralization nodus stain. The cells were treated with CGRP with 10 nM CGRP, and seeded into 12 multiwell dishes at 5×104 cells/well for 7 days. ALP was stained with an ALP stain kit. The cultured cells were rinsed in PBS, fixed in ice-cold 70% ethanol for 1 h at 4°C, rinsed with PBS, and overlaid with 0.5 ml of 0.15 mg/ml 5-bromo-4-chloro-3-indolylphosphate plus 0.3 mg/ml NBT (Nitro Blue Tetrazolium) chloride in 0.1 M Tris/HCl, followed by incubation at room temperature for 2 h in the dark.
Mineralization of the nodus was determined by Alizarin Red-S staining. Control group cells were incubated in mineralization medium containing 10% FBS, 50 g/ml ascorbic acid, and 10 mM 2-glycerophosphate, experimental groups contained 10 nM CGRP. After incubation for 14 days, cells were collected for Alizarin Red staining. The cells were washed with PBS and fixed in 70% ethanol for 1 h at 4°C. After additional washing in PBS, they were incubated in 0.4% Alizarin Red-S in water for 10 min at room temperature, followed by a final incubation in PBS for 15 min. Stained cells were dehydrated with 70% ethanol followed by absolute ethanol and air-dried.
2.7. Statistical analysis
The results are expressed as the means±S.E.M. The data were analysed using a one-way ANOVA and Newman–Keuls–Student's t test. P<0.05 or less was considered significant.
3. Results
3.1. Microscopic observation of OB-HUVEC co-culture
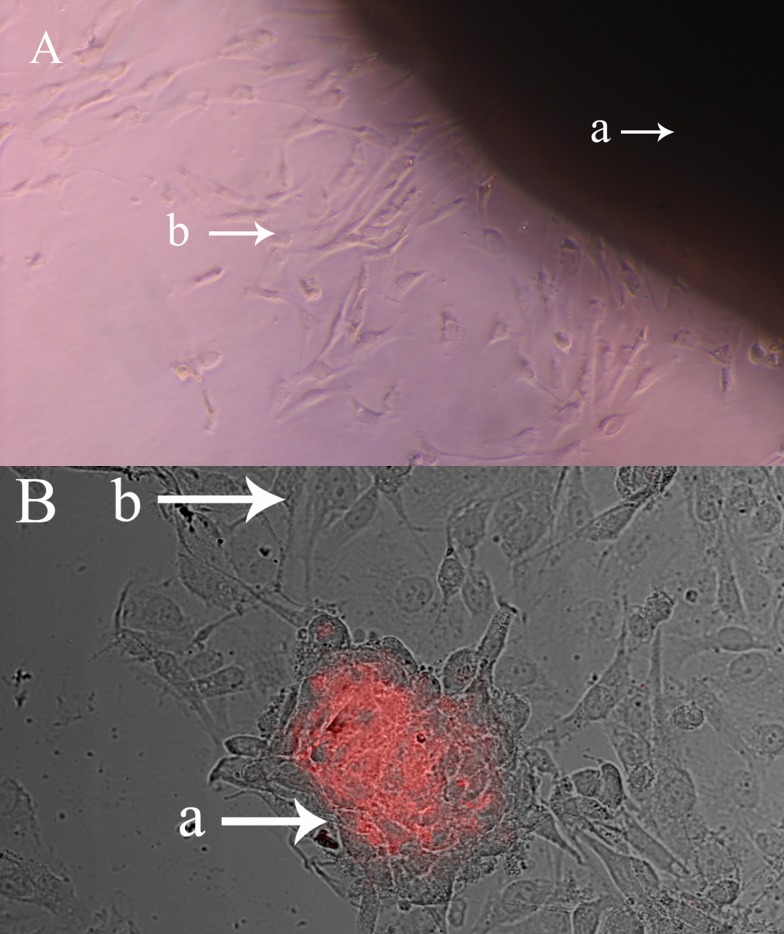
Both OB and HUVEC grew well (Figure 1) when OB and HUVEC were cultured in a 2:1 ratio (Figure 1). MG-63 and MOB cells are larger and spindle-shaped or triangular, whereas HUVEC are smaller and oval-shaped. After HUVEC were dyed with the marker DiI and imaged by confocal microscopy, they showed red fluorescence and grew well. After 1–24 h in co-culture, there was a two-cell intertwined distribution; after 24 h, HUVEC showed nodular aggregation, and MG-63 or MOB cells scattered around the nodules grew. By 48 h, HUVEC necrosis-like cell nodules cavity was seen, and at 96 h HUVEC were surrounded by tube formation.
Figure 1. Culture and co-culture of primary human MOB.
(A) MOB migrated from bone fragments after incubation in DMEM for 2 weeks (magnification, ×100). (a) Bone fragments, (b) Migrated MOB. (B) MOB were co-culture with HUVEC, which were dyed with DiI (magnification, ×200. (a) HUVEC, (b) MOB.
3.2. Effect of CGRP on OC production of OB in OB-HUVEC co-culture
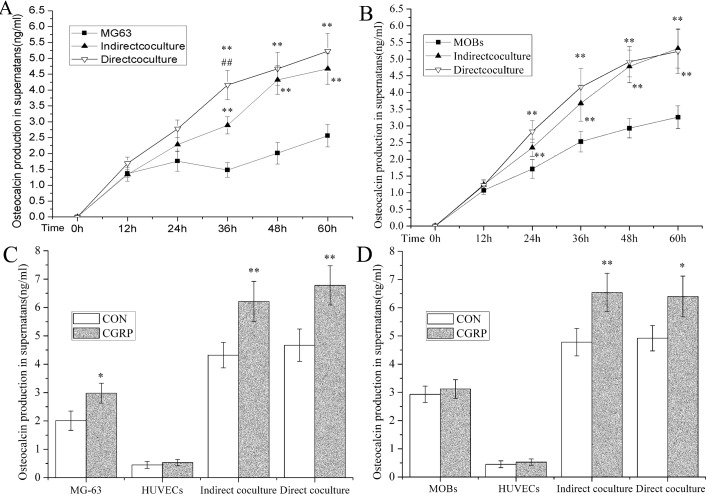
To evaluate OC production in OB in different culture systems, we measured OC concentrations in the cell supernatants via ELISA (Figures 2A and 2B). In MG-63 cells, after 48 h in culture OC production in supernatants of indirect-co-culture and direct-co-culture systems were all higher than MG-63 cells cultured alone (P<0.01), especially at 60 h, being 1.82 and 2.04 that of the control level. At 36 h, the OC of the direct-co-culture system was higher than the indirect-co-culture system (144%, P<0.01). In MOB at 24 h, OC production in supernatants of indirect-co-culture and direct-co-culture systems was higher than in MOB cultured alone (P<0.01), especially at 60 h being 1.63 and 1.60 that of the control levels. Interestingly, no difference in OC production between the direct-co-culture system and the indirect-co-culture system was detected.
Figure 2. ELISA detection of OC production of OB in osteoblast-HUVEC co-culture.
(A) Time-dependent OC concentration in supernatants of indirect and direct MG-63-HUVEC co-culture compared with MG-63. (B) Time-dependent OC concentration in supernatants of indirect and direct MOB-HUVEC co-culture compared with MOB. (C) Effect of CGRP on OC production in monoculture of MG-63 or HUVEC and direct or indirect MG-63-HUVEC co-culture compared with control groups at 48 h. (D) Effect of CGRP on OC production in monoculture of MOB or HUVEC and direct or indirect MOB-HUVEC co-culture compared with respective control groups at 48 h. OC concentrations in supernatants were detected using ELISA and measured on a microplate reader. Data are presented as the relative A (absorbance) value compared with control groups at 0 h and are expressed as the means±S.E.M. for each time point, n = 8; *P<0.05, **P<0.01.
To measure the effect of CGRP on OC production in different culture systems, control and experimental groups were designed with OB and HUVEC, using both indirect co-culture and direct co-culture (Figures 2C and 2D). In MG-63 cell experiments, OC production of the CGRP-induced group was significantly higher than in the control group of cells cultured alone. The indirect co-culture was 138% (P<0.05) and the direct co-culture systems, 144% (P<0.01) and 145% (P<0.01). There was no difference in HUVEC cultured alone. In the experiments using MOB, OC production of the CGRP-induced group was highly significant compared with the control group in indirect-co-culture 137% (P<0.01) and direct-co-culture 130% (P<0.05). There was no difference between MOB and HUVEC cultured alone.
3.3. Effect of CGRP on ALP mRNA of OB in OB-HUVEC co-culture
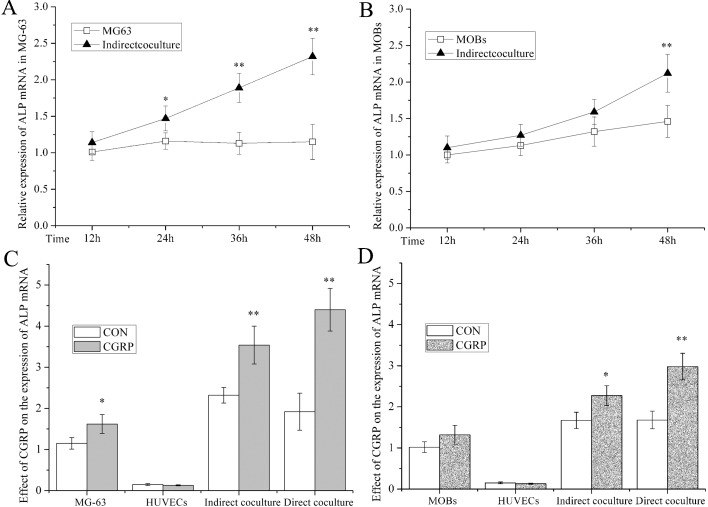
To measure ALP mRNA expression in OB in different culture systems, we used levels in the cell supernatants by quantitative PCR (Figures 3A and 3B). In MG-63 cells at 24 h in the indirect-co-culture system, the level was higher than in cells cultured alone (P<0.05 or 0.01). By 48 h, the levels were twice that of the controls. After only 48 h of culture was ALP mRNA in MOB of the indirect co-culture systems higher than in MOB cultured alone (145%, P<0.01).
Figure 3. ALP staining to detect ALP mRNA of OB in OB-HUVEC co-culture.
(A) Time-dependent ALP mRNA in indirect MG-63-HUVEC co-culture compared with MG-63 cells alone. (B) Time-dependent ALP mRNA in indirect MOB-HUVEC co-culture compared with MOB. (C) Effect of CGRP on ALP mRNA in monoculture of MG-63 or HUVEC, and direct or indirect MG-63-HUVEC co-culture compared with respective control groups after 48 h. (D) Effect of CGRP on ALP mRNA in monoculture of MOB or HUVEC, and direct or indirect MOB-HUVEC co-culture compared with respective control groups after 48 h. ALP mRNA in OB was detected by quantitative PCR. RQ% of target mRNA was calculated and expressed as the means±S.E.M., n = 6; *P<0.05, **P<0.01, compared with control groups.
Regarding the effect of CGRP on ALP mRNA in different culture systems, we designed control and experimental groups in OB, HUVEC, indirect co-culture, and direct co-culture (Figures 3C and 3D). In MG-63 cells, the ALP mRNA of the CGRP-induced group was higher than in the controls cultured alone and the indirect-co-culture and direct-co-culture systems, which were 130% (P<0.05), 153% (P<0.01) and 229% (P<0.01), respectively. There was no difference in the HUVEC cultured alone. In the experiments with MOB, ALP mRNA of the CGRP-induced group was higher than the control group in the indirect-co-culture and direct-co-culture systems, which were 135% (P<0.05) and 177% (P<0.01), respectively. There was no difference in the MOB and HUVEC cultured alone.
3.4. Effect of CGRP on collagen I mRNA of OB in OB-HUVEC co-culture
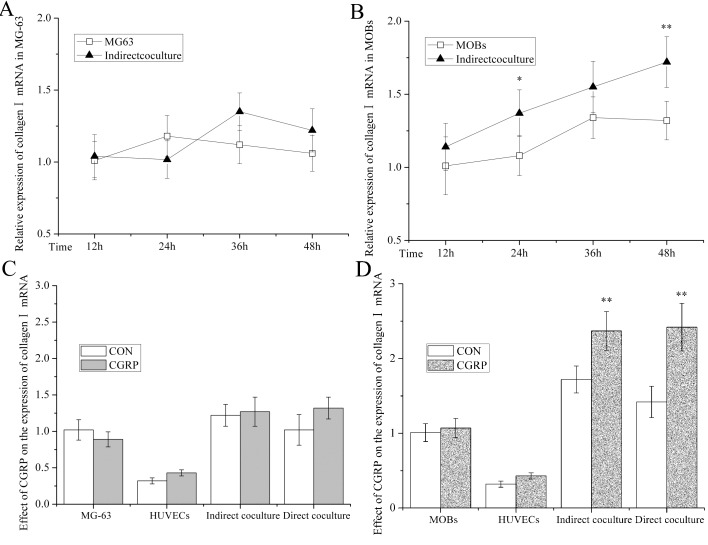
Collagen/mRNA expression levels in OB in different culture systems were measured in the cell supernatants by quantitative PCR (Figures 4A and 4B). In MG-63 cells, there was no time-dependent difference in single groups or between two groups. In MOB, the levels in indirect co-culture systems (127%, P<0.05 and 130%, P<0.01) were higher than in MOB cultured alone after only 24 and 48 h.
Figure 4. Collagen/mRNA in OB of OB-HUVEC co-culture.
(A) Time-dependent collagen/mRNA in indirect MG-63-HUVEC co-culture compared with MG-63 alone. (B) Time-dependent collagen/mRNA in indirect MOB-HUVEC co-culture compared with MOB. (C). Effect of CGRP on collagen/mRNA in monoculture of MG-63 or HUVEC and direct or indirect MG-63-HUVEC co-culture compared with respective control groups at 48 h. (D) Effect of CGRP on collagen/mRNA in monoculture of MOB or HUVEC and direct or indirect MOB-HUVEC co-culture compared with respective control groups after 48 h. Collagen/mRNA in OB were detected by quantitative PCR. RQ% of target mRNA was calculated and expressed as the means±S.E.M., n = 6; *P<0.05, **P<0.01, compared with control groups.
Regarding the effect of CGRP on Collagen/mRNA in different culture systems, the control and experimental groups in OB, HUVEC, indirect-co-culture and direct-co-culture are those given in Figures 4(C) and 4(D). In MG-63 cells, the CGRP-induced group was no different from the control groups in these culture systems. In MOB, the CGRP-induced group was higher than in the control group in indirect co-culture (138%; P<0.01) and the direct-co-culture systems (170%; P<0.01). There was no difference in level in MOB and HUVEC cultured alone.
3.5. Effect of CGRP on mineralization nodus of OB in OB-HUVEC co-culture
With regard to the effect of CGRP on the mineralization ability of OB in different culture systems, we detected mineralization nodus numbers in MG-63, MOB, MG-63-HUVEC and MOB-HUVEC in indirect-co-culture (Table 1). Except for MG-63 cultured alone, CGRP induced an increase in mineralization nodus numbers in MOB, MG-63-HUVEC and MOB-HUVEC indirect co-culture. In MOB cultured alone, the experimental group increased by 21.9% compared with the control group (P<0.05). In MG-63-HUVEC indirect co-culture, the experimental group increased by 60.5% compared with the control group (P<0.01). In MOB-HUVEC indirect co-culture, the experimental group increased by 59.94% compared with the control group (P<0.01).
Table 1. Summary of data on mineralization nodus of OB in the different groups.
Mineralization nodus of OB were stained with Alizarin Red-S, calculated and expressed as the means±S.E.M., n = 6; *P<0.05, **P<0.01, as compared with control groups.
| Groups | MG-63 | MOB | MG-63-HUVEC | MOB-HUVEC |
|---|---|---|---|---|
| Control | 12.6±2.51 | 14.09±2.21 | 13.22±3.17 | 15.93±2.63 |
| CGRP | 14.8±3.54 | 17.17±3.06* | 18.26±4.05** | 19.96±3.81** |
4. Discussion
After the suggested existence of a bone remodelling regulatory arm with neuronal characteristics from clinical observations of patients with head trauma, a number of in vitro and in vivo studies have confirmed that bone homoeostasis is under the influence of central and peripheral neuronal control (Elefteriou, 2005). In vitro studies have shown that CGRP stimulates OB proliferation by increasing intracellular cAMP and calcium, which leads to changes in cell morphology and function (Drissi et al., 1999; Lin et al., 2008). CGRP also increases the synthesis of growth factors and cytokines [including IGF-1 (insulin-like growth factor) 1], collagen synthesis and bone formation in vivo (Irie et al., 2002). However, the effect of CGRP on OB differentiation markers was seldom reported or debated. We have found moderate up-regulation of CGRP on OC and ALP in MG-63, a type of immature OB, but not in MOB. Up-regulation of Collagen I mRNA by CGRP did not occur in either MG-63 or MOB. Interestingly, contradictory results were seen in the detection of mineralization nodus and the up-regulation of CGRP, being effective in MOB but not in MG-63. These results may be related to the different maturity levels of these cell lines. These special protein markers of OB are expressed at different stages of maturity, and therefore the effect of CGRP cannot be seen throughout all stages of OB development.
Apart from this reason, other supporting cells in the callus also play an important role in the effect of CGRP on OB differentiation, for example, HUVEC (Villars et al., 2002; Guillotin et al., 2008). When HBMSC (human bone marrow stromal cells) were co-cultured with HUVEC, an increase in ALP was observed in HBMSC (Deckers et al., 2000; Mayr-Wohlfart et al., 2002). We also found an increase in ALP in MG-63 and MOB after co-culturing with HUVEC for 24 and 48 h (Figures 3A and 3B). Over the course of OB differentiation, ALP increases after the transition to mature OB and decreases when mineralization is well underway. This may explain why we could find such an early and significant increase in ALP mRNA expression in MG-63, but only a late and moderate increase in MOB. We also proved that HUVEC can secrete some OB maturation-promoting factors. Several studies have shown that there is a reciprocal regulatory and functional relationship between EC and OB-like cells during osteogenesis, in which systemic hormones and paracrine growth factors or cytokines play an active role (Villars et al., 2002). In particular, VEGF appears to be the main signal between OB cells and EC. With the other mature markers, we also found an increase of OC in direct co-culture or in indirect co-culture (Figures 1A and 1B). This difference between co-culture versus monoculture was the most significant, not only in MG-63 but also in MOB. There were even differences between the direct co-culture and indirect co-culture systems. These results suggest that communication between EC- and OB-like cells may not only require diffusible factors but also involve junction communication to form a multicellular network, a view supported by previous studies (Villars et al., 2002; Santos et al., 2009). For collagen/mRNA, we did not find an increase in MG-63 or any type of time-dependent regulation (Figures 2A and 2B), which may be related to low levels of its expression in immature OB. Some moderate increases in collagen/mRNA expression in MOB occurred after 24 and 48 h. These results suggest that collagen/mRNA is not the effector present in HUVEC. Due to technical constraints, we did not design immunomagnetic bead-separation technology to detect two types of cells in co-culture. In PCR experiments, we used Transwell to harvest the OB for detection. We detected OC production in direct co-culture and indirect co-culture in the experiment where we only harvested supernatants.
As we defined the change in the levels of these markers for OB differentiation, we were able to follow the effect of CGRP on OB in a co-culture system. No consistent regulation of CGRP on all differentiation markers when OB were cultured alone or treated with 10 nM of CGRP was seen; neither was there a significant change in HUVEC. However, in co-culture systems, the up-regulation of CGRP on differentiation was almost always observed. In the MG-63 co-culture system, OC and ALP, which were increased by CGRP under culture conditions alone, were also up-regulated in co-culture systems (Figures 2C and 3C), and these were more significant than in cells cultured alone. Collagen was still not up-regulated (Figure 4C). Compared with the negative results of the mineralization nodus, CGRP effectively increased the mineralization ability of MG-63. CGRP regulates the expression of VEGF through the CGRP receptor and ERK1/2 (extracellular-signal-regulated kinase 1/2) MAPK (mitogen-activated protein kinase) signalling pathway in human HaCaT keratinocytes (Yu et al., 2006). Thus, there may be indirect regulation of CGRP on OB differentiation via its effect on HUVEC and stimulation of some factors that may induce differentiation of OB, such as BMP and FGF (Santos et al., 2009). The same trend was also found in MOB co-culture systems, but it was more effective. We even found up-regulation of CGRP on collagen/mRNA in MOB (Figure 4D).
Overall, these investigations show a less than regular effect of CGRP on OB, but it did show an indirect control pathway via co-culturing with HUVEC. These results suggest that not only blood vessels but also the nervous system join in the differentiation of OB, consistent with in vivo studies. Further studies on the complex regulation of these cells and callus tissues should improve bone tissue engineering in the near future.
Footnotes
This work was funded by the National Natural Foundation of China [grant numbers A 30973334 and B 30772436] and by the Department of Health Foundation in Sichuan Province of China [grant number 100086].
References
- Carano RAD, Filvaroff EH. Angiogenesis and bone repair. Drug Discov Today. 2003;8:980–9. doi: 10.1016/s1359-6446(03)02866-6. [DOI] [PubMed] [Google Scholar]
- Choong CSN, Hutmacher DW, Triffitt JT. Co-culture of bone marrow fibroblasts and endothelial cells on modified polycaprolactone substrates for enhanced potentials in bone tissue engineering. Tissue Eng. 2006;12:2521–31. doi: 10.1089/ten.2006.12.2521. [DOI] [PubMed] [Google Scholar]
- Deckers MML, Karperien M, van der Bent C, Yamashita T, Papapoulos SE, Löwik CW. Expression of vascular endothelial growth factors and their receptors during osteoblast differentiation. Endocrinology. 2000;141:1667. doi: 10.1210/endo.141.5.7458. [DOI] [PubMed] [Google Scholar]
- Drissi H, Lieberherr M, Hott M, Marie PJ, Lasmoles F. Calcitonin gene-related peptide (CGRP) increases intracellular free Ca2+ concentrations but not cyclic AMP formation in CGRP receptor-positive osteosarcoma cells (OHS-4). Cytokine. 1999;11:200–7. doi: 10.1006/cyto.1998.0415. [DOI] [PubMed] [Google Scholar]
- Elefteriou F. Neuronal signaling and the regulation of bone remodeling. Cell Mol Life Sci. 2005;62:2339–49. doi: 10.1007/s00018-005-5175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillotin B, Bareille R, Bourget C, Bordenave L, Amédée J. Interaction between human umbilical vein endothelial cells and human osteoprogenitors triggers pleiotropic effect that may support osteoblastic function. Bone. 2008;42:1080–91. doi: 10.1016/j.bone.2008.01.025. [DOI] [PubMed] [Google Scholar]
- Ha E, Lemonnier J, Fromigué O, Marie PJ. Bone morphogenetic protein-2 promotes osteoblast apoptosis through a Smad-independent, protein kinase C-dependent signaling pathway. J Biol Chem. 2001;276:29028. doi: 10.1074/jbc.M011265200. [DOI] [PubMed] [Google Scholar]
- Imai S, Rauvala H, Konttinen YT, Tokunaga T, Maeda T, Hukuda S. Efferent targets of osseous CGRP-immunoreactive nerve fiber before and after bone destruction in adjuvant arthritic rat: an ultramorphological study on their terminal-target relations. J Bone Miner Res. 1997;12:1018–27. doi: 10.1359/jbmr.1997.12.7.1018. [DOI] [PubMed] [Google Scholar]
- Irie K, Hara-Irie F, Ozawa H, Yajima T. Calcitonin gene-related peptide (CGRP)-containing nerve fibers in bone tissue and their involvement in bone remodeling. Microsc Res Tech. 2002;58:85–90. doi: 10.1002/jemt.10122. [DOI] [PubMed] [Google Scholar]
- Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Sugimoto T. Metformin enhances the differentiation and mineralization of osteoblastic MC3T3-E1 cells via AMP kinase activation as well as eNOS and BMP-2 expression. Biochem Biophys Res Commun. 2008;375:414–9. doi: 10.1016/j.bbrc.2008.08.034. [DOI] [PubMed] [Google Scholar]
- Kawase T, Okuda K, Burns DM. Immature osteoblastic MG63 cells possess two calcitonin gene-related peptide receptor subtypes that respond differently to [Cys(Acm)2,7] calcitonin gene-related peptide and CGRP8-37. Am J Physiol Cell Physiol. 2005;289:C811. doi: 10.1152/ajpcell.00504.2004. [DOI] [PubMed] [Google Scholar]
- Lin IC, Smartt Jr JM, Nah HD, Ischiropoulos H, Kirschner RE. Nitric oxide stimulates proliferation and differentiation of fetal calvarial osteoblasts and dural cells. Plast Reconstr Surg. 2008;121:1554. doi: 10.1097/PRS.0b013e31816c3bd7. [DOI] [PubMed] [Google Scholar]
- Ma H, Young J, Cherng S. Calcitonin gene-related peptide and substance P significantly influence coronary flow rate in gene knockout mice. J Bacteriol Res. 2009;1:019–25. [Google Scholar]
- McDonald KR, Fudge NJ, Woodrow JP, Friel JK, Hoff AO, Gagel RF. Ablation of calcitonin/calcitonin gene-related peptide-{alpha} impairs fetal magnesium but not calcium homeostasis. Am J Physiol Endocrinol Metab. 2004;287:E218. doi: 10.1152/ajpendo.00023.2004. [DOI] [PubMed] [Google Scholar]
- Mayr-Wohlfart U, Waltenberger J, Hausser H, Kessler S, Günther KP, Dehio C. Vascular endothelial growth factor stimulates chemotactic migration of primary human osteoblasts. Bone. 2002;30:472–7. doi: 10.1016/s8756-3282(01)00690-1. [DOI] [PubMed] [Google Scholar]
- Santos MI, Unger RE, Sousa RA, Reis RL, Kirkpatrick CJ. Crosstalk between osteoblasts and endothelial cells co-cultured on a polycaprolactone-starch scaffold and the in vitro development of vascularization. Biomaterials. 2009;30:4407–15. doi: 10.1016/j.biomaterials.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Villa I, Mrak E, Rubinacci A, Ravasi F, Guidobono F. CGRP inhibits osteoprotegerin production in human osteoblast-like cells via cAMP/PKA-dependent pathway. Am J Physiol Cell Physiol. 2006;291:C529. doi: 10.1152/ajpcell.00354.2005. [DOI] [PubMed] [Google Scholar]
- Villars F, Guillotin B, Amedee T, Dutoya S, Bordenave L, Bareille R. Effect of HUVEC on human osteoprogenitor cell differentiation needs heterotypic gap junction communication. Am J Physiol Cell Physiol. 2002;282:C775. doi: 10.1152/ajpcell.00310.2001. [DOI] [PubMed] [Google Scholar]
- Yu XJ, Li CY, Wang KY, Dai HY. Calcitonin gene-related peptide regulates the expression of vascular endothelial growth factor in human HaCaT keratinocytes by activation of ERK1/2 MAPK. Regul Pept. 2006;137:134–9. doi: 10.1016/j.regpep.2006.07.001. [DOI] [PubMed] [Google Scholar]