Abstract
Objective
Premenstrual syndromes (PMS) affecting 20–40% of women of reproductive age. The aim of this double blind and placebo controlled trial was to investigate whether femicofort a supplement contains Vitamin B6, Vitamin E and evening primrose oil could relieve symptoms of PMS.
Method
This was a randomized and double blind clinical trial. The trial was conducted between November 2009 and April March 2010. Women aged 20 to 45 years with regular menstrual cycles and experience of PMS symptoms (According to the current diagnostic criteria proposed by the American College of Obstetrics and Gynecology) for at least 6 months were eligible for the study. Patients were randomized to receive femicomfort or placebo in a 1: 1 ratio using a computer-generated code. The assignments were kept in sealed, opaque envelopes until the point of analysis of data. In this double-blind, patients were randomly assigned to receive capsule of femicomfort (Group A) or capsule placebo for two menstrual cycles (cycles 3 and 4). The primary outcome measure was the Daily Symptom Report, a checklist of 17 premenstrual symptoms rated from 0 to 4 according to their severity throughout the menstrual cycle. Secondary outcome measure was Hamilton Depression Rating Scale (17-item).
Results
Femicomfort at this dose was found to be effective in relieving symptoms of PMS. The difference between the femicomfort and placebo in the frequency of side effects was not significant.
Conclusion
The results of this study indicate the efficacy of femicomfort in the treatment of PMS.
Keywords: Oenothera biennis, Premenstrual syndrome, Vitamin B6, Vitamin E
Premenstrual syndromes (PMS) are among the most common health problems reported by women, affecting 20–40% of women of reproductive age. Premenstrual dysphoric disorder (PMDD) is a severe subtype of PMS that occurs in 3–8% of women of reproductive age.(1) It is characterized by severe mood and behavioral changes. The hallmark of PMDD is its cyclical luteal-phase-related nature.(2) The etiology of the syndrome is multifactorial and not fully defined. Initially, a great role in the etiology was attributed to decreased levels of progesterone in the luteal phase. An American telephone survey suggested that up to 80% of self-medicating sufferers use complementary remedies.(3) It has been reported that herbal medicine and vitamin B6 and Vitamin E are useful in relieving PMS symptoms. Evening primrose (Oenothera biennis) is a commonly used alternative therapy for PMS and is a rich source of omega-6 essential fatty acids.(3) The aim of this double blind and placebo controlled trial was to investigate whether femicofort (a supplement) which contains Vitamin B6, Vitamin E and evening primrose oil could relieve PMS symptoms.
Materials and Methods
This was a randomized and double blind clinical trial. The trial was conducted between November 2009 and April March 2010.
Participants
Women aged 20 to 45 years with regular menstrual cycles and experience of PMS symptoms (According to the current diagnostic criteria proposed by the American College of Obstetrics and Gynecology) (4) for at least 6 months were eligible for the study. The exclusion criteria were as follows: pregnancy or lactation; menstrual cycle irregularity; unstable medical illness; seizure disorder within the past year; history of multiple drug reaction; menstrual cycle length shorter than 24 days or longer than 35 days; major psychiatric disorder; suicidal ideation or intent; use of psychoactive drugs, investigational drugs, or specific medication for PMS in the past two months and history of substance abuse in the previous 6 months.
The trial was performed in accordance with the Declaration of Helsinki and subsequent revisions. Written informed consents were obtained from the participants. Patients were recruited through an advertisement; and written informed consent was obtained. Although all the participants had been diagnosed with PMS by their gynecologist, they were interviewed again for two menstrual cycles (premenstrual stage) before medication started. This was done to complete the baseline daily symptom Ratings Report and Hamilton depression Rating Scale and to reconfirm the diagnosis of PMS.
Intervention
Patients were randomized to receive femicomfort or placebo in a 1: 1 ratio using a computer-generated code. The assignments were kept in sealed, opaque envelopes until the point of analysis of data. In this double-blind study, patients were randomly assigned to receive femicomfort capsules (Group A) or placebo capsules for two menstrual cycles (cycles 3 and 4). The randomization and allocation process were done by the principal investigator of the trial (S. Akhondzadeh). Two patients dropped out over the trial (one from the femicomfort group and one from the placebo group).
Measurements
The primary outcome measure was the Daily Symptom Report, a checklist of 17 premenstrual symptoms rated from 0 to 4 according to their severity throughout the menstrual cycle, and it consists of four subscale including mood (anxiety, irritability, depression, nervous tension, mood swings and out of control); behavior (poor coordination, insomnia, confusion, headache, crying and fatigue); pain (aches, cramps and tender breasts); and physical (food craving and swelling) subscales.(5) Secondary outcome measure was Hamilton Depression Rating Scale (17-item).(6) All the women who expressed interest to participate in the trial, underwent a preliminary screening telephone interview, and those who met the inclusion criteria were sent an information pack and Daily Symptom Ratings forms to be maintained for two menstrual cycles. A provisional diagnosis of premenstrual syndrome was made if the total premenstrual Daily Symptom Ratings score (over six days prior to the onset of menstruation) was at least 50 and at least 30% higher than the total postmenstrual Daily Symptom Ratings score (days 5-10 with day one being the first day of menstruation). These women were subsequently invited to a clinical screening visit mid-cycle, where a psychiatrist confirmed the premenstrual syndrome and excluded women with a major physical or psychiatric disorder or substance abuse in the previous six months. Participants returned for a second visit at the end of cycle 2 (premenstrual stage-as close as possible to two days prior to the onset of menstruation) to complete the baseline measures of the Hamilton depression rating scale. Daily Symptom Ratings were completed throughout the duration of the trial (menstrual cycles 1-4 by patients). Participants returned for two more visits (at the premenstrual stage of cycles 3 and 4) for assessing Hamilton Depression Rating Scale by a psychiatrist. The effect of treatment was assessed by comparing the baseline (cycle 2) premenstrual Daily Symptom Ratings and Hamilton Depression Rating Scale Scores with the premenstrual scores after one and two cycles of treatment with Crocus sativus (cycles 3 and 4).
Statistical analysis
A two-way repeated measures analysis of variance (time- treatment interaction) was used. The two groups as a between-subjects factor (group) and the three monthly measurements during treatment as the within-subjects factor (time) were considered. To compare the two groups at the baseline and to evaluate the outcome of the two groups at the end of the trial, an unpaired Student's t-test with a two-sided P value was used. Results are presented as mean±SD. Differences were considered significant with P<0.05. To consider, α=0.05, β=0.2, the final difference between the two groups at least score of five on the Daily Symptom Report, S=5 and power=0.8 (according to the pilot study of this research), the sample size was calculated at least 15 in each group. Intention to treat (ITT) analysis with the last observation carried forward (LOCF) procedures were performed.
Results
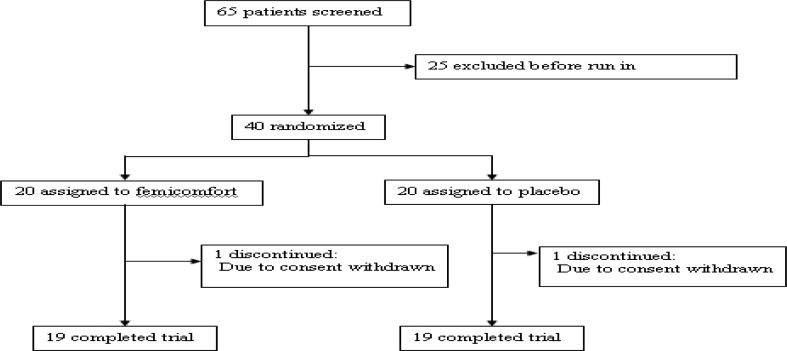
Sixty five patients were screened for the study and 40 were randomized to trial medication (20 patients in each group) (Fig. 1). No significant differences were identified between the patients.
Figure 1.
Trial profile
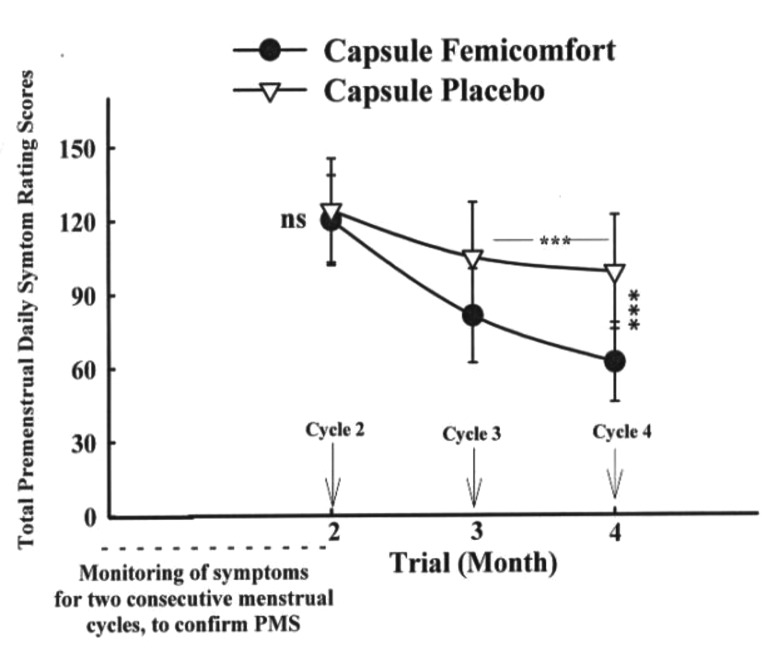
Figure 2.
Mean±SD scores of two groups of patients on the Total Premenstrual Daily Symptoms scores.
Hamilton Depression Rating Scale
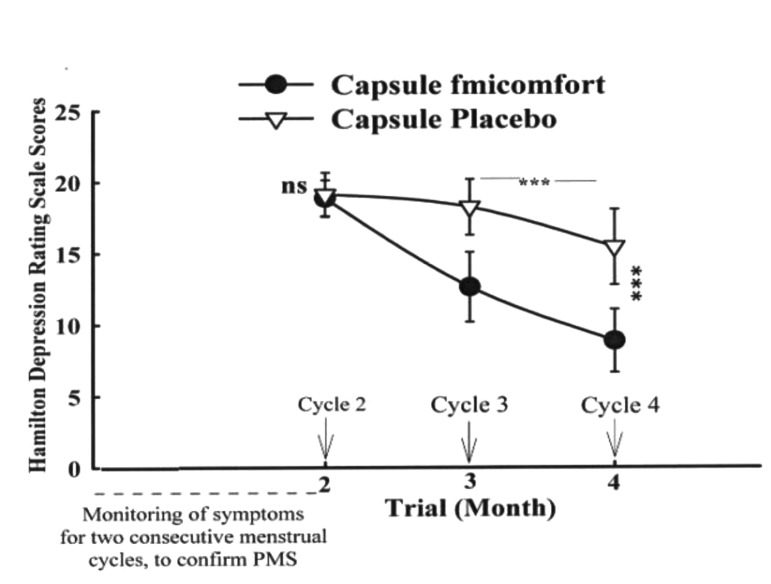
The mean±SD scores of the two groups are shown in Fig. 3. There were no significant differences between the two groups in month 2 (baseline, cycle 2) on the Hamilton Depression Rating Scale scores (t=1.26, d.f=38, P=0.31). The difference between the two protocols was significant as indicated by the effect of group, and the between-subjects factor (Greenhouse-Geisser corrected: F=85.36, d.f.=1, P=0.001). A significant difference between cycle three and four was observed in the femicomfort group (P<0.001). The difference between the two protocols was significant at the endpoint (cycle 4) (t=765, d.f.=38, P<0.001).
Figure 3.
Mean±SD scores of two groups of patients on the Hamilton Depression Rating Scale scores.
Clinical complications and side-effects
Seven side effects were observed over the trial. The difference between the femicomfort and placebo was not significant with regards to the frequency of side effects (Table 2).
Table 2.
Clinical complications and side effects were reported as number per group.
| Side Effects | Femicomfort | Placebo | P |
|---|---|---|---|
| Decreased | |||
| Appetite | 2 | 2 | ns |
| Increased Appetite | 3 | 2 | ns |
| Sedation | 2 | 1 | ns |
| Nausea | 0 | 2 | ns |
| Headache | 1 | 3 | ns |
| Vomiting | 0 | 1 | ns |
| Dizziness | 1 | 1 | ns |
ns=non-significant
Table 1.
Baseline data
| Femicomfort Group | Placebo Group | P | |
|---|---|---|---|
| Age (Mean ±SD) | 34.12±6.54 (year) | 35.35±6.68 (year) | ns |
| Marital status | Single: 16; Married: 3, Divorced: 1 | Single: 17, Married: 2, Divorced: 1 | ns |
| Level of education | Under diploma: 2, Diploma: 4, Higher diploma: 14 | Under diploma1; Diploma: 3; Higher diploma: 16 | ns |
ns=non-significant
Discussion
PMS are a group of menstrually related, chronic and cyclical disorders characterized by emotional, behavioral, and physical symptoms in the second half (luteal phase) of the menstrual cycle.(4) In this small preliminary double-blind and placebo controlled randomized trial, femicomfort at this dose was found to be effective in relieving symptoms of PMS. The clinical relevance of this finding was emphasized by the improvements seen in the Total Premenstrual Daily Symptoms and the Hamilton Depression Rating Scale.
The results of the present trial are in line with a reported study regarding the efficacy of evening primrose oil in the treatment of PMS.(7) The limitations of the present study, including the small number of participants and short period of follow up should be considered, and further research in this area, particularly comparison with an active agent such as fluoxetine, is needed.
Conclusion
The results of this study indicate the efficacy of femicomfort in the treatment of PMS.
Acknowledgment
This study was supported by a grant from Nature Gift to Shahin Akhondzadeh.
References
- 1.Halbreich U, Borenstein J, Pearlstein T, Kahn LS. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD) Psychoneuroendocrinology. 2003;28:1–23. doi: 10.1016/s0306-4530(03)00098-2. [DOI] [PubMed] [Google Scholar]
- 2.Kraemer GR, Kraemer RR. Premenstrual syndrome: diagnosis and treatment experiences. J Womens Health. 1998;7:893–907. doi: 10.1089/jwh.1998.7.893. [DOI] [PubMed] [Google Scholar]
- 3.Wyatt KM, Dimmock PW, Fischer M, Jones PW, O'Brien SP. Prescribing patterns in premenstrual syndrome. BMC Womens Health. 2002;2:4. doi: 10.1186/1472-6874-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ACOG (American College of Obstetricians and Gynecologist) 2000. Premenstrual syndrome. ACOG Practice Bulletin. [Google Scholar]
- 5.Cooper P, Osbom M, Gath D, Feggetter G. Evaluation of a modified self report measure of social adjustment. Br J Psychiatry. 1982;141:68–75. doi: 10.1192/bjp.141.1.68. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khoo SK, Munro C, Battistutta D. Evening primrose oil and treatment of premenstrual syndrome. Med J Aust. 1990;153:189–192. doi: 10.5694/j.1326-5377.1990.tb136857.x. [DOI] [PubMed] [Google Scholar]