Abstract
Background
Chylothorax is a rare but potentially lethal complication of esophagectomy. The study aims were to evaluate the rate of postesophagectomy chylothorax, identify associated risk factors and compare postoperative outcomes with patients who do not develop chylothorax.
Methods
We reviewed 892 consecutive patients undergoing esophagectomy (1997-2008). Preoperative, operative and postoperative details, including adverse outcomes and mortality, were analyzed.
Results
We identified postesophagectomy chylothorax in 34 patients (3.8%). Chylothorax was significantly associated with adverse outcomes, including 30-day major complications (85% vs. 46%; p<0.001) and mortality (17.7 vs. 3.9%, p<0.001). Patients with chylothorax were significantly more likely to develop sepsis (p=0.001), pneumonia (p=0.009), need reintubation (p=0.002) or require reoperation (p<0.001). Median length of stay was significantly longer (17 vs. 8 days; p=0.005). Median time to chylothorax diagnosis was 5 days. Thoracic duct ligation was performed in 62% (n=21; median 13 days after esophagectomy). Repeat duct ligation for persistent chylothorax was required in 2 patients. Squamous cell cancer histology (9/34; 26%) was an independent predictor of postoperative chylothorax (OR 4.18; 95% CI 1.39, 12.6). Odds of chylothorax were 36 times greater with average daily chest tube output >400 ml in the first 6 postoperative days (OR 35.9; 95% CI 8.2, 157.8).
Conclusions
Postoperative chylothorax is associated with significant postoperative morbidity and mortality. Patients with squamous cell cancer may be at increased risk. In addition, >400 ml average daily chest tube output in the early postoperative period should prompt fluid analysis for chylothorax to facilitate early diagnosis and consideration of thoracic duct ligation.
Keywords: Chyle, Esophageal surgery, Outcomes, Postoperative care, Thoracic duct, Surgery, complications
Introduction
Postesophagectomy chylothorax is a relatively rare but potentially lethal complication that results from traumatic injury to the thoracic duct and lymphatic tributaries during surgery. The proximity of the thoracic duct and collateral channels to the esophagus and a highly variable course contribute to an incidence of ductal injury during esophageal mobilization ranging from 1 to ~9%.(6, 7) Because chylothorax leads to malnutrition, immunosuppression through loss of lymphocytes, and respiratory compromise, it has a significant impact on postoperative morbidity and mortality. Due to the relatively infrequent occurrence of chylothorax after esophagectomy, the risk factors associated with postesophagectomy chylothorax and the indications for and timing of reoperation remain incompletely defined.
We evaluated factors associated with postoperative chylothorax and determined the associated postoperative and long-term morbidity and mortality. Early predictors of postesophagectomy chylothorax and failure of medical management were also evaluated.
Patients and Methods
Patient Selection
All patients (892 consecutive patients) undergoing esophagectomy at three hospitals in our center from January 1, 1997 to July 31, 2008 were stratified into two groups based on the identification of chylothorax during the 30 days following esophagectomy. Patients undergoing pharyngolaryngoesophagectomy were excluded as the airway manipulation during this operation carries additional potential for morbidity. The majority received minimally invasive esophagectomy. (Table 1) The remaining patients were treated with open esophagectomy [Ivor Lewis (n=22, 2.5%), modified McKeown (n=16, 1.8%), transhiatal (n=96, 11%), or other (n=9, 1%)] or a hybrid of open and minimally invasive techniques. This retrospective study was approved by our Institutional Review Board.
Table 1. Characteristics of patients undergoing esophagectomy.
| Preoperative Characteristics | Chylothoraxa | No Chylothoraxa | |||
|---|---|---|---|---|---|
|
|
|||||
| n=34 (3.8%) | n=858 (96.2%) | Crude OR (95% CI) | p-value | ||
| Age (median, IQR) | 67.5 (57-75) | 65 (56-73) | 1.04 (0.90, 1.21)b | 0.55 | |
| Male Sex (n, %) | 24 (71%) | 679 (79%) | 0.63 (0.2, 1.35) | 0.23 | |
| BMI | |||||
| BMI < 30 | 29 (88%) | 531 (67%) | 0.013 | ||
| BMI ≥ 30 | 4 (12%) | 257 (33%) | 3.51 (1.22, 10.13) | ||
| Albumin (mg/dl; median, IQR) | 3.7 (3.4, 4.0) | 4 (3.6, 4.2) | 0.94 (0.88, 0.997)c | 0.041 | |
| History of Tobacco Use: (n, %) | 28 (82%) | 620 (75%) | 1.87 (0.64, 3.82) | 0.321 | |
|
| |||||
| Comorbid Conditions | |||||
|
| |||||
| Malignant Disease | 30 (88%) | 792 (92%) | 0.63 (0.21, 1.83) | 0.391 | |
| History of gastroesophageal reflux disease | 17 (52%) | 574 (69%) | 0.49 (0.24, 0.98) | 0.043 | |
| Peptic ulcer disease | 7 (21%) | 73 (9%) | 2.77 (1.16, 6.57) | 0.021 | |
| COPD/Emphysema | 8 (24%) | 109 (13%) | 2.10 (0.93, 4.75) | 0.076 | |
| Previous antireflux surgery | 2 (6.1%) | 60 (7.5%) | 0.80 (0.19, 3.42) | 0.076 | |
| Previous chest surgery | 4 (12%) | 93 (11%) | 1.05 (0.36, 3.04) | 0.931 | |
|
| |||||
| Prior Therapy for Cancer or Barrett’s Esophagus | |||||
|
| |||||
| Induction chemotherapy | 9 (26%) | 244 (28%) | 0.91 (0.42, 1.97) | 0.803 | |
| Induction radiotherapy | 5 (15%) | 141 (16%) | 0.88 (0.33, 2.30) | 0.790 | |
| Endoscopic interventionsf | 10 (33%) | 181 (23%) | 1.69 (0.78, 3.67) | 0.187 | |
|
| |||||
| Operative Details | |||||
|
| |||||
| Approach to Esophagectomy | |||||
| Open | 5 (15%) | 138 (16%) | |||
| Hybridd | 2 (6%) | 54 (6%) | 1.02 (0.19, 5.43) | 0.98 | |
| MIEe | 27 (79%) | 666 (78%) | 1.12 (0.42, 2.96) | 0.42 | |
| Transthoracic esophageal mobilization | 33 (97%) | 747 (87%) | 4.9 (0.66, 36.4) | 0.085 | |
|
| |||||
| Tumor specific variables, if malignant | |||||
|
| |||||
| Proximal or middle esophagus tumor location | 5 (17%) | 57 (8%) | 2.47 (0.91, 6.72) | 0.078 | |
| Squamous tumor type | 9 (30%) | 90 (11%) | 3.3 (1.48, 7.56) | 0.002 | |
| Adenocarcinoma tumor type | 17 (57%) | 592 (75%) | 0.44 (0.21, 0.93) | 0.030 | |
| Tumor invasion at esophagectomy (T3 or T4) | 17 (59%) | 352 (45%) | 1.70 (0.80, 3.60) | 0.168 | |
| Number of lymph nodes examined (median, IQR) | 17 (12.5, 25) | 18 (12, 26) | 1.02 (0.99, 1.05) | 0.310 | |
| Nodal metastasis at esophagectomy | 16 (53%) | 343 (46%) | 1.27 (0.62, 2.57) | 0.511 | |
Not all criteria were in the record and able to be abstracted for every patient; therefore, the reported % are based on total patients for whom the criteria was recorded
For each increase of 5 years
For each increase of serum albumin of 0.1 mg/dl
Hybrid approach includes planned thoracotomy with laparoscopy or thoracoscopy with laparotomy
MIE consists of thoracoscopic mobilization of esophagus followed by laparoscopic creation of gastric conduit and mediastinal pull-through of the neoesophagus with cervical anastomosis or intrathoracic anastomosis at or above the level of the divided azygous vein
Preoperative endoscopic interventions included photodynamic therapy, esophageal stenting and dilation
CI, confidence interval; BMI, body mass index; COPD, chronic obstructive pulmonary disease; IQR, interquartile range; MIE, minimally invasive esophagectomy.
Database and Chylothorax-specific Postoperative Variables
Trained research personnel performed retrospective chart review of patients undergoing esophagectomy and entered the data into a surgical outcomes database with a standardized outcome protocol. Data abstracted routinely included standard observer-recorded measures, preoperative symptoms, laboratory and radiographic studies, operative details and tumor-specific variables. Surgical outcomes were abstracted, including length of stay (LOS), postoperative mortality, and postoperative in-hospital and 30-day adverse outcomes. Chylothorax was diagnosed by either a change in the quality of chest tube drainage to milky white drainage, regardless of chest tube output, or confirmation of chylomicrons or triglycerides in the pleural drainage in patients with high-volume drainage. Additional chylothorax-specific variables were collected, including chest tube drainage volume, for all chylothorax patients. Daily chest tube output was also abstracted for randomly selected patients who did not develop chylothorax (~3 non-chylothorax patients for every 1 chylothorax patient). The average daily outputs per patient were calculated as the sum of chest drainage per day divided by the total number of days for which a recorded value of chest drainage was available.
Statistical Analysis
Statistical analysis was performed using STATA SE 10.0 Corp software.(10) Descriptive statistics were summarized with frequencies and percentages for categorical variables and median with interquartile range (IQR) for continuous variables for the entire cohort and then stratified by presence or absence of chylothorax. Chi-square, Fischer’s exact, and Student’s t-tests, accounting for unequal variance, were used to describe differences between groups. To determine factors associated with an increased risk of chylothorax, crude and adjusted analyses were performed using univariate and multivariate logistic regression. In selecting variables for the multivariate logistic regression model, a cut-off p-value of 0.15 was used.
Results
Risk Factors for Postesophagectomy Chylothorax
Postesophagectomy chylothorax was identified in 34 patients (3.8%). Associations between postoperative chylothorax and preoperative patient characteristics, comorbid conditions, prior therapy for esophageal cancer, operative details, and tumor-specific properties were examined. (Table 1) Patient-specific risk factors for chylothorax identified in crude analysis included a body mass index (BMI) <30 and decreased serum albumin. There was a trend toward increased risk of chylothorax with transthoracic esophageal mobilization (0.085), but risk was not increased when comparing open versus minimally invasive approaches. Squamous tumor type, but not preoperative or postoperative malignant stage (p=0.364 and p=0.762, respectively), was associated with chylothorax in crude analysis. Using multivariate analysis, only squamous tumor type, body mass index <30 and age-adjusted Charlson Comorbidity Index score ≥3 remained independent prognostic factors for postesophagectomy chylothorax. (Table 2) In the immediate postoperative period (days 1 through 6), the average daily chest tube output was significantly greater in patients with the eventual diagnosis of chylothorax (730 ml/day compared with 265 ml/day; p=0.003) Odds of chylothorax were 36 times greater with an average daily chest tube output >400 ml in the first 6 postoperative days (OR 35.9; 95% CI 8.2, 157.8).
Table 2. Multivariate analysis of risk for postesophagectomy chylothorax adjusting for preoperative patient, treatment, and tumor factors.
| Adjusted ORa | (95% CI) | p-value | |
|---|---|---|---|
| BMI < 30 | 4.05 | (1.19, 13.7) | 0.025 |
| Charlson Comorbidity Index Score ≥ | 2.92 | (1.2, 7.25) | 0.020 |
| Peptic ulcer disease | 1.41 | (0.50, 4.09) | 0.512 |
| Transthoracic esophageal mobilization | 2.24 | (0.29, 17.2) | 0.439 |
| Proximal or middle esophagus tumor location | 0.56 | (0.14, 2.28) | 0.416 |
| Squamous tumor type | 4.18 | (1.39, 12.6) | 0.011 |
Odds ratio (OR) adjusted for each of the other factors CI, confidence interval; BMI, body mass index.
Morbidity and Mortality after Chylothorax
Postoperative morbidity and mortality were significantly higher in patients who developed chylothorax (Table 3). Length of initial hospital stay after esophagectomy was significantly longer. There were four patients with chylothorax who had persistent chylous drainage at the time of death.
Table 3. Postoperative morbidity and mortality.
| Chylothoraxa | No Chylothoraxa | p-value | |
|---|---|---|---|
| Any Major Complicationb (n, %) | 29 (85%) | 395 (46%) | <0.001 |
| Pneumonia | 10 (29%) | 107 (14%) | 0.009 |
| Reintubation | 13 (39%) | 143 (18%) | 0.002 |
| Empyema | 3 (9%) | 57 (7%) | 0.724 |
| Acute Respiratory Distress Syndrome | 5 (15%) | 26 (3%) | 0.001 |
| Renal failure | 4 (12%) | 55 (7%) | 0.279 |
| Sepsis | 8 (24%) | 62 (8%) | 0.001 |
| Septic Shock Req. Vasopressors | 6 (18%) | 27 (3%) | <0.001 |
| Clinically significant anastomotic leakc | 5 (15%) | 82 (10%) | 0.407 |
| Gastric tube necrosis | 0 (0%) | 21 (3%) | 0.342 |
|
| |||
| Length of initial postoperative hospital stay (median, IQR) | 17 (12-27) | 8 (6-16) | 0.005 |
| Readmission within 30 days | 10 (29%) | 139 (18%) | 0.092 |
| Reoperation in-hospital or within 30 days | 23 (68%) | 133 (17%) | <0.001 |
|
| |||
| In-hospital or 30-day mortalityd | 6 (17.7%) | 33 (3.9%) | <0.001 |
| Mortality within 90 days | 8 (24%) | 62 (7%) | 0.001 |
Not all criteria were in the record and able to be abstracted for every patient; reported % are based on total patients for whom the criteria was recorded
Morbidity defined according to the Society of Thoracic Surgery General Thoracic Surgery Database definitions
Clinically significant leak includes all patients requiring either neck drainage, endoscopic drain manipulation or reoperation with intrathoracic drainage.
Mortality determined using chart review and Social Security Death Index
Predictors for Failed Medical Management
The relationships between chylothorax-specific variables and the failure of medical management were assessed. Upon diagnosis of chylothorax, the majority of patients were instructed to take nothing by mouth. Medical management strategies also included additional drain placement to ensure complete drainage of the chylothorax (44%), use of total parenteral nutrition (63%), switching enteral feeds to elemental formula (52%), octreotide (31%) and bedside pleurodesis (16%). Unsuccessful medical management was significantly more likely in patients who were treated with total parenteral nutrition and bedside pleurodesis. (Table 4)
Table 4. Relationship between Chylothorax-specific Postoperative Variables and Failure of Medical Management.
| Variable | Failed Medical Management (n=21) | Successful Medical Management (n=13) | p-value |
|---|---|---|---|
| Time from esophagectomy to diagnosis (days; median, IQR) | 4(3-7) | 7 (4-13) | 0.152 |
|
| |||
| Late diagnosis (More than 6 days after esophagectomy; n) | 8 | 8 | 0.224 |
|
| |||
| Duration of initial chest tube (days; median, IQR) | 13 (4-19) | 7 (5-11) | 0.238 |
|
| |||
| Mode of presentation (n) | |||
| Chylous chest tube output | 14 | 7 | 0.055 |
| Pleural effusion on chest x-ray | 9 | 10 | 0.293 |
|
| |||
| Original chest tube in place at diagnosis | 12 | 8 | 0.581 |
|
| |||
| Additional procedure required for diagnosis | 0.859 | ||
| None | 9 | 6 | |
| Thoracentesis | 3 | 2 | |
| Chest tube or Pigtail placement | 6 | 6 | |
|
| |||
| Chest tube output on day of diagnosis (ml; median, IQR) | 1298 (800-2125) | 520 (384-1438) | 0.050 |
|
| |||
| Involved hemithorax at diagnosis | |||
| Right | 8 | 6 | 0.083 |
| Left | 8 | 3 | |
| Bilateral | 1 | 5 | |
|
| |||
| Late diagnosis (More than 6 days after esophagectomy; n, %) | 8 | 8 | 0.224 |
|
| |||
| Laboratory analysis of pleural drainage | |||
| Triglycerides (median, IQR) | 436 (240-806) | 311 (182-833) | 0.518 |
| Chylomicrons (median, IQR) | 189 (123-361) | 168 (66-258) | 0.741 |
|
| |||
| Initial Management | |||
| Nothing by mouth | 17 | 12 | 0.245 |
| Additional drain placement | 8 | 4 | 0.484 |
| Total parenteral nutrition | 14 | 5 | 0.013 |
| Change enteral feeds to elemental formula | 7 | 7 | 0.842 |
| Octreotide | 5 | 3 | 0.555 |
| Bedside pleurodesis | 5 | 0 | 0.032 |
Not all criteria were in the record and able to be abstracted for every patient; reported % are based on total patients for whom the criteria was recorded
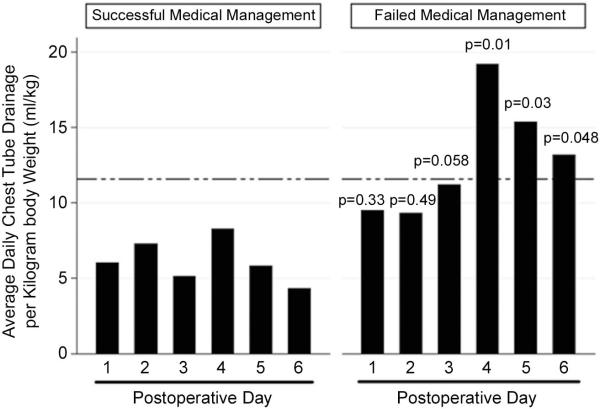
Chest tube output as a ratio of drainage to body weight (ml/kg) (7) in the immediate postoperative period was significantly greater in patients in whom medical management failed. (Figure 1) Furthermore, medical management was nearly 11 times more likely to fail in patients who had >11.6 ml/kg of pleural fluid drainage/day after the initiation of conservative therapy than in patients with ≤11.6 ml/kg output (OR 10.67; 95% CI 1.39, 82). Failure of medical management was not associated with the incidence of other major postesophagectomy complications, need for reoperation, or 30- or 90-day mortality.
Figure 1.
Chest tube output of patients with postesophagectomy chylothorax. The dashed line indicates the cut-off value of 11.6 ml/kg average daily chest tube output for high risk of failed medical therapy.
Operative Intervention in the Management of Postesophagectomy Chylothorax
Of the 34 patients with postesophagectomy chylothorax, 21 (62%) required operative intervention for definitive management. The surgeons’ preferences determined the operative approach [thoracoscopy in 6 (29%) and thoracotomy in 15 (71%)]. Mass ligation of the thoracic duct, inclusive of all tissue from lateral to the azygos to the vertebral body posteriorly and the aorta medially, was performed a median of 13.5 days (IQR 8-21) after esophagectomy and a median of 5.5 days (IQR 4-12) from the day of chylothorax diagnosis. Concomitant procedures included pleurodesis (7 patients) and pulmonary decortication (1 patient). Thoracic duct ligation resolved the chylothorax in the majority of patients (n=14). Persistent chylothorax required thoracentesis in 1 patient, pleurodesis in 1 patient, additional chest tube drainage in 3 patients and repeat thoracic duct ligation in 2 patients. There was no association between approach to thoracic duct ligation and persistent chylothorax (p=0.732). The use of thoracic duct ligation and the timing of operative intervention, from either esophagectomy or from the diagnosis of chylothorax, were not associated with prolonged postesophagectomy hospital stay, 30-day major morbidity or 30- or 90-day mortality. (Data not shown)
Comment
This study presents the incidence and impact of postesophagectomy chylothorax from a center with extensive experience in esophagectomy. These findings reinforce previous literature describing chylothorax as a rare but potentially lethal complication of esophagectomy. The morbidity and mortality associated with the diagnosis of chylothorax were significant. Major complications were nearly doubled and postesophagectomy length of stay increased substantially. Patients with chylothorax were nearly 5 times more likely to die in-hospital or within 30-days and had significantly increased mortality up to 90 days after esophagectomy. Pleural effusion and atelactasis from postesophagectomy chylothorax can lead to respiratory distress and our findings support a significant morbidity associated with pulmonary dysfunction, including increased incidence of pneumonia, reintubation, and ARDS, and sepsis. Squamous cell cancer was an independent risk factor for chylothorax and may delineate a group of patients for whom routine thoracic duct ligation at the time of esophagectomy is warranted.
Importantly, our analysis suggests that a high index of suspicion for chylothorax in patients with more than 4 ml of chest drainage per kilogram body weight (~400 ml), regardless of drainage appearance, may be useful for early diagnosis. Based on these findings, we now routinely leave the chest tube in place until the patient has received tube feeds at their goal rate for 24 hours. Chest tube output volume and quality are carefully evaluated. If the volume increases substantially following initiation of tube feeds or the quality becomes chylous, the chest tube is left in place, the feeds are stopped, and the patient allowed nothing by mouth. Pleural fluid analysis for triglycerides and chylomicrons is performed. In addition, persistence of high volume chest tube drainage, greater than 10-12 ml per kilogram of body weight per day after the initiation of medical therapy for treatment of chylothorax, predicted failure of medical therapy and may prove useful in guiding early operative intervention.
Assessment of Risk Factors for Postesophagectomy Chylothorax
In the esophagectomy patient, the underlying etiology of chylothorax is direct mechanical injury to the duct during dissection and identification of all possible risk factors is critically important to minimizing or eliminating this complication. In our study, we analyzed a large number of potential contributing factors and identified BMI<30, squamous cell cancer and age-adjusted Charlson Comorbidity Index score ≥3 as independent risk factors. The association with squamous cell cancer is highly plausible. Often located in the mid-esophagus, these lesions are in very close proximity to the duct as it crosses from right to left at the level of the T5 vertebral body.(20) Although not significantly associated in our study, lesions in the mid- to upper-esophagus were identified as a risk factor for postesophagectomy chylothorax in at least one report.(16) Rao and colleagues did not directly determine the risk of chylothorax by tumor type; however, 13 of 431 (3%) patients with squamous cell cancer developed postoperative chylothorax compared with 0/89 (0%) of the patients with adenocarcinoma. Awareness of increased risk for postesophagectomy chylothorax in patients with squamous cell cancer is warranted.
Predictors for Failed Medical Management
Our findings regarding the importance of chest tube drainage in the early postoperative period for both diagnosis of chylothorax and prediction of the need for operative intervention are consistent with published data. The volume of flow through the thoracic duct is directly related to body weight, with an estimated volume of 1.38 ml/kg of body weight per hour and increases after meals.(5) Given the relationship between body weight and normal thoracic duct flow, Dugue and colleagues investigated the relationship between chest tube drainage per kilogram of body weight and need for operative intervention in postesophagectomy chylothorax. They found a statistically and clinically significant difference in chest tube drainage on the 5th day after diagnosis of chylothorax that predicted failure of medical therapy and the need for thoracic duct ligation.(7). In their study, all patients who required reoperation had ≥10 ml/kg of drainage with an average day 5 output of 23.5 ml/kg body weight. Only 2 of 14 patients in the group with successful medical management had chest tube output ≥10 ml/kg (mean 6.7 ml/kg). Our findings are consistent those of Dugue. Because we were interested in determining factors that would facilitate early diagnosis and intervention, we analyzed chest tube drainage in the postoperative period prior to the diagnosis of chylothorax and found increased chest tube drainage through the initial postoperative period in patients who would eventually require reoperation for postesophagectomy chylothorax.
These findings suggest that early diagnosis may be improved through assiduous attention to the volume of chest tube drainage in the immediate postoperative period and prompt assessment of triglycerides and chylomicrons in the drainage if the chest tube output is greater than 400 ml daily in the first 3-4 postoperative days.(12, 17) This would allow early institution of conservative therapies. Persistent drainage of >10-12 ml/kg of chest tube drainage after institution of conservative measures would then be an indication for early thoracic duct ligation, which has been shown by others to improve mortality associated with chylothorax.(11,15) In patients with confirmed chylothorax but with <10 ml/kg/day drainage, medical management may have a high likelihood of success, making it reasonable to delay operative intervention for a trial of medical therapy.
The Importance of Operative Intervention in the Management of Postesophagectomy Chylothorax
Mortality rates after the development of chylothorax have varied significantly in the published series. Reported mortality rates are 0-82% (7, 13, 19) with conservative management and 0-50% in patients who required reoperation or were treated with an early thoracic duct ligation.(11, 14, 18) A planned early reoperation rather than delayed intervention after failed conservative management may significantly reduce mortality.(11, 14) Persistence of a high volume of chest drainage is a reasonable trigger for thoracic duct ligation.(2) Since the failure rate and mortality are high with conservative management,(13, 19) an expectant approach to thoracic ligation should be the standard and should only be avoided if the patient quickly responds to medical management.
With early reoperation, mortality can be less than 10%.(11, 14) In our series, however, we did not find that thoracic duct ligation was associated with reduced length of postoperative stay, morbidity or mortality. This is likely due to our aggressive management strategy toward chylothorax, particularly in patients with early, high-volume drainage. The median time from diagnosis of chylothorax to thoracic duct ligation (5.5 days), was similar to the interval of 4-6 days reported by others as an aggressive approach to treatment.(11, 14)
In some centers, percutaneous catheterization and embolization of the thoracic duct is an important option for management of chylothorax. Described by Cope and colleagues in 1999,(3,4) percutaneous embolization via the cisterna chyli can be an effective treatment, achieving resolution of the chylous drainage in up to 70% of patients in centers with extensive experience with the procedure.(9) Catheterization of the cisterna chyli can be challenging, however, and unsuccessful in up to 50% of patients.(8) In patients with failed catheterization or persistent chylous drainage despite successful catheterization (30%), thoracic duct ligation should be considered as an early next step. We do not favor percutaneous embolization and it was not used in this series of patients.
Study Limitations
This study has several strengths and weaknesses. As with all retrospective studies, comparisons between groups are limited by the completeness of the data and are biased by difficult-to-measure factors, such as surgeon experience and thoroughness of the documentation by care providers. We attempted to minimize the impact of these limitations by performing the chart review with consistent data definitions and validation of the data by a second abstractor.
Because prophylactic thoracic duct ligation is not performed by any of the surgeons in our group, we are unable to comment on the role of prophylactic thoracic duct ligation in preventing postesophagectomy chylothoraces. Prophylactic ligation may be a useful adjunct to transthoracic esophagectomy and is an approach that has been advocated by others. Cagol and colleagues reported on 323 patients undergoing transthoracic esophagectomy with concomitant thoracic duct ligation.(1) At their institution, there was a 0.9% incidence of postesophagectomy chylothorax between 1980 and 2000. Prophylactic thoracic duct ligation was then performed from 2001 to 2006 and no postesophagectomy chylothoraces were identified. In other studies, however, the incidence of chylothorax after prophylactic thoracic duct was as high as 2.7%.(7) While not a guarantee against postesophagectomy chylothorax, it may be reasonable to consider routine thoracic duct ligation in all patients undergoing transthoracic esophagectomy, especially in patients with squamous cell carcinoma.
Conclusions
In summary, postesophagectomy chylothorax is a rare complication which carries high morbidity and mortality. Patients with squamous cell cancer are at significantly increased risk, which may warrant prophylactic duct ligation. Early postesophagectomy chest tube output >400 ml per day or ≥10-12 ml/kg of body weight should raise suspicion of chylothorax and prompt laboratory analysis of the pleural fluid to avoid delay in diagnosis. In addition, the volume of drainage in the early postoperative period can be used to identify patients who will likely require thoracic duct ligation.
Acknowledgements
Supported by National Institutes of Health, NIH/NCRR/CTSA Grant UL1 RR024153
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentation: Southern Thoracic Surgical Association, 57th Annual Meeting, Orlando, FL November 3-6, 2010.
References
- 1.Cagol M, Ruol A, Castoro C, Alfieri R, Michieletto S, Ancona E. Prophylactic thoracic duct mass ligation prevents chylothorax after transthoracic esophagectomy for cancer. World journal of surgery. 2009;33:1684–1686. doi: 10.1007/s00268-009-0094-3. [DOI] [PubMed] [Google Scholar]
- 2.Cerfolio RJ, Allen MS, Deschamps C, Trastek VF, Pairolero PC. Postoperative chylothorax. J Thorac Cardiovasc Surg. 1996;112:1361–1365. doi: 10.1016/S0022-5223(96)70152-6. discussion 1365-1366. [DOI] [PubMed] [Google Scholar]
- 3.Cope C. Diagnosis and treatment of postoperative chyle leakage via percutaneous transabdominal catheterization of the cisterna chyli: a preliminary study. J Vasc Interv Radiol. 1998;9:727–734. doi: 10.1016/s1051-0443(98)70382-3. [DOI] [PubMed] [Google Scholar]
- 4.Cope C, Salem R, Kaiser LR. Management of chylothorax by percutaneous catheterization and embolization of the thoracic duct: prospective trial. J Vasc Interv Radiol. 1999;10:1248–1254. doi: 10.1016/s1051-0443(99)70227-7. [DOI] [PubMed] [Google Scholar]
- 5.Crandall LJ, Barker S, Graham D. A study of the lymph from a patient with thoracic duct fistula. Gastroenterology. 1943;1:1040–1042. [Google Scholar]
- 6.Dougenis D, Walker WS, Cameron EW, Walbaum PR. Management of chylothorax complicating extensive esophageal resection. Surg Gynecol Obstet. 1992;174:501–506. [PubMed] [Google Scholar]
- 7.Dugue L, Sauvanet A, Farges O, Goharin A, Le Mee J, Belghiti J. Output of chyle as an indicator of treatment for chylothorax complicating oesophagectomy. Br J Surg. 1998;85:1147–1149. doi: 10.1046/j.1365-2168.1998.00819.x. [DOI] [PubMed] [Google Scholar]
- 8.Hoffer EK, Bloch RD, Mulligan MS, Borsa JJ, Fontaine AB. Treatment of chylothorax: percutaneous catheterization and embolization of the thoracic duct. AJR Am J Roentgenol. 2001;176:1040–1042. doi: 10.2214/ajr.176.4.1761040. [DOI] [PubMed] [Google Scholar]
- 9.Itkin M, Kucharczuk JC, Kwak A, Trerotola SO, Kaiser LR. Nonoperative thoracic duct embolization for traumatic thoracic duct leak: experience in 109 patients. J Thorac Cardiovasc Surg. 2010;139:584–589. doi: 10.1016/j.jtcvs.2009.11.025. discussion 589-590. [DOI] [PubMed] [Google Scholar]
- 10.LP S. Stata Statistical Software: Release 10.1. StataCorp LP; College Station, TX: 2008. [Google Scholar]
- 11.Merigliano S, Molena D, Ruol A, et al. Chylothorax complicating esophagectomy for cancer: a plea for early thoracic duct ligation. Thorac Cardiovasc Surg. 2000;119:453–457. doi: 10.1016/s0022-5223(00)70123-1. [DOI] [PubMed] [Google Scholar]
- 12.Merrigan BA, Winter DC, O’Sullivan GC. Chylothorax. Br J Surg. 1997;84:15–20. [PubMed] [Google Scholar]
- 13.Milsom JW, Kron IL, Rheuban KS, Rodgers BM. Chylothorax: an assessment of current surgical management. J Thorac Cardiovasc Surg. 1985;89:221–227. [PubMed] [Google Scholar]
- 14.Orringer MB, Bluett M, Deeb GM. Aggressive treatment of chylothorax complicating transhiatal esophagectomy without thoracotomy. Surgery. 1988;104:720–726. [PubMed] [Google Scholar]
- 15.Orringer MB, Marshall B, Iannettoni MD. Transhiatal esophagectomy: clinical experience and refinements. Ann Surg. 1999;230:392–400. doi: 10.1097/00000658-199909000-00012. discussion 400-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rao DV, Chava SP, Sahni P, Chattopadhyay TK. Thoracic duct injury during esophagectomy: 20 years experience at a tertiary care center in a developing country. Dis Esophagus. 2004;17:141–145. doi: 10.1111/j.1442-2050.2004.00391.x. [DOI] [PubMed] [Google Scholar]
- 17.Romero S, Martin C, Hernandez L, et al. Chylothorax in cirrhosis of the liver: analysis of its frequency and clinical characteristics. Chest. 1998;114:154–159. doi: 10.1378/chest.114.1.154. [DOI] [PubMed] [Google Scholar]
- 18.Tam PC, Fok M, Wong J. Reexploration for complications after esophagectomy for cancer. J Thorac Cardiovasc Surg. 1989;98:1122–1127. [PubMed] [Google Scholar]
- 19.Wemyss-Holden SA, Launois B, Maddern GJ. Management of thoracic duct injuries after oesophagectomy. Br J Surg. 2001;88:1442–1448. doi: 10.1046/j.0007-1323.2001.01896.x. [DOI] [PubMed] [Google Scholar]
- 20.Williams KR, Burford TH. The Management of Chylothorax. Ann Surg. 1964;160:131–140. doi: 10.1097/00000658-196407000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]