Abstract
Introduction
Sleep disturbance is common during critical illness, yet little is known about its prevalence or role in post-discharge quality of life among high-risk acute lung injury (ALI) patients.
Methods
In a prospective cohort of 61 mechanically ventilated ALI patients, we examined the association between insomnia symptoms and quality of life six months after discharge. Subjects completed surveys rating quality of life (MOS SF-36), post-traumatic stress disorder (PCL), and depression (PHQ-9). Using an individual item from the PCL, we defined insomnia symptoms as moderate or greater trouble falling or staying asleep in the past month. We performed multivariable linear regression to examine the association between insomnia symptoms and SF-36 physical and mental component scores, adjusting for PTSD and depression.
Results
Forty subjects (85% of eligible) completed six-month questionnaires; 20 (50%) met criteria for insomnia symptoms. After adjustment for PTSD and depression, insomnia symptoms remained significantly associated with worse physical component scores (adjusted mean difference = -8.8; 95% CI: -15.0, -2.5; P<0.01).
Conclusions
Post-discharge insomnia symptoms were common and significantly associated with physical quality of life impairment among six-month ALI survivors, even after adjustment for PTSD and depression symptoms. Further studies are needed to validate these results and to characterize sleep disturbance after ALI using sleep-specific metrics.
Keywords: Respiratory Distress Syndrome, Adult/therapy, Quality of Life, Insomnia, Sleep Initiation and Maintenance Disorders, Intensive Care Units
Introduction
Sleep in the intensive care unit (ICU) is poor [1]. Recent studies suggest that specific abnormalities, including a “burst suppression” pattern on EEG, may be pathological and predict mortality [2]. However, little is known about sleep disturbance after critical illness.
Critical illness is increasingly regarded as a life-altering event [3]. Patients with acute lung injury (ALI) are among the most severely ill and at particular risk for poor long-term outcomes including a spectrum of long-term physical, cognitive, and psychological sequelae [3]. Randomized trials have failed to identify an effective strategy that improves long-term outcomes [4,5], and there remains a critical need to identify modifiable factors for intervention. Chronic sleep disturbance, known to impair many quality of life (QoL) domains, has been relatively unexplored in this population. ALI survivors may be at risk for chronic sleep disturbance as a result of medical and psychiatric sequelae [6] or ICU exposures including mechanical ventilation [7], sedation [8] and an abnormal sleep environment [1], yet there are no published studies that specifically address the role of post-discharge insomnia symptoms in long-term QoL after ALI.
To begin understanding the clinical relevance of post-discharge insomnia symptoms, we used data from an existing cohort of ALI survivors to examine the association between insomnia and QoL concurrently measured at six months post-discharge. We also evaluated whether patient or ICU factors might identify subjects at risk for post-discharge insomnia symptoms.
Methods
Study Subjects
We performed a secondary analysis of a prospective cohort study enrolling 61 mechanically ventilated ALI patients at a Seattle tertiary care hospital from November 2006 to November 2008 [9]. Eligible subjects were critically ill patients enrolled within 7-10 days after ALI onset. Subjects were excluded for inability to obtain patient/surrogate consent or preexisting neuromuscular disease. Surrogates provided consent at enrollment and patients provided consent at follow-up. This study was approved by the University of Washington Institutional Review Board.
Data Collection
We obtained patient and hospitalization data via systematic chart abstraction from the electronic medical record. We used physician admission notes and nurse intake forms to identify baseline smoking status, chronic health problems, and psychiatric disorders.
At six months, patients completed surveys including the MOS 36-item Short Form Health Survey (SF-36) [10], the post-traumatic stress disorder (PTSD) checklist (PCL) [11], and the PHQ-9 depression scale [12]. The SF-36 measures QoL across eight domains, each ranging from 0 (worst) – 100 (best). These eight domains are summarized by physical and mental component summary scores that are standardized to a mean of 50 (SD 10). Scores below 50 represent below average QoL [10]. The 17-item PCL rates severity of PTSD symptoms over the preceding month from one (not at all) to five (extremely severe). The nine-item PHQ-9 depression scale rates the frequency of depression-related symptoms over the past two weeks from zero (not at all) to three (every day) [12].
Definition of Insomnia Symptoms
We defined insomnia symptoms as a symptom score >=3 (moderate or higher severity) using an individual item from the PCL: “In the last month, how much have you been bothered by trouble falling or staying asleep?”
Statistical Analysis
All analyses were conducted using Stata 11 SE (Stata, College Station, Texas, USA). We performed linear regression to compare mean physical and mental component scores by the presence or absence of insomnia symptoms. We adjusted a priori for PTSD and depression using previously validated cutoffs (PCL score >=45 for PTSD, and PHQ-9 score >=10 for depression) [12,13] while excluding sleep items. An expanded model evaluated age and chronic health problems as additional adjustment variables using likelihood ratio testing to compare goodness of fit to our primary model. In exploratory analyses we created separate regression models looking for associations between insomnia symptoms and each of the eight SF-36 QoL domains.
Results
Post-discharge insomnia symptoms at six-month follow-up
Of the 61 patients enrolled, 14 were unable to complete follow-up (12 died, two remained non-verbal). Of the 47 eligible survivors, 40 (85%) completed follow-up questionnaires (median 236.5 days after discharge, range 174 – 425 days). Twenty patients (50% of respondents) met criteria for post-discharge insomnia symptoms.
Association between patient and ICU factors and post-discharge insomnia symptoms
Respondents were predominantly white (95%) patients, admitted for trauma (73%), and had at least one chronic health problem (68%). We identified no baseline or ICU factors associated with post-discharge insomnia symptoms, including: smoking status, chronic health problem, baseline psychiatric disorder, APACHE III score, ventilator or ICU days, average lung compliance, or average ICU blood glucose.
Association between insomnia symptoms and QoL at six months post-discharge
QoL was globally impaired at follow-up, with a mean physical component score of 37.1 (SD 9.9) and a mean mental component score of 43.1 (SD 12.9). In unadjusted analyses insomnia symptoms were associated with significant decrements in both mental and physical QoL (Table 1). The association between insomnia symptoms and mental QoL did not remain significant after adjustment for PTSD and depression. However, subjects with insomnia symptoms had significantly worse physical QoL independent of these factors (adjusted difference =-8.8; 95% CI: -15.0, -2.5; P<0.01) (Table 1). The addition of age and chronic health problems did not change our results.
Table 1.
Association between insomnia symptoms and SF-36 quality of life component scores among six-month ALI survivors
| Unadjusted Difference (95% CI) | P | Adjusted Differencea (95% CI) | P | |
|---|---|---|---|---|
| Mean Physical Component Score | -10.8 (-16.5, -5.1) | <0.01 | -8.8 (-15.0, -2.5) | <0.01 |
| Mean Mental Component Score | -10.3 (-18.5, -2.2) | 0.01 | -6.4 (-14.5, 1.8) | 0.12 |
Adjusted for PTSD (total PCL score >=45 excluding sleep item) and depression (total PHQ-9 score >=10 excluding sleep item)
Secondary Analyses
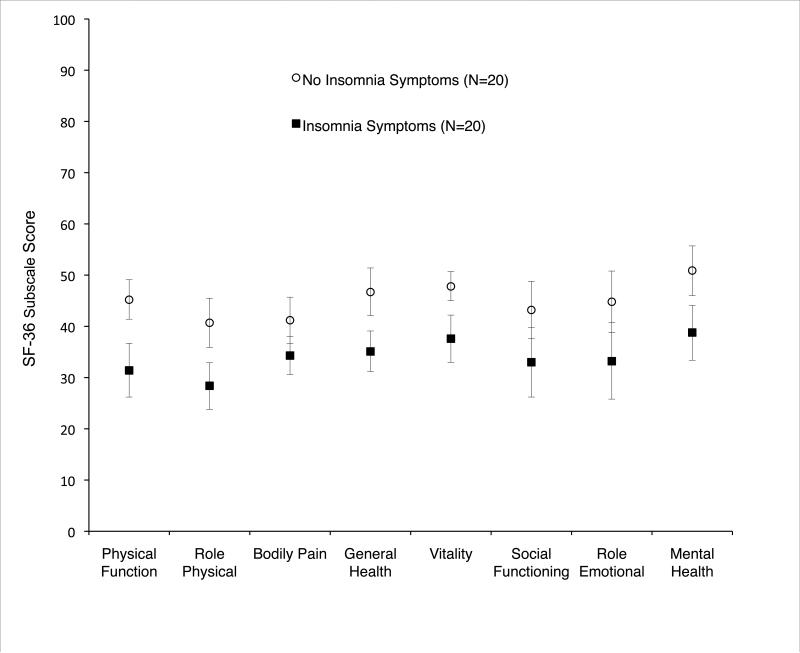
Insomnia symptoms were associated with worse unadjusted QoL across multiple SF-36 domains (Figure 1). Adjusted analyses revealed the most significant decrements in physical function (difference = -9.9, 95% CI: -17.6, -2.2; P=0.01), general health (difference = -9.4, 95% CI: -16.0, -2.8; P<0.01), vitality (difference = -7.6, 95% CI: -13.5, -1.7; P=0.01), and mental health (difference = -9.5, 95% CI: -16.4, -2.6; P<0.01).
Figure 1. Unadjusted SF-36 quality of life domain scores by insomnia symptoms among six-month ALI survivors.
Points represent mean values, with vertical bars reflecting 95% confidence intervals. Non-overlapping vertical bars reflect significant differences in unadjusted QoL scores (P value <0.05).
We performed several sensitivity analyses. Four subjects were delayed in their first follow-up. Repeating our analysis among the 36 subjects completing follow-up questionnaires within the expected six-month follow-up period did not change our results. We also examined the prevalence of insomnia symptoms among the 18 subjects who completed a second, twelve-month follow-up. There was no significant difference in the prevalence of insomnia symptoms between the first and second assessments (56% vs. 44%; P=0.19).
Discussion
The purpose of this study was to determine the prevalence of insomnia symptoms among six-month ALI survivors and the symptoms relationship to QoL. Half of the ALI survivors in our cohort reported insomnia symptoms, and these subjects scored worse in both mental and physical QoL. After adjustment for co-morbid PTSD and depression, there remained a significant association between insomnia symptoms and physical QoL impairment. This finding has particular relevance to ALI patients, in whom physical impairment is the most common, severe, and persistent area of disability [3].
The high prevalence of sleep disturbance in our cohort is consistent with previously published data in general ICU patients [14]. While two prior studies have reported some aspects of sleep as part of larger long-term outcome assessments in ALI survivors [15,16], none have systematically examined insomnia symptoms in relationship to long-term functional outcomes. Our data provide preliminary evidence linking insomnia to long-term physical function impairment. Insomnia may result from physical conditions such as pain (either pre-existing or as a result of critical illness). Less well recognized, but equally supported by the literature, is the potential for insomnia to impact QoL. Insomnia-related sleep deprivation may contribute to poor physical function through daytime sleepiness, pain exacerbation [17], or cognitive impairment hindering activities of daily living and other integrative tasks [18].
The high prevalence of insomnia in ALI survivors is potentially important regardless of its etiology or chronicity. Treatment of insomnia has been shown to improve QoL, even when insomnia exists co-morbidly with other medical conditions, such as chronic pain [20]. Given the disappointing results of physically-directed interventions in survivors of critical illness [4], the potential role of sleep disturbance is enticing. Designing effective interventions to aid recovery may require a broader paradigm that recognizes bidirectional relationships between physical and non-physical factors, including sleep, social, psychological, and cognitive impairment.
Our study has several limitations. The secondary nature of our analysis allowed us to capture only insomnia symptoms reported in a single survey item from the PCL [11]. In addition, our small sample size did not allow us to rigorously examine the association between insomnia symptoms and QoL domains or to explore potential modifiers (such as trauma). We also cannot establish the chronicity of insomnia symptoms because we measured them at a single time point post-discharge, and, thus, we cannot rule out the possibility that insomnia was a pre-existing condition. It is plausible that insomnia symptoms may result from a variety of insults in the prehospitalization, ICU, and recovery periods. However, understanding the chronicity of sleep disturbance may be more important in understanding mechanisms than in determining which patients to target for intervention, because even longstanding sleep disorders may improve with treatment.
Despite its limitations, our study highlights an intriguing association between insomnia and physical QoL that we hope will encourage the design of future studies examining the complex relationship between sleep and long-term QoL after ALI. Insomnia is a clinical diagnosis that may be readily captured and potentially treated. Cognitive behavioral therapy has been shown to improve not only sleep but other QoL factors as well, including pain [20]. Further investigation is warranted to rigorously explore insomnia in ALI survivors using validated sleep metrics [19], to explore longitudinal relationships with QoL, and to evaluate the potential value of sleep-targeted interventions.
Acknowledgments
Financial Support
This work was supported by the National Institutes of Health [Grants HL007287-31, K23HL74294].
Abbreviations
- ARDS
Acute respiratory distress syndrome
- ICU
Intensive care unit
- PCL
PTSD checklist
- PHQ-9
Patient Health Questionnaire – 9
- PTSD
Post-traumatic stress disorder
- QoL
quality of life
- RALI
Recovery After Lung Injury
- MOS SF-36
Medical Outcomes Study 36-item Short Form Health Survey
- SOFA
Sequential Organ Failure Assessment
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Freedman NS, Gazendam J, Levan L, Pack AI, Schwab RJ. Abnormal sleep/wake cycles and the effect of environmental noise on sleep disruption in the intensive care unit. Am J Respir Crit Care Med. 2001;163:451–7. doi: 10.1164/ajrccm.163.2.9912128. [DOI] [PubMed] [Google Scholar]
- 2.Watson PL, Shintani AK, Tyson R, Pandharipande PP, Pun BT, Ely EW. Presence of electroencephalogram burst suppression in sedated, critically ill patients is associated with increased mortality. Crit Care Med. 2008;36:3171–7. doi: 10.1097/CCM.0b013e318186b9ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herridge MS, Tansey CM, Matte A, Tomlinson G, Diaz-Granados N, Cooper A, et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 4.Elliott D, McKinley S, Alison J, Aitken LM, King M, Leslie GD, et al. Health-related quality of life and physical recovery after a critical illness: a multi-centre randomised controlled trial of a home-based physical rehabilitation program. Crit Care. 2011;15:R142. doi: 10.1186/cc10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuthbertson BH, Rattray J, Campbell MK, Gager M, Roughton S, Smith A, et al. The PRaCTICaL study of nurse led, intensive care follow-up programmes for improving long term outcomes from critical illness: a pragmatic randomised controlled trial. BMJ. 2009;339:b3723. doi: 10.1136/bmj.b3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz DA, McHorney CA. The relationship between insomnia and health-related quality of life in patients with chronic illness. J Fam Pract. 2002;51:229–35. [PubMed] [Google Scholar]
- 7.Chishti A, Batchelor AM, Bullock RE, Fulton B, Gascoigne AD, Baudouin SV. Sleep-related breathing disorders following discharge from intensive care. Intensive Care Med. 2000;26:426–33. doi: 10.1007/s001340051177. [DOI] [PubMed] [Google Scholar]
- 8.Borbely AA, Mattmann P, Loepfe M, Strauch I, Lehmann D. Effect of benzodiazepine hypnotics on all-night sleep EEG spectra. Hum Neurobiol. 1985;4:189–94. [PubMed] [Google Scholar]
- 9.Hough C, Swersie H, Caldwell E, Robinson L. Hospital and 6 month outcomes of ICU-acquired neuromuscular dysfunction in patients with acute lung injury. Am J Respir Crit Care Med. 2010:A5357. [Google Scholar]
- 10.Ware JE, Jr, Kosinski M, Bjorner JB, Turner-Bowker DM, Gandek B, Maruish ME. User's Manual for the SF-36v2 Health Survey. 2nd ed. QualityMetric Incorporated; Lincoln, RI: 2007. [Google Scholar]
- 11.Weathers FW, Keane TM, Davidson JR. Clinician-administered PTSD scale: a review of the first ten years of research. Depress Anxiety. 2001;13:132–56. doi: 10.1002/da.1029. [DOI] [PubMed] [Google Scholar]
- 12.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruggiero KJ, Del Ben K, Scotti JR, Rabalais AE. Psychometric properties of the PTSD Checklist-Civilian Version. J Trauma Stress. 2003;16:495–502. doi: 10.1023/A:1025714729117. [DOI] [PubMed] [Google Scholar]
- 14.Orwelius L, Nordlund A, Nordlund P, Edell-Gustafsson U, Sjoberg F. Prevalence of sleep disturbances and long-term reduced health-related quality of life after critical care: a prospective multicenter cohort study. Crit Care. 2008;12:R97. doi: 10.1186/cc6973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee CM, Herridge MS, Gabor JY, Tansey CM, Matte A, Hanly PJ. Chronic sleep disorders in survivors of the acute respiratory distress syndrome. Intensive Care Med. 2009;35:314–20. doi: 10.1007/s00134-008-1277-3. [DOI] [PubMed] [Google Scholar]
- 16.Masclans JR, Roca O, Munoz X, Pallisa E, Torres F, Rello J, et al. Quality of life, pulmonary function, and tomographic scan abnormalities after ARDS. Chest. 2011;139:1340–6. doi: 10.1378/chest.10-2438. [DOI] [PubMed] [Google Scholar]
- 17.Roehrs T, Hyde M, Blaisdell B, Greenwald M, Roth T. Sleep loss and REM sleep loss are hyperalgesic. Sleep. 2006;29:145–51. doi: 10.1093/sleep/29.2.145. [DOI] [PubMed] [Google Scholar]
- 18.Van Dongen HP, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26:117–26. doi: 10.1093/sleep/26.2.117. [DOI] [PubMed] [Google Scholar]
- 19.Bastien CH, Vallieres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 20.Vitiello MV, Rybarczyk B, Von Korff M, Stepanski EJ. Cognitive behavioral therapy for insomnia improves sleep and decreases pain in older adults with co-morbid insomnia and osteoarthritis. J Clin Sleep Med. 2009;5:355–62. [PMC free article] [PubMed] [Google Scholar]