Abstract
Multiple laboratories have recently demonstrated that long-term dopaminergic transplants form Lewy bodies in patients with Parkinson’s disease. Debate has arisen as to whether these Lewy bodies form from the transfer of alpha synuclein from the host to the graft or whether they form from intrinsic responses of the graft from being placed into what was, or became, an inflammatory focus. To test whether the former hypothesis was possible, we grafted fetal rat ventral mesencephalon into the dopamine depleted striatum of rats that had previously received 6-hydroxydopamine lesions. One month after the transplant, rats received viral over expression of human alpha synuclein (AAV2/6 - alpha synuclein) or green fluorescent protein (AAV2/6-GFP) into the striatum rostral to the grafts. Care was taken to make sure the AAV injections were sufficiently distal to the graft so no cells would be directly transfected. All rats were sacrificed five weeks after the virus injections. Double label immunohistochemistry combined with confocal microscopy revealed that a small number of grafted tyrosine hydroxylase (TH) neurons (5.7%+ 1.5% (mean + SEM) of grafted dopamine cells) expressed host derived alpha synuclein but none of the grafted cells expressed host-derived GFP. The alpha synuclein in a few of these cells was misfolded and failed to be digested with proteinase K. These data indicate that it is possible for host derived alpha synuclein to transfer to grafted neurons supporting the concept that this is one possible mechanism by which grafted dopamine neurons form Lewy bodies in Parkinson’s disease patients.
INTRODUCTION
/body/ Excess alpha synuclein, either due to genetic aberrations or experimental manipulations induces degeneration of substantia nigra dopamine neurons in both experimental and human Parkinson’s disease. In addition to neurons within the substantia nigra degenerating, we (Chu and Kordower, 2010; Kordower et al., 2008a, 2008b) and others (Li et al., 2008, 2010) have recently demonstrated that human nigral neurons grafted into the putamen of patients with Parkinson’s disease (PD) for greater than 10 years, display Lewy bodies that are indistinguishable from the Lewy bodies seen in host brain regions including the substantia nigra. In this regard, they display the morphological features of Lewy bodies as seen in hemotoxylin and eosin stained sections (4,5) express alpha synuclein (Chu and Kordower, 2010; Kordower et al., 2008a, 2008b; Li et al., 2008; Li et al., 2010) and ubiquitin (Chu and Kordower, 2010; Kordower et al., 2008a, 2008b; Li et al., 2010) and are thioflavin S positive (Kordower et al., 2008b; Li et al., 2010), the most definitive light microscopic marker for Lewy bodies.
While the presence of Lewy bodies in grafted neurons is incontrovertible, the means by which they occur have been subject to debate. Some have argued that the presence of Lewy bodies is a response to grafts being placed into an environment altered by aging, or a result of grafting techniques that are conducive to producing immune or inflammatory responses (6). Others have argued that alpha synuclein can transfer from the host to the graft in a prion-like process (Angot et al., 2010; Brundin et al., 2008) analogous to the proposed spread of PD pathology in the brain from the olfactory bulb and caudal brainstem, which then spreads caudally and rostrally from these two regions respectfully. This model has been termed the Braak hypothesis (Braak et al., 2006).
The present study was performed to test a single hypothesis: can grafted neurons take up host-derived alpha synuclein and express this protein within their cell soma in a manner seen in patients with long-term fetal nigral grafts? Towards this end, fetal nigral allografts were placed within the striatum of dopamine denervated rats. Subsequent to the grafting, alpha synuclein or a reporter gene were virally over-expressed within the striatum in regions distal to the graft using viral vectors. Only alpha synuclein was seen within grafted nigral perikarya with some appearing to be aggregated. These data demonstrate that grafted nigral neurons can uptake and transfer alpha synuclein from the host and this may be a mechanism by which Lewy bodies form in grafted dopamine neurons.
MATERIALS AND METHODS
Animals
Animals were housed and treated following the National Institutes of Health guidelines. Young adult male F344 rats (3 months of age) were used as host animals for transplant experiments. Rats were obtained from Harlan Animal Research Laboratory (Indianapolis, IN) and housed in a temperature (21 degrees Celsius) and humidity (40–50%) controlled room with a 12 hour light/dark cycle (6 AM lights on). Rats had free access to food and water. The animal facility was accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care and complied with all Federal animal care and use guidelines. Protocols utilized in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Cincinnati.
Surgery
Male F344 rats were anesthetized (30 mg/kg, pentobarbital, i.p.) and placed into a stereotaxic device. Experimental subjects underwent a series of three surgical interventions. First, 6-OHDA (8μg/2μl) was infused unilaterally into the medial forebrain bundle (AP −3.6mm, ML +2.0mm, DV −8.3mm from skull) and rostral substantia nigra (AP −4.8mm, ML +1.7mm, DV −8.0mm from skull) at a rate of 0.5μl/minute to produce severe depletions of striatal dopamine. 6-OHDA was dissolved in a 0.2% ascorbic acid-physiological saline solution. Second, five weeks later, subjects received cell implants into the dopamine-depleted striatum. Cell implants consisted of dissociated embryonic day (E)15 F344 rat ventral mesencephalon (VM). The ventral portion of the mesencephalic flexure was dissected with the isthmus, the sulcus limitans and the rostral limit of the flexure serving as boundaries. The tissue pieces were pooled and dissociated into a cell suspension as previously described (Steece-Collier et al., 2009) 300,000 cells/2μl was infused along a single track in the striatum (AP +0.3mm, ML +3.0mm, DV −5.5, −4.5mm from skull) at a rate of 0.5μl/min. Finally, four weeks later, subjects received an infusion of 2.0μl of AAV6- human alpha synuclein or AAV6–GFP (synapsin promoter for both; titers for both were 5 × 1012) into the striatum rostral to the cell graft (AP +1.5, ML +2.5, DV −5.5, −4.5) at a rate of 0.5μl/min. Five weeks after vector infusion, animals were sacrificed via pentobarbital overdose (60mg/kg) and perfused intracardially with saline followed by ice cold 4% PFA (0.1 M PO4 buffer). Brains were extracted and post-fixed in 4% PFA for 24 hours and sunk in 30% sucrose.
Histology
All brains were cut frozen (40μm) in the coronal plane on a sliding knife microtome. Sections were stained for tyrosine hydroxylase (TH) (Chemicon inc: 1:20,000), alpha synuclein (Invitrogen inc, 1:500), adeno-associated virus 6 (AAV6) (Acris antibodies inc, 1:20) and green fluorescent protein (GFP) (CloneTech, 1:1,000) using standard staining procedures (Chu and Kordower, 2007) Double labeling was performed using double immunoperoxidase (Kordower et al., 1994) and double immunofluorescence (Chu et al., 2009) procedures.
Stereology
Estimates of the number of human alpha synuclein and TH positive grafted neurons were made using the optical fractionator procedure as previously described (Chu and Kordower, 2007) Briefly stereological estimates for TH-ir and TH-ir/human alpha synuclein double labeled cells were performed on all cases using Stereoinvestigator (Micro Bright Field [MBF] Bioscience, Colchester, VT) following optical fractionator principles (West, 1993). A systematic random sampling strategy of the area occupied by graft was made by the Stereoinvestigator software. With 300μm × 300μm grid size and 90μm × 90μm counting frame, neuronal estimates were performed. The section thickness was determined empirically on each section. Using a uniform, systematic and random design procedure double labeled neurons were sampled. Numerical density for these neurons was measured by the Stereoinvestigator software. The coefficients of error (C.E.) were calculated according to the procedure of Gundersen and colleagues (Gundersen and Jensen, 1987)
RESULTS
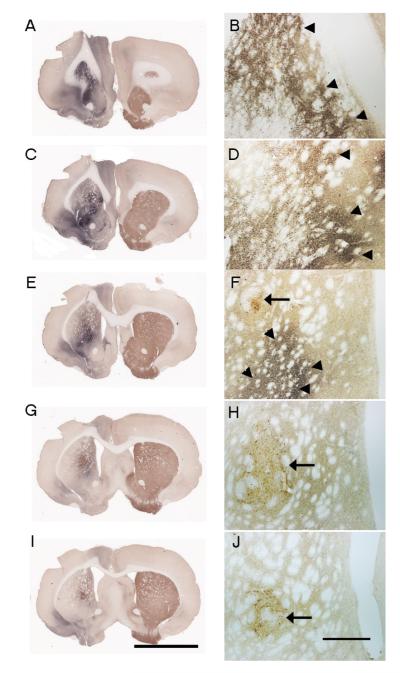
Double-label immunoperoxidase stained sections revealed a segregation of alpha synuclein staining from the injection site and the somata of tyrosine hydroxylase – immunoreactive (TH-ir) grafted neurons in the striatum of all animals (Figure 1). Thus the grafted neurons were not directly transfected by the vector and any human alpha synuclein seen within grafted neurons occurred via retrograde transport of the transgene from the host to grafted neurons. Human alpha synuclein immunoreactivity was observed rostral to the ventral mesencephalic transplant and extended close to, but not within the borders of the graft (Fig. 1A, C, E, G, I). A similar pattern of GFP-immunoreactivity was seen in control injected rats (see Figure 3A-C). Grafts displaying the typical cytoarchitecture of ventral mesecephalic transplants in which TH-ir perikarya were preferentially distributed towards the periphery of the graft was observed (Fig 1B, 1D, 1F, 1G). Eight of the nine rats injected with ventral mesencephalic transplants displayed viable grafts with an average of 2418+ 847 (Mean+ SEM) cells per case. Fibers emanating from transplanted cells were observed to cross the graft-host interface and innervate the host striatum.
Figure 1.
Photomicrograph displaying human alpha synuclein (Blue-black) and TH (Brown) double peroxidase immunostained sections. A, C, E, G, I represents low power photomicrographs, while B, D, F, H and J represents high power photomicrographs showing TH-ir grafted cells and virally expressed human alpha synuclein protein. Arrowheads represent the human alpha synuclein vector injection and arrows represent the grafted cells injection site. Note the presence of grafted cells in the absence of human alpha synuclein immunoreactivity directly from the injection indicating that the grafted cells were not directly transfected by the vector. Scale bar in I represents 5mm and applies to (A, C, E, G, I). Scale bar in J represents 500μm and applies to (B, D, F, H, J) respectively.
Figure 3.
Low power magnification of confocal images (A – C) illustrating GFP injection site (A), TH-ir grafted cells (B) and merged GFP and TH (C). Panels D, E, F, and G represent high power micrographs of TH grafted cells (D, F) and GFP injection site (E, G). Note TH immunoreactive fiber innervation (G) from grafted cells at GFP injection site. However, GFP never colocalized with TH grafted cells. Scale bar in C represents 100μm and applies to (A, B, C). Scale bar in G represents 50μm and applies to (D, E, F, G) respectively.
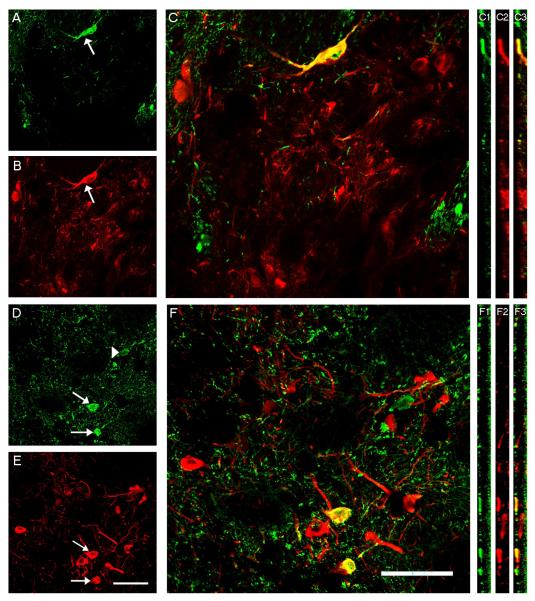
Systematic assessments of dual stained sections revealed that a subset of grafted TH-ir neurons co-expressed alpha synuclein (Fig 2). Intermingled among human alpha synuclein negative TH-ir positive somata were grafted TH-ir neurons that did express human alpha synuclein. To ensure that the grafted neurons were double labeled, each putative double labeled cell was both optically rotated and optically dissected through the z plane and only those cells in which both labels could be found within the same plane were quantified. Quantitative confocal microscopy using unbiased counting techniques revealed that between 1.6% to 15% (5.7% + 1.5% (mean + SEM); excluding the one animal that did not have a viable graft) of the grafted neurons co-expressed TH and human alpha synuclein. Only a rare non-TH-ir neuron expressed human alpha synuclein. No TH-ir or non-TH-ir grafted neurons expressed GFP (Figure 3). Critical to the interpretation of this study is the assurance that the grafted cells were not directly transfected in any manner. Even though AAV6-GFP treated rats failed to display GFP in grafted neurons, it remained possible that fibers in the hist picked up the vector, retrogradely transported it to the perikarya of grafted cells, where the transgene was transcribed. To rule out this possibility, we stained striatal sections for the AAV6 capsid. As expected, we saw robust staining for AAV6 in the striatum using both immunoperoxidase and immunofluorescence (Figure 4) techniques. In contrast, but no such staining was seen in any grafted neurons
Figure 2.
High magnification confocal images of grafted cells from rat striatum visualized with human alpha synuclein (green) and TH (red) double immunofluorescence. Arrow in A and B point to the same cells which is double labeled in the merged image (C) Arrows in D and E point to double labeled cells which were confirmed in the merged image (F). Arrowhead in D points to human alpha synuclein labeled grafted cell that is not TH-ir. Panels C1-C3 and F1-F3 are X-Z rotated images confirming the colocalization of human alpha synuclein with TH-ir grafted cells. Scale bar in E and F represents 50μm and applies to all.
Figure 4.
(A,B,C) Low (A, B) and high (C) power photomicrographs illustrating robust expression of AAV6-immunoractivity in the striatum of rats injected with AAV6-human alpha synuclein in regions distal to the graft using both immunoperoxidase (A) and immunofluorescence (B, C) techniques. (D) Immunoperoxidase and (E) immunofluorescence photomicrographs illustrating the presence of TH-immunoreactive nigral transplants. (F) No AAV6 was observed in any grafted cell illustrating that the virus was not uptaken by grafte-derived fibers and the cells were not directed transfected by the virus. Scale bar in E represents 200μm and applies to (A, B, D, E). Scale bar in F represents 50 μm and applies to C and F.
DISCUSSION
The present study clearly demonstrates that fetal dopamine neurons grafted into the striatum of 6-OHDA lesioned rats can retrogradely transfer human alpha synuclein from the host into the graft. This suggests that this is one possible mechanism by which alpha synuclein accumulates and aggregates in Parkinson’s disease patients with long-term (>10 year) fetal nigral transplants. Although not abundant, some of the host-derived human alpha synuclein failed to digest following proteinase K treatment (data not shown) suggesting that not only is human alpha synuclein retrogradely transported from host-to graft but misfolds in a manner seen in the parkinsonian nigra (Chu and Kordower, 2007) and in long-term transplants (Kordower et al., 2008b). The failure for aggregation to be more abundant can be due to many reasons, but may be related in part to the short post-grafting interval (5 weeks) examined in our study.
What needs to be demonstrated with clarity is the fact that the double-labeling seen in grafted neurons is due to mechanisms other than direct transfection of grafted neurons or mechanisms intrinsic to the grafted cell. Firstly, there was clear anatomical segregation between the human alpha synuclein signal and the grafted neurons. Secondly, a relatively small percentage of grafted neurons co-expressed human alpha synuclein and these cells were intermingled with non-alpha synuclein expressing grafted cells. If the expression of human alpha synuclein occurred by direct transfection, then one would expect all grafted neurons located in the same cluster to be transfected. This was not the case. Furthermore, the antibody used to visualize human alpha synuclein only recognizes the human epitotpe (Jakes et al., 1999) and thus would only stain human alpha synuclein derived from the AAV6 vector and would not stain rodent alpha synuclein that may be augmented in response to the transplantation procedure. Indeed if the later scenario was true, one would expect to see human alpha synuclein in grafted neurons from animals receiving AAV6-GFP but this was not the case. Lastly, it is theoretically possible that fibers emanating from the graft could pick up and transport the vector back to grafted somata where the transgene could then be transcribed. Again, GFP expression was never seen in grafted neurons in which AAV6-GFP was injected into the striatum even though the same promoter and titers were employed suggesting that this scenario did not occur. More directly, we stained striatal section for AAV6 and while numerous striatal neuron expressed this markers no grafted cells did. These data, taken together clearly support the concept that fibers emanating from grafted nigral neurons can uptake host-derived human alpha synuclein protein, and not vector, and retrogradely transport this protein to grafted dopaminergic somata.
Because of its lack of a signal sequence, and its predominant localization in the cytosol, alpha synuclein has been generally considered an exclusively intracellular protein. However a number of groups have recently challenged this assumption and have demonstrated conclusively that a small percentage of newly synthesized alpha synuclein can be rapidly secreted from neuronal cells via an endoplasmic/Golgi independent exocytosis (Lee et al., 2005). Interestingly, Lee and coworkers (Lee et al., 2005) also demonstrated that intravesicular alpha synuclein is more prone to aggregation than the cystolic protein and that the aggregated form can be secreted from cells. It has been proposed that this release can cause neuroinflammation and be responsible for the propagation of alpha synuclein between neurons. The present study supports this view. One can make the argument that the viral over-expression of human alpha synuclein is so non-physiological that its utility in understanding mechanisms is limited. However, others have grafted nondopaminergic cells into the hippocampus (Desplats et al., 2009) or striatum (Hansen et al., 2011) of alpha synuclein over-expressing mice, a more physiological system, and found transfer of the alpha synuclein from the host to the graft as well
So what are the implications of these data? From a cell replacement strategy perspective, likely little. In the human graft cases our group (Kordower et al., 2008a, 2008b) and others (Li et al., 2008, 2010) observe that a mean of between 2% and 8 % of the grafted dopamine neurons exhibit Lewy bodies. It is interesting to note that approximately 6% of grafted neurons displayed detectable alpha synuclein in the present study. However, given that the vast majority of grafted neurons in these human cases still appeared morphologically robust even more than a decade after transplantation, albeit some with phenotypic changes (Kordower et al., 2008a, 2008b), they likely remain functional even after this time. In contrast, from a pathogenesis point of view, these data may be important in understanding the sequence and progression of Parkinson’s disease pathology. Brundin and colleagues have posited that Parkinson’s disease is a prion-like disorder with transfer of pathology from one neuronal system to another (Angot et al., 2010; Brundin et al., 2010). This concept is based, in part, upon the Braak hypothesis (Braak et al., 2006) that stipulates that Parkinson’s disease begins in the myenteric plexus and olfactory system. It is proposed that from the myenteric plexus alpha synuclein pathology is retrogradely transported to the caudal brainstem from where it then marches over time to include a variety of more rostrally located structures. From the olfactory system pathology is proposed to transfer to more caudally located structures. In Parkinson’s disease, alpha synuclein positive Lewy bodies can be found in the striatum (Duda et al., 2002) much like the over-expression of alpha synuclein created in the present study. The present data support the hypothesis that grafted neurons exposed to this secreted pathological protein, especially late in the disease, may transfer pathology from the host to the graft. Thus, in this respect, the graft is likely subject to a process of parkinsonian pathology that is similar to what occurs in the ungrafted parkinsonian brain and may provide critical cues to the pathogenic cascade in this disease.
Highlights.
This paper demonstrates human alpha synuclein can be transferred to grafted neurons.
This paper provides a mechanism for the formation in Lewy bodies in grafted neurons in PD.
This paper provides input towards the pathophysiology of Parkinson’s disease
ACKNOWLEDGMENT
This study is funded by private donations (JHK) and NS055295 and NS58830; The Udall Center of Excellence at Michigan State University (TJC).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Angot E, Steiner JA, Hansen C, Li JY, Brundin P. Are synucleinopathies prion-like disorders? Lancet Neurol. 2010;9:1128–38. doi: 10.1016/S1474-4422(10)70213-1. [DOI] [PubMed] [Google Scholar]
- Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett. 2006;396:67–72. doi: 10.1016/j.neulet.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Brundin P, Li JY, Holton JL, Lindvall O, Revesz T. Research in motion: the enigma of Parkinson’s disease pathology spread. Nat Rev Neurosci. 2008;9:741–5. doi: 10.1038/nrn2477. [DOI] [PubMed] [Google Scholar]
- Brundin P, Melki R, Kopito R. Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol. 2010;11:301–7. doi: 10.1038/nrm2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Y, Dodiya H, Aebischer P, Olanow CW, Kordower JH. Alterations in lysosomal and proteasomal markers in Parkinson’s disease: relationship to alpha-synuclein inclusions. Neurobiol Dis. 2009;35:385–98. doi: 10.1016/j.nbd.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Chu Y, Kordower JH. Age-associated increases of alpha-synuclein in monkeys and humans are associated with nigrostriatal dopamine depletion: Is this the target for Parkinson’s disease? Neurobiol Dis. 2007;25:134–49. doi: 10.1016/j.nbd.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Chu Y, Kordower JH. Lewy body pathology in fetal grafts. Ann N Y Acad Sci. 2010;1184:55–67. doi: 10.1111/j.1749-6632.2009.05229.x. [DOI] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106:13010–5. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda JE, Giasson BI, Mabon ME, Lee VM, Trojanowski JQ. Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Ann Neurol. 2002;52:205–10. doi: 10.1002/ana.10279. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–63. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Hansen C, Angot E, Bergstrom AL, Steiner JA, Pieri L, Paul G, Outeiro TF, Melki R, Kallunki P, Fog K, Li JY, Brundin P. alpha-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest. 2011;121:715–25. doi: 10.1172/JCI43366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakes R, Crowther RA, Lee VM, Trojanowski JQ, Iwatsubo T, Goedert M. Epitope mapping of LB509, a monoclonal antibody directed against human alpha-synuclein. Neurosci Lett. 1999;269:13–6. doi: 10.1016/s0304-3940(99)00411-5. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chen EY, Sladek JR, Jr., Mufson EJ. trk-immunoreactivity in the monkey central nervous system: forebrain. J Comp Neurol. 1994;349:20–35. doi: 10.1002/cne.903490103. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med. 2008a;14:504–6. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Olanow CW, Freeman TB. Transplanted dopaminergic neurons develop PD pathologic changes: a second case report. Mov Disord. 2008b;23:2303–6. doi: 10.1002/mds.22369. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Patel S, Lee SJ. Intravesicular localization and exocytosis of alpha-synuclein and its aggregates. J Neurosci. 2005;25:6016–24. doi: 10.1523/JNEUROSCI.0692-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Bjorklund A, Widner H, Revesz T, Lindvall O, Brundin P. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–3. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- Li JY, Englund E, Widner H, Rehncrona S, Bjorklund A, Lindvall O, Brundin P. Characterization of Lewy body pathology in 12- and 16-year-old intrastriatal mesencephalic grafts surviving in a patient with Parkinson’s disease. Mov Disord. 2010;25:1091–6. doi: 10.1002/mds.23012. [DOI] [PubMed] [Google Scholar]
- Steece-Collier K, Soderstrom KE, Collier TJ, Sortwell CE, Maries-Lad E. Effect of levodopa priming on dopamine neuron transplant efficacy and induction of abnormal involuntary movements in parkinsonian rats. J Comp Neurol. 2009;515:15–30. doi: 10.1002/cne.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ. New stereological methods for counting neurons. Neurobiol Aging. 1993;14:275–85. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]