Abstract
Parkinson’s disease (PD) is a progressive, neurodegenerative disorder for which there is currently no effective neuroprotective therapy. Patients are typically treated with a combination of drug therapies and/or receive deep brain stimulation to combat behavioral symptoms. The ideal candidate therapy would be the one which prevents neurodegeneration in the brain, thereby halting the progression of debilitating disease symptoms. Neurotrophic factors have been in the forefront of PD research, and clinical trials have been initiated using members of the GDNF family of ligands (GFLs). GFLs have been shown to be trophic to ventral mesencephalic cells, thereby making them good candidates for PD research. This paper examines the use of GDNF and neurturin, two members of the GFL, in both animal models of PD and clinical trials.
Keywords: neurotrophic factors, Parkinson’s disease, glial cell line-derived neurotrophic factor family ligands, GDNF, neurturin, gene therapy, clinical trials
Parkinson’s disease and neurotrophic factors
Parkinson’s disease (PD) is a progressive neurodegenerative disorder characterized by the cardinal motor symptoms of tremor, rigidity, bradykinesia, and postural instability (Parkinson’s Disease Foundation, 2009). In PD, there is a loss of dopamine in the striatum and degeneration of dopaminergic neurons within the substantia nigra pars compacta (Hwang et al., 2003). This causes dysfunction in the basal ganglia, ultimately resulting in impoverished thalamocortical innervation and the manifestation of the cardinal motor symptoms of the disease. While the pathology of PD is not limited to the nigrostriatal circuitry, it has been, to date, the focus of most therapeutic interventions. Due to the progressive nature of PD, many researchers have focused their efforts on the use of neuroprotective agents to rescue vulnerable nigral neurons before they are lost to disease. One particularly exciting novel therapy that has gained interest in recent decades has been the use of neurotrophic factors, molecules typically characterized for their role in neuronal development and maintenance. Neurotrophic factors have allowed researchers to expand their therapeutic reach, beyond merely augmenting dopaminergic function to replacing neurons lost to disease and rescuing intrinsic neuronal systems before they succumb.
The neurotrophic factors that have been commonly explored for use in the therapy of PD patients include the glial cell line-derived neurotrophic factor (GDNF) family ligands (GFLs), neurotrophins, and cytokines. GDNF and neurturin (NTN) are the two main members of the GFLs that have been widely tested in animal models of PD and clinically tested in PD patients.
Glial cell line-derived neurotrophic factor
GDNF supports the survival of several different neuronal populations, in both the central and the peripheral nervous system. GDNF’s potential therapeutic value for PD was first recognized in 1993 when it was purified and shown to promote the growth and survival of midbrain embryonic dopaminergic neurons (Lin et al., 1993). GDNF signaling is mediated via a multicomponent receptor complex consisting of a binding receptor (GDNF family receptor alpha, GFRα) that forms a ligand–receptor complex, which then is retrogradely transported from the target to the cell soma where it signals through a second receptor called Ret receptor tyrosine kinase (Sariola and Saarma, 2003). Serendipitously for PD therapy, all components of the GDNF signaling pathway are expressed at high levels in the striatum and substantia nigra pars compacta while the Ret receptor is in abundance only in the nigra. Therefore, GDNF can be injected into the striatum, and still provide trophic influence at the level of the midbrain.
GDNF is essential for the survival of dopaminergic neurons as shown in a conditional GDNF knockout mouse model. Down-regulation of GDNF, even by only 40% in adulthood, causes a marked reduction in dopaminergic neurons in the substantia nigra, the locus coeruleus, and ventral tegmental area (VTA). This neuronal loss is accompanied by a detectable hypokinetic movement disorder in mice (Pascual et al., 2008). These findings, illustrating that GDNF is protective for dopaminergic neurons, prompted several animal studies in PD models.
Injections in animal PD models
Initial studies using GDNF involved direct bolus injections of the trophic factor either into the striatum or lateral ventricle, or directly into the substantia nigra. Most of these studies initially used the 6-hydroxydopamine (6-OHDA) lesion model. 6-OHDA, when administered to the striatum, causes a progressive dying back of nigrostriatal fibers and eventually leads to cell loss in the substantia nigra (Rosenblad et al., 1999). In rats receiving lesions to either the striatum or the substantia nigra, GDNF administered to the nigra has differential effects on neuronal survival (Kearns and Gash, 1995). When injected directly into the striatum, GDNF preserves nigral neurons destined to die following the administration of 6-OHDA. In a second study, administration of GDNF to the region just above the substantia nigra 1-week post lesion results in a partial but significant protection of tyrosine hydroxylase (TH)-positive nigral neurons (Sauer et al., 1995). However, the neurons that do remain appear significantly atrophied, indicating that administering GDNF far from the site of lesion may cause a decrease in functionality of protected neurons. Furthermore, these studies did not examine the effects on TH-positive fibers in the nigrostriatal system, a critical component in preserving motor function.
Single bolus injections of GDNF have been used in other studies of rats receiving 6-OHDA lesions. As mentioned above, protection of nigral neurons is irrelevant if it is not accompanied by a preservation of function. A crucial study compared both the motor and the cellular benefits of administering GDNF to the striatum, nigra, or lateral ventricle prior to 6-OHDA delivery (Kirik et al., 2000a). When GDNF is administered to the striatum, both cell bodies in the nigra and TH-positive fibers in the striatum are significantly protected. More importantly, this neuroprotection is accompanied by a preservation of motor function, a far more relevant barometer of trophic factor efficacy. When administered directly to the nigra, GDNF protected nigral cell bodies and caused some local axonal sprouting, but did not promote the preservation of striatal TH levels. Furthermore, it did not significantly protect motor function in these rats. Finally, when GDNF is infused into the lateral ventricles, there is inefficient diffusion of the trophic factor from the cerebrospinal fluid. This causes GDNF-treated rats to be indistinguishable behaviorally from untreated rats receiving 6-OHDA.
Infusions in animal PD models
While the therapeutic value of GDNF was evident in the above-mentioned studies, it was recognized that the previously used methods of administration were inefficient. Researchers began to explore more long-term, sustainable ways of getting GDNF into target regions. Catheters were inserted into the brain and GDNF was infused over prolonged periods of time using pumps. In one such study, a catheter was inserted into the putamen of aged rhesus monkeys (Ai et al., 2003). This catheter was connected to a pump that was implanted subcutaneously in the abdominal region. The pump was programmed to continuously infuse GDNF into the putamen over 8 weeks. This delivery method effectively distributed the trophic factor up to 11 mm away from the site of catheter insertion. GDNF diffused to the rostral putamen, internal capsule, external capsule, caudate nucleus, and globus pallidus. Additionally, retrograde transport of GDNF was seen in nigral cell bodies. This transport of GDNF into adjacent areas translated into a significant improvement in the overall motor performance of these aged monkeys in the last 3 weeks of the study compared to controls (Maswood et al., 2002). Additionally, there was a 50% increase in dopamine levels in the ipsilateral caudate nucleus and a 390% increase in dopamine in the ipsilateral globus pallidus. Similar encouraging results were seen in a PD model using 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned rhesus monkeys that received continuous infusion of GDNF to either the lateral ventricle or the putamen (Grondin et al., 2002). GDNF promoted a significant anti-parkinsonian effect in monkeys that received the trophic factor to both the ventricle and the striatum. This positive motor effect was brought on by a very modest increase in overall TH-positive fiber density throughout the striatum. However, TH-positive fiber density was increased five-fold only in the immediate area surrounding the lateral ventricles, indicating that small areas that are efficiently delivered GDNF can experience robust trophic effects.
Experimental administration in PD human subjects
Based on the seemingly encouraging results from animal studies, the first clinical trial using GDNF in PD patients was initiated in 1996. This was a randomized, double-blinded study administering recombinant GDNF protein into the lateral ventricle using mechanical pumps (Nutt et al., 2003). Fifty patients, between the ages of 35 and 75 years, with moderate or advanced idiopathic PD were chosen for this study. Patients received either placebo or doses of GDNF varying between 25 and 4000 μg into the ventricles once a month over 8 months. Sixteen of these patients then received 4000 μg of GDNF for an additional 20 months in an open-labeled trial. When the study was unblinded after the first 8 months, results were disappointing. Not only did patients not improve but they also experienced several adverse events including nausea, vomiting, and anorexia for several days after GDNF administration. Patients who received higher doses of GDNF also experienced weight loss and depression symptoms. Even patients who received 4000 μg of GDNF in the 20-month open-label continuation of the trial did not show any improvements in either the “on” or “off” Unified Parkinson’s Disease Rating Scale (UPDRS) scores. The trial initiated by AMGEN was halted in September 2004 (Slevin et al., 2007). The lack of symptomatic relief seen in these patients may have been attributed to the inadequate penetration of GDNF from the cerebrospinal fluid into the adjacent striatum. Postmortem analysis in one patient from this study demonstrated that GDNF did not efficiently diffuse out of the lateral ventricles and thus was unable to elicit any effect in the striatum or the nigra (Kordower et al., 1999). It was evident that administering direct bolus injections of GDNF into the lateral ventricles was an inadequate method of trophic factor delivery. Studies have shown that intraputamental injection of GDNF in rhesus monkeys causes a variable distribution of the trophic factor with only 2–9% of the area receiving GDNF infusion (Salvatore et al., 2006). Furthermore, it has been shown that even convection-enhanced delivery of GDNF to slowly diffuse the factor into the target region is not desirable. There is a great deal of variability in GDNF diffusion using this method (Gash et al., 2005). There is little consistency in the distribution of GDNF in MPTP-treated rhesus monkeys even using the convection-enhanced delivery method. Researchers saw a volume of GDNF ranging anywhere from 59 to 325 mm3 in the putamen. This may be because GDNF easily binds to receptor sites in the extracellular matrix, impeding its even distribution (Hamilton et al., 2001). Despite these varying results in animal models using intraputamenal infusion, Amgen optimistically conducted an initial Phase I open-labeled trial using this method of delivery in five patients (Gill et al., 2003). All but one of the patients received bilateral infusion of GDNF into the posterior putamen for 43 months. The dose of GDNF was increased from 14.4 to 28.8 μg/putamen/day because of a decrease in benefit. This increased dose resulted in a sustained and progressive improvement (Patel et al., 2005). No adverse side effects were reported after 1 year of treatment, and in fact significant decreases were reported in both “on” and “off” UPDRS scores. Additionally, there was a 39% decrease in the off-medication motor score, a 61% improvement in the activities of daily living sub-score, a 20% decrease in severe immobility, a decrease in medication-induced dyskinesias, and a 28% increase in fluoro-dopa (18F-dopa) uptake in the posterior putamen. One of the five patients, who started receiving unilateral (right putamen) infusions of GDNF at the age of 62, died from a myocardial infarct 3 months after drug withdrawal (Love et al., 2005). Postmortem analyses of brain tissue indicated that there was a more than five-fold increase in tyrosine hydroxylase immunoreactivity in the right versus left putamen. However, due to the asymmetrical pathology seen in most PD patients, there was a higher level of TH immunoreactivity and higher numbers of neurons were seen in the left versus the right substantia nigra. Interestingly, there was an increase in growth-associated protein 43 (GAP43) staining in the right putamen, indicating that GDNF induces sprouting in substantia nigra fibers. The researchers note that the increase in TH staining in the putamen may either be a result of sprouting of fibers or an upregulation of the enzyme in spared fibers. These exciting results prompted a double-blinded, placebo-controlled study using 34 subjects, half of whom received placebo and the other half received 15 μg/putamen/day of GDNF. Unfortunately, bilateral infusion of GDNF into the putamen in this study did not significantly reduce UPDRS scores even after 6 months of treatment. Surprisingly, there was a 23% increase in 18F-dopa uptake in the posterior putamen. The discrepancy between the increase in 18F-dopa uptake and a lack of clinical benefit might indicate that although there is an increase in dopamine in the putamen as a result of GDNF treatment, it is not being efficiently released. The researchers state that they used a different-sized catheter to administer GDNF in this study compared to the initial open-labeled trial. Results from both the ventricular and the putamenal infusion studies indicate that direct administration of GDNF to the brain is not an efficient method of treatment. Therefore, a novel vehicle is needed to aid in the administration of GDNF to the striatal parenchyma.
Gene therapy in animal PD models
As trophic factor therapy in PD models was evolving, gene therapy approaches were evolving in parallel. Gene therapy employs viral vectors, which provide a safe and robust way to deliver trophic factors such as GDNF uniformly over very long periods of time.
Adenoviral vector-mediated gene therapy
One of the first vehicles to be used for the administration of GDNF was the adenoviral (Ad) vector. Marty Bohn and colleagues showed that a single injection of Ad-GDNF near the rat substantia nigra could sustain trophic factor expression for at least 7 weeks (Choi-Lundberg et al., 1997). Additionally, Ad-GDNF significantly protected TH-positive neurons in the substantia nigra from 6-OHDA-induced toxicity. It did not however, alter the expression of TH-positive fibers in the striatum, indicating that there may not have been any therapeutic consequences from solely treating the nigra. They repeated this study but this time administered the Ad-GDNF to the striatum, the site of dopamine fiber loss (Choi-Lundberg et al., 1998). Again they protected 40% of cells in the substantia nigra but did not maintain levels of TH in fibers of the striatum. Interestingly, Ad-GDNF administration to the striatum improved motor performance in treated rats, indicating that either a preservation of striatal TH-positive fibers was not necessary for behavioral improvement in this model or TH levels were increased to a level undetected by the methods used. A second group conducted a similar study and found that both nigral cells and striatal fibers were protected (Bilang-Bleuel et al., 1997). They also showed an attenuation of behavioral deficits as seen using the amphetamine-induced rotational paradigm. Adenoviral delivery of GDNF was the first to be used in animal studies of PD to successfully transfect cells with GDNF and protect both nigral neurons and fibers. However, the original version of this vector caused severe immune responses in the injected region (Bilang-Bleuel et al., 1997). Thus alternative, less immunogenic vectors had to be developed and tested. The two vectors that emerged were the lentiviral (LV) and the adeno-associated viral (AAV) vectors.
Lentiviral vector-mediated gene therapy
Subsequent to the adenoviral era, recombinant lentiviral (rLV) vectors were used to express GDNF in both the striatum and the substantia nigra in a 6-OHDA model of PD (Georgievska et al., 2002b). When expressed in the striatum, GDNF was successfully transported to nigral neurons where it protected 65–77% of these cells. This protection was dose-dependent and rats receiving a higher dose of GDNF showed a greater magnitude of cellular protection. However, fibers in the striatum were not significantly protected. Encouragingly though, fibers along the striato-nigral pathway were conserved as seen by intact fibers in the globus pallidus. Additionally, sprouting was seen in areas where GDNF was expressed at very high levels like in the globus pallidus and the immediately surrounding striatum. Irrespective of a lack of striatal fiber preservation, deficits in amphetamine-induced rotational behavior were prevented in rLV-GDNF rats compared to lesioned controls, indicating an increase in dopamine function on the GDNF-treated side. The lack of striatal preservation may have been due to the short time course of GDNF treatment. To confirm this theory, this group repeated this study and expressed GDNF in the striatum for 9 months using the rLV vector (Georgievska et al., 2002a). Unfortunately, they saw similar results, namely, neuroprotection in the nigra, striato-nigral fiber protection through the globus pallidus, but no fiber protection in the striatum. They reported a lack of functional recovery, which was attributed to a lack of dopamine in the striatum.
While the use of rodent models in the testing of therapies for PD is essential, ultimately any potential therapy likely must be tested in nonhuman primates before it can be used in the clinic. Therefore, the efficacy of LV-GDNF was tested both in aged (Fig. 1) rhesus monkeys and in monkeys lesioned 1 week before using the toxin MPTP (Kordower et al., 2000). In aged monkeys, LV-GDNF enhanced dopaminergic function. Aged monkeys receiving LV-GDNF treatment to the striatum showed an enhanced 18F-dopa uptake ipsilaterally. These monkeys had an increase in TH immunoreactivity in the striatum, an 85% increase in the number of TH-immunoreactive neurons within the substantia nigra, and a 35% increase in the volume of these neurons (Fig. 2). In MPTP-treated monkeys, LV-GDNF reversed functional deficits and completely prevented nigrostriatal degeneration. Monkeys receiving striatal LV-GDNF showed significant improvements in clinical rating scale scores during the 3-month period after GDNF treatment. Additionally, LV-GDNF treatment reversed motor deficits in an operant hand-reach task. LV-GDNF-treated monkeys also showed robust increases in 18F-dopa uptake on the impaired side compared to untreated controls. All LV-GDNF-treated monkeys displayed enhanced striatal TH levels and 32% more TH-positive nigral neurons compared to the intact side (Fig. 3).
Fig. 1.
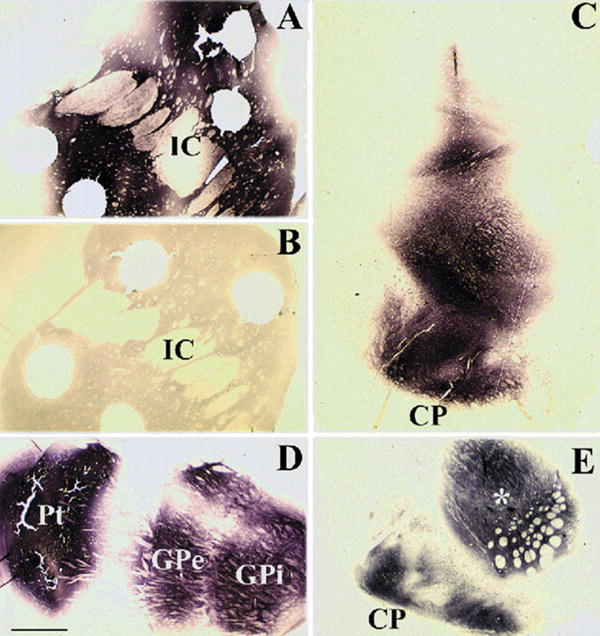
GDNF immunohistochemistry in aged monkeys receiving LV-GDNF or LV-βGal (control) in the striatum and substantia nigra. (A) Robust GDNF immunoreactivity is seen within the caudate and putamen in a LV-GDNF-treated aged monkey. (B) In contrast, no GDNF immunoreactivity is observed in a control LV-βGal-treated animal (IC, internal capsule). (C) Robust GDNF immunoreactivity is also observed within the midbrain of a LV-GDNF-treated monkey. (D) GDNF immunoreactivity within the forebrain of a LV-GDNF-treated monkey. Staining is seen within the injection site in the putamen (Pt) and within both segments of the globus pallidus (GPe and GPi) from anterograde transport. (E) GDNF immunohistochemistry is also seen in the substantia nigra pars reticulata from anterograde transport. Holes in the tissue are from postmortem for HPLC analysis. Asterisk in (E) represents a LV-GDNF injection site (CP, cerebral peduncle). Scale bar in (D) represents 1600 μm for panels A, B, and D; 1150 μm for panel C; and 800 μm for panel E.
Fig. 2.
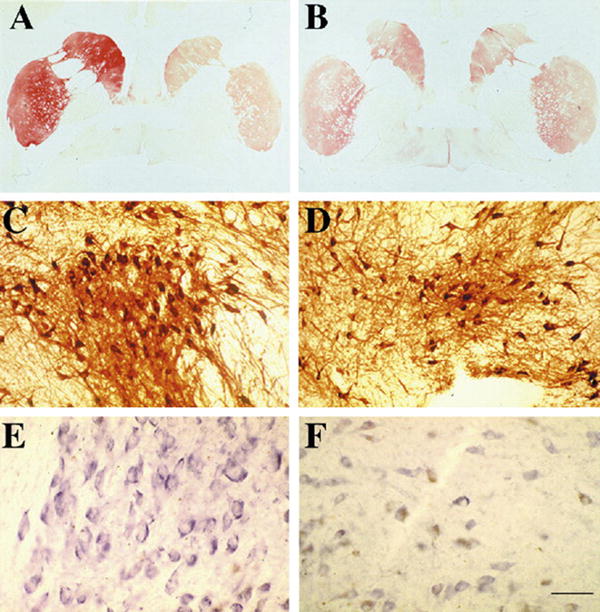
Tyrosine hydroxylase staining in aged monkeys receiving LV-GDNF to the right striatum. (A) LV-GDNF administration to the right striatum increases TH immunoreactivity within the right caudate and putamen in aged monkeys. (B) In monkeys receiving control LV-βGal injections to the right striatum, there is symmetrical and less intense staining for TH. (C) There are greater numbers and larger TH-immunoreactive neurons within the substantia nigra (SN) of LV-GDNF-treated animals relative to (D) a LV-βGal-treated monkeys. (E) LV-GDNF-treated aged monkeys display increased TH mRNA relative to (F) LV-βGal-treated monkeys in the SN. Scale bar in (F) represents 4500 μm for panels A and B; 250 μm for panels C and D; and 100 μm for panels E and F.
Fig. 3.
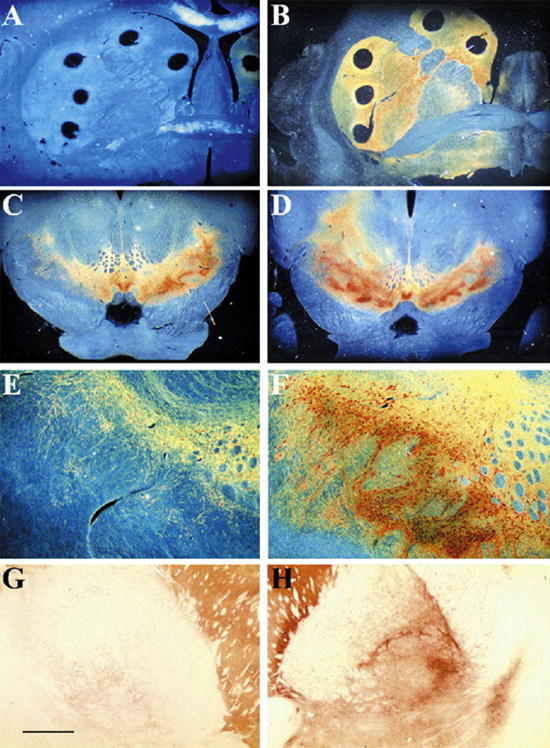
TH immunoreactivity in unilaterally MPTP-lesioned young monkeys. (A and B) Low-power dark-field photomicrographs through the right striatum of TH-immunostained sections of MPTP-treated monkeys treated with (A) LV-βGal or (B) LV-GDNF. (A) There is a comprehensive loss of TH immunoreactivity in the caudate and putamen of LV-βGal-treated animal. In contrast, near normal level of TH immunoreactivity is seen in LV-GDNF-treated animals. Low-power (C and D) and intermediate-power (E and F) photomicrographs of TH-immunostained section through the substantia nigra of animals treated with LV-βGal (C and E) and LV-GDNF (D and F). There is a loss of TH-immunoreactive neurons in the LV-βGal-treated animals on the side of the MPTP injection. TH-immunoreactive sprouting fibers as well as a supranormal number of TH-immunoreactive nigral perikarya are seen in LV-GDNF-treated animals on the side of the MPTP injection. (G and H) Bright-field low-power photomicrographs of a TH-immunostained section from a LV-GDNF-treated monkey. (G) Note the normal TH-immunoreactive fiber density through the globus pallidus on the intact side, which was not treated with LV-GDNF. (H) In contrast, an enhanced network of TH-immunoreactive fibers is seen on the side treated with both MPTP and LV-GDNF. Scale bar in (G) represents the following magnifications: panels A–D at 3500 μm and panels E–H at 1150 μm.
References
- Ai Y, Markesbery W, Zhang Z, Grondin R, Elseberry D, Gerhardt GA, et al. Intraputamenal infusion of GDNF in aged rhesus monkeys: distribution and dopaminergic effects. The Journal of Comparative Neurology. 2003;461:250–261. doi: 10.1002/cne.10689. [DOI] [PubMed] [Google Scholar]
- Akerud P, Alberch J, Eketjall S, Wagner J, Arenas E. Differential effects of glial cell line-derived neurotrophic factor and neurturin on developing and adult substantia nigra dopaminergic neurons. Journal of Neurochemistry. 1999;73:70–78. doi: 10.1046/j.1471-4159.1999.0730070.x. [DOI] [PubMed] [Google Scholar]
- Bilang-Bleuel A, Revah F, Colin P, Locquet I, Robert JJ, Mallet J, et al. Intrastriatal injection of an adenoviral vector expressing glial-cell-line-derived neurotrophic factor prevents dopaminergic neuron degeneration and behavioral impairment in a rat model of Parkinson disease. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:8818–8823. doi: 10.1073/pnas.94.16.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burazin TC, Gundlach AL. Localization of GDNF/neurturin receptor (c-ret, GFRalpha-1 and alpha-2) mRNAs in postnatal rat brain: differential regional and temporal expression in hippocampus, cortex and cerebellum. Brain Research Molecular Brain Research. 1999;73:151–171. doi: 10.1016/s0169-328x(99)00217-x. [DOI] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Lin Q, Chang YN, Chiang YL, Hay CM, Mohajeri H, et al. Dopaminergic neurons protected from degeneration by GDNF gene therapy. Science. 1997;275:838–841. doi: 10.1126/science.275.5301.838. [DOI] [PubMed] [Google Scholar]
- Choi-Lundberg DL, Lin Q, Schallert T, Crippens D, Davidson BL, Chang YN, et al. Behavioral and cellular protection of rat dopaminergic neurons by an adenoviral vector encoding glial cell line-derived neurotrophic factor. Experimental Neurology. 1998;154:261–275. doi: 10.1006/exnr.1998.6887. [DOI] [PubMed] [Google Scholar]
- Dowd E, Monville C, Torres EM, Wong LF, Azzouz M, Mazarakis ND, et al. Lentivector-mediated delivery of GDNF protects complex motor functions relevant to human Parkinsonism in a rat lesion model. The European Journal of Neuroscience. 2005;22:2587–2595. doi: 10.1111/j.1460-9568.2005.04414.x. [DOI] [PubMed] [Google Scholar]
- Eslamboli A, Cummings RM, Ridley RM, Baker HF, Muzyczka N, Burger C, et al. Recombinant adeno-associated viral vector (rAAV) delivery of GDNF provides protection against 6-OHDA lesion in the common marmoset monkey (Callithrix jacchus) Experimental Neurology. 2003;184:536–548. doi: 10.1016/j.expneurol.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Gash DM, Zhang Z, Ai Y, Grondin R, Coffey R, Gerhardt GA. Trophic factor distribution predicts functional recovery in parkinsonian monkeys. Annals of Neurology. 2005;58:224–233. doi: 10.1002/ana.20549. [DOI] [PubMed] [Google Scholar]
- Gasmi M, Brandon EP, Herzog CD, Wilson A, Bishop KM, Hofer EK, et al. AAV2-mediated delivery of human neurturin to the rat nigrostriatal system: long-term efficacy and tolerability of CERE-120 for Parkinson’s disease. Neurobiology of Disease. 2007a;27:67–76. doi: 10.1016/j.nbd.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Gasmi M, Herzog CD, Brandon EP, Cunningham JJ, Ramirez GA, Ketchum ET, et al. Striatal delivery of neurturin by CERE-120, an AAV2 vector for the treatment of dopaminergic neuron degeneration in Parkinson’s disease. Molecular Therapy. 2007b;15:62–68. doi: 10.1038/sj.mt.6300010. [DOI] [PubMed] [Google Scholar]
- Georgievska B, Kirik D, Bjorklund A. Aberrant sprouting and downregulation of tyrosine hydroxylase in lesioned nigrostriatal dopamine neurons induced by long-lasting overexpression of glial cell line derived neurotrophic factor in the striatum by lentiviral gene transfer. Experimental Neurology. 2002a;177:461–474. doi: 10.1006/exnr.2002.8006. [DOI] [PubMed] [Google Scholar]
- Georgievska B, Kirik D, Rosenblad C, Lundberg C, Bjorklund A. Neuroprotection in the rat Parkinson model by intrastriatal GDNF gene transfer using a lentiviral vector. Neuroreport. 2002b;13:75–82. doi: 10.1097/00001756-200201210-00019. [DOI] [PubMed] [Google Scholar]
- Gill SS, Patel NK, Hotton GR, O’Sullivan K, McCarter R, Bunnage M, et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nature Medicine. 2003;9:589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- Grondin R, Zhang Z, Yi A, Cass WA, Maswood N, Andersen AH, et al. Chronic, controlled GDNF infusion promotes structural and functional recovery in advanced parkinsonian monkeys. Brain. 2002;125:2191–2201. doi: 10.1093/brain/awf234. [DOI] [PubMed] [Google Scholar]
- Hamilton JF, Morrison PF, Chen MY, Harvey-White J, Pernaute RS, Phillips H, et al. Heparin coinfusion during convection-enhanced delivery (CED) increases the distribution of the glial-derived neurotrophic factor (GDNF) ligand family in rat striatum and enhances the pharmacological activity of neurturin. Experimental Neurology. 2001;168:155–161. doi: 10.1006/exnr.2000.7571. [DOI] [PubMed] [Google Scholar]
- Herzog CD, Dass B, Holden JE, Stansell J, III, Gasmi M, Tuszynski MH, et al. Striatal delivery of CERE-120, an AAV2 vector encoding human neurturin, enhances activity of the dopaminergic nigrostriatal system in aged monkeys. Movement Disorders. 2007;22:1124–1132. doi: 10.1002/mds.21503. [DOI] [PubMed] [Google Scholar]
- Horger BA, Nishimura MC, Armanini MP, Wang LC, Poulsen KT, Rosenblad C, et al. Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons. The Journal of Neuroscience. 1998;18:4929–4937. doi: 10.1523/JNEUROSCI.18-13-04929.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang DY, Ardayfio P, Kang UJ, Semina EV, Kim KS. Selective loss of dopaminergic neurons in the substantia nigra of Pitx3-deficient aphakia mice. Brain Research Molecular Brain Research. 2003;114:123–131. doi: 10.1016/s0169-328x(03)00162-1. [DOI] [PubMed] [Google Scholar]
- Kearns CM, Gash DM. GDNF protects nigral dopamine neurons against 6-hydroxydopamine in vivo. Brain Research. 1995;672:104–111. doi: 10.1016/0006-8993(94)01366-p. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Bjorklund A. Preservation of a functional nigrostriatal dopamine pathway by GDNF in the intrastriatal 6-OHDA lesion model depends on the site of administration of the trophic factor. The European Journal of Neuroscience. 2000a;12:3871–3882. doi: 10.1046/j.1460-9568.2000.00274.x. [DOI] [PubMed] [Google Scholar]
- Kirik D, Rosenblad C, Bjorklund A, Mandel RJ. Long-term rAAV-mediated gene transfer of GDNF in the rat Parkinson’s model: intrastriatal but not intranigral transduction promotes functional regeneration in the lesioned nigrostriatal system. The Journal of Neuroscience. 2000b;20:4686–4700. doi: 10.1523/JNEUROSCI.20-12-04686.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Emborg ME, Bloch J, Ma SY, Chu Y, Leventhal L, et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290:767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Herzog CD, Dass B, Bakay RA, Stansell J, III, Gasmi M, et al. Delivery of neurturin by AAV2 (CERE-120)-mediated gene transfer provides structural and functional neuroprotection and neurorestoration in MPTP-treated monkeys. Annals of Neurology. 2006;60:706–715. doi: 10.1002/ana.21032. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Palfi S, Chen EY, Ma SY, Sendera T, Cochran EJ, et al. Clinicopathological findings following intraventricular glial-derived neurotrophic factor treatment in a patient with Parkinson’s disease. Annals of Neurology. 1999;46:419–424. doi: 10.1002/1531-8249(199909)46:3<419::aid-ana21>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Lang AE, Gill S, Patel NK, Lozano A, Nutt JG, Penn R, et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Annals of Neurology. 2006;59:459–466. doi: 10.1002/ana.20737. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Liu WG, Lu GQ, Li B, Chen SD. Dopaminergic neuroprotection by neurturin-expressing c17.2 neural stem cells in a rat model of Parkinson’s disease. Parkinsonism & Related Disorders. 2007;13:77–88. doi: 10.1016/j.parkreldis.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Lo BC, Deglon N, Pralong W, Aebischer P. Lentiviral nigral delivery of GDNF does not prevent neurodegeneration in a genetic rat model of Parkinson’s disease. Neurobiology of Disease. 2004;17:283–289. doi: 10.1016/j.nbd.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Lo BC, Ridet JL, Schneider BL, Deglon N, Aebischer P. α-Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:10813–10818. doi: 10.1073/pnas.152339799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love S, Plaha P, Patel NK, Hotton GR, Brooks DJ, Gill SS. Glial cell line-derived neurotrophic factor induces neuronal sprouting in human brain. Nature Medicine. 2005;11:703–704. doi: 10.1038/nm0705-703. [DOI] [PubMed] [Google Scholar]
- Mandel RJ, Spratt SK, Snyder RO, Leff SE. Midbrain injection of recombinant adeno-associated virus encoding rat glial cell line-derived neurotrophic factor protects nigral neurons in a progressive 6-hydroxydopamine-induced degeneration model of Parkinson’s disease in rats. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14083–14088. doi: 10.1073/pnas.94.25.14083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks WJ, Jr, Ostrem JL, Verhagen L, Starr PA, Larson PS, Bakay RA, et al. Safety and tolerability of intraputaminal delivery of CERE-120 (adeno-associated virus serotype 2-neurturin) to patients with idiopathic Parkinson’s disease: an open-label, phase I trial. Lancet Neurology. 2008;7:400–408. doi: 10.1016/S1474-4422(08)70065-6. [DOI] [PubMed] [Google Scholar]
- Maswood N, Grondin R, Zhang Z, Stanford JA, Surgener SP, Gash DM, et al. Effects of chronic intraputamenal infusion of glial cell line-derived neurotrophic factor (GDNF) in aged Rhesus monkeys. Neurobiology of Aging. 2002;23:881–889. doi: 10.1016/s0197-4580(02)00022-2. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Burchiel KJ, Comella CL, Jankovic J, Lang AE, Laws ER, Jr, et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60:69–73. doi: 10.1212/wnl.60.1.69. [DOI] [PubMed] [Google Scholar]
- Oiwa Y, Yoshimura R, Nakai K, Itakura T. Dopaminergic neuroprotection and regeneration by neurturin assessed by using behavioral, biochemical and histochemical measurements in a model of progressive Parkinson’s disease. Brain Research. 2002;947:271–283. doi: 10.1016/s0006-8993(02)02934-7. [DOI] [PubMed] [Google Scholar]
- Parkinson’s Disease Foundation. 2009 Available at www.pdf.org.
- Pascual A, Hidalgo-Figueroa M, Piruat JI, Pintado CO, Gomez-Diaz R, Lopez-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nature Neuroscience. 2008;11:755–761. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- Patel NK, Bunnage M, Plaha P, Svendsen CN, Heywood P, Gill SS. Intraputamenal infusion of glial cell line-derived neurotrophic factor in PD: a two-year outcome study. Annals of Neurology. 2005;57:298–302. doi: 10.1002/ana.20374. [DOI] [PubMed] [Google Scholar]
- Rosenblad C, Kirik D, Devaux B, Moffat B, Phillips HS, Bjorklund A. Protection and regeneration of nigral dopaminergic neurons by neurturin or GDNF in a partial lesion model of Parkinson’s disease after administration into the striatum or the lateral ventricle. The European Journal of Neuroscience. 1999;11:1554–1566. doi: 10.1046/j.1460-9568.1999.00566.x. [DOI] [PubMed] [Google Scholar]
- Salvatore MF, Ai Y, Fischer B, Zhang AM, Grondin RC, Zhang Z, et al. Point source concentration of GDNF may explain failure of phase II clinical trial. Experimental Neurology. 2006;202:497–505. doi: 10.1016/j.expneurol.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Sariola H, Saarma M. Novel functions and signalling pathways for GDNF. Journal of Cell Science. 2003;116:3855–3862. doi: 10.1242/jcs.00786. [DOI] [PubMed] [Google Scholar]
- Sauer H, Rosenblad C, Bjorklund A. Glial cell line-derived neurotrophic factor but not transforming growth factor beta 3 prevents delayed degeneration of nigral dopaminergic neurons following striatal 6-hydroxydopamine lesion. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:8935–8939. doi: 10.1073/pnas.92.19.8935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slevin JT, Gash DM, Smith CD, Gerhardt GA, Kryscio R, Chebrolu H, et al. Unilateral intraputamenal glial cell line-derived neurotrophic factor in patients with Parkinson disease: response to 1 year of treatment and 1 year of withdrawal. Journal of Neurosurgery. 2007;106:614–620. doi: 10.3171/jns.2007.106.4.614. [DOI] [PubMed] [Google Scholar]
- Tseng JL, Bruhn SL, Zurn AD, Aebischer P. Neurturin protects dopaminergic neurons following medial forebrain bundle axotomy. Neuroreport. 1998;9:1817–1822. doi: 10.1097/00001756-199806010-00027. [DOI] [PubMed] [Google Scholar]
- Wang L, Muramatsu S, Lu Y, Ikeguchi K, Fujimoto K, Okada T, et al. Delayed delivery of AAV-GDNF prevents nigral neurodegeneration and promotes functional recovery in a rat model of Parkinson’s disease. Gene Therapy. 2002;9:381–389. doi: 10.1038/sj.gt.3301682. [DOI] [PubMed] [Google Scholar]
- Ye M, Wang XJ, Zhang YH, Lu GQ, Liang L, Xu JY, et al. Transplantation of bone marrow stromal cells containing the neurturin gene in rat model of Parkinson’s disease. Brain Research. 2007;1142:206–216. doi: 10.1016/j.brainres.2006.12.061. [DOI] [PubMed] [Google Scholar]


