Abstract
Transfer RNA's were isolated from Euglena gracilis. Chloroplast cistrons for tRNA were quantitated by hybridizing tRNA to ct DNA. Species of tRNA hybridizing to ct DNA were partially purified by hybridization-chromatography. The tRNA's hybridizing to ct DNA and nuclear DNA appear to be different. Total cellular tRNA was hybridized to ct DNA to an equivalent of approximately 25 cistrons. The total cellular tRNA was also separated into 2 fractions by chromatography on dihydroxyboryl substituted amino ethyl cellulose. Fraction I hybridized to both nuclear and ct DNA. Hybridizations to ct DNA indicated approximately 18 cistrons. Fraction II-tRNA hybridized only to ct DNA, saturating at a level of approximately 7 cistrons. The tRNA from isolated chloroplasts hybridized to both chloroplast and nuclear DNA. The level of hybridization to ct DNA indicated approximately 18 cistrons. Fraction II-type tRNA could not be detected in the isolated chloroplasts.
Full text
PDF





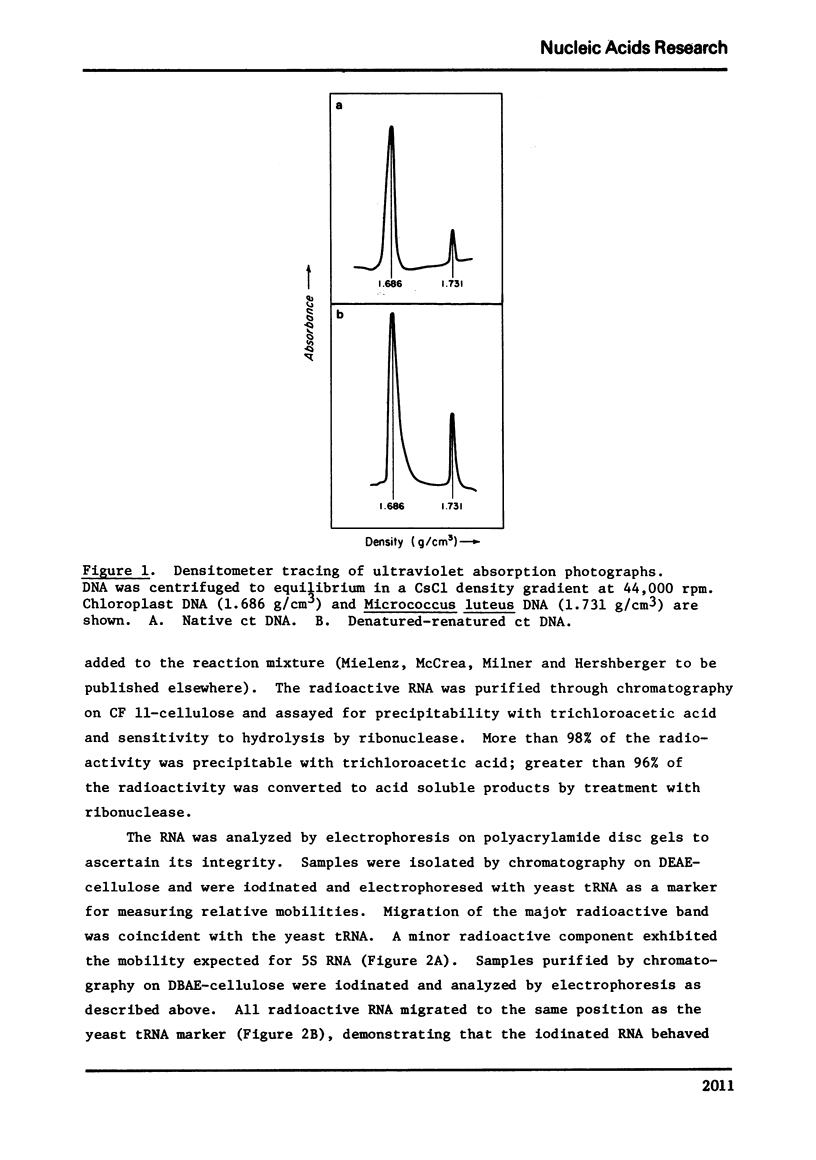
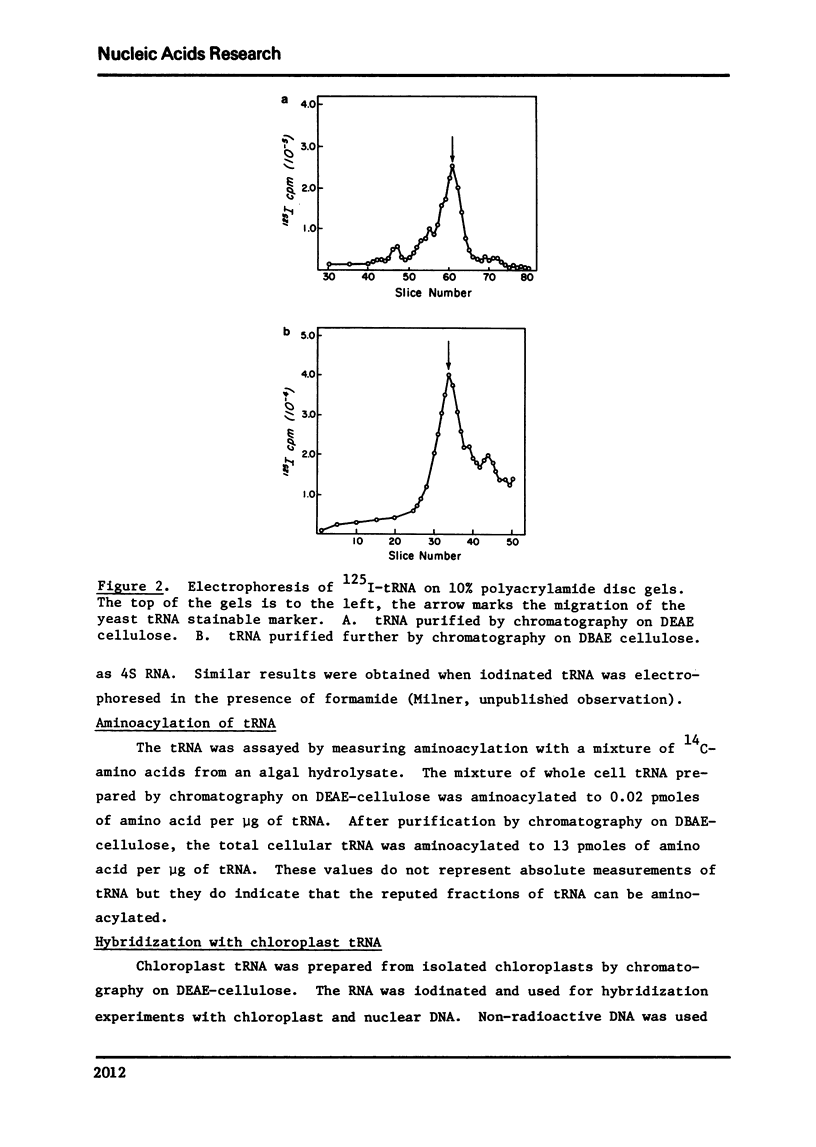
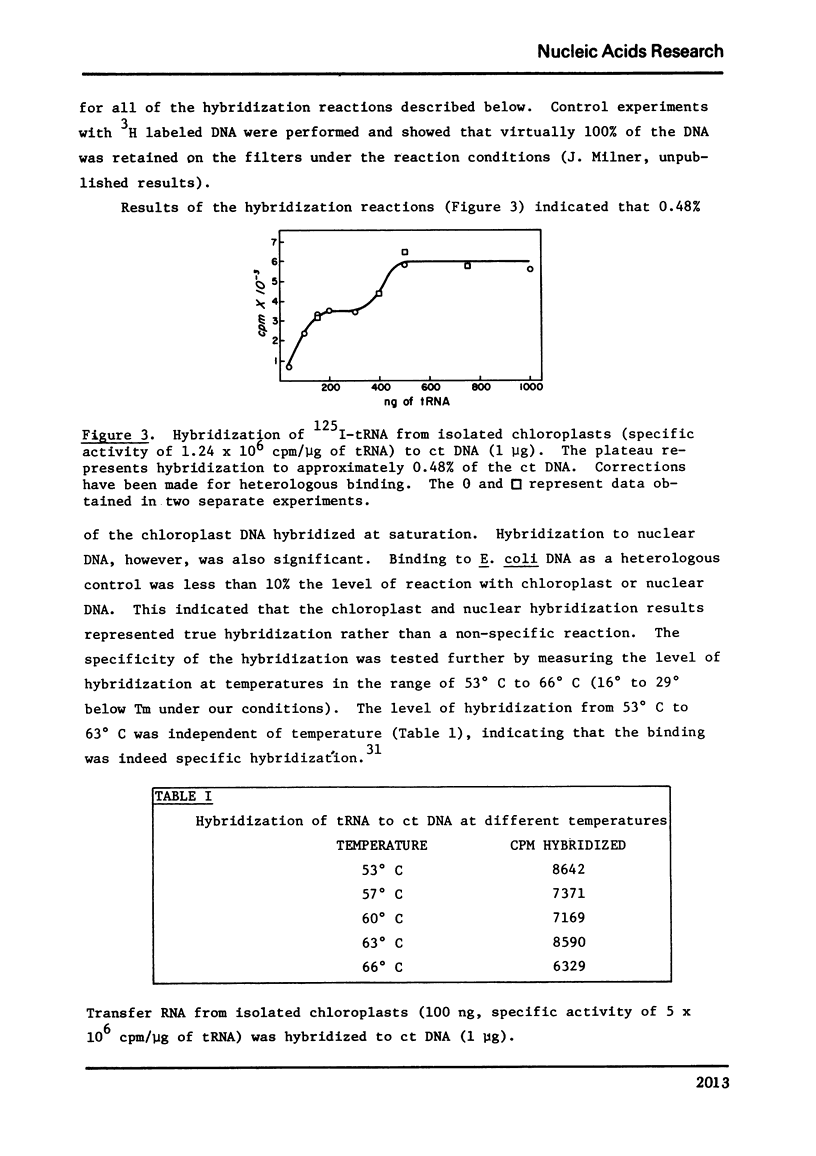

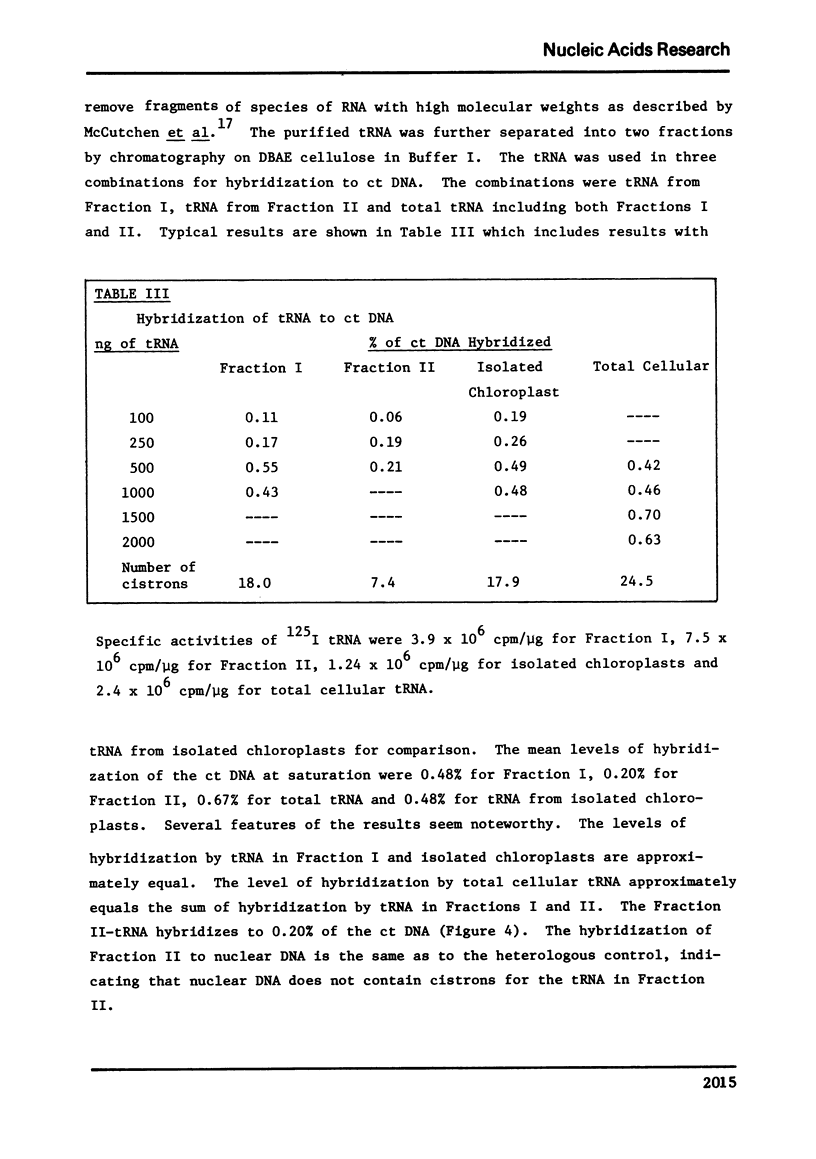


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett W. E., Pennington C. J., Jr, Fairfield S. A. Induction of euglena transfer RNA's by light. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1261–1268. doi: 10.1073/pnas.63.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkard G., Eclancher B., Weil J. H. Presence of N-formyl-methionyl-transfer RNA in bean chloroplasts. FEBS Lett. 1969 Aug;4(4):285–287. doi: 10.1016/0014-5793(69)80257-7. [DOI] [PubMed] [Google Scholar]
- Burkard G., Guillemaut P., Weil J. H. Comparative studies of the tRNA's and the aminoacyl-tRNA synthetases from the cytoplasm and the chloroplasts of Phaseolus vulgaris. Biochim Biophys Acta. 1970 Nov 12;224(1):184–198. doi: 10.1016/0005-2787(70)90632-5. [DOI] [PubMed] [Google Scholar]
- Carnevali F., Falcone C., Frontali L., Leoni L., Macino G., Palleschi C. Informational content of mitochondrial DNA from a "low density" petite mutant of yeast. Biochem Biophys Res Commun. 1973 Apr 2;51(3):651–658. doi: 10.1016/0006-291x(73)91364-8. [DOI] [PubMed] [Google Scholar]
- Casey J. W., Hsu H. J., Getz G. S., Rabinowitz M. Transfer RNA genes in mitochondrial DNA of grande (wild-type) yeast. J Mol Biol. 1974 Oct 5;88(4):735–747. doi: 10.1016/0022-2836(74)90396-9. [DOI] [PubMed] [Google Scholar]
- Casey J., Cohen M., Rabinowitz M., Fukuhara H., Getz G. S. Hybridization of mitochondrial transfer RNA's with mitochondrial and nuclear DNA of grande (wild type) yeast. J Mol Biol. 1972 Feb 14;63(3):431–440. doi: 10.1016/0022-2836(72)90438-x. [DOI] [PubMed] [Google Scholar]
- Chiu N., Chiu A., Suyama Y. Native and imported transfer RNA in mitochondria. J Mol Biol. 1975 Nov 25;99(1):37–50. doi: 10.1016/s0022-2836(75)80157-4. [DOI] [PubMed] [Google Scholar]
- Clarkson S. G., Birnstiel M. L., Serra V. Reiterated transfer RNA genes of Xenopus laevis. J Mol Biol. 1973 Sep 15;79(2):391–410. doi: 10.1016/0022-2836(73)90013-2. [DOI] [PubMed] [Google Scholar]
- Cohen M., Casey J., Rabinowitz M., Getz G. S. Hybridization of mitochondrial transfer RNA and mitochondrial DNA in petite mutants of yeast. J Mol Biol. 1972 Feb 14;63(3):441–451. doi: 10.1016/0022-2836(72)90439-1. [DOI] [PubMed] [Google Scholar]
- Cohen M., Rabinowitz M. Analysis of grande and petite yeast mitochondrial DNA by tRNA hybridization. Biochim Biophys Acta. 1972 Oct 11;281(2):192–201. doi: 10.1016/0005-2787(72)90171-2. [DOI] [PubMed] [Google Scholar]
- Doolittle W. F., Pace N. R. Transcriptional organization of the ribosomal RNA cistrons in Escherichia coli. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1786–1790. doi: 10.1073/pnas.68.8.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISENSTADT J. M., BRAWERMAN G. THE PROTEIN-SYNTHESIZING SYSTEMS FROM THE CYTOPLASM AND THE CHLOROPLASTS OF EUGLENA GRACILIS. J Mol Biol. 1964 Dec;10:392–402. doi: 10.1016/s0022-2836(64)80060-7. [DOI] [PubMed] [Google Scholar]
- Fonty G., Crouse E. J., Stutz E., Bernardi G. The mitochondrial genome of Euglena gracilis. Eur J Biochem. 1975 Jun;54(2):367–372. doi: 10.1111/j.1432-1033.1975.tb04147.x. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Guillemaut P., Steinmetz A., Burkard G., Weil J. H. Aminoacylation of tRNA-Leu species from Escherichia coli and from the cytoplasm, chloroplasts and mitochondria of Phaseolus vulgaris by homologous and heterologous enzymes. Biochim Biophys Acta. 1975 Jan 6;378(1):64–72. doi: 10.1016/0005-2787(75)90137-9. [DOI] [PubMed] [Google Scholar]
- Halbreich A., Rabinowitz M. Isolation of Saccharomyces cerevisiae mitochondrial formyltetrahydrofolic acid:methionyl-tRNA transformylase and the hybridization of mitochondrial fMet-tRNA with mitochondrial DNA. Proc Natl Acad Sci U S A. 1971 Feb;68(2):294–298. doi: 10.1073/pnas.68.2.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy B. J., Church R. B. The specificity of molecular hybridization reactions. Annu Rev Biochem. 1970;39:131–150. doi: 10.1146/annurev.bi.39.070170.001023. [DOI] [PubMed] [Google Scholar]
- McCutchan T. F., Gilham P. T., Söll D. An improved method for the purification of tRNA by chromatography on dihydroxyboryl substituted cellulose. Nucleic Acids Res. 1975 Jun;2(6):853–864. doi: 10.1093/nar/2.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orosz J. M., Wetmur J. G. In vitro iodination of DNA. Maximizing iodination while minimizing degradation; use of buoyant density shifts for DNA-DNA hybrid isolation. Biochemistry. 1974 Dec 31;13(27):5467–5473. doi: 10.1021/bi00724a003. [DOI] [PubMed] [Google Scholar]
- Peacock A. C., Dingman C. W. Resolution of multiple ribonucleic acid species by polyacrylamide gel electrophoresis. Biochemistry. 1967 Jun;6(6):1818–1827. doi: 10.1021/bi00858a033. [DOI] [PubMed] [Google Scholar]
- Poonian M. S., Schlabach A. J., Weissbach A. Covalent attachment of nucleic acids to agarose for affinity chromatography. Biochemistry. 1971 Feb 2;10(3):424–427. doi: 10.1021/bi00779a011. [DOI] [PubMed] [Google Scholar]
- Roe B. A. Studies on human tRNA. I. The rapid, large scale isolation and partial fractionation of placenta and liver tRNA. Nucleic Acids Res. 1975 Jan;2(1):21–42. doi: 10.1093/nar/2.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santi D. V., Anderson R. T., Jr A deae-cellulose filter disk assay for aminoacyl-tRNA. Anal Biochem. 1974 Mar;58(1):175–182. doi: 10.1016/0003-2697(74)90455-2. [DOI] [PubMed] [Google Scholar]
- Schwartz J. H., Meyer R., Eisenstadt J. M., Brawerman G. Involvement of N-formylmethionine in initiation of protein synthesis in cell-free extracts of Euglena gracilis. J Mol Biol. 1967 May 14;25(3):571–574. doi: 10.1016/0022-2836(67)90210-0. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Martin M. A. A general method of gene isolation. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1697–1700. doi: 10.1073/pnas.70.6.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolarsky L., Walfield A. M., Birch R. A., Hershberger C. L. Light-stimulated synthesis of chloroplast DNA. Biochim Biophys Acta. 1976 Apr 2;425(4):438–450. doi: 10.1016/0005-2787(76)90008-3. [DOI] [PubMed] [Google Scholar]
- Williams G. R., Williams A. S., George S. A. Hybridization of leucyl-transfer ribonucleic Acid isoacceptors from green leaves with nuclear and chloroplast deoxyribonucleic Acid. Proc Natl Acad Sci U S A. 1973 Dec;70(12 Pt 1-2):3498–3501. doi: 10.1073/pnas.70.12.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]



