Abstract
Although dystonias are a common group of movement disorders the mechanisms by which brain dysfunction results in dystonia are not understood. Rapid-onset Dystonia-Parkinsonism is a hereditary dystonia caused by mutations in the ATP1A3 gene. Affected subjects can be symptom free for years but rapidly develop persistent dystonia and parkinsonism-like symptoms after a stressful experience. Using a mouse model here we show that an adverse interaction between the cerebellum and basal ganglia can account for the symptoms of the patients. The primary instigator of dystonia is the cerebellum whose aberrant activity alters basal ganglia function which in turn causes dystonia. This adverse interaction between the cerebellum and basal ganglia is mediated through a di-synaptic thalamic pathway which when severed is effective in alleviating dystonia. Our results provide a unifying hypothesis for the involvement of cerebellum and basal ganglia in generation of dystonia and suggest therapeutic strategies for the treatment of RDP.
Introduction
Dystonias, characterized by prolonged co-contraction of the opposing agonist and antagonist muscles, comprise the third most common movement disorder after Parkinson’s disease and essential tremor 1. Although most dystonias are idiopathic 2, mutations in at least seventeen genes have been implicated in its hereditary forms 3. Rapid-onset Dystonia-Parkinsonism (RDP), DYT12, is a hereditary dystonia caused by loss of function mutations in the α3 isoform of the sodium-potassium ATPase pump (sodium pump) 4. Subjects carrying these mutations show few symptoms prior to the sudden onset of the disease which is often triggered by an extremely stressful event 4,5. The stressful event rapidly produces a combination of dystonia and parkinsonism (primarily akinesia) frequently accompanied with dysarthria, dysphagia, slurred speech, postural instability, and wide stance 5. The symptoms are permanent although in some cases improve slightly with time 4,5.
There is currently no treatment for RDP 5. Moreover, despite our detailed appreciation of the role of the sodium pump in the generation and maintenance of intracellular ionic gradients how and why the mutations cause dystonia is not understood. Even the identities of the brain regions affected remain elusive. Scrutiny of hereditary dystonia and exploration of their therapeutic options in general have been limited by the fact that their genetic animal models have routinely failed to reproduce their pathophysiology 2,6. In the case of RDP, neither of the two available genetic mouse models show dystonia or dyskinesia 7–9, although rodents are fully capable of manifesting dystonia. The reason for the inability of available genetic animal models of dystonia to fully capture the human symptoms is not established although it may stem from differences in compensatory mechanisms during brain development in rodents compared with humans.
In contrast to most hereditary dystonias where the function of the mutated protein is poorly understood, in the case of RDP the role of the sodium pump in the generation and maintenance of intracellular ionic gradients is well established. Moreover, the function of the sodium pump can be pharmacologically manipulated using its high affinity and exquisitely selective blocker, ouabain 10, which has ≈ 1000× higher selectivity for the mutated α3 isoform vs. the other neuronal α1 isoform 11. This allowed for generation of a pharmacologic animal model of RDP and bypassing the concerns and complications associated with compensatory mechanisms in the genetic models. We found that dysfunction of sodium pumps in the both the cerebellum (CB) and the basal ganglia (BG) was required to replicate the salient features of RDP. Mice whose cerebellum and basal ganglia were simultaneously perfused with ouabain showed mild symptoms which rapidly transformed to persistent dystonia and rigidity after stress. We found that involuntary dystonic movements were caused by aberrant cerebellar activity and that both pharmacologically reducing cerebellar activity and silencing cerebellar output with selective electrical lesions of its output nuclei were effective in alleviating dystonia. Lastly, to reconcile the fact that dystonia is primarily associated with basal ganglia function we tested the hypothesis that aberrant cerebellar activity adversely affected basal ganglia function which in turn caused dystonia. In agreement with this hypothesis, we found that severing the di-synaptic link between the cerebellum and basal ganglia by selectively lesioning the centro-lateral nucleus of the thalamus was remarkably effective in alleviating cerebellar-induced dystonia. This data provide a unifying hypothesis to account for the involvement of cerebellum and basal ganglia in the generation of dystonia, and inspire therapeutic approaches for the treatment of RDP.
Results
To identify the neural substrates of RDP, we stereotaxically implanted guide canula into select brain regions of mice and chronically or acutely perfused low amounts of ouabain to partially block sodium pumps. This permitted us to pharmacologically replicate the loss-of-function sodium pump mutations that afflict RDP patients and to examine consequences for motor function.
BG sodium pump dysfunction causes parkinsonism
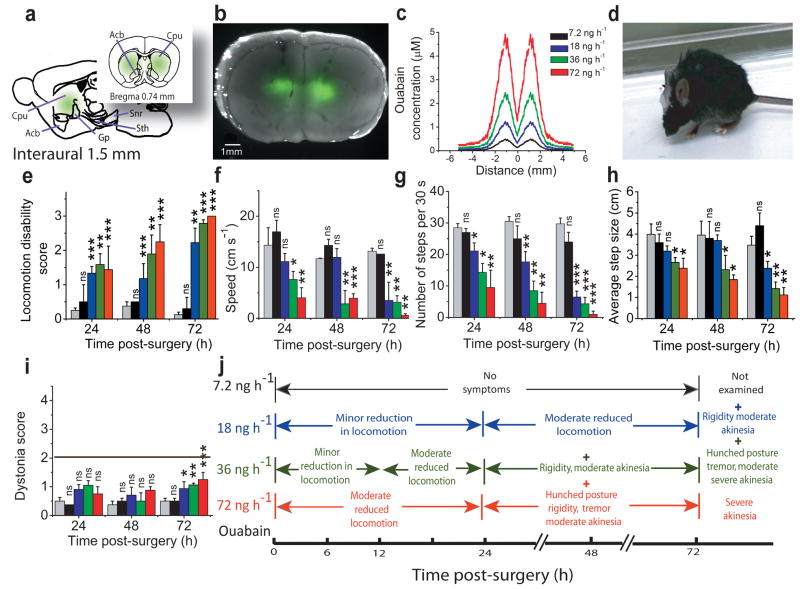
We first bilaterally targeted the basal ganglia (n=35). These subcortical structures are involved in the selection and execution of voluntary movements and their dysfunction is the main cause of Parkinsonism 12 and nonidiopathic dystonias 1. The ouabain-perfused region contained the caudate, putamen, globus pallidus, and nucleus accumbens (Figures 1a-c, Supplementary Data 1). The main consequence of perfusion of ouabain into the basal ganglia was reduced locomotor activity, rigidity, hunched posture, postural instability and tremor (Figure 1d,j & Supplementary Video 1).
Figure 1. Chronic partial blockade of basal ganglia sodium pumps induces Parkinsonism-like symptoms.
(a) Sagittal and coronal schematics of the basal ganglia showing the anatomical structures targeted by chronic bilateral perfusion of ouabain. Use of bodipy-FL-ouabain, a fluorescent analogue of ouabain, allowed determination of the extent of its diffusion in vivo.
(b) Merge of the bright field picture of a coronal section of the basal ganglia with the fluorescence intensity profile obtained after perfusion of 18 ng/h bodipy-FL-ouabain for 72 h.
(c) The concentration of ouabain in the tissue was estimated by quantitative fluorescence microscopy using the fluorescence emitted by Bodipy-FL-ouabain. The highest concentration of ouabain was obtained close to the canula and dropped monotonically as a function of lateral distance. The color key legend applies to all panels in this figure.
(d) Hunched posture, rigidity and akinesia in a mouse after 72 h chronic perfusion of 36 ng/h ouabain.
(e–h) Effects of chronic perfusion of 0–72 ng/h ouabain into the basal ganglia on overall locomotion (e), speed (f), average number of steps (g) and step size (h) as a function of time after start of ouabain perfusion. (mean±s.e.m; n= 4 for vehicle; 4 for 7.2 ng/h ouabain; 13 for 18 ng/h; 11 for 36 ng/h; and 3 for 72 ng/h).
(i) Assessment of the impact of bilateral perfusion of ouabain into the basal ganglia on motor function using a dystonia rating scale for the same animals described above. With the scale used, only a score of 2 or more denotes dystonia. mean±s.e.m.
(j) Summary of symptoms associated with chronic bilateral perfusion of ouabain into the basal ganglia for 24, 48 and 72 hours.
The impact of partial dysfunction of basal ganglia sodium pumps on locomotion was quantified by adapting a locomotion disability rating scale used for Parkinson’s disease in humans 13 for use in mice. Perfusion of ouabain into the basal ganglia reduced locomotion (Figure 1e) in both a time- and concentration-dependent manner (F=6.7; P=0.0043). This reduced mobility was manifest as decreases in all motor parameters examined (Figures 1f–h): number of steps taken in 30 s (F=15.07; P<0.001), the average step size (F=7.04; P=0.0018), and locomotion speed (F=9.8; P<0.0001). In contrast, perfusion of vehicle into the basal ganglia for extended periods (up to 8 days) did not produce any detectable symptoms.
The symptoms produced by perfusion of ouabain into the basal ganglia (Figure 1j) such as rigidity, akinesia, and tremor are hallmarks of parkinsonism-like symptoms in mice 14 and thus mimic a number of symptoms seen in RDP patients 4,5. Concurrent with manifestation of these symptoms the mice also showed a pronounced hunched posture. This hunched posture closely resembled the forward flexion of the thoracolumbar spine (camptocormia) seen in patients suffering from Parkinson’s disease. In humans parkinsonism camptocormia is not typically considered to be caused by dystonia 15.
Despite producing clear parkinsonism-like symptoms and the hunched posture, perfusion of ouabain into the basal ganglia did not cause involuntary dystonic-like movements in any of the animals examined at any time or concentration even when monitored for >8 days (F=3.06; P=0.064; Fig 1I,J). Similarly, chronic perfusion of high amounts of ouabain into the lateral ventricles for several days (360 ng/h), or primarily targeting individual basal ganglia output nuclei (globus pallidus, substantia nigra, and the entopeduncular nucleus) did not produce any form of dystonic postures (Supplementary Data 2).
CB sodium pump dysfunction produces ataxia and dystonia
We next examined the potential role of the cerebellum in RDP since i) a role for the cerebellum in producing dystonia is not unprecedented 16,17, and ii) its principal neurons, the Purkinje cells, exclusively express the α3 isoform of the sodium pumps that is mutated in RDP 18. Without affecting the neighboring brain regions, cerebellar sodium pumps were selectively targeted by midline chronic perfusion of ouabain in vivo (n= 51; Figures 2a–c). Cerebellar perfusion of ouabain resulted in ataxia and clear dystonic-like postures (Figure 2d and Supplementary video 2) in a time and concentration-dependent manner. This was quantitatively reflected as reduced locomotion (F=25.43; P<0.0001), and as high dystonia scores (F=7.8; P<0.0003; Figures 2e,f). The motor symptoms appeared initially as ataxia which in time transformed to dystonic-like postures and (with a higher concentration of ouabain) to generalized dystonia (Figures 2d,g). Electromyogram (EMG) recordings in agonist and antagonist muscles of the hind limb and examination of the corresponding cross-correlations of the EMG signals confirmed that the dystonic-like postures were caused by prolonged co-contraction of the opposing muscle groups (n=3; Supplementary data 3). These dystonic episodes were not caused by seizures since electroencephalogram (EEG) recordings showed that they were not accompanied with epileptic activity in the motor cortex (n=5; Supplementary data 3). Moreover, targeting perfusion of ouabain to only one of the cerebellar hemispheres generated comparable but unilateral symptoms.
Figure 2. Chronic partial blockade of cerebellar sodium pumps results in ataxia and dystonic-like postures.
(a) Coronal schematic of the cerebellum showing the regions affected by chronic perfusion of ouabain.
(b) Merge of the bright field picture of a coronal section of the cerebellum with the fluorescence intensity profile obtained after perfusion of 18 ng/h bodipy-FL-ouabain for 18 h.
(c) Estimated concentration of ouabain in the cerebellum as a function of lateral distance from the center of the canula with different concentrations of perfused ouabain.
(d) Dystonic postures in a mouse caused by perfusion of 36 ng/h ouabain into the cerebellum for 18 hours. Arrows point to the commonly observed hyperextensions of anterior and posterior limbs.
(e) Effects of chronic perfusion of 0–72 ng/h ouabain into the cerebellum on locomotor activity in mice at 24, 48 and 72 hours. The decreases in locomotion reflected in the locomotion disability scores were a reflection of the ataxia and dystonia in these mice and not akinesia and rigidity as seen when ouabain was perfused into the basal ganglia. mean±s.e.m.
(f) Severity of ouabain-induced dystonia in mice chronically perfused with 0–72 ng/h ouabain into the cerebellum at 4, 12, 24, 48 and 72h. mean±s.e.m.
(g) Symptoms associated with chronic perfusion of ouabain into the cerebellum.
Stress-induced dystonia with dysfunction of both BG & CB
The data presented so far are consistent with the involvement of both the basal ganglia and cerebellum in generation of RDP symptoms. However, a hallmark of RDP is that subjects carrying the defective gene do not fully manifest the symptoms until a very stressful event which then abruptly produces the disease phenotype. We sought to explore whether we could replicate this aspect of the disorder.
With concomitant perfusion of low (18 ng/h) concentrations of ouabain into both the basal ganglia and the cerebellum the mice showed mild, but stable dyskinesia but not dystonia (n=7; Supplementary data 4; Supplementary video 3). This is somewhat similar to the pre-stress condition in RDP patients. Individuals carrying the mutated gene are not always completely free of symptoms and show, to a varying extent, parkinsonism, cramping and mild focal dystonic spasms in arms and limbs 5. We then tested whether subjecting these mice to stress could precipitate the permanent RDP symptoms.
In RDP the stressful event can be both psychological and physical in nature with physical exertion and elevated body temperature being common triggers 5. Following brief chronic perfusion of 18 ng/h ouabain (typically for 10–24 hours) mice were stressed for two hours by random exposure to electric foot shocks in a warm environment (≈38 °C). In 10 out of 14 animals examined stress immediately increased the severity of dyskinesia and resulted in generation of mild to severe dystonia (Figures 3a,b,d; P<0.001; Supplementary video 4). Similar to that seen in RDP patients, behavioral observations and EMG recordings confirmed that the dystonia in these animals persisted for as long as monitored although sometimes the severity lessened (Figure 3d). In 4 of the 14 animals examined stress did not affect the dyskinesia even when repeated a second time.
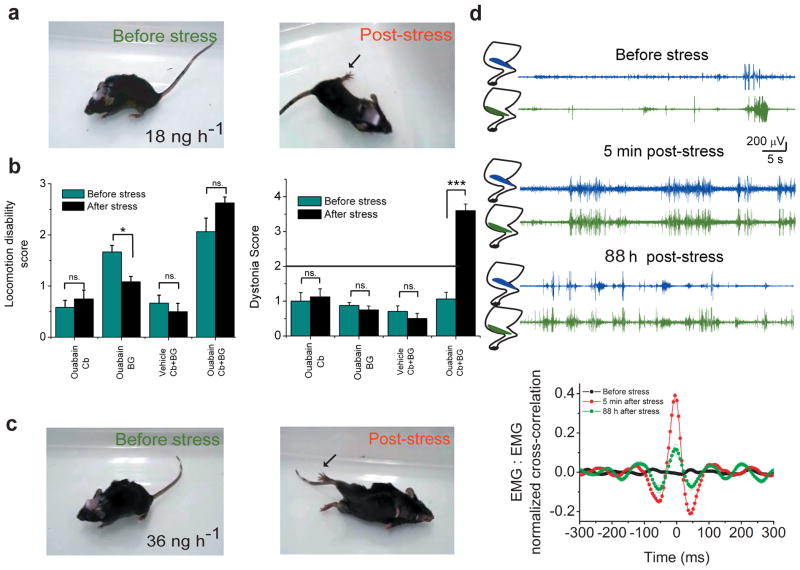
Figure 3. Stress-induced dystonia in mice requires interaction between cerebellar and basal ganglia motor control loops.
(a) Concomitant perfusion of 18 ng/h ouabain into the cerebellum and basal ganglia for 24 h produces reduced locomotion, and mild gait disturbance (before stress). Immediately after exposing the animal to severe stress the mouse developed persistent dystonic postures. Arrow points to hyperextension of posterior limb.
(b) The effect of stress on locomotion and dystonia in mice chronically perfused with 18 ng/h ouabain only in the cerebellum or basal ganglia, or concomitantly in both structures. Stress induces dystonia only in animals in which both the cerebellum and basal ganglia were concomitantly perfused with ouabain.
(c) EMGs recorded from agonist and antagonist anterior cranial tibial and gastrocnemius muscles in a mouse in which the cerebellum and basal ganglia were concurrently perfused with 18 ng/h ouabain for 24 h. Before exposure to stress, the mouse showed reduced locomotion but rarely co-contraction of the two muscles. Five minutes post-stress persistent co-contraction of the two muscles could be seen for several seconds. These co-contractions reduced in frequency and intensity, but nonetheless were notable even 3 days later. The graph at the bottom shows normalized cross-correlation of the two EMG signals (mean±s.e.m.). Significant cross-correlation in the activity of the two muscles was observed after, but not before stress. Note that the EMG traces used for analysis corresponded to times at which significant activity in at least one muscle was detected.
(d) More severe symptoms in the form of reduced locomotion, and gait disturbance/mild ataxia in a mouse concomitantly perfused with 36 ng/h ouabain into the cerebellum and basal ganglia for 5 h (before stress). Subjecting the mouse to the stress paradigm resulted in generalized dystonia (post stress) including the distortion of the lower jaw.
We also performed experiments in mice in which the cerebellum and basal ganglia were concomitantly perfused with a higher concentration of ouabain (36 ng/h; n=9). In these cases within 4–6 hours of perfusion the mice were subjected to the stress paradigm. Prior to the stressful episode the mice showed gait disturbance, mild ataxia, and reduced locomotion, but no detectable signs of dystonia. Immediately after exposure to the stress paradigm, the mice showed severe generalized dystonia (Figure 3c, Supplementary video 5). These symptoms did not show signs of improvement for the duration of the observation (in one case up to 30 hours).
Strikingly, in none of the mice examined when the cerebellum or basal ganglia was perfused in isolation with 18ng/hr ouabain (even for as long as 36 hours) did stress produce dystonia or significantly worsen the motor symptoms (n= 23 for cerebellum and 12 for basal ganglia; Figure 3B; Supplementary Videos 6 and 7). In some animals, the stress paradigm was repeated twice. Thus concomitant perfusion of both the cerebellum and the basal ganglia is absolutely required for stress to produce dystonia suggesting that an interaction between the two dysfunctional structures is essential for stress-induced dystonia in RDP. An adverse interaction between dysfunctional cerebellum and basal ganglia has been reported previously 19.
Dystonia is associated with aberrant cerebellar activity
The results presented demonstrate that concurrent perfusion of low concentrations of ouabain into the cerebellum and basal ganglia replicates the salient features of RDP; the mice have few motor symptoms prior to a stressful event and stress rapidly triggers persistent dystonia. Since perfusion of higher concentrations of ouabain into the cerebellum alone causes dystonia, we initially used this paradigm to explore the mechanism by which dysfunction of cerebellar sodium pumps can cause dystonia. Because several lines of evidence suggest that aberrant cerebellar activity can cause dystonia 16,20,21, we examined whether the dystonic postures in our animals were accompanied with abnormal cerebellar activity.
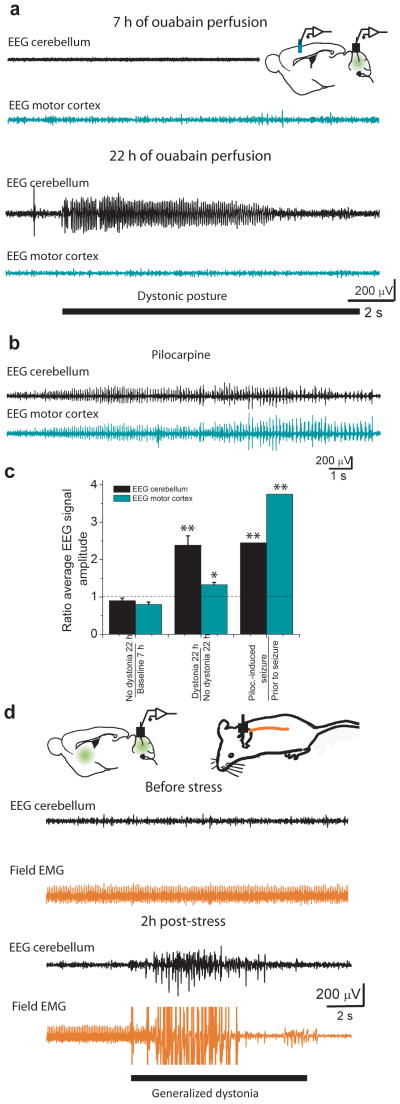
We first simultaneously recorded EEGs from the motor cortex and the cerebellum of mice in which the cerebellum alone was perfused with concentrations of ouabain that produced dystonia. In the absence of motor dysfunction EEGs from the cerebellum and motor cortex showed no sign of overt activity. However, once the animals developed intermittent dystonic postures we found a tight correlation between dystonic postures and abnormal cerebellar, but not cortical EEG activity (n=4; Figure 4a). During dystonic postures the mean cerebellar EEG signal amplitude increased by 138% compared to its average in the absence of dystonia (Figure 4c, P<0.001). In contrast, during the same dystonic episodes there was little hyperactivity present in the simultaneously recorded motor cortex EEG (Figure 4a) and on average its mean amplitude during dystonia was only ≈ 33% higher than its average in the absence of dystonia (Figures 4c, P=0.023). To ascertain accurate electrode positioning and to examine the relative sensitivities of the cortical and cerebellar EEG electrodes we induced status epilepticus in the same animals with pilocarpine. Both the cortical and cerebellar EEGs reported significant activity during epilepsy with the average cortical EEG signal amplitude increasing by more than 175% (Figure 4b,c, P<0.001). We next examined whether the stress-induced dystonia when both the cerebellum and basal ganglia were perfused with ouabain was also accompanied with abnormal cerebellar activity and in all cases found it to be so (n=3; Figures 4d).
Figure 4. Dystonic postures correlate with abnormal cerebellar activity.
(a) EEG recordings from the motor cortex and the cerebellum of a mouse whose cerebellum was chronically perfused with 36 ng/h ouabain. The first pair of traces (7 h perfusion) was obtained before any noticeable dystonic postures. Neither the cerebellar EEG (black) nor the motor cortex EEG (cyan) show abnormal activity. The second pair of traces (22 h perfusion) was recorded concurrent with a dystonic posture in the mouse and shows an abnormal cerebellar EEG signal whereas the motor cortex EEG was unremarkable.
(b) Abnormal electrical activity in both the cerebellum and the motor cortex of the same mouse during status epilepticus induced by 300 mg/kg pilocarpine.
(c) The ratios of average cerebellar and motor cortex EEG signal amplitude under various conditions. The average cerebellar EEG signal amplitude was about 2.5-fold larger when the animal manifested dystonic postures compared to time periods straddling dystonia. At exactly the same time periods, the corresponding change in the average motor cortex EEG signal amplitude was much smaller. Both the cerebellar and motor cortex EEG signal amplitudes increased substantially during pilocarpine-induced seizures. mean±s.e.m.
(d) EEG activity in the cerebellum (black) and field EMG signals from the back muscles (orange) were recorded in a mouse in which the cerebellum and basal ganglia were concomitantly perfused with 36 ng/h ouabain. The low impedance of the electrode used for field EMG recording permitted the detection of concerted activity of large group of muscle fibers as an accurate marker of the episodes of generalized dystonia. Prior to stress, the mouse showed mild ataxia/gait disturbance but no dystonic postures. The heart rate is noted in the field EMG with no apparent signs of abnormal activity. The cerebellar EEG signal also did not show any abnormal activity. Exposure of the same mouse to stress (the pair of traces labeled as 2 h post-stress) precipitated repeated dystonic postures, the timings of which were reflected in the field EMG signal. The cerebellar EEG showed abnormal hyperactivity of the cerebellum concurrent with the dystonic postures. In this instance the episode of generalized dystonia partly dislodged the field EMG wire which reduced the amplitude of the EKG to within noise levels.
Modest role of motor cortex in cerebellar-induced dystonia
The small increase in the amplitude of the cortical EEG signal during cerebellar-induced dystonic postures could, in part, be accounted for by feedback within the cortico-cerebellar and cortico-striatal loops and it is therefore plausible that motor cortex plays only a modest role in the generation of dystonia. To directly test this possibility we bilaterally silenced motor cortex by blocking voltage-gated sodium channels with TTX and examined the consequences for cerebellar-induced dystonia. The efficacy of TTX in silencing the motor cortex was ascertained by EEG recordings (Supplementary Data 5). In all 8 mice examined silencing the motor cortex made the animals flaccid, and reduced the occurrence of spontaneous dystonia. However, sometimes spontaneously, and more often with physical perturbation the mice showed clear dystonia although the severity was reduced (average dystonia score reduced from 3.7±0.1 to 2.8±0.3, P=0.041, n=8; Supplementary Video 8). These findings suggest that while cortical activity clearly contributes to the severity and frequency of cerebellar-induced dystonic postures, dystonia can nonetheless manifest in the absence of overt cortical activity.
Curbing aberrant cerebellar output alleviates dystonia
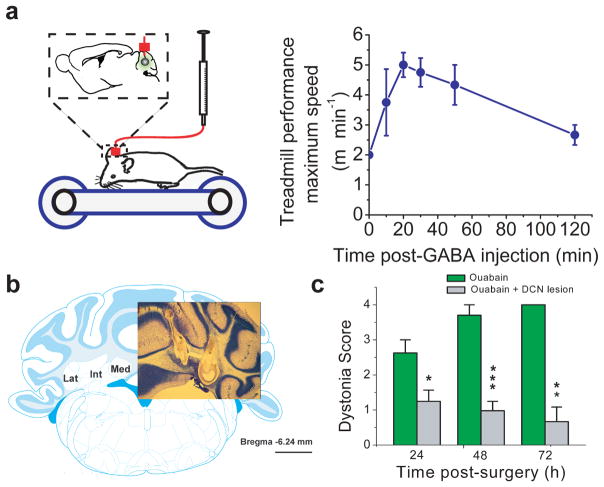
If ouabain-induced aberrant cerebellar activity is the main cause of dystonia then reducing aberrant activity or eliminating cerebellar output should lessen dystonia. The sodium pump is electrogenic and contributes to the resting membrane potential. We reasoned that the main cause of the aberrant cerebellar activity when sodium pumps were partially blocked was depolarization of cerebellar neurons. If this were the case, pharmacologically hyperpolarizing the cells with GABA should partially restore their activity to normalcy and thus one would predict that acute perfusion of GABA into the cerebellum of ouabain-perfused animals might lessen dystonia. To test this hypothesis we induced dystonia in mice by chronic cerebellar perfusion of ouabain using a canula which also permitted acute perfusions using a secondary port. Acute perfusion of GABA into the cerebellum of dystonic mice lessened the frequency and severity of dystonic postures and significantly improved their performance on a treadmill (n=4, Figure 5a, Supplementary Video 9).
Figure 5. Reducing aberrant cerebellar activity or silencing cerebellar output lessons dystonia.
(a) Mice whose cerebella were chronically perfused with 36 ng/h ouabain showed clear signs of dystonia and were unable to walk on a treadmill at its lowest speed setting of 2 m/min. Acute injection of GABA into their cerebellum using the auxiliary port of the same canula used for ouabain perfusion reduced the severity of their dystonic postures such that, on average, the mice could walk on the treadmill at a pace of 5 m/min. (n=4; mean±s.e.m.)
(b) Deep cerebellar nuclei (DCN) were electrically lesioned in both cerebellar hemispheres at either at one or two sites (the two site lesion is shown here). Comparable data was obtained with both approaches. Scale bar corresponds to 1 mm.
(c) Chronic perfusion of 36ng/h ouabain into the cerebellum of DCN-lesioned mice did not produce dystonia. (n=7; mean±s.e.m.)
We also examined whether silencing cerebellar output was capable of preventing cerebellar-induced dystonia. The majority of the cerebellar output is routed through the cerebellar nuclei. In agreement with our working hypothesis, we found that bilateral lesioning of cerebellar nuclei significantly reduced the severity of the dyskinesia produced by chronic perfusion of ouabain into the cerebellum (n=7, F=4.01; P=0.03, Figure 5b,c; Supplementary Video 10).
The data presented are consistent with the hypothesis that in RDP aberrant cerebellar activity makes a major contribution to generation of involuntary dystonic movements.
Role of CB→BG di-synaptic connection in cerebellar dystonia
Given the predominance of reports implicating basal ganglia as the primary instigator in most dystonias 1, it would be of value if a unifying hypothesis could be formulated to account for the potential contribution of each of these two brain regions, the basal ganglia and the cerebellum, in the induction of dystonia. In an attempt to put forth such a hypothesis we postulated that in cerebellar-induced dystonia the aberrant cerebellar activity may cause dystonia by dynamically forcing the dysfunction of the basal ganglia. This could be the case, for example, if the cerebellar output has a significant impact on the activity and function of the basal ganglia. In support of such a supposition it is reported that altering the activity of the cerebellar output nuclei alters the neuronal firing rates 22,23 and the dopamine levels in the basal ganglia 19,24. Indeed a direct substrate for an interaction between the cerebellum and the basal ganglia motor loops might be the di-synaptic pathways that connect the two structures 25,26. One approach to test this hypothesis would be to sever the link between the cerebellum and the basal ganglia: if the cerebellum induces dystonia by altering basal ganglia function then breaking the connection should alleviate dystonia. We sought to experimentally test this hypothesis.
One of the most prominent di-synaptic connections from the cerebellum to the basal ganglia is routed via the centrolateral (CL) nucleus of the thalamus 26. We optimized the parameters needed for selective electrical lesioning of CL (Figure 6a) and found that, remarkably, its selective ablation (confirmed by histology post-mortem) significantly reduced the motor symptoms associated with chronic perfusion of ouabain into the cerebellum (Figure 6b, n=14). The CL-lesioned animals in general did not show dystonia even when monitored for more than 90 hours after start of cerebellar ouabain perfusion (F=3.51, P=0.023, Figure 6b, Supplementary video 11). We found that if the lesions were off target and affected other thalamic nuclei the dystonia did not improve and in most cases it worsened. This suggests that the beneficial effects of CL lesions were not simply as the consequence of generalized thalamotomy which can sometimes improve dystonia 27. Based on these results, it is plausible that inactivation of the CL nucleus of the thalamus, or the output of the cerebellum, either by lesioning or perhaps by deep brain stimulation might constitute plausible therapeutic approaches for RDP patients. Indeed such an approach might be of value in other cerebellar-induced dystonias.
Figure 6. Severing the link between the cerebellum and basal ganglia alleviates dystonia.
(a) Using bilateral electrical lesions, the CL nucleus of the thalamus was selectively ablated. The photograph on the left shows Nissl stain of one such lesion, and the schematic on right shows the relevant brain structures with the lesion area marked in red. The scale bar corresponds to 1 mm. Legends correspond to: GP: Globus Palidus, CPu; Caudate/Putaman, and the following nuclei of the thalamus: CM: centrolmedian, CL: centrolateral, VL: ventral lateral, VP: ventral posterior, VM: ventral medial, and Pc: Paracentral.
(b) The consequences of CL lesions (or sham operations noted as “No CL lesion”) on the motor symptoms associated with chronic perfusion of 36 ng/h ouabain into the cerebellum of mice was determined by assessing their locomotion and dystonia scores. Lesioning the CL significantly reduced ouabain-induced motor dysfunction and prevented generation of dystonia.
Discussion
Dystonia is a movement disorder frequently accompanied with devastating symptoms. However, our understanding of its pathophysiology remains incomplete. A major handicap in dystonia research is the limited availability of animal models of identified human dystonia which faithfully mimic the dystonic symptoms experienced by patients 2,28. This shortcoming persists even in genetic mouse models where the engineered mice express the mutated genes that are known to be the cause of various hereditary dystonias 2. While these transgenic mice are informative 2,28 the need for animal models which actually show dystonia and mimic human symptoms cannot be overstated. In this context, RDP presents a challenge since the patients not only have dystonia, but also show Parkinsonism combined with a number of other motor symptoms. Moreover the symptoms are precipitated by an episode of severe stress.
Remarkably, the ouabain-based pharmacological animal model described here reproduces all the salient features of RDP. We show that when sodium pumps are partially blocked both in the cerebellum and in the basal ganglia the mice show mild signs of motor dysfunction. This condition mimics the mild symptoms seen in some subjects affected with the mutated RDP gene prior to experiencing severe stress. Moreover, in the model stress rapidly caused severe motor dysfunction including dystonia and Parkinsonism-like symptoms such as akinesia similar to that seen in RDP patients. Paralleling the human disorder, these symptoms outlasted the stress paradigm and persisted until the animals were euthanized. Thus, the results presented suggest that the ouabain-based model is a reasonable animal model of RDP, implicate the basal ganglia and cerebellum as the primary sites of dysfunction in this disorder, and offer potential therapeutic interventions for alleviating or lessening the severity of the motor symptoms in patients.
A role for both the basal ganglia and the cerebellum in RDP
Our data implicate two structures in the pathophysiology of RDP: the basal ganglia and the cerebellum. A role for the basal ganglia in RDP was not unexpected; RDP patients show Parkinsonism-like symptoms, a hallmark of basal ganglia dysfunction. Our data clearly corroborate the hypothesis that a reduction in the overall function of sodium pumps in the basal ganglia, as is likely to occur in RDP, may be the cause of the Parkinsonism-like symptoms seen in patients. Considering a wider context, the crouched posture, akinesia, and tremor seen in mice following partial blockade of basal ganglia sodium pumps closely resemble the actual symptoms of patients that suffer from Parkinsonism and provide an attractive animal model of this disorder.
What remains to be established is the mechanism by which partial dysfunction of sodium pumps in the basal ganglia causes Parkinsonism. One possibility is that reduced activity of sodium pumps might have affected presynaptic dopaminergic axons and nerve terminals. Because sodium pumps are electrogenic and contribute to maintenance of the membrane resting potential their dysfunction can depolarize nerve endings and render the axon incapable of supporting an action potential or liable to propagation failure 29. Alternatively, the symptoms may have been caused by reduced expression of the D1 dopamine receptors and altered phosphorylation levels which can occur with chronic ouabain perfusion 30,31. The reduced dopamine signaling in the basal ganglia caused by either of these mechanisms may account for the parkinsonism-like symptoms seen in our animal model and in RDP patients although this issue requires further scrutiny.
Our studies also implicate cerebellar dysfunction in RDP and suggest that it makes a major contribution to dystonia. There are a number of patient case reports that implicate cerebellar dysfunction in dystonia 32–34. Surgically lesioning the dentate cerebellar nuclei in some of these patients improved dystonia 32,33, suggesting that in these and some other cases 35–40 the cerebellum might have been a major contributing factor to dystonia. Indeed, abnormal cerebellar activity has been noted frequently in dystonic patients although these observations have routinely been interpreted in the context of cerebellar compensation of dysfunctional basal ganglia 17.
The evidence amassed in patients in support of a direct role of the cerebellum in some dystonias is also corroborated with observations in animal models of dystonia. Injection of Kainic acid into the cerebellum results in dystonia 16, and abnormal cerebellar activity has been implicated in several rodent strains afflicted with spontaneous mutations that render them dystonic 21,41–43. In some of these animals cerebellectomy, or Purkinje cell degeneration, has been shown to alleviate dystonia alas at the expense of producing ataxia 43,44. Thus, as summarized recently 17, it might be important and timely to re-evaluate the role of the cerebellum in dystonia in general.
The mechanisms by which partial dysfunction of cerebellar sodium pumps result in aberrant cerebellar activity are not understood. However, it is noteworthy that in contrast to most neurons that express a combination of different isoforms of the sodium pump, cerebellar Purkinje cells exclusively express the α3 isoform 18 (the isoform affected in RDP patients 4).Thus while neurons can upregulate other pump isoforms to compensate for a dysfunctional α3 protein, Purkinje cells lack this option. It is plausible that the outward current contributed by the sodium pumps might functionally be an integral part of pacemaking in these neurons. In the absence of any compensatory mechanisms, the reduction in the sodium pump current that occurs as a consequence of the RDP mutations may affect the activity of Purkinje cells and possibly cause the aberrant cerebellar activity reported here.
Interaction between the cerebellum and the basal ganglia
The observations reported here point toward an intricate interaction between the cerebellar and basal ganglia motor control systems. There is a strong body of anatomical and functional evidence in support of reciprocal interactions between these two structures. In the cat, electrical stimulation of the cerebellar output nuclei (dentate) alters the rate of firing of neurons in the caudate nucleus 22 and to a lesser extent, in globus pallidus 23. Electrical stimulation of the dentate nuclei was also shown to alter dopamine levels in both the substantia nigra and caudate of the cat 24. A significant change in dopamine levels in the basal ganglia of mice after acute cerebellar injection of kainic acid has also been noted 19. These observations are supported by anatomical studies that show the presence of direct and indirect projections from the cerebellum to the basal ganglia 25,26.
Stress-induced dystonia
An intriguing aspect of the work reported here is the ability to reproduce the stress-induced dystonia seen in RDP patients. At the present we do not understand why dysfunction of both the basal ganglia and the cerebellum is required for stress-induced dystonia, nor do we know the mechanism by which stress results in generation of permanent symptoms. Both of these questions require further examination. Speculatively, one potential mechanism by which stress might exacerbate partial dysfunction of sodium pumps is by increasing endo-ouabain levels in the brain. Endo-ouabain is produced by adrenal cortex and the hypothalamus and is indistinguishable from ouabain 45. Interestingly, stress and exercise increase endo-ouabain levels by as much as 18 fold 46,47. As delineated earlier we found that perfusion of higher concentrations of ouabain into the cerebellum and basal ganglia precipitate the symptoms seen in RDP in the absence of stress. Thus, by increasing the concentration of endo-ouabain, stress and activities commonly associated with triggers of RDP are likely to increase the fraction of “dysfunctional” sodium pumps in the cerebellum and the basal ganglia thus prompting the symptoms.
Limitations of our study
Our model suffers from a number of caveats one of which is the suitability of rodents as an accurate model of human neurological or behavioral disorders. In this context it is likely that motor control in quadrupeds is different from that of primates including humans. This concern, however, is balanced by the preceding discussions which highlight consistency of our findings with many prior observations in patients.
Another caveat is that in our studies we targeted a limited number of specific brain regions with a blocker that despite its selectivity for the α3 sodium pumps can nevertheless block other isoforms. In RDP, in contrast, only the α3 sodium pump isoform is dysfunctional and the mutated protein is expressed throughout the brain. At face value the best pharmacological approach might be to perfuse ouabain into the ventricles. However, our preliminary attempts corroborated the finding that perfusion of ouabain into the ventricles results in seizures 48,49, and using lower concentrations of ouabain we failed to detect any motor symptoms prior and post-stress. Because it is tremendously difficult to chronically perfuse two brain regions and simultaneously monitor EEG and EMG signals in mice it was not practical to survey additional brain regions within a reasonable timeframe. Despite this shortcoming, we believe that the choice of the structures examined were well justified. Nonetheless we look forward to the development of a genetic mouse model of RDP to reaffirm our findings. Obviously, a genetic RDP mouse model which shows a clear phenotype will be of immense value because in addition to allowing for confirmation of our findings it will provide a far less laborious model than ours for exploring potential therapeutic approaches for this disorder.
Potential implications for treatment of RDP
Two sets of observations made here have potential implications for treatment of RDP. First, our data suggest that lesions or deep brain stimulation of structures that mediate adverse interactions between the basal ganglia and the cerebellum might be of some therapeutic value. Second, our data also suggests that aberrant activity of the cerebellum might be a major contributing factor to dystonia in RDP. Accordingly, pharmacological approaches that restore cerebellar activity to normalcy, or lesions/deep brain stimulation of its output nuclei might also lessen associated symptoms in patients. Obviously both of these approaches require rigorous scrutiny.
A unifying hypothesis for the roles of cerebellum and basal ganglia in the generation of dystonia
Our observations which demonstrate that aberrant cerebellar activity can have an adverse effect on the basal ganglia via the thalamic di-synaptic pathways provide a plausible unifying working hypothesis to account for the role of each of these two structures in the generation of dystonia. Concurrently our findings underscore the importance of basal ganglia dysfunction in generation of dystonia even in cases where the primary instigator is elsewhere in the brain (in this case the cerebellum). It is thus plausible that aberrant activity of other structures that innervate the basal ganglia might also be capable of disrupting basal ganglia function and causing dystonia. Because a neuron can burst and fire erratically in the absence of any significant change in its average firing rate aberrant activity does not necessarily translate into hyper- or hypo-activity when assayed with imaging techniques. Therefore it might be fruitful to explore the potential dysfunction of basal ganglia input structures (such as the cerebellum) in other hereditary and idiopathic dystonias even in cases where imaging data have not specifically pointed to their hypo- or hyperactivity.
Methods
Experiments were performed on 8 to 10 week old C57/BL6 mice in accord with the guidelines set by Albert Einstein College of Medicine. The behavior of each animal before and after surgery, perfusion of drugs, or exposure to the stress paradigm was documented by video recordings. To minimize pain experienced by the animals, mice which exhibited severe dystonia or parkinsonism symptoms were only monitored for a few days and then euthanized. To lessen the pain of these animals, they were given the long lasting NSAID pain reliever Flunixin. It is important to note that in these instances we did not detect any symptoms aside form the movement disorders described suggesting that long term perfusion of ouabain with the methods employed here did not have nonspecific toxic effects on the CNS.
Chronic perfusion of the Basal ganglia
To chronically perfuse the basal ganglia, bilateral canula (Plastics One) were stereotaxically implanted (AP: 0.74 mm from bregma, ML:1.5 mm and DV: 4 mm), and were connected to two osmotic pumps (model 1007D, 0.25 μl/h, Alzet) which were placed under the skin on the back of the mice. The concentration of ouabain solution was set such that 7.2–82 ng ouabain was dispensed by the pump every hour. Ouabain was dissolved in water and 0.01% methylene blue was added to the solution to allow visual confirmation of the perfusion site post-mortem. Immediately after each surgical procedure, and every 12 hours thereafter Flunixin was administered subcutaneously.
Chronic perfusion of the Cerebellum
The cerebellum was perfused at the midline (AP: −6.90 mm from bregma and DV: 3 mm) following the procedures described above. For some experiments a perfusion canula with a second port was used to perfuse the cerebellum. This auxiliary port allowed for acute injection of drugs directly into the cerebellum. For acute injections 5 μl of the desired solution was injected over a period of 15 minutes using an automated pump.
Chronic perfusion of lateral ventricles
The lateral ventricles were perfused using a single canula with 360 ng/h ouabain using the coordinates (AP: −0.5 mm from bregma, ML:1.0 mm and DV: −1.6 mm).
Concurrent perfusion of the basal ganglia and the cerebellum
For concurrent perfusion of the basal ganglia and the cerebellum we used the same coordinates delineated above. The procedure for perfusion of the cerebellum was identical. However, because mice can maximally carry only two osmotic pumps, perfusion of the basal ganglia required the use of a “Y” bifurcation canula (Plastics One) which was connected to a single pump filled with double the concentration of ouabain used for cerebellum.
Acute injections of ouabain into the cerebellum and select basal ganglia nuclei
For acute injections, guide canula were stereotaxically implanted at the target location and 2.5 or 5 μl of the desired solution was injected over a period of 15 minutes using an automated pump. The same coordinate used for chronic perfusion were used in the acute injection of 5 μl of solution into the midline cerebellum. To target select basal ganglia output nuclei guide canula were bilaterally positioned and 2.5 μl of the solution was independently injected to each side using the following coordinates: the entopeduncular nucleus (AP: −1.34 mm, ML: 1.5 mm, DV: 4.5 mm), the globus pallidus (AP: −0.58 mm, ML: 1.9 mm, DV: 4 mm), and the substantia nigra (AP: −3.28 mm, ML: 1.5 mm, DV: 4.5 mm).
EMG and EEG recordings
For EMG recordings electrodes were surgically inserted into the gastronemius (extensor) and cranial tibial muscle (flexor) respectively. Thin Teflon-coated EMG wires were routed underneath the skin and connected to a connector secured on the skull. For field EMG recordings a wire underneath the skin on the back of the animal was positioned to terminate 2/3 of the way between the neck and the tail. The low impedance of the exposed end of the wire permitted pick up of electrical signals over relatively long distances. Additionally, also as a consequence of its very low impedance, the wire only registered large electrical changes and thus a detectable signal correlated with concerted activity of large groups of excitable elements (in this case mainly muscle fibers). This technique is analogous to extracellular field recordings in the CNS where a low impedance electrode is positioned above a region and registers concurrent activity of a large number of neurons.
To perform EEG recordings a bipolar electrode (MS 303; Plastics One) or a screw was stereotaxically implanted into the motor cortex using the coordinates: AP: −1.6mm from bregma, ML: 1mm and DV: 1mm. To record the cerebellar EEG, or in experiments where motor cortex EEG was recorded while TTX was injected into the cortex, the same canula used to deliver ouabain into the cerebellum or TTX into the cortex was used as the EEG electrode (the canula is conductive throughout its length). EEG, EMG and field EMG signals were monitored by attaching the headstage of a Pinnacle Technology EEG/EMG recording system (4100 USB Data acquisition and conditioning system) to the connection platform secured on the skull just prior to the recording session. In all cases the segments of data used for analysis contained at least one channel in which significant muscle activity was evident. To calculate cross correlation signals were normalized to their respective standard deviation such that two identical input signals or signals which are scaled versions of one another yielded a cross correlation value of 1 50.
To induce seizures animals were injected subcutaneously with 300 mg/kg pilocarpine hydrochloride (Sigma). Methyl scopolamine nitrate (Sigma) 1mg/kg was injected subcutaneously 30 minutes prior to pilocarpine to minimize peripheral cholinergic effects.
Stress paradigm
Electric foot shocks were delivered via the grid floor of a custom made plastic box (30 cm × 22 cm × 30 cm). Electric shocks of 250 ms duration (6 mA maximal limiting current) were applied randomly at 10–60 sec intervals over a two hour-period and under an elevated temperature of 38–40 °C. Right before and after the stress paradigm animals were video taped and EMGs and EEGs were recorded.
Locomotion disability score
Locomotion disability was quantified using a scale based on the unified Parkinson’s disease rating scale in humans (UPRDS), but modified for rodents 13. With this adapted scale, 0= Normal motor behavior, 1= Slightly slow movements, 2= Limited and slow ambulation even when persistently disturbed, but disturbance rarely resulted in the animal losing balance and falling, and 3= No ambulation even when repeatedly disturbed with the animal usually losing balance and falling after being disturbed.
The assessment of the severity of locomotion disability (and the Dystonia rating described in the next section) was made independently by four members of the laboratory (none of the authors). All four colleagues were first “trained”: they were shown a set of exactly the same training videos from mice with movement disorders, asked to identify specific abnormal movement features, and told what score each behavior should receive. Then, the same four colleagues reviewed and scored all the mice reported in the present study by viewing video clips of individual mice. All four reviewers saw exactly the same video clips. Moreover, all four reviewers were blind to the procedures done. Their scores was averaged and decoded by the authors.
Dystonia rating scale
The presence and severity of ouabain-induced dystonia in mice was quantified using a modification of a previously published scale 16 where 0= normal motor behavior, 1= abnormal motor behavior, no dystonic postures, 2= Mild motor impairment; dystonic-like postures when disturbed, 3= Moderate impairment; frequent spontaneous dystonic postures. 4= Severe impairment; sustained dystonic postures. As described above, the scores given by four colleagues who were blind to the treatment that each mouse had received were averaged.
Number of steps
We quantified the number of steps taken with the forelimb during a 30 s trial 14. The analysis was done by two observers which were blind to the treatment that each animal had received.
Average step size
We quantified the average step size by measuring the distance between the toe of the posterior limb and the hill of the forelimb.
Open field test
Mice were placed into an arena of (50 cm length × 35 cm width × 25 cm height) for at least 5 minutes to monitor spontaneous locomotor activity. The Viewer2 software (Biobserve) was used to calculate the speed of animals.
Treadmill
Mice were individually placed on a treadmill which was advanced at a constant rate starting from 2 m/min. The maximum speed that the mice could walk on the treadmill for at least 30 s was noted.
Estimation of ouabain concentration in tissue
To estimate the concentration of ouabain in the tissue a fluorescent analogue of ouabain, Bodipy-FL-ouabain (Molecular Probes), was perfused into the brain instead of ouabain. The fluorescently tagged ouabain, Bodipy-FL-ouabain, is active and clear symptoms were observed in the mice used for these experiments. Examination of the perfused tissue confirmed that the perfusion site could be well described by a sphere centered on the tip of the canula. The intensity profile of bodipy-FL-oubain was quantified using IP Lab software and was then used to calculate the concentration of ouabain as a function of lateral distance from the center of the canula. To estimate the concentration of ouabain, the total amount of ouabain delivered during the perfusion period was adjusted for its clearance in the brain. Due to lack of data, we conservatively used a τ½ of 60 min based on the fact that after an acute increase in the concentration of brain endo-ouabain its levels return to baseline in less than one hour. The actual τ½ is likely to be much less than 60 minutes. Because each molecule of ouabain is conjugated with a fluorescent tag, the total ouabain was taken to be distributed within the tissue based on the intensity profile of bodipy-FL-oubain. Based on this distribution profile, the concentration of ouabain was calculated for concentric spherical shells by dividing its amount in each shell by the volume of each shell. Given the value used for the rate of breakdown of ouabain in the brain the concentration of ouabain is likely to be significantly over-estimated.
Electrical Lesion of the CL nucleus
To bilaterally lesion the CL, an electrode was sequentially positioned at AP: −1.45 mm, ML: −0.8 mm, and DV 3.5 mm and 300 μA current pulses of 45 s duration were delivered to each side. As a control, the same surgery was done, the electrode was lowered in place, but no current was delivered. The extent and location of lesions were determined histologically using Nissl staining. Only animals in which the location and specificity of the lesion for CL were histologically confirmed were included in the data.
Electrical lesion of the cerebellar nuclei and subsequent perfusion of ouabain into the cerebellum
Lesions of the cerebellar nuclei were performed by one of either two methods. Comparable results were obtained with both approaches. With the first method, a single lesion was bilaterally at AP: −6.24 mm, ML: 1.37 mm, and DV: 3.00 mm using a 45 second long, 0.75 mA current at each site. In the second method a total of 4 lesions (two lesions bilaterally) were made using a 45 second, 0.50 mA current at AP: −6.24 mm, ML: 1.15 mm and 1.75, and DV: 3.00 mm on each side. After lesions, many mice exhibited signs of severe ataxia. In time, however, the mice recovered such that little overt motor symptoms were discernable (analogues to published experiments in which the entire cerebellum is removed). Approximately 1 week post lesion surgery, once the gross motor behavior of mice returned to normalcy, the cerebellum of was chronically perfused with 36 ng/h ouabain as described earlier.
Statistical analysis
We used a “mixed-design analysis of variance” model to analyze changes in akinesia and dystonia using the REML estimation method of JMP software (version 8.0.1; SAS software). The statistical analysis scrutinized differences in animals perfused with different ouabain concentrations (0–72ng/h), and provided repeated measures of these variables at times 4–72 h post-drug perfusion. In this mixed effects model, one factor is between-subjects variable (or fixed effect) and the other is within-subjects variable (or random effect). This analysis was followed by individual ANOVAs at each time point and a posthoc Dunnet’s t-test the results of which are reported in the figures shown. Thus, data were considered not to be statistically different (ns) from vehicle if P>0.05. Subsequently, if deemed statistically significant, in all figures “*” is used to denote P<0.05; “**” to denote P<0.01; and “***” to denote P<0.001. Throughout the manuscript, data are presented as Mean±S.E.M. For other cases, when appropriate, we used a paired t test.
Supplementary Material
Acknowledgments
We thank the members of Khodakhah lab for invaluable discussions and comments on the manuscript, and for evaluating and scoring motor dysfunction. We also thank Drs. Dominick Purpura, Roy Sillitoe and Stacey Reeber for help and advice.
Reference List
- 1.Stacy MA. Handbook of Dystonia. Informa Health care; New York: 2006. [Google Scholar]
- 2.Breakefield XO, et al. The pathophysiological basis of dystonias. Nat Rev Neurosci. 2008;9:222–234. doi: 10.1038/nrn2337. [DOI] [PubMed] [Google Scholar]
- 3.Muller U. The monogenic primary dystonias. Brain. 2009;132:2005–2025. doi: 10.1093/brain/awp172. [DOI] [PubMed] [Google Scholar]
- 4.De Carvalho AP, et al. Mutations in the Na+/K+-ATPase alpha3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron. 2004;43:169–175. doi: 10.1016/j.neuron.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 5.Brashear A, et al. The phenotypic spectrum of rapid-onset dystonia-parkinsonism (RDP) and mutations in the ATP1A3 gene. Brain. 2007;130:828–835. doi: 10.1093/brain/awl340. [DOI] [PubMed] [Google Scholar]
- 6.Tanabe LM, Kim CE, Alagem N, Dauer WT. Primary dystonia: molecules and mechanisms. Nat Rev Neurol. 2009;5:598–609. doi: 10.1038/nrneurol.2009.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moseley AE, et al. Deficiency in Na, K-ATPase alpha isoform genes alters spatial learning, motor activity, and anxiety in mice. J Neurosci. 2007;27:616–626. doi: 10.1523/JNEUROSCI.4464-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clapcote SJ, et al. Mutation I810N in the alpha3 isoform of Na+,K+-ATPase causes impairments in the sodium pump and hyperexcitability in the CNS. Proc Natl Acad Sci U S A. 2009;106:14085–14090. doi: 10.1073/pnas.0904817106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deandrade MP, Yokoi F, van Groen T, Lingrel JB, Li Y. Characterization of Atp1a3 mutant mice as a model of rapid-onset dystonia with parkinsonism. Behav Brain Res. 2010 doi: 10.1016/j.bbr.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allen JC, Lindenmayer GE, Schwartz A. An allosteric explanation for ouabain-induced time-dependent inhibition of sodium, potassium-adenosine triphosphatase. Arch Biochem Biophys. 1970;141:322–328. doi: 10.1016/0003-9861(70)90138-4. [DOI] [PubMed] [Google Scholar]
- 11.Urayama O, Sweadner KJ. Ouabain sensitivity of the alpha 3 isozyme of rat Na, K-ATPase. Biochem Biophys Res Commun. 1988;156:796–800. doi: 10.1016/s0006-291x(88)80914-8. [DOI] [PubMed] [Google Scholar]
- 12.Jankovic J. Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79:368–376. doi: 10.1136/jnnp.2007.131045. [DOI] [PubMed] [Google Scholar]
- 13.Fahn S, Elton RL. Recent developments in Parkinsons disease. 1987. [Google Scholar]
- 14.Sotnikova TD, et al. Dopamine-independent locomotor actions of amphetamines in a novel acute mouse model of Parkinson disease. PLoS Biol. 2005;3:e271. doi: 10.1371/journal.pbio.0030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lepoutre AC, et al. A specific clinical pattern of camptocormia in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2006;77:1229–1234. doi: 10.1136/jnnp.2005.083998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pizoli CE, Jinnah HA, Billingsley ML, Hess EJ. Abnormal cerebellar signaling induces dystonia in mice. J Neurosci. 2002;22:7825–7833. doi: 10.1523/JNEUROSCI.22-17-07825.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jinnah HA, Hess EJ. A new twist on the anatomy of dystonia: the basal ganglia and the cerebellum? Neurology. 2006;67:1740–1741. doi: 10.1212/01.wnl.0000246112.19504.61. [DOI] [PubMed] [Google Scholar]
- 18.McGrail KM, Phillips JM, Sweadner KJ. Immunofluorescent localization of three Na, K-ATPase isozymes in the rat central nervous system: both neurons and glia can express more than one Na, K-ATPase. J Neurosci. 1991;11:381–391. doi: 10.1523/JNEUROSCI.11-02-00381.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neychev VK, Fan X, Mitev VI, Hess EJ, Jinnah HA. The basal ganglia and cerebellum interact in the expression of dystonic movement. Brain. 2008;131:2499–2509. doi: 10.1093/brain/awn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown LL, Lorden JF. Regional cerebral glucose utilization reveals widespread abnormalities in the motor system of the rat mutant dystonic. J Neurosci. 1989;9:4033–4041. doi: 10.1523/JNEUROSCI.09-11-04033.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Richter A, Brotchie JM, Crossman AR, Loscher W. [3H]-2-deoxyglucose uptake study in mutant dystonic hamsters: abnormalities in discrete brain regions of the motor system. Mov Disord. 1998;13:718–725. doi: 10.1002/mds.870130419. [DOI] [PubMed] [Google Scholar]
- 22.Ratcheson RA, Li CL. Effect of dentate stimulation on neuronal activity in the caudate nucleus. Exp Neurol. 1969;25:268–281. doi: 10.1016/0014-4886(69)90050-8. [DOI] [PubMed] [Google Scholar]
- 23.Li CL, Parker LO. Effect of dentate stimulation on neuronal activity in the globus pallidus. Exp Neurol. 1969;24:298–309. doi: 10.1016/0014-4886(69)90023-5. [DOI] [PubMed] [Google Scholar]
- 24.Nieoullon A, Cheramy A, Glowinski J. Release of dopamine in both caudate nuclei and both substantia nigrae in response to unilateral stimulation of cerebellar nuclei in the cat. Brain Res. 1978;148:143–152. doi: 10.1016/0006-8993(78)90384-0. [DOI] [PubMed] [Google Scholar]
- 25.Hoshi E, Tremblay L, Feger J, Carras PL, Strick PL. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8:1491–1493. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- 26.Ichinohe N, Mori F, Shoumura K. A di-synaptic projection from the lateral cerebellar nucleus to the laterodorsal part of the striatum via the central lateral nucleus of the thalamus in the rat. Brain Res. 2000;880:191–197. doi: 10.1016/s0006-8993(00)02744-x. [DOI] [PubMed] [Google Scholar]
- 27.Andrew J, Fowler CJ, Harrison MJ. Stereotaxic thalamotomy in 55 cases of dystonia. Brain. 1983;106 ( Pt 4):981–1000. doi: 10.1093/brain/106.4.981. [DOI] [PubMed] [Google Scholar]
- 28.Jinnah HA, et al. Rodent models for dystonia research: characteristics, evaluation, and utility. Mov Disord. 2005;20:283–292. doi: 10.1002/mds.20364. [DOI] [PubMed] [Google Scholar]
- 29.Kim JH, Sizov I, Dobretsov M, von Gersdorff H. Presynaptic Ca2+ buffers control the strength of a fast post-tetanic hyperpolarization mediated by the alpha3 Na(+)/K(+)-ATPase. Nat Neurosci. 2007;10:196–205. doi: 10.1038/nn1839. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Y, Yuan Z, Ge H, Ren Y. Effects of long-term ouabain treatment on blood pressure, sodium excretion, and renal dopamine D(1) receptor levels in rats. J Comp Physiol B. 2010;180:117–124. doi: 10.1007/s00360-009-0391-z. [DOI] [PubMed] [Google Scholar]
- 31.Zhang YR, Yuan ZY. Dopamine-mediated inhibition of renal Na+/K+-ATPase in HK-2 cells is reduced by ouabain. Clin Exp Pharmacol Physiol. 2010;37:613–618. doi: 10.1111/j.1440-1681.2010.05364.x. [DOI] [PubMed] [Google Scholar]
- 32.Fraioli B, Guidetti Effects of stereotactic lesions of the dentate nucleus of the cerebellum in man. Appl Neurophysiol. 1975;38:81–90. doi: 10.1159/000102647. [DOI] [PubMed] [Google Scholar]
- 33.Zervas NT, Horner FA, Pickren KS. The treatment of dyskinesia by stereotxic dentatectomy. Confin Neurol. 1967;29:93–100. doi: 10.1159/000103685. [DOI] [PubMed] [Google Scholar]
- 34.Heimburger RF. Dentatectomy in the treatment of dyskinetic disorders. Confin Neurol. 1967;29:101–106. doi: 10.1159/000103686. [DOI] [PubMed] [Google Scholar]
- 35.Rousseaux M, et al. Dystonia and tremor in bilateral lesion of the posterior mesencephalon and the vermis. Rev Neurol (Paris) 1996;152:732–737. [PubMed] [Google Scholar]
- 36.Yoon JH, Lee PH, Yoon SN. Subtraction brain SPECT imaging in a patient with paroxysmal exercise-induced dystonia: role of the primary somatosensory cortex. Arch Neurol. 2007;64:1652–1656. doi: 10.1001/archneur.64.11.1652. [DOI] [PubMed] [Google Scholar]
- 37.Le BI, et al. Predominant dystonia with marked cerebellar atrophy: a rare phenotype in familial dystonia. Neurology. 2006;67:1769–1773. doi: 10.1212/01.wnl.0000244484.60489.50. [DOI] [PubMed] [Google Scholar]
- 38.Zadro I, Brinar VV, Barun B, Ozretic D, Habek M. Cervical dystonia due to cerebellar stroke. Mov Disord. 2008;23:919–920. doi: 10.1002/mds.21981. [DOI] [PubMed] [Google Scholar]
- 39.Rumbach L, Barth P, Costaz A, Mas J. Hemidystonia consequent upon ipsilateral vertebral artery occlusion and cerebellar infarction. Mov Disord. 1995;10:522–525. doi: 10.1002/mds.870100424. [DOI] [PubMed] [Google Scholar]
- 40.Manto MU. The wide spectrum of spinocerebellar ataxias (SCAs) Cerebellum. 2005;4:2–6. doi: 10.1080/14734220510007914. [DOI] [PubMed] [Google Scholar]
- 41.LeDoux MS, Lorden JF. Abnormal cerebellar output in the genetically dystonic rat. Adv Neurol. 1998;78:63–78. [PubMed] [Google Scholar]
- 42.Lorden JF, Lutes J, Michela VL, Ervin J. Abnormal cerebellar output in rats with an inherited movement disorder. Exp Neurol. 1992;118:95–104. doi: 10.1016/0014-4886(92)90026-m. [DOI] [PubMed] [Google Scholar]
- 43.Campbell DB, North JB, Hess EJ. Tottering mouse motor dysfunction is abolished on the Purkinje cell degeneration (pcd) mutant background. Exp Neurol. 1999;160:268–278. doi: 10.1006/exnr.1999.7171. [DOI] [PubMed] [Google Scholar]
- 44.LeDoux MS, Lorden JF, Ervin JM. Cerebellectomy eliminates the motor syndrome of the genetically dystonic rat. Exp Neurol. 1993;120:302–310. doi: 10.1006/exnr.1993.1064. [DOI] [PubMed] [Google Scholar]
- 45.Bagrov AY, Shapiro JI. Endogenous digitalis: pathophysiologic roles and therapeutic applications. Nat Clin Pract Nephrol. 2008;4:378–392. doi: 10.1038/ncpneph0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weidemann H, et al. Diverse effects of stress and additional adrenocorticotropic hormone on digitalis-like compounds in normal and nude mice. J Neuroendocrinol. 2004;16:458–463. doi: 10.1111/j.1365-2826.2004.01181.x. [DOI] [PubMed] [Google Scholar]
- 47.Bauer N, et al. Ouabain-like compound changes rapidly on physical exercise in humans and dogs: effects of beta-blockade and angiotensin-converting enzyme inhibition. Hypertension. 2005;45:1024–1028. doi: 10.1161/01.HYP.0000165024.47728.f7. [DOI] [PubMed] [Google Scholar]
- 48.Ruktanonchai DJ, El Mallakh RS, Li R, Levy RS. Persistent hyperactivity following a single intracerebroventricular dose of ouabain. Physiol Behav. 1998;63:403–406. doi: 10.1016/s0031-9384(97)00457-5. [DOI] [PubMed] [Google Scholar]
- 49.Donaldson J, Minnich JL, Barbeau A. Ouabain-induced seizures in rats: regional and subcellular localization of 3 H-ouabain associated with Na +-K +-ATPase in brain. Can J Biochem. 1972;50:888–896. doi: 10.1139/o72-124. [DOI] [PubMed] [Google Scholar]
- 50.Lampl I, Reichova I, Ferster D. Synchronous membrane potential fluctuations in neurons of the cat visual cortex. Neuron. 1999;22:361–374. doi: 10.1016/s0896-6273(00)81096-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.